Abstract
Esophageal carcinoma cuniculatum is a rare histology and can be difficult to diagnose prior to resection. To date, there have been 28 cases of resected esophageal carcinoma cuniculatum reported. Herein we describe a case found in the stomach of a patient who previously underwent a Roux-en-Y gastric bypass surgery. We report the preoperative, intraoperative, and postprocedural care. We review gross and histologic pathology.
Carcinoma cuniculatum is a rare subtype of well-differentiated squamous cell carcinoma. Histologically, it is characterized by burrowing channels of well-differentiated squamous epithelium, and may include hyperkeratosis/dyskeratosis, acanthosis, neutrophilic inflammation, microabscess, focal atypia, and koilocyte-like cells.1 Carcinoma cuniculatum has been reported in prior case reports on tumors from various sites, including skin, oral cavity, and esophagus.2,3 A review in 2023 identified 28 cases of esophageal carcinoma cuniculatum (ECC).4 With this case report, we aim to contribute to the growing literature on this rare histology and present a case of ECC discovered in a gastric mass, and furthermore in a patient who had previously undergone Roux-en-Y gastric bypass.
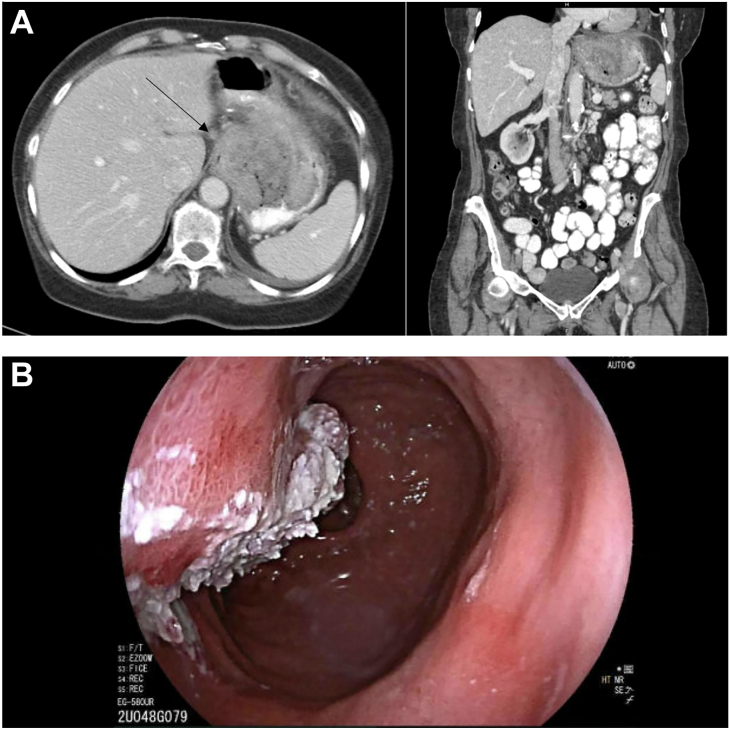
The patient is a 71-year-old white woman with a history of Roux-en-Y gastric bypass who presented with complaints of shortness of breath and fatigue. A computed tomography scan of her chest demonstrated fat stranding surrounding her gastric pouch and wall thickening of the distal esophagus and gastric pouch. A 6.4 x 9.2 x 11 cm heterogeneously enhancing mass with irregular ulceration was noted stemming from the region of the gastroesophageal junction into the level of the gastrojejunostomy, with multiple adjacent subcentimeter lymph nodes (Figure 1A).
Figure 1.
(A) Computed tomography scan of gastroesophageal mass with arrow noting lymph node. (B) Endoscopic view of proximal extent of gastric mass.
An endoscopy was performed which found ulceration on the jejunal side of her prior gastrojejunal anastomosis. Biopsies of the ulcer were nondiagnostic, revealing epidermoid metaplasia in squamous epithelium. A repeat endoscopy 7 weeks later revealed a different ulcer just above the esophagogastric junction, resolution of the prior ulcer, and a friable lesion at the gastric pouch. Multiple biopsies taken at this time demonstrated no evidence of malignancy. A third endoscopy a week later identified a 5-cm mass at the stomach extending into the esophagogastric junction, with new biopsies demonstrated low-to-focally high-grade squamous intraepithelial neoplasia without invasive carcinoma.
