Abstract
Esophageal bronchial fistula after Ivor Lewis esophagectomy is a challenging complication. Surgical treatment is definitive, but it carries high morbidity and mortality, whereas esophageal stents have been shown to be temporary measures. We highlight the case of a patient who was treated with endoluminal wound vacuum therapy. The fistula healed after 9 days of therapy. The likely reason for success was the presence of an omentum between the esophagus and the bronchus that was placed during esophagectomy and that provided adequate tissue to close the fistula. In a patient with appropriate anatomy, esophageal bronchial fissure can be successfully treated with endoluminal wound vacuum therapy.
Postoperative esophageal bronchial fistula after Ivor Lewis esophagectomy remains a rare but challenging condition with high morbidity and mortality.1 The most suitable treatment approach remains controversial. Historically, operative treatment with esophageal diversion and a muscle flap to the bronchus has been associated with significant morbidity and mortality.2,3 The use of esophageal stents is associated with low rates of success and is often considered more of a temporizing measure to surgery.4 In recent years, endoluminal wound vacuum therapy (EWVT) has emerged as a promising form of treatment for luminal defects such as anastomotic leaks and perforations in the esophagus or colon.5, 6, 7 We present a case of a patient who experienced esophageal bronchial fistula after Ivor Lewis esophagectomy and was successfully treated with EWVT.
A 67-year-old White man was admitted to our hospital with painless rectal bleeding. The workup included a computed tomographic (CT) scan of the abdomen and pelvis that showed esophageal thickening; subsequent endoscopy showed a 5-cm polypoid mass 37 cm from the incisors, and a snare mucosal resection was attempted but was incomplete because of a broad base. Further staging workup with positron emission tomography and CT and with endoscopic ultrasound revealed stage IIB (cT2 N0 M0) poorly differentiated adenocarcinoma. The patient underwent induction chemoradiation therapy with subsequent restaging and showed no signs of cancer progression. The patient underwent a robot-assisted Ivor Lewis esophagectomy with a modified Orringer anastomosis in the chest with V-Loc suture (Medtronic) and an omental flap to the anastomosis and with jejunostomy tube placement. The patient’s pathology report showed ypT0 N0 M0 or a pathologic complete response. The patient was discharged home on postoperative day 7 while tolerating goal-tube feeding and ingesting nothing by mouth.
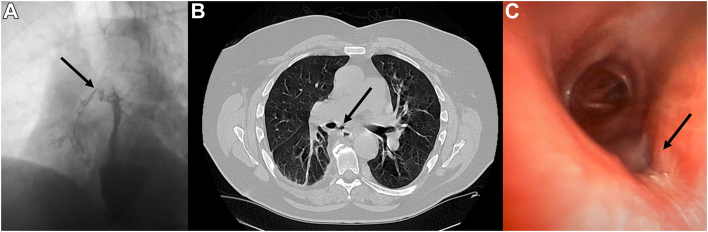
On postoperative day 13, the patient was examined in the clinic with an esophagram, which showed focal outpouching at the anastomosis concerning for a small, contained leak. Initially, the patient was treated with antibiotics after a short hospital stay but returned on postoperative day 21 with severe coughing and emesis. The patient had a CT scan of the chest that showed aspiration pneumonia, which was treated with intravenous antibiotics. The patient continued to have coughing episodes that led to a repeat esophagogram, which showed a fistula from the anastomosis to the right main bronchus (Figure 1A). CT evaluation showed an omentum between the esophagus and the right bronchus, and there was no major exposed vascular structure around the area of the fistula (Figure 1B).
Figure 1.
Esophagobronchial fistula. (A) Esophagogram showing contrast medium in the right main bronchus (arrow). Computed tomographic scan showing the fistula between the esophagus and the bronchus with the omentum (arrow) between them without a major vessel near the fistula. (C) Bronchoscopic image showing a 5-mm defect in the bronchus intermedius (arrow).
