Abstract
Embryo culture with and without human serum supplementation, previously common practice in assisted reproductive technologies (ARTs), have been associated with increased heart weight in early and late gestation in the sheep fetus. The present study aimed to determine whether the effects of embryo culture and transfer on cardiac growth and associated signalling pathways persist after birth. Embryos were either transferred to an intermediate ewe (ET) or cultured in vitro in the absence (IVC) or presence of human serum (IVCHS) and with methionine supplementation (IVCHS+M) for 6 days after mating. Naturally mated (NM) ewes were used as controls. There was an increase in the number of cardiomyocytes in the left ventricle of IVC and IVCHS+M compared to IVCHS lambs, but only in males. There were no differences in birth weight, body weight, relative heart weight, left ventricular weight, signalling molecules involved in hypertrophy, apoptosis or fibrosis at 6 months of age between the treatment groups. However, there was increased protein abundance of signalling molecules involved in ribosomal biogenesis, in male offspring from the IVC and IVCHS+M groups compared to the IVCHS group. In conclusion, the composition of the culture media used for in vitro embryo culture altered the abundance of proteins involved in ribosomal biogenesis as well as cardiomyocyte endowment in a sex specific manner. Our data suggest that male embryos cultured in the presence of human serum leads to molecular and structural changes that may detrimentally impact cardiovascular health across the life-course.
Keywords: Assisted reproductive technologies, In vitro embryo culture, Cardiac, Hypertrophy
Graphical abstract
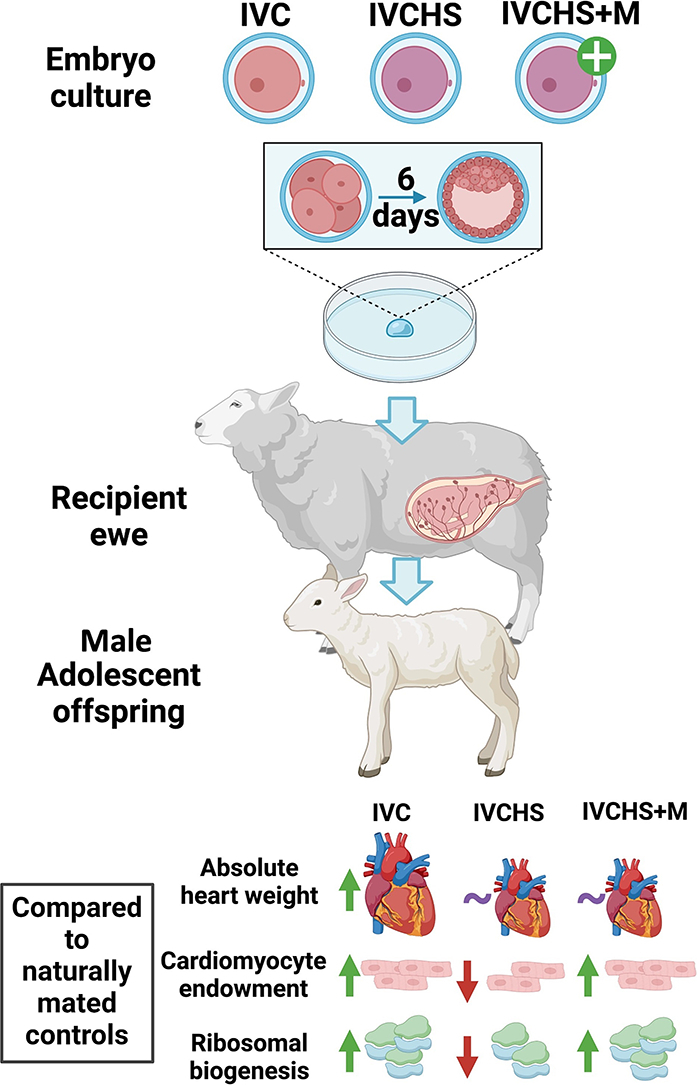
Summary of major changes in the IVC, IVCHS and IVCHS + M treatment groups relative to NM and ET controls. IVC adolescent male offspring displayed an increase in absolute heart weight compared to NM and ET controls. The IVCHS group has significantly reduced cardiomyocyte endowment and reduced RPS6 expression (a marker of ribosomal biogenesis) compared to IVC and IVCHS+M groups. NM, natural mate; ET, embryo transfer; IVC, in vitro embryo culture; IVCHS, in vitro embryo culture with human serum; IVCHS+M, in vitro embryo culture with human serum and methionine supplementation.
Highlights
-
•
In vitro embryo culture (IVC) with or without human serum is associated with aberrant cardiac growth during fetal life.
-
•
We aimed to determine whether aberrant cardiac growth and the associated molecular changes persist into postnatal life.
-
•
There was increased absolute heart weight in the 6 month old males conceived using in vitro embryo culture.
-
•
Conception utilising in vitro embryo culture was associated with more cardiomyocytes and increased ribosomal biogenesis.
-
•
This study provides evidence for a sex-specific effect of in vitro embryo culture for heart health after birth.
1. Introduction
Changes in substrate availability during the periconceptional period, often observed during cases of maternal undernutrition and overnutrition as well as in the use of Assisted Reproductive Technologies (ARTs), have been associated with altered cardiovascular development and function [[1], [2], [3], [4], [5], [6], [7]]. There is contrasting evidence that offspring conceived utilizing ARTs have an increased risk of heart disease and poor cardiovascular outcomes highlighting that small changes to the periconceptional environment can have a large and, importantly, variable impact on postnatal life. Though an increased risk of congenital heart disease has been documented [8,9], studies assessing the long-term health of offspring conceived utilizing ARTs are highly variable. Most studies have found no change in vascular function: however, there are a number of studies that have identified elevated blood pressure in in vitro fertilization (IVF) children in childhood to early adulthood as well as vascular dysfunction in children at ∼11 years of age and right ventricular dysfunction in 12 year olds [[10], [11], [12], [13]]. These negative outcomes have been confirmed in animal studies, with mice conceived using IVF displaying altered renin-angiotensin-system, miRNA expression and reduced methylation in myocardial tissue at 3 weeks, 10 weeks and 1.5 years old [14]. The duration of embryo culture is an additional risk factor in animal studies, with male but not female offspring having adverse cardiovascular outcomes associated with prolonged culture to blastocyst stage, but mostly cardiac metabolic dysfunction if embryo transfer occurred at early cleavage stage [15]. In contrast to these, a number of studies found no change in cardiometabolic outcomes in childhood to adulthood of individuals conceived through ART [[16], [17], [18], [19], [20]]. Although this is a reassuring outcome, continued observation is required as well as further investigation to tease apart and identify the mechanisms underlying cardiometabolic health in ART adults.
The link between ARTs and detrimental cardiometabolic outcomes is not fully understood. However, preclinical animal studies have identified mechanisms by which ARTs may alter cardiovascular physiology [15,[21], [22], [23]]. For example, embryo culture and transfer increase heart weight during fetal life in both sheep and cows [[24], [25], [26]] and cause large changes to cardiac gene expression in mice [23]. However, whether in vitro embryo culture has lasting consequences on postnatal cardiac development and growth is yet to be determined in an animal model where cardiac development parallels that of the human [27,28]. In vitro derived calves had increased heart weight and this persisted into postnatal life in the absence of changes in body weight [29]. We have also shown that there is no change in blood pressure as a result of ARTs in adolescent sheep [20]; however, no studies have investigated the effect of in vitro embryo culture and transfer on cardiomyocyte endowment, which is set at birth in humans and sheep [27,[30], [31], [32]], or the molecular pathways responsible for cardiac development and hypertrophy in postnatal life.
Supplementation of IVF culture media with human serum was widely used throughout the 1990s in an attempt to more closely mimic in vivo culture conditions. Although serum free medium is now the gold standard for effective preimplantation embryo culture in all species [33,34], there remains a population of individuals that were exposed to human serum during the periconceptual period that are now entering early adulthood. In ruminants, in vitro embryo culture in the presence of serum has shown to increase heart weight in fetal life, possibly due to decreased methylation [22]. This suggests that addition of methionine to the media may restore methylation status and thus normalize cardiac growth. Therefore, in this study, we aimed to investigate the impact of embryo transfer and in vitro embryo culture in the absence or presence of human serum along with methionine supplementation (intervention aimed at replenishing the methylation levels) on both cardiomyocyte endowment and cardiac growth in male and female adolescent sheep.
2. Materials and methods
2.1. Animal ethics and housing
All procedures were approved by the IMVS/University of South Australia and the Primary Industries and Resources South Australia Animal Ethics Committee and comply with the Australian code of practice for the care and use of animals for scientific purposes (IMVS 110/07, PIRSA 12/09). All investigators understood and followed the ethical principles outlined in Grundy et al [35] and the principles of the 3Rs, specifically the reduction of the use of animals in research [36].
2.2. Animals and experimental design
Ewes were randomly assigned as either donor, intermediate or final recipients as previously described [20]. All ewes received 100 % of nutritional requirements (7.6 MJ/day for the maintenance of a 64 kg non-pregnant ewe) as defined by the Agricultural and Food Research Council (1993) [37]. Donor ewes were supplemented with 350 g of peas/day for 14 days prior to ovulation to increase ovulation rates, which increased the metabolizable energy of the diet to 10.6 MJ/kg DM and 151 g/kg of crude protein. Synchronization and superovulation of donor ewes was performed following standard procedures [[38], [39], [40], [41]]. Ewes were treated with progestagen pessaries (45 mg flugestone acetate; Intervet, France) for 12 days and 9.5 mL of follicle stimulating hormone was administered 48 h before removal of pessary (FSH; 190 mg NIH-FSH-P1 standard, Follotropin, Bioniche Inc., Canada) as two daily injections along with 500 IU of equine chorionic gonadotropin (eCG, Pregnecol, Bioniche Inc.) at the time of the first FSH treatment. Twenty-seven hours after pessary removal, synthetic gonadotrophin releasing hormone (GnRH, Fertagyl; 30 μg; Intervet, France) was administered. Both intermediate and recipient ewes were synchronized using progestagen pessaries and were injected with 400 IU eCG (Pregnecol, Bioniche Inc., Canada) at the time of pessary removal [42]. Ovulatory cycles of the recipient ewes were scheduled to synchronize with the ovulatory cycles of donor animals (±12 h).