The patient then underwent an endoscopic ultrasound which demonstrated a fungating mass at the gastroesophageal junction. Additionally, there was a second large, fungating mass found in the cardia, fundus, and gastric body (Figure 1B), with sonographic evidence of invasion into the serosa. Several abnormal lymph nodes in the lower paraoesophageal mediastinum, gastrohepatic ligament, and perigastric region were noted. Pathology identified well-differentiated invasive squamous cell carcinoma of the stomach, with atypical focal verruciform squamous proliferation in the esophagus, which was PDL1 positive. Throughout this period she experienced a 30- to 40-pound unintentional weight loss.
After multidisciplinary discussion, the patient underwent 8 cycles of fluorouracil/leucovorin/ oxaliplatin/docetaxel for a presumptive diagnosis of gastric squamous cell carcinoma. Posttreatment computed tomography demonstrated increased extension of the mass with persistent abdominal lymph nodes. At this point, the decision was made to proceed with surgery.
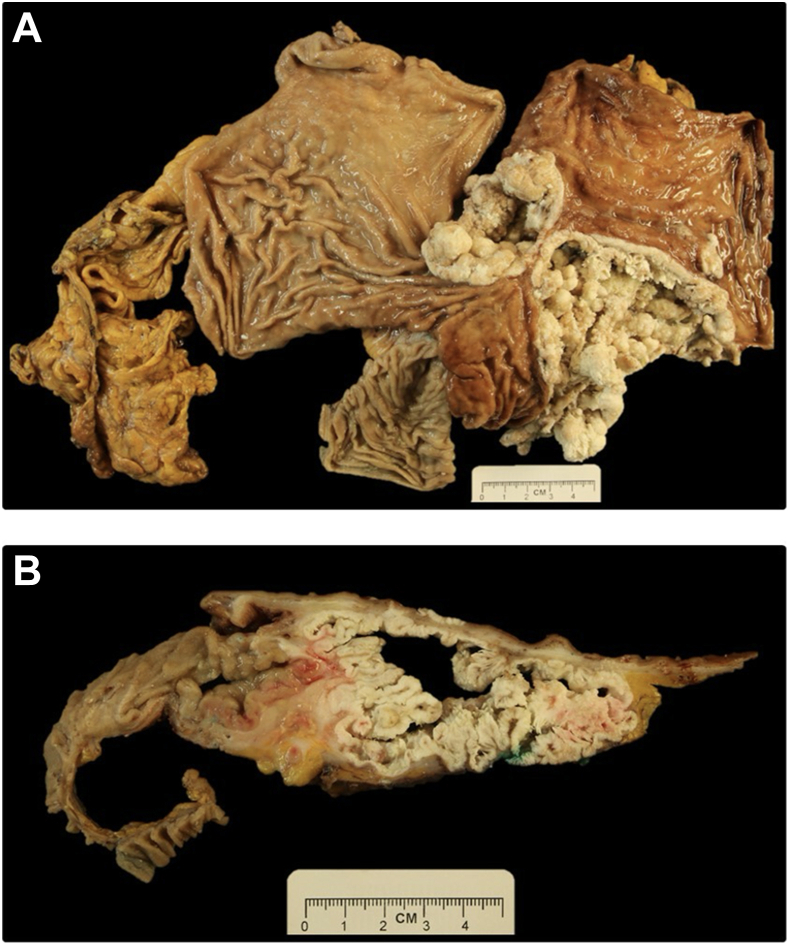
She underwent a total gastrectomy via a thoracoabdominal incision, with resection of her gastric remnant and pouch, Roux-en-Y reconstruction, and placement of a jejunostomy tube. The specimen included the Roux-en-Y gastric bypass, remnant stomach, Roux limb, distal esophagus, and portion of the diaphragm with attached epigastric fat and omentum (Figures 2A, 2B). The tumor (15.2 cm in greatest dimension) appeared to arise at the esophagogastric junction and grow distally. It was tan-white in color and friable, with characteristic burrowing pattern undermining the gastric mucosa and a marked verrucoid exophytic gross appearance. The gastric serosa was pink-red, nodular, and firm, with multiple foci grossly suspicious for transmural invasion. The diaphragm was adhesed to the stomach, with gross evidence of invasion.
Figure 2.
Postfixation gross photographs of the opened and complete Roux-en-Y esophagogastrectomy specimen with attached omentum, overall prior to sectioning (A), and on cross-section demonstrating the tunneling and verrucoid gross architecture of the tumor (B); transmural invasion is inked green.
