Abstract
Background
The objective of this study was to investigate whether lung cancer screening low-dose computed tomography (LDCT) can be used to identify features associated with increased risk of hospitalization during the subsequent year.
Methods
Patients who underwent lung cancer screening between 2015 and 2020 with at least 1-year follow-up were identified. Patient charts were examined and LDCT scans were analyzed using body segmentation software to identify characteristics potentially associated with frailty and injury. Hospitalization was defined as an admission >48 hours within 1 year of the LDCT scan; admissions for elective procedures were excluded.
Results
There were 1606 LDCT scans that met inclusion criteria. The cohort median age was 65 years (interquartile range, 61-70 years), with 54% (875/1606) female, 50% (804/1606) current smokers, and median smoking history of 40 pack-years (interquartile range, 34-50 pack-years). There were 107 hospitalizations within 1 year of the LDCT scan. On univariate analysis, cardiomegaly (odds ratio [OR], 2.83; 95% CI, 1.33-6.04; P < .01), emphysema (OR, 1.67; 95% CI, 1.09-2.56; P = .02), pulmonary artery enlargement (OR, 2.72; 95% CI, 1.09-6.62; P = .03), and coronary artery calcification (OR, 1.59; 95% CI, 1.07-2.41; P = .02) were associated with increased risk of hospitalization. On multivariate analysis, after controlling for age and sex, cardiomegaly (OR, 2.41; 95% CI, 1.05-4.97; P = .03), emphysema (OR, 1.88; 95% CI, 1.19-2.93; P < .01), and body mass index >30 kg/m2 (OR, 1.55; 95% CI, 1.02-2.36; P = .04) were associated with increased risk of hospitalization.
Conclusions
In lung cancer screening patients, features extractable from LDCT scans are associated with increased risk of hospitalizations during the subsequent year.
In Short.
-
▪
Low-dose computed tomography scans can provide additional clinically relevant health information outside of early cancer detection.
-
▪
Features extracted from lung cancer screening low-dose computed tomography scans are associated with hospitalizations.
-
▪
Lung cancer screening may offer the ability to prognosticate specific health outcomes as annual screening is currently recommended.
As the leading cause of cancer-related death worldwide, lung cancer accounted for approximately 133,000 cancer deaths in 2022 in the United States.1 Low-dose computed tomography (LDCT) lung cancer screening has been shown to identify early-stage lung cancers and to reduce lung cancer death2; however, the potential for LDCT scans to provide additional health-related information remains largely unexplored. Understanding the potential added utility of LDCT may help with overall screening uptake and retention.
In the United States, there are an estimated 9 to 10 million patients who are eligible for lung cancer screening based on US Preventive Services Task Force guidelines, whereas estimates suggest that only 5% to 20% of patients are screened.3 Individuals who qualify for lung cancer screening often have comorbidities that result in increased health care system utilization and cost. Cardiovascular disease and chronic obstructive pulmonary disease (COPD) are the most commonly observed comorbidities in this population. In 2020, an estimated $49 billion was spent on medical treatments for COPD alone, with 45% to 50% of that cost coming directly from hospitalization.4 Additional comorbidities frequently found in this population of patients include interstitial lung disease, diabetes, and renal insufficiency, which complicates the management of these patients and increases their health care utilization.4
Studies have assessed the ability of LDCT to quantify sarcopenia, coronary artery calcification (CAC), emphysema, and bone mineral density (BMD).5,6 We hypothesize that radiographic features extractable from LDCT scans can be associated with annual risk of hospitalization. This knowledge could potentially facilitate early intervention and measures to decrease hospitalizations, to reduce health care expenditure, and to improve overall lung cancer screening utilization.
Material and Methods
Study Population
Patients who underwent lung cancer screening between 2015 and 2020 with at least 1-year follow-up were identified from an institutional database. All patients met the 2013 lung cancer screening guidelines established by the US Preventive Services Task Force, which included patients who were 55 to 80 years old, patients who were current smokers or had quit in the past 15 years, and patients who had at least a 30 pack-year smoking history. Exclusion criteria included patients who were lost to follow-up and those whose LDCT scans had low image resolution resulting in an incomplete data set. All data were deidentified and stored in an encrypted database.