Eighteen hours after GnRH treatment, donor ewes were inseminated with fresh semen (20 × 106 spermatozoa) that was collected from one ram of proven fertility. The same ram was used for all replicates and breeding of naturally mated (NM) ewes. Uterine flushings were performed ∼12–17 h after the expected median time of ovulation for the collection of zygotes [38,42]. Zygotes from donor ewes were either transferred to an intermediate recipient (ET) or cultured in a defined synthetic oviductal fluid medium in the absence of human serum (in vitro culture, IVC) [43], presence of human serum (IVCHS) or presence of human serum with methionine supplementation (IVCHS+M) until day 6 (day 0 = day of IVF). A group of ewes were naturally mated to result in 5 experimental groups [20]. Zygotes selected for in vivo culture were transferred to intermediate recipients by mid-ventral laparotomy to a single intermediate recipient per replicate in groups of 7 to 22 embryos [42]. Synthetic oviduct fluid medium was prepared as previously described by Walker and co-workers [43,44]. IVC medium was a mixture of synthetic oviductal fluid containing bovine serum albumin (4 mg/mL) and amino acids at oviduct fluid concentrations. IVCHS medium was a mixture of IVC medium and 20 % human serum (HS). HS was collected and prepared freshly for each replicate. Whole venous blood (10 mL) was collected and immediately centrifuged (2000g for 20 min), plasma was allowed to clot and serum was harvested by compression of the clot. Serum was then heat inactivated at 56.0 °C for 30 min, filtered and stored at 4.0 °C. IVC medium (16 mL) was mixed with HS (4 mL) to give a final concentration of 20 % HS. IVCHS+M medium was supplemented with 5000 μM of methionine (Sigma) and is the maximum amount which can be dissolved in the medium. Zygotes allocated to embryo culture groups (IVC, IVCHS and IVCHS+M) were washed three times in their respective medium, transferred to wells of a culture dish (Nunc Inc., USA) containing 600 μL of medium (IVC, IVCHS or IVCHS+M), covered with 300 μL sterile mineral oil (Sigma) and cultured for 6 days. In vitro embryo culture was performed in an atmosphere of 5 % CO2, 2.5 % O2 and 90 % N2 at 38.5 °C in groups of 12–15 embryos. The culture medium was changed every 48 h. On day 6, embryos from the ET, IVC, IVCHS and IVCHS+M groups were transferred to synchronized final recipients (1 embryo per recipient) [20]. The feed allowance per singleton pregnancy was increased by 15 % every 10 days from gestational day 115 until the ewes lambed spontaneously.
2.3. Post mortem
Lambs were humanely killed with an overdose of sodium pentobarbitone (Virbac Pty Limited, Australia) at 6 months of age. The heart was dissected, weighed, and samples were snap frozen in liquid nitrogen and stored at −80 °C for subsequent mRNA and protein analysis or fixed in 4 % paraformaldehyde for determination of cardiomyocyte number.
2.4. Estimation of cardiomyocyte number
Fixed left ventricle tissue was serially sectioned into 2 mm slabs for determination of cardiomyocyte number as previously described [[45], [46], [47]]. Briefly, slabs were sampled using the smooth fractionator technique and 7–8 tissue sections were chosen for calculation of numerical density of cardiomyocyte nuclei using the optical disector technique. The tissue sections were embedded in glycolmethacrylate (Technovit 7100, Ax-lab, Denmark), a 30 mm-thick section was mounted on Superfrost Plus slides (Menzel-Glaser) and stained with Mayer's hematoxylin and 0.15 % basic fuchsin. Thirty-two unbiased counting frames of 3000 μm2 surface area were randomly assigned by newCAST software (Visiopharm) software to each piece of the ventricle for point counting. A disector height of 10 μm in the centre of each section determined after a z-axis analysis was used to determine the numerical density of nuclei in a minimum of seven left ventricle (LV) pieces per animal. The numerical density of nuclei per μm3 of left ventricle (NV (nuclei/LV)) and total number of nuclei per left ventricle, N (nuclei, LV) was determined using previously published formulae [45,46]. Previous studies have shown that ∼99 % of cardiomyocytes are binucleated in the left ventricle of the 9 week old sheep [48], therefore we assumed that almost all of the cardiomyocytes in the heart of 24 week old lambs (6 months) would be binucleated. The number of cardiomyocytes in the LV (N (cm,lv)) was determined by dividing the NV (nuclei/lv) by the average number of nuclei per cardiomyocyte (which is 2 in this study).
2.5. mRNA quantification
RNA was extracted from left ventricle samples using TRIzol reagent (Invitrogen, Netherlands) using a tissue homogeniser (TissueLyser LT, Qiagen) and purified using RNeasy Mini Kit (Qiagen, Switzerland). The quality and concentration of the RNA was determined by measuring absorbance at 260 and 280 nm and the integrity of the RNA was confirmed by agarose gel electrophoresis. cDNA was synthesized using 1 μg RNA by reverse transcription using Superscript III with random hexamers (Invitrogen Australia Pty Ltd., Australia). Negative controls containing no RNA or Superscript III were used to test for DNA contamination and reagent contamination [49]. All essential information regarding our procedure is included as per the MIQE guidelines [50]. Quantitative real-time reverse transcription-PCR was used to measure the expression of target genes relative to three housekeeper genes, hypoxanthine phosphoribosyltransferase 1 (HPRT) [51], phosphoglycerate kinase 1 (PGK1) [46] and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) [46] on a ViiA7 Fast Real-time PCR system (Applied Biosystems, USA). These housekeeper genes were selected from a candidate list of 10 housekeeper genes based on their stability across treatment groups using GeNorm [51,52]. The primer sets for target genes included activation of PI3K pathway: IGF1 [53]; IGF2 [53]; IGF1R [53]; PI3K pathway: mTOR [54]; PI3K(p110 α) [54]; hypertrophic makers: ANP [6]; MHCβ [55]; apoptosis and fibrosis: TGFβ [56]; Bax [46]; BCL-2 [46]. Each qRT-PCR reaction well contained 3 μL of Fast SYBR Green Master Mix (Applied Biosystems), 0.8 μL H2O, 0.6 μL each of forward and reverse primer (GeneWorks, SA, Australia) for the target genes and 1 μL of diluted relevant cDNA. Three replicates of each cDNA were performed for each gene and controls containing no cDNA were also used to check for reagent contamination in each run. The data was analysed using DataAssist Software v3.0 (Applied Biosystems) and expressed as mean normalised expression (MNE) [57,58].
2.6. Protein extraction and Western blotting
Left ventricle samples (∼50 mg) were sonicated (John Morris Scientific, Australia) in lysis buffer (1 mL/100 mg tissue; 1 mmol/L Tris HCl pH = 8, 5 mol/L NaCl, 1 % NP-40, 1 mmol/L Na Orthovanadate, 30 mmol/L NaF, 10 mmol/L Na Tetrapyrophosphate, 10 mmol/L EDTA and a protease inhibitor tablet (cOmplete Mini; Roche)). The suspensions were centrifuged (Eppendorf Centrifuge 5415, Crown Scientific, Australia) for 14 min at 14,300g and 4 °C. Protein content of extracts was determined using a Micro BCA Protein Assay Kit (PIERCE, Thermo Fisher Scientific Inc., USA) and bovine serum albumin (2 mg/mL stock solution) as a standard curve. Extracted protein (50 μg) was subjected to SDS-PAGE. Gels were stained with Coomassie blue and there were no differences in abundance of the major proteins present in each sample [51,54,59]. Due to the large number of samples, males and females were run on different gels, and one sample per animal were run per gel. Proteins were transferred onto a nitrocellulose membrane (Hybond ECL, GE Health Care, Australia). Nitrocellulose membranes were stained with Ponceau S (0.5 % Ponceau in 1 % acetic acid) to assess the efficacy of the transfer. The membranes were blocked with 5 % BSA or 5 % milk in Tris-Buffered Saline with 1 % Tween (TBS-T) for 1 h at room temperature. The membranes were then washed 3 times with TBS-T and then incubated with the respective primary antibody overnight at 4 °C. The membranes were then incubated with the respective secondary antibody with agitation for 1 h to determine protein abundance of key molecules in the IGF-PI3K signalling pathway: protein kinase B (Akt) and phospho-Akt (Ser-478) (Cell Signaling Technology, Inc., USA), mTOR and phospho-mTOR (Ser-2448) (Cell Signaling Technology) and RPS6 and phospho-RPS6 (Ser-235/236) (Cell Signaling Technology) [54,60]. Membranes were imaged using enhanced chemiluminescence (SuperSignal West Pico Chemiluminescent Substrate, Thermo Scientific, USA) on a ImageQuant LAS 4000 (GE Healthcare, Australia) and the protein abundance was quantified by densitometry using Image quant software (GE Healthcare, Australia). Samples were removed if there was a visible defect within the region of the protein of interest. The abundance of each protein was expressed relative to the loading control, β-actin.
2.7. Statistical analysis
Due to samples from males and females being run on different gels the evaluation of the effect of treatment on protein abundance were analysed within each sex and thus no comparison between sexes for protein data was performed. For consistency, the effect of treatment on other parameters such as birth weight, heart weight, cardiac gene expression and cardiomyocyte number were also analysed separately in males and females. All statistical analyses were performed in GraphPad Prism version 9, USA. Normality of the data was assessed using Shapiro-Wilk test for each measure. The effect of treatment within each sex was determined using one-way ANOVA. Turkey post hoc test was used to determine if there was a significant difference between treatment groups. Outliers were identified using the ROUT method with a Q = 1 %. Data are presented as mean ± Standard deviation. A probability level of 5 % (P < 0.05) was taken as significant. For gene expression studies, all available samples were included resulting in; 5 NM, 6 ET, 9 IVC, 4 IVCHS and 7 IVCHS+M male offspring and 4 NM, 5 ET, 11 IVC, 7 IVCHS and 4 IVCHS+M female offspring. For protein studies, samples were randomly selected resulting in; 4 NM, 5 ET, 5 IVC, 4 IVCHS and 5 IVCHS+M male offspring and 4 NM, 4 ET, 6 IVC, 5 IVCHS and 4 IVCHS+M female offspring.
3. Results
3.1. Body and heart weight
There was no effect of in vitro culture and/or transfer of the embryo on birth weight or body weight, relative heart weight (heart weight/body weight) and relative left ventricular weight (left ventricle weight/heart weight) in either males or females at 6 months of age. However, there was an increase in absolute heart weight in male offspring from the IVC group compared to the NM, IVCHS and IVCHS+M groups, but not compared to the ET group (Table 1).
Table 1.