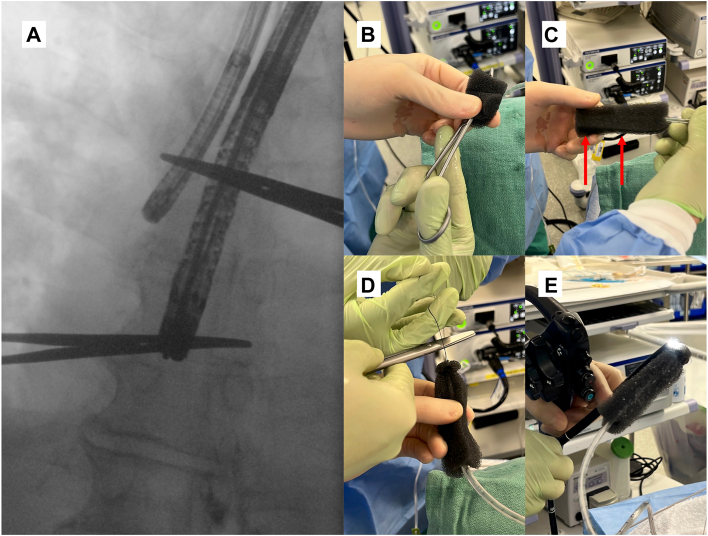
The patient underwent bronchoscopy, which showed a 5-mm defect in the medial aspect of the right bronchus intermedius (Figure 1C), and endoscopy showed no obvious opening; however, the V-Loc suture was loose. The locations of the bronchial defect and anastomosis were marked with a hemostat on the patient’s chest by using fluoroscopy (Figure 2A). We decided to perform EWVT by placing a nasogastric tube (NGT) through the patient’s nose with the tip pulled out through his mouth. A black wound vacuum-assisted closure (VAC) sponge was trimmed and placed over the tip of the NGT, covering all holes (Figure 2B), and was secured with a 3-0 silk suture at the tip and middle of the sponge (Figure 2C). A loop was created at the end of the NGT with 2-0 nylon (Figure 2D), which was grasped with rat tooth forceps through the endoscope (Figure 2E). The NGT tip with a VAC sponge was maneuvered to the area marked with a paper clip on fluoroscopy. Once the position was confirmed, we bridled the NGT to prevent dislodgment. The NGT was connected to the pump by cutting standard suction tubing, attaching it between the 2, and tying off the sump in the NGT. This allowed the VAC machine to hold a 125 mm Hg pressure.
Figure 2.
Endoluminal wound vacuum therapy. (A) The image shows the location of the defect in the bronchus with the tip of the bronchoscope and anastomosis marked by the endoscope. A vacuum sponge was cut to 10 cm × 2.5 cm × 3.2 cm. (B) We placed the hemostat in the middle and pulled the nasogastric tube through the sponge with the hemostat covering the side holes. (C) We sutured 3-0 silk through the sponge and the nasogastric tube and secured it in 2 places (marked by red arrows). (D) We sutured 2-0 nylon at the tip. (E) The nylon was held with a rat tooth grasper, which was pulled into the endoscope. The sponge was centered around the anastomosis.
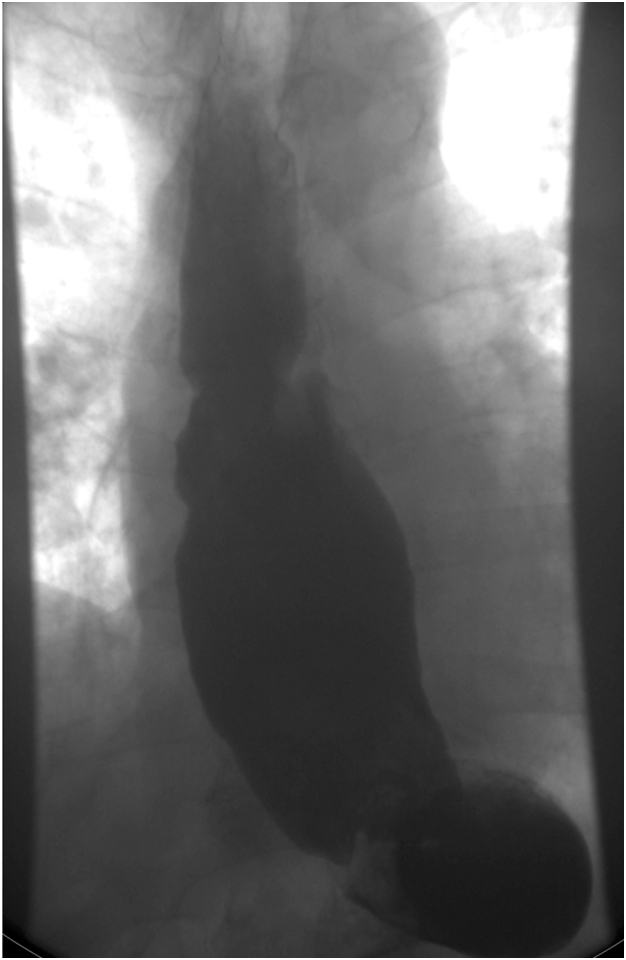
After the procedure, the patient’s cough and leukocytosis had resolved. EWVT was replaced 4 days after the first placement by removing the old VAC sponge and placing a new VAC sponge. Nine days after the initial placement, the patient returned to the operating room for the removal of the VAC sponge and NGT. Irrigation with normal saline solution resulted in no fluid in the airway. Next, we performed an on-table thin barium esophagram, which showed no fistula on fluoroscopy and no barium in the airway. The patient underwent a formal esophagram the next day (Figure 3), which revealed resolution of the fistula. The patient started on a full-liquid diet and advanced to a regular diet.