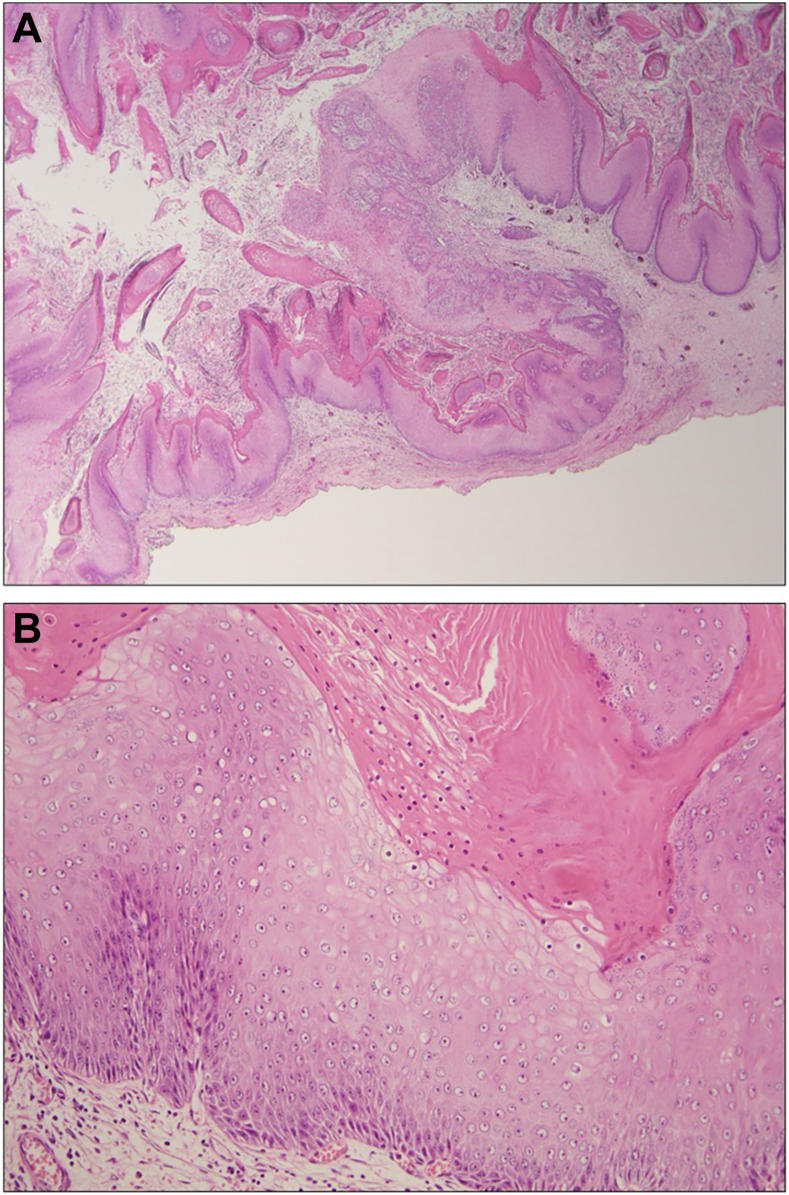
Microscopic examination revealed keratinizing, well-differentiated squamous cell carcinoma with marked pushing-type invasion and superficial furrows and burrowing. These findings are characteristic of ECC. Verrucoid architecture with spire-like peaks of hyperkeratosis, acanthosis, and dyskeratosis were noted. Superficial koilocyte-like cells were observed. Abundant keratinaceous debris superficially and within burrows and pools was noted in the main surgical resection as well as on frozen section analysis.
The surrounding mucosa displayed chronic inactive gastritis; immunohistochemistry for H. pylori was negative. Final pathology demonstrated pT3N0 well-differentiated esophageal squamous cell carcinoma with features consistent with esophageal carcinoma cuniculatum with negative margins (1.5 cm proximally, 10.2 cm distally) (Figures 3A, 3B). Fifteen lymph nodes were identified, all of which were negative for malignancy.
Figure 3.
Photomicrograph of hematoxylin and eosin–stained representative section of tumor, demonstrating tunneling architecture with pushing invasion and keratin formation on (A) low power (20x magnification), and (B) well-differentiated carcinoma with preserved squamous cell maturation, parakeratosis, dyskeratosis, and koilocyte-like cytology on 200x magnification.