Biometry of 6 month old lambs. Values are mean ± SD. NM, natural mate; ET, embryo transfer; IVC, in vitro embryo culture; IVCHS, in vitro embryo culture with human serum; IVCHS+M, in vitro embryo culture with human serum and methionine supplementation. Data were analysed using a one-way ANOVA (treatment group). Different superscripts denote mean values that are significantly different from each other (P < 0.05).
| NM | ET | IVC | IVCHS | IVCHS + M | P | ||
|---|---|---|---|---|---|---|---|
| Males | n | 5 | 6 | 9 | 4 | 7 | |
| Birth weight | 5.64 ± 0.26 | 5.47 ± 0.30 | 5.85 ± 0.16 | 5.76 ± 0.45 | 6.14 ± 0.41 | 0.7534 | |
| Body weight | 44.8 ± 1.6 | 47.0 ± 1.9 | 49.3 ± 1.2 | 42.4 ± 1.8 | 45.5 ± 1.8 | 0.2566 | |
| Absolute heart weight | 207 ± 9 a | 219 ± 10 ab | 239 ± 5 b | 193 ± 9 a | 208 ± 11 a | 0.0450 | |
| Relative heart weight | 4.63 ± 0.13 | 4.66 ± 0.13 | 4.87 ± 0.15 | 4.56 ± 0.11 | 4.57 ± 0.16 | 0.4552 | |
| Relative LV weight | 0.34 ± 0.01 | 0.31 ± 0.01 | 0.33 ± 0.02 | 0.31 ± 0.01 | 0.34 ± 0.02 | 0.8730 | |
| Females | n | 4 | 5 | 11 | 7 | 4 | |
| Birth weight | 5.76 ± 0.14 | 4.78 ± 0.17 | 5.12 ± 0.17 | 5.63 ± 0.46 | 5.22 ± 0.41 | 0.5193 | |
| Body weight | 39. 38 ± 1.23 | 35.90 ± 1.14 | 40.68 ± 1.36 | 38.43 ± 1.47 | 38.75 ± 2.90 | 0.3122 | |
| Absolute heart weight | 182 ± 5 | 174 ± 14 | 180 ± 8 | 181 ± 10 | 187 ± 14 | 0.2451 | |
| Relative heart weight | 4.63 ± 0.07 | 4.82 ± 0.27 | 4.45 ± 0.14 | 4.71 ± 0.17 | 4.87 ± 0.38 | 0.7883 | |
| Relative LV weight | 0.34 ± 0.01 | 0.30 ± 0.02 | 0.34 ± 0.01 | 0.32 ± 0.01 | 0.34 ± 0.03 | 0.8696 | |
3.2. Cardiomyocyte endowment
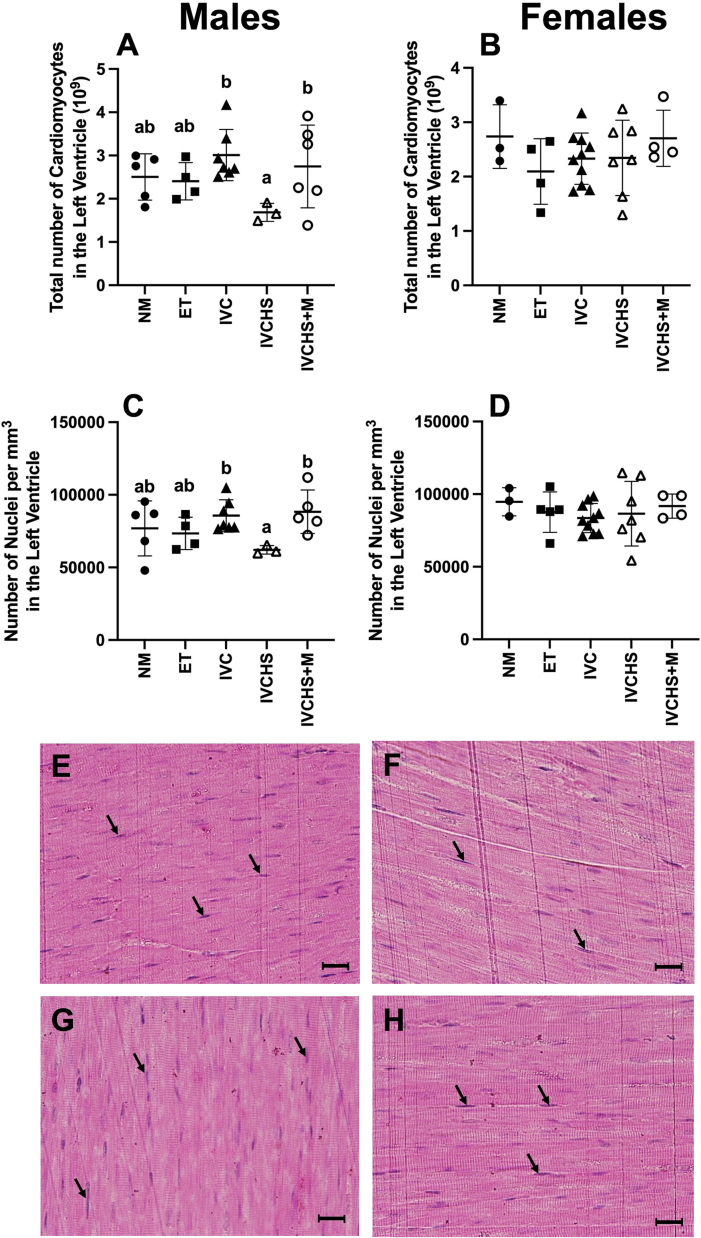
IVC and IVCHS+M groups had an increased number of cardiomyocytes and cardiac nuclei density in the left ventricle compared to the IVCHS group, but none of these groups were different from the NM and ET groups (Fig. 1). This effect of treatment was present only in males (P = 0.0486, P = 0.0472).
Fig. 1.
In vitro embryo culture containing human serum decreases the total number of cardiomyocytes, but only in male sheep offspring. There was a decreased total number of cardiomyocytes in the left ventricle of IVCHS when compared to IVC and IVCHS+M IVCHS only in the males (A; P = 0.0486) of 6 months old lambs with no change in females (B), these same changes were also present in the density of cardiomyocytes (C; P = 0.0472, D). Micrographs E, F, G, H are representative Mayer's hematoxylin and basic fuchsin stain from NM, IVC, IVCHS and IVCHS+M groups respectively (Arrows indicated cardiomyocyte nuclei, scale bar = 10 μm). Values are mean ± SD. NM, natural mate (Males n = 5, Females n = 3); ET, embryo transfer (Males n = 4, Females n = 4); IVC, in vitro embryo culture (Males n = 7, Females n = 11); IVCHS, in vitro embryo culture with human serum (Males n = 3, Females n = 7); IVCHS+M, in vitro embryo culture with human serum and methionine supplementation (Males n = 6, Females n = 4). Thirty-two unbiased counting frames of 3000 μm2 surface area were assessed per animal. mRNA expression was run in triplicate and one sample per animal was run per western blot. Different superscript letters denote mean values that are significantly different from each other (P < 0.05). Data were analysed using a one-way ANOVA (treatment group).
3.3. PI3K pathway gene and protein expression
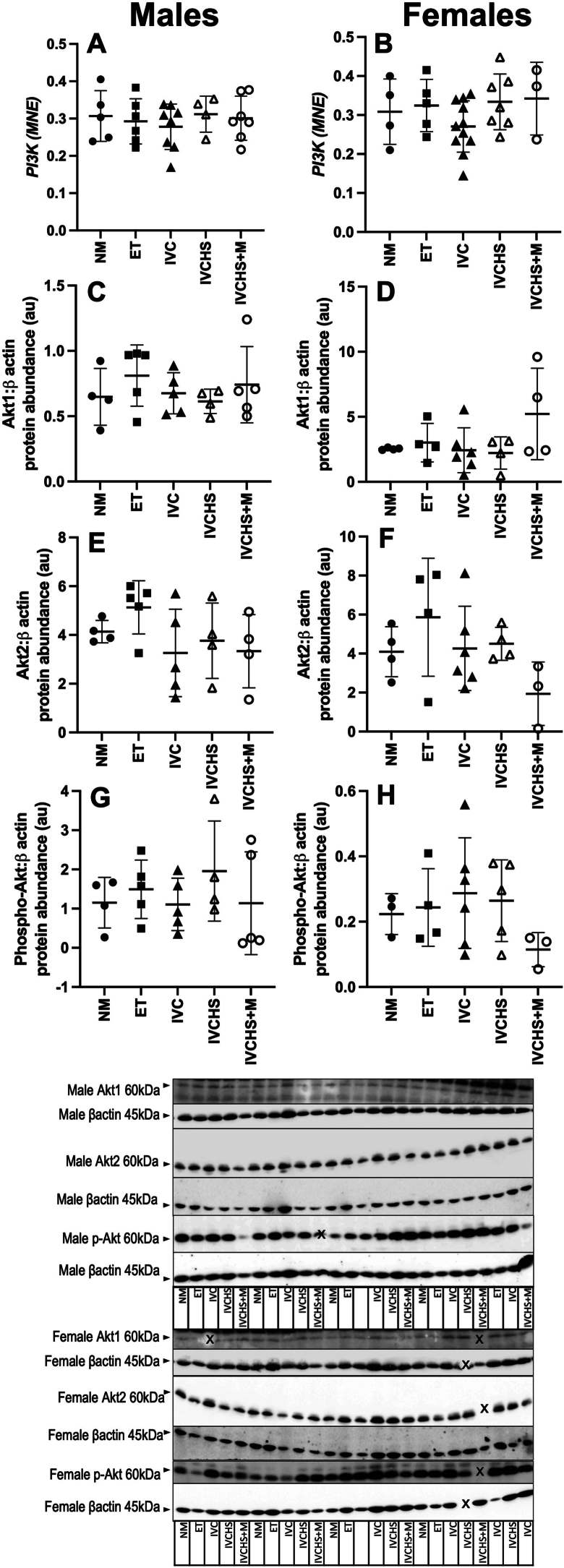
Embryo transfer and embryo culture in the absence and presence of human serum as well as with methionine supplementation did not alter the gene expression of IGF-1, IGF-2, IGF-1R, IGF-2R (Table 2) or PI3K (Fig. 2) or the protein abundance of Akt and its phosphorylated form in males or females (Fig. 2).
Table 2.