Variables
Covariates were selected for analysis on the basis of prior studies suggesting an association with hospitalization or frailty.5,7 All variables were defined in accordance with the Fleischner Society, Society of Thoracic Radiology, American College of Radiology, and Society of Cardiovascular Computed Tomography guidelines. The independent variables selected for analysis were age, sex, cardiomegaly, pulmonary artery diameter, skeletal muscle index (SMI), body mass index (BMI), BMD, emphysema, and CAC. Patient data were collected using the electronic medical record, radiologic diagnostic viewer (GE Centricity PACS Radiology RA1000 Workstation), and image segmentation analysis software (TomoVision sliceOmatic 5.0 rev 17.0).
Cardiomegaly was defined as a cardiothoracic ratio >0.5 calculated by dividing the maximal cardiac width by the maximal thoracic width at the same level. Pulmonary artery enlargement was determined by the ratio of the pulmonary artery to aortic diameter >1. The image segmentation software allowed skeletal muscle quantification using the standard tissue attenuation range for muscle (–29 HU to +150 HU) and collected cross-sectional area of muscle tissue. SMI was calculated from cross-sectional area of skeletal muscle at T4.8 Sarcopenia was defined by SMI cutoff values of <41 cm2/m2 for women, <43 cm2/m2 for men with BMI <25 kg/m2, and <53 cm2/m2 for men with BMI ≥25 kg/m2.7 BMI was dichotomized as ≤30 kg/m2 or >30 kg/m2, consistent with the National Institutes of Health and World Health Organization definition of obesity class I. The threshold for normal BMD established by the American College of Radiology as >120 mg/cm3 was assessed with the diagnostic viewer at the T11 or T12 level, depending on the lowest fully viewable vertebra in the sagittal plane.9 LDCT quantification of BMD was conducted through use of an elliptical region of interest located in the trabecular portion of the vertebral body.5 Severity of emphysema and CAC was classified as none, mild, moderate, or severe on the basis of the criteria detailed in Table 1. Emphysema and CAC were dichotomized as moderate or severe (score of 2 or 3) vs mild or none (score of 1 or 0). The dependent variable, hospitalization, was defined as an admission ≥48 hours within 1 year of the LDCT, which was documented in the electronic medical record at the primary institution or within network; admissions for elective procedures and any admission directly related to lung cancer or screening were excluded. Hospitalizations <48 hours were excluded with the purpose of filtering out single-day admissions that may be less likely to reflect significant adverse health events that could be assessed by LDCT. All data that required interpretation of imaging were verified by a thoracic radiologist.
Table 1.
Stratification for Coronary Artery Calcification and Emphysema
| Severity | Coronary Artery Calcium | Emphysema |
|---|---|---|
| None–0 | Agatston score of 0 | No evidence of emphysema |
| Mild–1 | Agatston score of 1-99 | Emphysema that composes 0.5%-5.0% of the lung zone |
| Moderate–2 | Agatston score of 100-299 | Emphysema that composes >5% of the lung zone |
| Severe–3 | Agatston score of >300 | Advanced destructive emphysema with vascular distortion |
Data were derived from10.
Statistical Analysis
Univariate and multivariate logistic regression analyses were used to identify clinical and radiographic features associated with hospitalizations. Statistical calculations were performed with R (version 4.1) statistical software (R Foundation for Statistical Computing).
Results
In total, 1871 lung cancer screening scans were performed between 2015 and 2020. Of those, 1766 of 1871 (94%) met inclusion criteria and 160 of 1766 (9%) were excluded from analysis because of poor LDCT imaging quality. Overall, 1606 LDCT scans from 1056 unique patients were included in the analysis. Because lung cancer screening is performed annually, some patients had multiple screening scans. Each scan was analyzed to synthesize body composition data and correlated to recorded hospitalizations for the year in which the screening was obtained; thus, a single patient could contribute multiple unique data sets.
The cohort had a median age of 65 years (interquartile range, 61-70 years; Table 2). There was a slight predominance of women at 54% (875/1606). The median smoking history was 40 pack-years, and 50% of the cohort (804/1606) were current smokers. During the study period, 107 hospitalizations (6.7% of scans) occurred within 1 year of the LDCT imaging date. The most common causes of hospitalization were related to pulmonary disease (COPD exacerbation, pneumonia, COVID-19 [28/107]) and cardiovascular diseases (congestive heart failure, acute coronary syndrome, peripheral artery disease [27/107]; Table 3). On univariate analysis, cardiomegaly (odds ratio [OR], 2.83; 95% CI, 1.33-6.04; P < .01), severe emphysema (OR, 1.67; 95% CI, 1.09-2.56; P = .02), pulmonary artery enlargement (OR, 2.72; 95% CI, 1.09-6.62; P = .03), and severe CAC (OR, 1.59; 95% CI, 1.07-2.41; P = .02) were associated with increased risk of hospitalization (Table 4). After controlling for age and sex on multivariate analysis, cardiomegaly (OR, 2.41; 95% CI, 1.05-4.97; P = .03), severe emphysema (OR, 1.88; 95% CI, 1.19-2.93; P < .01), and BMI >30 kg/m2 (OR, 1.55; 95% CI, 1.02-2.36; P = .04) were independently associated with increased risk of hospitalization during the subsequent year.