She was discharged to rehabilitation on hospital day 25. She has been seen in clinic with no radiologic evidence of disease recurrence, is no longer requiring tube feeds, and has maintained a stable weight. She underwent 1 endoscopic dilation of her esophagojejunal anastomosis 4 months postoperatively.
Comment
In this report we have reviewed a case of ECC that occurred in the setting of prior Roux-en-Y gastric bypass, with significant tumor also noted in the stomach. There is a complex relationship between bariatric surgery and the development of esophageal cancer.5 It is possible that some of the ulcers noted on her initial endoscopy were related to her bypass. These may have complicated the diagnosis of the mass which became more obvious on subsequent endoscopy.
This case did, however, have other features in common with prior reports of ECC, including difficultly obtaining an accurate preoperative tissue diagnosis and lack of lymph node involvement in the final specimen. Mucosal biopsies in the setting of ECC are often insufficient for diagnosis and show epidermoid metaplasia in squamous epithelium and nonspecific inflammatory changes. Endoscopic ultrasound tends to show reactive hyperplastic lymph nodes that can mimic metastatic disease.6
In our patient, these diagnostic findings on biopsy and endoscopy suggested well-differentiated squamous cell carcinoma. This led to treatment with fluorouracil/leucovorin/ oxaliplatin/docetaxel for presumed gastric cancer, to which her tumor ultimately demonstrated no response. The correct pathologic diagnosis upfront may have prevented her from undergoing unnecessary chemotherapy. Thus, if the diagnosis of ECC is suspected clinically, there may be a role for more aggressive tissue biopsy so that these patients can undergo upfront surgery instead of neoadjuvant therapy. Improved diagnostic and pathologic markers of ECC would be of strong clinical interest. An accurate preoperative pathologic diagnosis might impact the surgical approach to this disease. Knowing the indolent course and lack of spread to lymph nodes, it may allow for a resection with smaller margins and a smaller lymph node harvest, which could improve surgical morbidity in these large operations.
In summary, ECC remains a difficult diagnostic challenge in the preoperative setting. Furthermore, the etiology and risk factors for ECC remain unclear. Associations with gastroesophageal reflux and smoking have been proposed but there have been no studies large enough to demonstrate a clear relationship. This case occurred in a patient after bariatric surgery, with the lesion primarily found in the stomach despite the final pathology demonstrating esophageal origin.
Acknowledgments
Funding Sources
The authors have no funding sources to disclose.
Disclosures
The authors have no conflicts of interest to disclose.
Patient Consent
Obtained.
References
- 1.Liu X., Yang D., Zhang X., Oduntan O. Esophageal carcinoma cuniculatum diagnosed on mucosal biopsies using a semiquantitative histologic schema: report of two esophagectomy-confirmed cases. Gastroenterology Res. 2020;13:44–51. doi: 10.14740/gr1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yadav S., Bal M., Rane S., Mittal N., Janu A., Patil A. Carcinoma cuniculatum of the oral cavity: a series of 6 cases and review of literature. Head Neck Pathol. 2022;16:213–223. doi: 10.1007/s12105-021-01340-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shwe-Daniel S., Cox S.V., Kraus C.N., Elsensohn A.N. Epithelioma cuniculatum (plantar verrucous carcinoma): a systematic review of treatment options. Cutis. 2023;111:E19–E24. doi: 10.12788/cutis.0720. [DOI] [PubMed] [Google Scholar]
- 4.Enofe I., Venkataraj H., Hong P., Ding X., Haseeb A. Esophageal carcinoma cuniculatum: a narrative review to understand this rare and commonly misdiagnosed variant of well-differentiated esophageal squamous cell carcinoma. Transl Gastroenterol Hepatol. 2023;8:20. doi: 10.21037/tgh-22-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bevilacqua L.A., Obeid N.R., Yang J., et al. Incidence of GERD, esophagitis, Barrett’s esophagus, and esophageal adenocarcinoma after bariatric surgery. Surg Obes Relat Dis. 2020;16:1828–1836. doi: 10.1016/j.soard.2020.06.016. [DOI] [PubMed] [Google Scholar]
- 6.Dick T.M., El Hag M., Mallery J.S., Amin K. Esophageal carcinoma cuniculatum associated with non-necrotizing granulomatous inflammation and lymphadenopathy: clinicopathologic features and diagnostic challenges. Am J Case Rep. 2018;19:790–795. doi: 10.12659/AJCR.908116. [DOI] [PMC free article] [PubMed] [Google Scholar]