Cardiac gene and protein expression of markers of hypertrophy, apoptosis and protein translation in 6 month old lambs. Values are mean ± SD. NM, natural mate; ET, embryo transfer; IVC, in vitro embryo culture; IVCHS, in vitro embryo culture with human serum; IVCHS+M, in vitro embryo culture with human serum and methionine supplementation. MNE, mean normalised expression. Data were analysed using a one-way ANOVA (treatment group).
| NM | ET | IVC | IVCHS | IVCHS + M | P | ||
|---|---|---|---|---|---|---|---|
| Males | n | 5 | 6 | 9 | 4 | 7 | |
| PI3K (MNE) | 0.307 ± 0.068 | 0.293 ± 0.061 | 0.278 ± 0.061 (n = 8) | 0.312 ± 0.048 | 0.301 ± 0.059 | 0.8741 | |
| mTOR (MNE) | 0.344 ± 0.029 (n = 4) | 0.258 ± 0.071 | 0.379 ± 0.134 | 0.328 ± 0.059 | 0.336 ± 0.108 | 0.2840 | |
| ANP (MNE) | 0.387 ± 0.344 (n = 4) | 0.468 ± 0.379 (n = 5) | 0.190 ± 0.234 (n = 7) | 0.170 ± 0.071 | 0.378 ± 0.311 | 0.3594 | |
| MHCβ (MNE) | 19.78 ± 6.408 | 11.86 ± 5.771 | 13.39 ± 3.851 (n = 8) | 21.27 ± 11.40 | 14.66 ± 3.765 | 0.0797 | |
| TGFβ (MNE) | 0.087 ± 0.022 | 0.105 ± 0.024 | 0.093 ± 0.024 (n = 8) | 0.111 ± 0.026 (n = 3) | 0.090 ± 0.017 | 0.4604 | |
| BAX:BCL2 (MNE) | 3.144 ± 1.179 | 2.499 ± 1.485 | 2.766 ± 1.426 | 2.670 ± 0.380 | 2.838 ± 0.928 | 0.9336 | |
| IGF1 (MNE) | 0.056 ± 0.021 | 0.074 ± 0.031 (n = 5) | 0.070 ± 0.039 (n = 8) | 0.058 ± 0.027 (n = 3) | 0.082 ± 0.033 | 0.6893 | |
| IGF2 (MNE) | 1.454 ± 0.345 | 1.466 ± 0.135 | 1.411 ± 0.382 (n = 8) | 1.629 ± 0.244 (n = 3) | 1.185 ± 0.344 (n = 6) | 0.3438 | |
| IGF1R (MNE) | 0.366 ± 0.057 | 0.373 ± 0.104 | 0.432 ± 0.092 (n = 8) | 0.406 ± 0.135 | 0.350 ± 0.057 (n = 6) | 0.4842 | |
| IGF2R (MNE) | 0.559 ± 0.030 | 0.536 ± 0.082 | 0.619 ± 0.111 (n = 8) | 0.617 ± 0.049 | 0.500 ± 0.127 (n = 6) | 0.1523 | |
| 4EBP1 (MNE) | 0.254 ± 0.110 | 0.195 ± 0.043 | 0.208 ± 0.066 | 0.257 ± 0.092 | 0.240 ± 0.082 | 0.5901 | |
| n | 4 | 5 | 5 | 4 | 5 | ||
| Akt1 (AU) | 0.649 ± 0.218 | 0.811 ± 0.234 | 0.676 ± 0.157 | 0.614 ± 0.093 | 0.742 ± 0.292 | 0.6567 | |
| Akt2 (AU) | 4.136 ± 0.459 | 5.133 ± 1.093 | 3.264 ± 1.795 | 3.764 ± 1.545 | 3.335 ± 1.505 (n = 4) | 0.2554 | |
| Phospho-Akt (AU) | 1.153 ± 0.647 | 1.495 ± 0.746 | 1.108 ± 0.668 | 1.957 ± 1.276 | 1.138 ± 1.309 | 0.6701 | |
| mTOR (AU) | 1.048 ± 0.621 | 0.929 ± 0.258 | 0.987 ± 0.228 | 0.718 ± 0.160 | 1.177 ± 0.700 | 0.6583 | |
| Phospho-mTOR (AU) | 6.479 ± 4.847 (n = 3) | 20.33 ± 4.447 | 22.73 ± 12.45 (n = 3) | 5.522 ± 2.790 | 32.56 ± 17.58 (n = 3) | 0.0097 | |
| RSP6 (AU) | 3.84 ± 3.22 | 3.36 ± 2.72 | 9.09 ± 5.11 (n = 3) | 2.59 ± 0.569 | 11.23 ± 3.91 (n = 3) | 0.0105 | |
| Phospho-RSP6 (AU) | 0.064 ± 0.039 | 0.100 ± 0.028 | 0.275 ± 0.134 | 0.044 ± 0.034 | 0.163 ± 0.109 | 0.0073 | |
| Females | n | 4 | 5 | 11 | 7 | 4 | |
| PI3K (MNE) | 0.309 ± 0.084 | 0.324 ± 0.067 | 0.271 ± 0.066 | 0.334 ± 0.071 | 0.342 ± 0.093 (n = 3) | 0.3396 | |
| mTOR (MNE) | 0.388 ± 0.067 | 0.309 ± 0.061 | 0.284 ± 0.095 | 0.273 ± 0.097 | 0.320 ± 0.181 | 0.4423 | |
| ANP (MNE) | 0.184 ± 0.129 (n = 3) | 0.224 ± 0.163 | 0.440 ± 0.550 (n = 9) | 0.550 ± 0.516 (n = 6) | 1.102 ± 1.087 | 0.2066 | |
| MHCβ (MNE) | 22.21 ± 4.953 (n = 3) | 15.79 ± 4.385 (n = 4) | 14.49 ± 2.831 (n = 9) | 16.76 ± 5.925 (n = 6) | 19.74 ± 12.11 | 0.3547 | |
| TGFβ (MNE) | 0.103 ± 0.024 | 0.087 ± 0.030 | 0.096 ± 0.020 | 0.095 ± 0.016 | 0.097 ± 0.036 | 0.8922 | |
| BAX:BCL2 (MNE) | 2.382 ± 1.314 | 3.131 ± 0.438 | 2.296 ± 0.808 | 3.123 ± 0.491 | 3.385 ± 0.818 | 0.0678 | |
| IGF1 (MNE) | 0.044 ± 0.030 (n = 3) | 0.068 ± 0.039 | 0.057 ± 0.025 | 0.052 ± 0.021 | 0.047 ± 0.029 | 0.7162 | |
| IGF2 (MNE) | 1.359 ± 0.834 | 1.208 ± 0.170 (n = 4) | 1.317 ± 0.355 | 1.394 ± 0.271 | 1.920 ± 1.208 | 0.4301 | |
| IGF1R (MNE) | 0.516 ± 0.131 | 0.415 ± 0.085 | 0.369 ± 0.076 | 0.469 ± 0.155 | 0.516 ± 0.290 | 0.2839 | |
| IGF2R (MNE) | 0.651 ± 0.012 (n = 3) | 0.548 ± 0.112 | 0.602 ± 0.083 | 0.560 ± 0.157 | 0.507 ± 0.153 | 0.4754 | |
| 4EBP1 (MNE) | 0.264 ± 0.112 | 0.270 ± 0.101 | 0.233 ± 0.086 | 0.200 ± 0.053 | 0.269 ± 0.117 | 0.6065 | |
| n | 4 | 4 | 6 | 5 | 4 | ||
| Akt1 (AU) | 2.537 ± 0.083 | 3.015 ± 1.482 | 2.431 ± 1.731 | 2.223 ± 1.238 (n = 4) | 5.221 ± 3.510 | 0.2012 | |
| Akt2 (AU) | 4.095 ± 1.284 | 5.860 ± 3.025 | 4.265 ± 2.161 | 4.500 ± 0.844 (n = 4) | 1.941 ± 1.630 (n = 3) | 0.2010 | |
| Phospho-Akt (AU) | 0.223 ± 0.063 (n = 3) | 0.244 ± 0.119 | 0.288 ± 0.169 | 0.264 ± 0.125 | 0.115 ± 0.052 (n = 3) | 0.4421 | |
| mTOR (AU) | 0.943 ± 0.501 (n = 3) | 1.137 ± 0.172 | 0.632 ± 0.494 (n = 5) | 0.552 ± 0.339 (n = 3) | 1.328 ± 1.039 (n = 3) | 0.2877 | |
| Phospho-mTOR (AU) | 0.058 ± 0.045 (n = 3) | 0.057 ± 0.010 (n = 3) | 0.112 ± 0.075 (n = 5) | 0.052 ± 0.025 (n = 4) | 0.159 ± 0.128 | 0.2597 | |
| RSP6 (AU) | 1.242 ± 0.776 | 1.639 ± 1.350 | 2.966 ± 1.931 | 1.655 ± 1.096 | 3.178 ± 2.089 (n = 3) | 0.4588 | |
| Phospho-RSP6 (AU) | 0.058 ± 0.045 | 0.057 ± 0.010 | 0.112 ± 0.075 | 0.052 ± 0.025 | 0.159 ± 0.128 | 0.2597 | |
Fig. 2.
In vitro culture and/or transfer of sheep embryos does not change PI3K expression or cardiac protein abundance of AKT in either males or females. There was no change in mRNA expression of PI3K (A and B) or the protein abundance of Akt1 (C and D), Akt2 (E and F) and phospho-Akt (G and H) across the treatment groups. Values are mean ± SD. NM, natural mate (mRNA: Males n = 5, Females n = 4; Protein: Males n = 4, Females n = 4); ET, embryo transfer (mRNA: Males n = 6, Females n = 5; Protein: Males n = 5, Females n = 4); IVC, in vitro embryo culture (mRNA: Males n = 9, Females n = 11; Protein: Males n = 5, Females n = 6); IVCHS, in vitro embryo culture with human serum (mRNA: Males n = 4, Females n = 7; Protein: Males n = 4, Females n = 5); IVCHS+M, in vitro embryo culture with human serum and methionine supplementation (mRNA: Males n = 7, Females n = 4; Protein: Males n = 5, Females n = 4). mRNA expression was run in triplicate and one sample per animal was run per western blot. Different superscript letters denote mean values that are significantly different from each other (P < 0.05). Data were analysed using a one-way ANOVA (treatment).
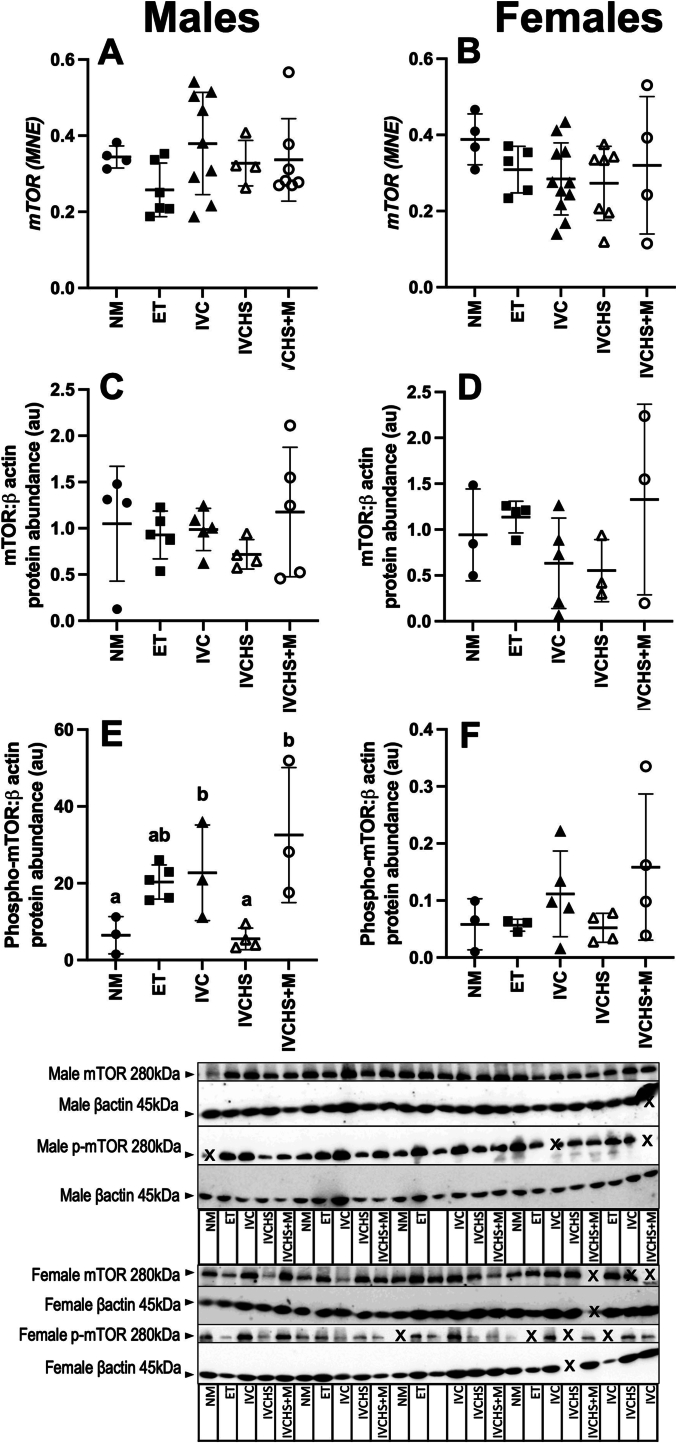
3.4. mTOR expression
The cardiac gene and protein abundance of mTOR were not different in any treatment groups compared to NM. However, there was increased protein abundance of phospho-mTOR in the males of the IVC group and IVCHS+M group when compared to NM and IVCHS group (P = 0.0097; Fig. 3).