Table 2.
Demographic Characteristics of Study Cohort
| Demographics | Value |
|---|---|
| No. of scans | 1606 |
| Age, y | 65 (61-70) |
| Female (% total) | 875 (54) |
| Race | |
| Black or African American (% total) | 558 (35) |
| White (% total) | 951 (59) |
| Other (% total) | 97 (6) |
| Current smoker (% total) | 804 (50) |
| Pack-years | 40 (34-50) |
Categorical variables are presented as number (percentage). Continuous variables are presented as median (interquartile range).
Table 3.
Number of Hospitalizations by Cause
| Cause | No. of Hospitalizations |
|---|---|
| Pulmonary | 28 |
| Cardiovascular | 27 |
| Gastrointestinal | 12 |
| Infection | 11 |
| Renal | 7 |
| Musculoskeletal | 6 |
| Endocrine | 5 |
| Genitourinary | 5 |
| Neurologic | 4 |
| Other | 2 |
Table 4.
Univariate and Multivariate Associations With Hospitalizations (n = 1606)
| Variable | Unadjusted Odds Ratio (95% CI) | P Value | Adjusted Odds Ratio (95% CI) | P Value |
|---|---|---|---|---|
| Age | 1.01 (0.98-1.03) | .38 | 1.10 (0.72-1.67) | .66 |
| Male | 1.51 (1.01-2.25) | .04 | 1.66 (1.08-2.55) | .02 |
| Cardiomegaly | 2.83 (1.33-6.04) | <.01 | 2.41 (1.05-4.97) | .03 |
| Sarcopenia | 1.18 (0.78-1.80) | .46 | 1.29 (0.83-1.99) | .26 |
| Emphysema | 1.67 (1.09-2.56) | .02 | 1.88 (1.19-2.93) | <.01 |
| Body mass index >30 kg/m2 | 1.31 (0.87-1.96) | .19 | 1.55 (1.02-2.36) | .04 |
| Pulmonary artery enlargement | 2.72 (1.09-6.62) | .03 | 2.03 (0.73-4.83) | .14 |
| Bone mineral density >120 mg/cm3 | 0.90 (0.55-1.48) | .70 | 0.97 (0.60-1.64) | .91 |
| Coronary artery calcifications | 1.59 (1.07-2.41) | .02 | 1.34 (0.88-2.04) | .18 |
Boldface P values represent statistical significance.
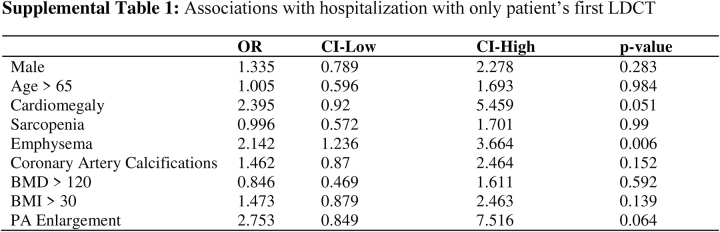
As a sensitivity analysis, only the first LDCT scan obtained by each patient was examined, with emphysema (OR, 2.14; 95% CI, 1.24-3.67; P < .01) remaining significant, whereas BMI <30 kg/m2 (OR, 1.47; 95% CI, 0.88-2.46; P = .14) and cardiomegaly (OR, 2.36; 95% CI, 0.92-5.46; P = .51) were no longer associated with hospitalization during the subsequent year (Supplemental Table). The results of the sensitivity analysis emphasize the importance of an annual screening.
Comment
This study identified radiographic features extractable from LDCT scans that are associated with hospitalization. After controlling for age and sex, cardiomegaly, emphysema, and class I obesity or greater were independently associated with hospitalization during the subsequent year. Whereas CAC and pulmonary artery enlargement were associated with hospitalization on univariate analysis, this association was lost on multivariate analysis. Similarly, class I obesity was associated with hospitalization only on the multivariate analysis, suggesting that the interplay between these 3 conditions has important but not fully understood roles in their association with hospitalization.