Figure 3.
Barium esophagram showing resolution of the esophagobronchial fistula.
Comment
Our case illustrates the successful use of EWVT in a patient with a postesophagectomy leak and an associated esophagobronchial fistula. Compared with esophageal diversion or open repair, endoscopic options allowed us to treat the patient on the surgical floor with minimal pain and discomfort, with active removal of exudates and infectious material to promote wound healing and preservation of normal anatomy. This therapy also allowed aspiration pneumonia to resolve by preventing the esophageal contents from entering the airway. In this patient, a small fistula with an omentum between the esophagus and the bronchus may have contributed to the successful closure with EWVT.
Other studies have shown potential complications of sponge migration, which can be mitigated by a bridle, esophageal stricture that can be treated with endoluminal balloon dilation, and very rare massive bleeding from the vascular structure, which may be avoided by careful assessment of the CT scan and lower suction pressure if there is a vascular structure close to the VAC sponge.7 Finally, EWVT exposes the patient to the risks of frequent general anesthesia and intubation; however, this risk is lower than that associated with alternative treatment.
EWVT represents an effective approach for the management of esophageal bronchial fistula, a rare but serious complication after Ivor Lewis esophagectomy. However, further research is required to identify the optimal duration and intensity of vacuum therapy, potential complications, and long-term outcomes.
Acknowledgments
Funding Sources
The authors have no funding sources to disclose.
Disclosures
Min P. Kim has received honoraria for teaching technology use for Intuitive Surgical, Medtronic; and Olympus; and has consulted for Medtronic. The other authors have no conflicts of interest to disclose.
Patient Consent
Obtained.
References
- 1.Zheng B., Zeng T., Yang H., et al. The clinical characteristics, treatments and prognosis of post-esophagectomy airway fistula: a multicenter cohort study. Transl Lung Cancer Res. 2022;11:331–341. doi: 10.21037/tlcr-22-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Groot E.M., Kingma B.F., Goense L., et al. Surgical treatment of esophago-tracheobronchial fistulas after esophagectomy. Dis Esophagus. 2024;37 doi: 10.1093/dote/doad054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li Y., Wang Y., Chen J., et al. Management of thoracogastric airway fistula after esophagectomy for esophageal cancer: a systematic literature review. J Int Med Res. 2020;48 doi: 10.1177/0300060520926025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chaddha U., Hogarth D.K., Murgu S. Perspective on airway stenting in inoperable patients with tracheoesophageal fistula after curative-intent treatment for esophageal cancer. J Thorac Dis. 2019;11:2165–2174. doi: 10.21037/jtd.2018.12.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moore C.B., Almoghrabi O., Hofstetter W., Veeramachaneni N. Endoluminal wound vac: an evolving role in treatment of esophageal perforation. J Visc Surg. 2020;6 43-43. [Google Scholar]
- 6.Pournaras D.J., Hardwick R.H., Safranek P.M., et al. Endoluminal vacuum therapy (E-Vac): a treatment option in oesophagogastric surgery. World J Surg. 2018;42:2507–2511. doi: 10.1007/s00268-018-4463-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Laukoetter M.G., Mennigen R., Neumann P.A., et al. Successful closure of defects in the upper gastrointestinal tract by endoscopic vacuum therapy (EVT): a prospective cohort study. Surg Endosc. 2017;31:2687–2696. doi: 10.1007/s00464-016-5265-3. [DOI] [PubMed] [Google Scholar]