Fig. 3.
Increased cardiac protein abundance of phospho-mTOR in male sheep offspring in the IVC and IVCHS + M groups, but not the IVCHS group. There was no change in the cardiac gene or protein expression of mTOR across treatment groups (A & C, males; B & D, females). However, there was increased protein abundance of phospho-mTOR in the males (E; P = 0.0097), but not females (F) in the IVC and IVCHS+M. Values are mean ± SD. NM, natural mate (mRNA: Males n = 4, Females n = 4; Protein: Males n = 4, Females n = 4); ET, embryo transfer (mRNA: Males n = 6, Females n = 5; Protein: Males n = 5, Females n = 4); IVC, in vitro embryo culture (mRNA: Males n = 9, Females n = 11; Protein: Males n = 5, Females n = 6); IVCHS, in vitro embryo culture with human serum (mRNA: Males n = 4, Females n = 7; Protein: Males n = 4, Females n = 5); IVCHS+M, in vitro embryo culture with human serum and methionine supplementation (mRNA: Males n = 7, Females n = 4; Protein: Males n = 5, Females n = 4). mRNA expression was run in triplicate and one sample per animal was run per western blot. Different superscript lettters denote mean values that are significantly different from each other (P < 0.05). Data were analysed using a one-way ANOVA (treatment).
3.5. Ribosomal biogenesis
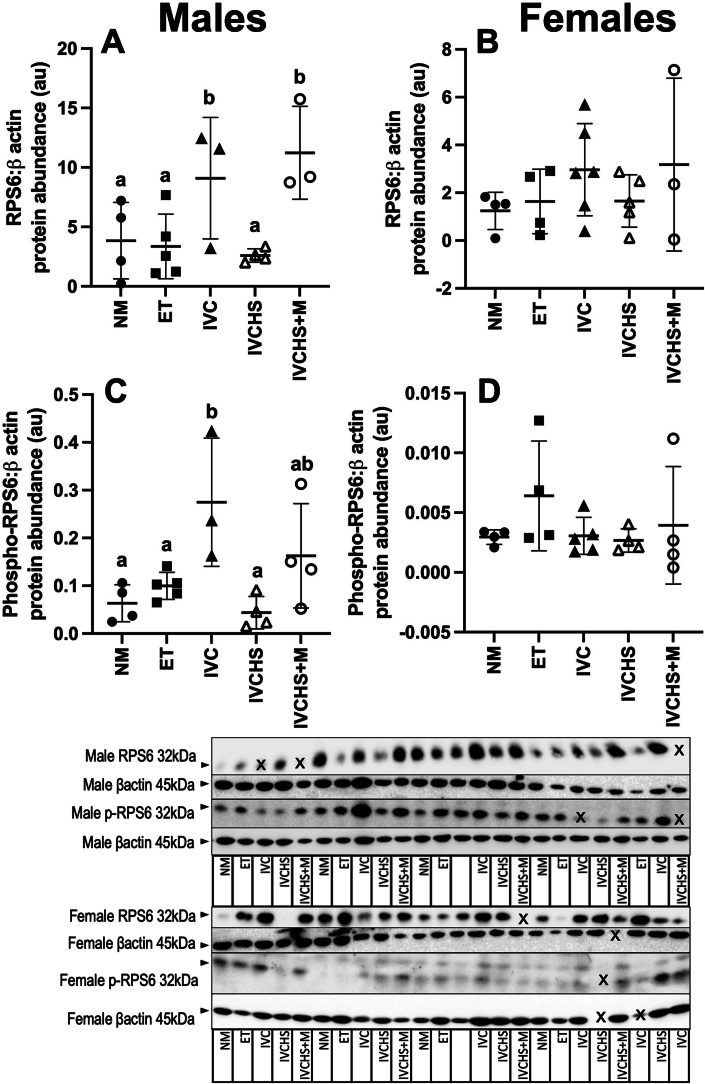
The protein abundance of RPS6 (P = 0.0105) was upregulated in the IVC and IVCHS+M groups, but only in males. However, the protein abundance of phospho-RPS6 (P = 0.0073) was increased in the IVC compared to the NM, ET and IVCHS groups, again only in males (Fig. 4).
Fig. 4.
Differential regulation of cardiac RPS6 abundance in IVC and IVCHS + M groups. Culture of sheep embryos in the absence of human serum and presence of human serum with group resulted in an increased protein abundance of RPS6 in males (A; P = 0.0105), but not in females (B). The protein abundance of phospho-RPS6 was upregulated in males in the IVC group (C; P = 0.0073), but not in the females (D). Values are mean ± SD. NM, natural mate (Males n = 4, Females n = 4); ET, embryo transfer (Males n = 5, Females n = 4); IVC, in vitro embryo culture (Males n = 3, Females n = 6); IVCHS, in vitro embryo culture with human serum (Males n = 4, Females n = 5); IVCHS+M, in vitro embryo culture with human serum and methionine supplementation (Males n = 3, Females n = 3). One sample per animal was run per western blot. Different superscript letters denote mean values that are significantly different from each other (P < 0.05). Data were analysed using a one-way ANOVA (treatment).
3.6. Cardiac protein translation
The mRNA expression of 4EBP1 was not different in any of the treatment groups compared to the NM group in both males and females (Table 2).
3.7. Markers of hypertrophy, fibrosis and apoptosis
There was no significant difference in the mRNA expression of markers of hypertrophy such as ANP and MHCβ. There was also no difference in mRNA expression of TGFβ and the ratio of the pro-apoptotic gene, Bax, to the anti-apoptotic gene, Bcl-2, which are key markers for fibrosis and apoptosis, respectively, in the ET, IVC, IVCHS or IVCHS+M compared to the NM group in both males and females (Table 2).
4. Discussion
Contrary to earlier reports, this study found that embryo culture and transfer did not result in large offspring syndrome (also known as Beckwith-Wiedemann Syndrome in humans) in the ET, IVC, IVCHS and IVCHS+M groups at birth [44,61]. We also found no change in lamb weight at 6 months in any treatment group. In addition to overgrowth of the body, it has been reported that in vitro culture with and without human serum supplementation alters growth of major organs, including the heart, liver and kidney [22,24]. We found an increase in absolute heart weight of male offspring in the IVC group, but no increase in relative heart weight or relative left ventricular weight. This is in contrast to previous studies demonstrating increased body weight in sheep at birth and increased heart weight at 1 year, despite similar body weight to naturally mated controls [29,62,63]. A possible explanation may be due to procedural differences or the culture media used [63]. The observed changes in only male offspring are similar to a mouse model of in vitro embryo culture where systolic blood pressure was increased in only males in postnatal life [64]. In another study, in vitro fertilization resulted in increased glucose intolerance in only males [65]. Male fetuses are in general more vulnerable to nutritional deprivation during pregnancy whereas females are more vulnerable in cases of overnutrition [66,67]. These dimorphic responses to manipulations during embryonic and fetal development may arise from differences in the ability of each sex to respond and adapt to specific challenges [67,68]. These sex-differences are also observed in later life where pre-menopause females are at a lower risk of cardiovascular diseases possibly due to the presence of estrogen [69,70]. However, males are exposed to higher levels of androgens such as testosterone, which are pro-hypertensive, and males are more susceptible to various cardiovascular diseases [69,70]. The results of this study therefore are consistent with the larger context of programmed cardiovascular disease, with males being more affected than females.
Heart development is particularly vulnerable to perturbations during pregnancy impacting cardiomyocyte endowment at birth and in early postnatal life [28,[71], [72], [73], [74]]. For example, early gestation onset fetal growth restriction results in fewer cardiomyocytes in late gestation [46] that this persists from 9 weeks after birth and into adulthood in sheep [48,75] and into adolescence in guinea pigs [47]. In this study, we found a sexually dimorphic response to the different culture media used where male embryos cultured in the presence of human serum (IVCHS) had less cardiomyocytes in comparison to offspring that were cultured without the addition of human serum (IVC). Furthermore, the addition of methionine (IVCHS+M) restored cardiomyocyte number to that of the IVC group. The differential cardiomyocyte endowment across the cultured groups may be due to cardiac remodelling processes such as an increase in autophagy or decreased proliferation earlier in gestation and requires further investigation [76,77]. We have previously shown that this protocol did not change blood pressure or its regulation by the sympathetic system [20], hence this change was in the absence of vascular stress. We therefore sought to understand the impact of IVC on molecular regulation of cardiac growth.