This study examined data from LDCT to assess patients’ risk of hospitalization. Prior studies demonstrated that coronary artery calcium scoring as a part of LDCT lung cancer screening can be used as an independent predictor of all-cause death and cardiovascular events5,6 LDCT-measured bone density is independently associated with all-cause death in lung cancer screening patients.5 Low skeletal muscle mass and sarcopenia are powerful prognostic factors that have been shown to predict falls, fractures, postsurgical complications, and all-cause death.7 The presence and severity of emphysema identified through computed tomography–based visual classification are associated with increased risk of death.10 The results of this study suggest that like standard computed tomography, LDCT can be used to obtain additional health-related information outside of lung cancer screening.
There are several limitations to this study. Overall, 9% of scans could not be analyzed because of image noise. LDCT protocols ensure accurate assessment of the lungs, but certain covariates in our analysis, such as SMI and BMD, are not standard measurements in lung cancer screening, and image noise prevented reliable measurements from a small number of scans. The patients included in this study are from a single institution. Whereas lung cancer screening patients have common medical history of age and smoking history, the results of a large, multicenter study from multiple regions in the country would increase the external validity.
The results of this study demonstrate that radiographic features available from LDCT scans are associated with annual risk of hospitalization. Ultimately, LDCT scans could offer clinicians a cost-effective and powerful screening tool for numerous health-related outcomes in addition to lung cancer screening. If clinicians gained more health-related information from LDCT scans, it may increase their likelihood of recommending lung cancer screening for those who are eligible and increase screening uptake and follow-up. Because LDCT scans are low cost, can be read and interpreted quickly, and introduce minimal radiation exposure, they should garner further attention for their use beyond lung cancer screening.
Acknowledgments
The Supplemental Table can be viewed in the online version of this article [https://doi.org/10.1016/j.atssr.2023.06.011] on http://www.annalsthoracicsurgery.org.
Funding Sources
The authors have no funding sources to disclose.
Disclosures
The authors have no conflicts of interest to disclose.
Supplementary Data
Supplementary Table.
References
- 1.Cancer Facts & Figures 2022. https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2022.htm Accessed December 13, 2022.
- 2.The National Lung Screening Trial Research Team Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.US Preventive Services Task Force; Krist A.H., Davidson K.W., Mangione C.M., et al. Screening for lung cancer: US Preventive Services Task Force recommendation statement. JAMA. 2021;325:962. doi: 10.1001/jama.2021.1117. [DOI] [PubMed] [Google Scholar]
- 4.Guarascio A.J., Ray S.M., Finch C.K., Self T.H. The clinical and economic burden of chronic obstructive pulmonary disease in the USA. Clinicoecon Outcomes Res. 2013;5:235–245. doi: 10.2147/CEOR.S34321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buckens C.F., van der Graaf Y., Verkooijen H.M., et al. Osteoporosis markers on low-dose lung cancer screening chest computed tomography scans predict all-cause mortality. Eur Radiol. 2015;25:132–139. doi: 10.1007/s00330-014-3361-0. [DOI] [PubMed] [Google Scholar]
- 6.Jacobs P.C., Gondrie M.J., van der Graaf Y., et al. Coronary artery calcium can predict all-cause mortality and cardiovascular events on low-dose CT screening for lung cancer. AJR Am J Roentgenol. 2012;198:505–511. doi: 10.2214/AJR.10.5577. [DOI] [PubMed] [Google Scholar]
- 7.Cao Q., Xiong Y., Zhong Z., Ye Q. Computed tomography–assessed sarcopenia indexes predict major complications following surgery for hepatopancreatobiliary malignancy: a meta-analysis. Ann Nutr Metab. 2019;74:24–34. doi: 10.1159/000494887. [DOI] [PubMed] [Google Scholar]
- 8.Martin L., Birdsell L., MacDonald N., et al. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol. 2013;31:1539–1547. doi: 10.1200/JCO.2012.45.2722. [DOI] [PubMed] [Google Scholar]
- 9.American College of Radiology ACR-SPR-SSR Practice Parameter for the Performance of Musculoskeletal Quantitative Computed Tomography (QCT). 2018. https://www.acr.org/-/media/ACR/Files/Practice-Parameters/qct.pdf?la=en Accessed December 15, 2022.
- 10.Lynch D.A., Moore C.M., Wilson C., et al. CT-based visual classification of emphysema: association with mortality in the COPDGene study. Radiology. 2018;288:859–866. doi: 10.1148/radiol.2018172294. [DOI] [PMC free article] [PubMed] [Google Scholar]