We explored signalling pathways that play a pivotal role in regulating cardiac growth in both fetal and postnatal life [28,78,79] and found that there was no difference in expression of Insulin-like growth factor (IGF1, IGF2, IGF1R, IGF2R) or PI3K in any of the treatment groups. Protein abundance of Akt1, Akt2 and phospho-Akt also remained unaltered, providing evidence that PI3K is not activated as a result of ET or IVC. However, the protein abundance of phospho-mTOR was upregulated in males in the IVC and IVCHS+M groups, with no change in mRNA and protein abundance of mTOR. This suggests that mTOR is not regulated by the traditional upstream PI3K pathway [80,81]. Evidence suggests that amino acid signalling can activate mTOR independent of the PI3K pathway and regulate mTOR through the Class III PI3K and mammalian Vps (vacuolar protein sorting) 34 homologue (hVps34) [[82], [83], [84], [85]]. In addition, mTOR can also be activated by amino acids through other intermediate signalling molecules such as Rag GTPases and Rheb [[85], [86], [87]]. Therefore, culture of embryos in media containing a variety of amino acids may increase the activation of mTOR and this effect persists into postnatal life.
mTOR activates two downstream molecules that contribute to hypertrophic growth; 4EBP1, which increases translational rate and efficiency, and RPS6, which increases translational capacity by enhancing biogenesis of ribosomes [88,89]. Although there was no change in 4EBP1 mRNA expression; we observed an increase in RPS6 expression in the IVC and IVCHS+M groups as well as phospho-RPS6 in IVC, but only in male offspring. This suggests an enhanced translational capacity may be present in males in the IVC group, potentially increasing hypertrophic growth in later life. An increase in translational efficiency of existing ribosomes is required for the initial and rapid response to stimuli for hypertrophic growth [90,91], but not sufficient for sustained hypertrophic growth, which is achieved by synthesis of new functional ribosomes through ribosomal biogenesis [[92], [93], [94]]. However, there was no change in relative heart weight in any of the treatment groups, which suggests that the increased expression of signalling molecules for ribosomal biogenesis may not have reached the required threshold for phenotypic changes in heart weight at this age. There was no difference in the expression of hypertrophic markers ANP and MHCβ suggesting that embryo transfer and culture does not result in pathological hypertrophy through activation of Gαq signalling. In vitro culture and transfer of embryos also had no effect on markers of fibrosis and apoptosis, such as TGFβ and the ratio of Bax to Bcl-2. This is in contrast to the findings from a study where periconceptional undernutrition resulted in increased activation of pathways leading to fibrosis and apoptosis in the twins of late gestation sheep fetuses [54]. Consistent with our study, changes in periconceptional nutrition also result in no changes in hypertrophic markers [6,95].
Serum free medium is now the standard for effective preimplantation embryo culture for all species [33,34]. However, serum is known to increase the effects of in vitro culture on embryo development and was used within this study to provide a model for assessing the effects of ART procedures that have already occurred prior to the transition to serum free medium being the gold standard. As we are studying the impact of different ART approaches on adolescent sheep, this data applies to procedures that occurred in the past where the human offspring are now more than twenty years old. Human serum is a complex mixture of a variety of substances such as proteins and peptides, including albumins, enzymes, hormones, nutrients, electrolytes, growth factors and other small organic molecules. Therefore, it is difficult to dissect the effects of each individual component on embryo development [96]. The highly variable nature of human serum in different batches and individuals also makes it difficult to pinpoint any specific component of the serum or a combination of components that may be inhibiting or normalising the effects of embryo culture media on different signalling molecules in the current study [97,98]. Although methylation was not measured within the present study, the general lack of change between the IVCHS and IVCHS+M groups also suggests that there may be either no effect of and/or no alteration in methylation levels with the addition of human serum. It is also possible that the additional methionine content in the medium may have a negative impact, as high levels of methionine can suppress the activity of DNA methyltransferase [99]. One suggestion is that oocytes and embryos at early stages may not have the proper machinery to metabolise excess methionine [100]. These intracellular mechanisms can result in suppression of the methylation cycle and alteration in normal methylation levels [99]. We did however find that methionine restored the deficiency in cardiomyocytes within the human serum treated offspring, implying a small but protective role in cardiac development.
It is possible that the chosen age (6 months old) within this study captured the beginnings of an upregulation to hypertrophic signalling and alterations in heart growth may be seen in later life. Six months of age is equivalent to adolescence in the sheep. Assessing the cardiovascular consequences of ARTs in adolescence allows us to begin to understand how in vitro embryo manipulation may impact the cardiovascular health of offspring not only in adulthood but across the life course. For example, previous evidence in six months old sheep suggested no change in basal blood pressure or alteration to the baroreflex in response to ART [20]. However, clinically a number of studies demonstrate elevated blood pressure in IVF children to early adulthood as well as vascular dysfunction in children at ∼11 years of age and right ventricular dysfunction in 12 year olds [[10], [11], [12], [13]]. These findings have a direct impact on ART procedures and the clinical follow-up of individuals from ART pregnancies at younger ages. A weakness of this study was that we were unable to assess cardiac function within the offspring. But given the lack of change in blood pressure or baroreflex [20] along with the lack of change in relative heart and LV weight within the current study support the notion of no cardiac functional change at adolescence in offspring of ART, but cardiovascular health may be detrimentally affected in later life.
5. Conclusion
The present study provides evidence for an increased rate of ribosomal biogenesis in IVC males, which may affect protein translational capacity as a result of embryo transfer and/or embryo culture. However, the increase in ribosomal biogenesis did not result in hypertrophic growth of the heart, which may be due to the young age of the lambs. It is possible that the present study has captured the beginnings of an upregulation to hypertrophic signalling and alterations in heart growth may be seen in later life as this process progresses. There was also evidence to suggest that the addition of human serum during IVC to male embryos was detrimental to later life cardiomyocyte endowment and that this was prevented by the addition of methionine to the culture medium. We speculate that upregulation of the pathways involving ribosomal biogenesis may lead to cardiac hypertrophy in later life and that this effect may be particularly evident in the male offspring from the IVCHS group due to their decreased cardiomyocyte endowment. Long-term cardiovascular health assessment is therefore warranted in those male individuals conceived with the aid of ART, especially those exposed to human serum.
Translational perspectives
The study shows that manipulations as early as the periconceptional period can result in differences in molecular mechanisms that are responsible for the alteration in heart development as a result of ART procedures and these alterations persist into postnatal life. This warrants the need for life course studies that are able to demonstrate the effects of ART/IVF in later stages of life.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
We acknowledge the support of members of the Early Origins of Adult Health Research Group, particularly Stacey Holman and Bang Hoang, for their expert assistance with sheep surgery and the conduct of the protocols using the pregnant ewes and lambs in this study.
CRediT authorship contribution statement
ICMcM, SMM, DOK, SW and JLM were responsible for the conception and design of the experiments.
MP, SZ, KJB, DOK, SW, SR, JK and JLM were each involved in data acquisition.
MP, MCL, SZ, KJB, JRN and JLM were involved in analysis and interpretation of the data.
MP, MCL, SZ and JLM drafted the article and all authors contributed to and approved the final version.
Funding
The animal component of this project was funded by a NHMRC Project Grant (ICMcM & JLM). The molecular analysis component of this project was funded by a South Australian Cardiovascular Research Network Fellowship (CR10A4988). JLM was funded by a South Australian Cardiovascular Research Network Fellowship (CR10A4988) and a NHMRC Career Development Fellowship (APP1066916). Centre for Stochastic Geometry and Advanced Bioimaging is supported by Villum Foundation.
Data availability
All data supporting the results are presented in the manuscript.
References
- 1.Watkins A.J., Platt D., Papenbrock T., Wilkins A., Eckert J.J., Kwong W.Y., et al. Mouse embryo culture induces changes in postnatal phenotype including raised systolic blood pressure. PNAS. 2007;104(13):5449–5454. doi: 10.1073/pnas.0610317104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fernández-Gonzalez R., Moreira P., Bilbao A., Jiménez A., Pérez-Crespo M., Ramírez M.A., et al. Long-term effect of in vitro culture of mouse embryos with serum on mRNA expression of imprinting genes, development, and behavior. PNAS. 2004;101(16):5880–5885. doi: 10.1073/pnas.0308560101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Celermajer D.S. Manipulating nature: might there be a cardiovascular price to pay for the miracle of assisted conception? Circulation. 2012;125(15):1832–1834. doi: 10.1161/CIRCULATIONAHA.112.100479. [DOI] [PubMed] [Google Scholar]
- 4.Ceelen M., van Weissenbruch M.M., Vermeiden J.P.W., van Leeuwen F.E., Delemarre-van de Waal H.A. Growth and development of children born after in vitro fertilization. Fertil Steril. 2008;90(5):1662–1673. doi: 10.1016/j.fertnstert.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 5.Padhee M., Zhang S., Lie S., Wang K.C., Botting K.J., McMillen I.C., et al. The periconceptional environment and cardiovascular disease: does in vitro embryo culture and transfer influence cardiovascular development and health? Nutrients. 2015;7(3):1378–1425. doi: 10.3390/nu7031378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lie S., Sim S.M., McMillen I.C., Williams-Wyss O., MacLaughlin S.M., Kleemann D.O., et al. Maternal undernutrition around the time of conception and embryo number each impact on the abundance of key regulators of cardiac growth and metabolism in the fetal sheep heart. J Dev Orig Health Dis. 2013;4(05):377–390. doi: 10.1017/S2040174413000354. [DOI] [PubMed] [Google Scholar]
- 7.Vaughan O.R., Rosario F.J., Chan J., Cox L.A., Ferchaud-Roucher V., Zemski-Berry K.A., et al. Maternal obesity causes fetal cardiac hypertrophy and alters adult offspring myocardial metabolism in mice. J Physiol. 2022;600(13):3169–3191. doi: 10.1113/JP282462. [DOI] [PubMed] [Google Scholar]
- 8.Källén B., Finnström O., Lindam A., Nilsson E., Nygren K.-G., Otterblad P.O. Congenital malformations in infants born after in vitro fertilization in Sweden. Birth Defects Res A Clin Mol Teratol. 2010;88(3):137–143. doi: 10.1002/bdra.20645. [DOI] [PubMed] [Google Scholar]
- 9.Hansen M., Kurinczuk J.J., Bower C., Webb S. The risk of major birth defects after intracytoplasmic sperm injection and in vitro fertilization. N Engl J Med. 2002;346(10):725–730. doi: 10.1056/NEJMoa010035. [DOI] [PubMed] [Google Scholar]
- 10.Ceelen M., van Weissenbruch M.M., Vermeiden J.P.W., van Leeuwen F.E., Delemarre-van de Waal H.A. Cardiometabolic differences in children born after in vitro fertilization: follow-up study. J Clin Endocrinol Metab. 2008;93(5):1682–1688. doi: 10.1210/jc.2007-2432. [DOI] [PubMed] [Google Scholar]
- 11.Scherrer U., Rimoldi S.F., Rexhaj E., Stuber T., Duplain H., Garcin S., et al. Systemic and pulmonary vascular dysfunction in children conceived by assisted reproductive technologies. Circulation. 2012;125:1890–1896. doi: 10.1161/CIRCULATIONAHA.111.071183. [DOI] [PubMed] [Google Scholar]
- 12.Guo X.Y., Liu X.M., Jin L., Wang T.T., Ullah K., Sheng J.Z., et al. Cardiovascular and metabolic profiles of offspring conceived by assisted reproductive technologies: a systematic review and meta-analysis. Fertil Steril. 2017;107(3):622–631.e5. doi: 10.1016/j.fertnstert.2016.12.007. [DOI] [PubMed] [Google Scholar]
- 13.von Arx R., Allemann Y., Sartori C., Rexhaj E., Cerny D., de Marchi S.F., et al. Right ventricular dysfunction in children and adolescents conceived by assisted reproductive technologies. J Appl Physiol. 2015;118(10):1200–1206. doi: 10.1152/japplphysiol.00533.2014. [DOI] [PubMed] [Google Scholar]
- 14.Wang Q., Zhang Y., Le F., Wang N., Zhang F., Luo Y., et al. Alteration in the expression of the renin-angiotensin system in the myocardium of mice conceived by in vitro fertilization. Biol Reprod. 2018;99(6):1276–1288. doi: 10.1093/biolre/ioy158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aljahdali A., Airina R., Velazquez M.A., Sheth B., Wallen K., Osmond C., et al. The duration of embryo culture after mouse IVF differentially affects cardiovascular and metabolic health in male offspring. Hum Reprod. 2020;35(11):2497–2514. doi: 10.1093/humrep/deaa205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elhakeem A., Taylor A.E., Inskip H.M., Huang J.Y., Mansell T., Rodrigues C., et al. Long-term cardiometabolic health in people born after assisted reproductive technology: a multi-cohort analysis. Eur Heart J. 2023;44:1464–1473. doi: 10.1093/eurheartj/ehac726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shiloh S.R., Sheiner E., Wainstock T., Walfisch A., Segal I., Landau D., et al. Long-term cardiovascular morbidity in children born following fertility treatment. J Pediatr. 2019;204:84–88.e2. doi: 10.1016/j.jpeds.2018.08.070. [DOI] [PubMed] [Google Scholar]
- 18.Wijs L.A., Doherty D.A., Keelan J.A., Burton P., Yovich J.L., Beilin L., et al. Comparison of the cardiometabolic profiles of adolescents conceived through ART with those of a non-ART cohort. Hum Reprod. 2022;37(8):1880–1895. doi: 10.1093/humrep/deac122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuiper D., Hoek A., la Bastide-van Gemert S., Seggers J., Mulder D.J., Haadsma M., et al. Cardiovascular health of 9-year-old IVF offspring: no association with ovarian hyperstimulation and the in vitro procedure. Hum Reprod. 2017;32(12):2540–2548. doi: 10.1093/humrep/dex323. [DOI] [PubMed] [Google Scholar]
- 20.Padhee M., McMillen I.C., Zhang S., MacLaughlin S.M., Armitage J.A., Head G.A., et al. Impact of in vitro embryo culture and transfer on blood pressure regulation in the adolescent lamb. J Dev Orig Health Dis. 2021;12(5):731–737. doi: 10.1017/S2040174420001014. [DOI] [PubMed] [Google Scholar]
- 21.Young L.E., Sinclair K.D., Wilmut I. Large offspring syndrome in cattle and sheep. Rev Reprod. 1998;3(3):155–163. doi: 10.1530/ror.0.0030155. [DOI] [PubMed] [Google Scholar]
- 22.Young L.E., Fernandes K., McEvoy T.G., Butterwith S.C., Gutierrez C.G., Carolan C., et al. Epigenetic change in IGF2R is associated with fetal overgrowth after sheep embryo culture. Nat Genet. 2001;27(2):153–154. doi: 10.1038/84769. [DOI] [PubMed] [Google Scholar]
- 23.Chen H., Zhang L., Yue F., Cui C., Li Y., Zhang Q., et al. Effects of assisted reproductive technology on gene expression in heart and spleen tissues of adult offspring mouse. Front Endocrinol. 2023;14:1035161. doi: 10.3389/fendo.2023.1035161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sinclair K.D., McEvoy T.G., Maxfield E.K., Maltin C.A., Young L.E., Wilmut I., et al. Aberrant fetal growth and development after in vitro culture of sheep zygotes. J Reprod Fertil. 1999;116(1):177–186. doi: 10.1530/jrf.0.1160177. [DOI] [PubMed] [Google Scholar]
- 25.Farin P.W., Farin C.E. Transfer of bovine embryos produced in vivo or in vitro: survival and fetal development. Biol Reprod. 1995;52(3):676–682. doi: 10.1095/biolreprod52.3.676. [DOI] [PubMed] [Google Scholar]
- 26.Sinclair K.D., McEvoy T.G., Carolan C., Maxfield E.K., Maltin C.A., Young L.E., et al. Conceptus growth and development following in vitro culture of ovine embryos in media supplemented with bovine sera. Theriogenology. 1998;49(1):218. [Google Scholar]
- 27.Morrison J.L., Berry M.J., Botting K.J., Darby J.R.T., Frasch M.G., Gatford K.L., et al. Improving pregnancy outcomes in humans through studies in sheep. Am J Physiol Regul Integr Comp Physiol. 2018;315(6):R1123–r1153. doi: 10.1152/ajpregu.00391.2017. [DOI] [PubMed] [Google Scholar]
- 28.Lock M.C., Tellam R.L., Botting K.J., Wang K.C.W., Selvanayagam J.B., Brooks D.A., et al. The role of miRNA regulation in fetal cardiomyocytes, cardiac maturation and the risk of heart disease in adults. J Physiol. 2018;596(23):5625–5640. doi: 10.1113/JP276072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McEvoy T.G., Sinclair K.D., Broadbent P.J., Goodhand K.L., Robinson J.J. Post-natal growth and development of Simmental calves derived from in vivo or in vitro embryos. Reprod Fertil Dev. 1998;10(6):459–464. doi: 10.1071/rd98126. [DOI] [PubMed] [Google Scholar]
- 30.Jonker S.S., Zhang L., Louey S., Giraud G.D., Thornburg K.L., Faber J.J. Myocyte enlargement, differentiation, and proliferation kinetics in the fetal sheep heart. J Appl Physiol. 2007;102(3):1130–1142. doi: 10.1152/japplphysiol.00937.2006. [DOI] [PubMed] [Google Scholar]
- 31.Burrell J.H., Boyn A.M., Kumarasamy V., Hsieh A., Head S.I., Lumbers E.R. Growth and maturation of cardiac myocytes in fetal sheep in the second half of gestation. Anat Rec A Discov Mol Cell Evol Biol. 2003;274A(2):952–961. doi: 10.1002/ar.a.10110. [DOI] [PubMed] [Google Scholar]
- 32.Botting K.J., Wang K.C., Padhee M., McMillen I.C., Summers-Pearce B., Rattanatray L., et al. Early origins of heart disease: low birth weight and determinants of cardiomyocyte endowment. Clin Exp Pharmacol Physiol. 2012;39(9):814–823. doi: 10.1111/j.1440-1681.2011.05649.x. [DOI] [PubMed] [Google Scholar]
- 33.Chronopoulou E., Harper J.C. IVF culture media: past, present and future. Hum Reprod Update. 2015;21(1):39–55. doi: 10.1093/humupd/dmu040. [DOI] [PubMed] [Google Scholar]
- 34.Swain J.E. In: In vitro fertilization: a textbook of current and emerging methods and devices. Nagy Z.P., Varghese A.C., Agarwal A., editors. Springer International Publishing; Cham: 2019. Culture media in IVF: Decisions for the laboratory; pp. 105–119. [Google Scholar]
- 35.Grundy D. Principles and standards for reporting animal experiments in The Journal of Physiology and Experimental Physiology. J Physiol. 2015;593(Pt 12):2547–2549. doi: 10.1113/JP270818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Russell W.M.S., Burch R.L. Methuen; 1959. The principles of humane experimental technique. [Google Scholar]
- 37.Lawes C.M.M., Bennett D.A., Feigin V.L., Rodgers A. Blood pressure and stroke: an overview of published reviews. Stroke. 2004;35(3):776–785. doi: 10.1161/01.STR.0000116869.64771.5A. [DOI] [PubMed] [Google Scholar]
- 38.Walker S.K., Smith D.H., Seamark R.F. Timing of multiple ovulations in the ewe after treatment with FSH or PMSG with and without GnRH. J Reprod Fertil. 1986;77(1):135–142. doi: 10.1530/jrf.0.0770135. [DOI] [PubMed] [Google Scholar]
- 39.Holm P., Walker S.K., Seamark R.F. Embryo viability, duration of gestation and birth weight in sheep after transfer of in vitro matured and in vitro fertilized zygotes cultured in vitro or in vivo. Reproduction. 1996;107(2):175–181. doi: 10.1530/jrf.0.1070175. [DOI] [PubMed] [Google Scholar]
- 40.Kakar M.A., Maddocks S., Lorimer M.F., Kleemann D.O., Rudiger S.R., Hartwich K.M., et al. The effect of peri-conception nutrition on embryo quality in the superovulated ewe. Theriogenology. 2005;64(5):1090–1103. doi: 10.1016/j.theriogenology.2004.12.017. [DOI] [PubMed] [Google Scholar]
- 41.Kelly J.M., Kleemann D.O., Walker S.K. Enhanced efficiency in the production of offspring from 4- to 8-week-old lambs. Theriogenology. 2005;63(7):1876–1890. doi: 10.1016/j.theriogenology.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 42.Kleemann D.O., Walker S.K., Hartwich K.M., Fong L., Seamark R.F., Robinson J.S., et al. Fetoplacental growth in sheep administered progesterone during the first three days of pregnancy. Placenta. 2001;22(1):14–23. doi: 10.1053/plac.2000.0594. [DOI] [PubMed] [Google Scholar]
- 43.Walker S.K., Hill J.L., Kleemann D.O., Nancarrow C.D. Development of ovine embryos in synthetic oviductal fluid containing amino acids at oviductal fluid concentrations. Biol Reprod. 1996;55(3):703–708. doi: 10.1095/biolreprod55.3.703. [DOI] [PubMed] [Google Scholar]
- 44.Walker S.K., Heard T.M., Seamark R.F. In vitro culture of sheep embryos without co-culture: successes and perspectives. Theriogenology. 1992;37(1):111–126. [Google Scholar]
- 45.Brüel A., Nyengaard J.R. Design–based stereological estimation of the total number of cardiac myocytes in histological sections. Basic Res Cardiol. 2005;100(4):311–319. doi: 10.1007/s00395-005-0524-9. [DOI] [PubMed] [Google Scholar]
- 46.Botting K.J., McMillen I.C., Forbes H., Nyengaard J.R., Morrison J.L. Chronic hypoxemia in late gestation decreases cardiomyocyte number but does not change expression of hypoxia-responsive genes. J Am Heart Assoc. 2014;3(4) doi: 10.1161/JAHA.113.000531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Botting K.J., Loke X.Y., Zhang S., Andersen J.B., Nyengaard J.R., Morrison J.L. IUGR decreases cardiomyocyte endowment and alters cardiac metabolism in a sex- and cause-of-IUGR-specific manner. Am J Physiol Regul Integr Comp Physiol. 2018;315(1):R48–r67. doi: 10.1152/ajpregu.00180.2017. [DOI] [PubMed] [Google Scholar]
- 48.Stacy V., De Matteo R., Brew N., Sozo F., Probyn M.E., Harding R., et al. The influence of naturally occurring differences in birthweight on ventricular cardiomyocyte number in sheep. Anat Rec. 2009;292(1):29–37. doi: 10.1002/ar.20789. [DOI] [PubMed] [Google Scholar]
- 49.Wang K.C., Zhang L., McMillen I.C., Botting K.J., Duffield J.A., Zhang S., et al. Fetal growth restriction and the programming of heart growth and cardiac insulin-like growth factor 2 expression in the lamb. J Physiol. 2011;589(Pt 19):4709–4722. doi: 10.1113/jphysiol.2011.211185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bustin S.A., Benes V., Garson J.A., Hellemans J., Huggett J., Kubista M., et al. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem. 2009;55(4):611. doi: 10.1373/clinchem.2008.112797. [DOI] [PubMed] [Google Scholar]
- 51.Wang K.C., Lim C.H., McMillen I.C., Duffield J.A., Brooks D.A., Morrison J.L. Alteration of cardiac glucose metabolism in association to low birth weight: experimental evidence in lambs with left ventricular hypertrophy. Metabolism. 2013;62(11):1662–1672. doi: 10.1016/j.metabol.2013.06.013. [DOI] [PubMed] [Google Scholar]
- 52.Vandesompele J., De Preter K., Pattyn F., Poppe B., Van Roy N., De Paepe A., et al. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3(7) doi: 10.1186/gb-2002-3-7-research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McGillick E.V., Morrison J.L., McMillen I.C., Orgeig S. Intrafetal glucose infusion alters glucocorticoid signalling and reduces surfactant protein mRNA expression in the lung of the late gestation sheep fetus. Am J Physiol Regul Integr Comp Physiol. 2014;307:R538–R545. doi: 10.1152/ajpregu.00053.2014. [DOI] [PubMed] [Google Scholar]
- 54.Lie S., Morrison J.L., Williams-Wyss O., Ozanne S.E., Zhang S., Walker S.K., et al. Impact of embryo number and periconceptional undernutrition on factors regulating adipogenesis, lipogenesis, and metabolism in adipose tissuein the sheep fetus. Am J Physiol Endocrinol Metab. 2013;305(8):E931–E941. doi: 10.1152/ajpendo.00180.2013. [DOI] [PubMed] [Google Scholar]
- 55.Lock M.C., Darby J.R.T., Soo J.Y., Brooks D.A., Perumal S.R., Selvanayagam J.B., et al. Differential response to injury in fetal and adolescent sheep hearts in the immediate post-myocardial infarction period. Front Physiol. 2019;10:208. doi: 10.3389/fphys.2019.00208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang S., Rattanatray L., MacLaughlin S.M., Cropley J.E., Suter C.M., Molloy L., et al. Periconceptional undernutrition in normal and overweight ewes leads to increased adrenal growth and epigenetic changes in adrenal IGF2/H19 gene in offspring. FASEB J. 2010;24(8):2772–2782. doi: 10.1096/fj.09-154294. [DOI] [PubMed] [Google Scholar]
- 57.Hellemans J., Mortier G., De Paepe A., Speleman F., Vandesompele J. qBase relative quantification framework and software for management and automated analysis of real-time quantitative PCR data. Genome Biol. 2007;8(2):1–14. doi: 10.1186/gb-2007-8-2-r19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Soo P.S., Hiscock J., Botting K.J., Roberts C.T., Davey A.K., Morrison J.L. Maternal undernutrition reduces P-glycoprotein in guinea pig placenta and developing brain in late gestation. Reprod Toxicol. 2012;33(3):374–381. doi: 10.1016/j.reprotox.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 59.Nicholas L.M., Rattanatray L., MacLaughlin S.M., Ozanne S.E., Kleemann D.O., Walker S.K., et al. Differential effects of maternal obesity and weight loss in the periconceptional period on the epigenetic regulation of hepatic insulin-signaling pathways in the offspring. FASEB J. 2013;27(9):3786–3796. doi: 10.1096/fj.13-227918. [DOI] [PubMed] [Google Scholar]
- 60.Zhang S., Morrison J.L., Gill A., Rattanatray L., MacLaughlin S.M., Kleemann D., et al. Maternal dietary restriction during the periconceptional period in normal-weight or obese ewes results in adrenocortical hypertrophy, an up-regulation of the JAK/STAT and down-regulation of the IGF1R signaling pathways in the adrenal of the postnatal lamb. Endocrinology. 2013;154(12):4650–4662. doi: 10.1210/en.2013-1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Filippi G., McKusick V.A. The Beckwith-Wiedmann syndrome. Medicine (Baltimore) 1970;49(4):279–298. doi: 10.1097/00005792-197007000-00002. [DOI] [PubMed] [Google Scholar]
- 62.Walker S.K., Hartwich K.M., Seamark R.F. The production of unusually large offspring following embryo manipulation: concepts and challenges. Theriogenology. 1996;45(1):111–120. [Google Scholar]
- 63.Wilson J.M., Williams J.D., Bondioli K.R., Looney C.R., Westhusin M.E., McCalla D.F. Comparison of birth weight and growth characteristics of bovine calves produced by nuclear transfer (cloning), embryo transfer and natural mating. Anim Reprod Sci. 1995;38(1–2):73–83. [Google Scholar]
- 64.Watkins A.J., Fleming T.P. Blastocyst environment and its influence on offspring cardiovascular health: the heart of the matter. J Anat. 2009;215(1):52–59. doi: 10.1111/j.1469-7580.2008.01033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Donjacour A., Liu X., Lin W., Simbulan R., Rinaudo P.F. In vitro fertilization affects growth and glucose metabolism in a sex-specific manner in an outbred mouse model. Biol Reprod. 2014;90(4):80. doi: 10.1095/biolreprod.113.113134. 1-10-80, 1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Aiken C.E., Ozanne S.E. Sex differences in developmental programming models. Reproduction. 2013;145(1):R1–13. doi: 10.1530/REP-11-0489. [DOI] [PubMed] [Google Scholar]
- 67.Gilbert J.S., Banek C.T. INTECH Open Access Publisher; 2012. Sex differences in the developmental programming of adult disease. [Google Scholar]
- 68.Meakin A.S., Cuffe J.S.M., Darby J.R.T., Morrison J.L., Clifton V.L. Let’s talk about placental sex, baby: understanding mechanisms that drive female- and male-specific fetal growth and developmental outcomes. Int J Mol Sci. 2021;22(12) doi: 10.3390/ijms22126386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Intapad S., Ojeda N.B., Dasinger J.H., Alexander B.T. Sex differences in the developmental origins of cardiovascular disease. Physiology. 2014;29(2):122–132. doi: 10.1152/physiol.00045.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Armeni E., Lambrinoudaki I. Androgens and cardiovascular disease in women and men. Maturitas. 2017;104:54–72. doi: 10.1016/j.maturitas.2017.07.010. [DOI] [PubMed] [Google Scholar]
- 71.Goh J.M., Bensley J.G., Kenna K., Sozo F., Bocking A.D., Brien J., et al. Alcohol exposure during late gestation adversely affects myocardial development with implications for postnatal cardiac function. Am J Physiol Heart Circ Physiol. 2011;300(2):H645–H651. doi: 10.1152/ajpheart.00689.2010. [DOI] [PubMed] [Google Scholar]
- 72.Ream M., Ray A.M., Chandra R., Chikaraishi D.M. Early fetal hypoxia leads to growth restriction and myocardial thinning. Am J Physiol Regul Integr Comp Physiol. 2008;295(2):R583–R595. doi: 10.1152/ajpregu.00771.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Louey S., Jonker S.S., Giraud G.D., Thornburg K.L. Placental insufficiency decreases cell cycle activity and terminal maturation in fetal sheep cardiomyocytes. J Physiol. 2007;580(2):639–648. doi: 10.1113/jphysiol.2006.122200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Corstius H.B., Zimanyi M.A., Maka N., Herath T., Thomas W., van der Laarse A., et al. Effect of intrauterine growth restriction on the number of cardiomyocytes in rat hearts. Pediatr Res. 2005;57(6):796–800. doi: 10.1203/01.PDR.0000157726.65492.CD. [DOI] [PubMed] [Google Scholar]
- 75.Vranas S., Heinemann G.K., Liu H., De Blasio M.J., Owens J.A., Gatford K.L., et al. Small size at birth predicts decreased cardiomyocyte number in the adult ovine heart. J Dev Orig Health Dis. 2017;8(5):618–625. doi: 10.1017/S2040174417000381. [DOI] [PubMed] [Google Scholar]
- 76.Porrello E.R., Widdop R.E., Delbridge L.M.D. Early origins of cardiac hypertrophy: does cardiomyocyte attrition programme for pathological ‘catch-up’ growth of the heart? Clin Exp Pharmacol Physiol. 2008;35(11):1358–1364. doi: 10.1111/j.1440-1681.2008.05036.x. [DOI] [PubMed] [Google Scholar]
- 77.Hill J. Autophagy in cardiac plasticity and disease. Pediatr Cardiol. 2011;32(3):282–289. doi: 10.1007/s00246-010-9883-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Luo J., McMullen J.R., Sobkiw C.L., Zhang L., Dorfman A.L., Sherwood M.C., et al. Class IA phosphoinositide 3-kinase regulates heart size and physiological cardiac hypertrophy. Mol Cell Biol. 2005;25(21):9491–9502. doi: 10.1128/MCB.25.21.9491-9502.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Engelman J.A., Ji L., Cantley L.C. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet. 2006;7(8):606–619. doi: 10.1038/nrg1879. [DOI] [PubMed] [Google Scholar]
- 80.Wullschleger S., Loewith R., Hall M.N. TOR signaling in growth and metabolism. Cell. 2006;124(3):471–484. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 81.Laplante M., Sabatini D.M. mTOR signaling at a glance. J Cell Sci. 2009;122(20):3589–3594. doi: 10.1242/jcs.051011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nobukuni T., Joaquin M., Roccio M., Dann S.G., Kim S.Y., Gulati P., et al. Amino acids mediate mTOR/raptor signaling through activation of class 3 phosphatidylinositol 3OH-kinase. PNAS. 2005;102(40):14238–14243. doi: 10.1073/pnas.0506925102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Byfield M.P., Murray J.T., Backer J.M. hVps34 is a nutrient-regulated lipid kinase required for activation of p70 S6 kinase. J Biol Chem. 2005;280(38):33076–33082. doi: 10.1074/jbc.M507201200. [DOI] [PubMed] [Google Scholar]
- 84.Smith E.M., Finn S.G., Tee A.R., Browne G.J., Proud C.G. The tuberous sclerosis protein TSC2 is not required for the regulation of the mammalian target of rapamycin by amino acids and certain cellular stresses. J Biol Chem. 2005;280(19):18717–18727. doi: 10.1074/jbc.M414499200. [DOI] [PubMed] [Google Scholar]
- 85.Takahara T., Amemiya Y., Sugiyama R., Maki M., Shibata H. Amino acid-dependent control of mTORC1 signaling: a variety of regulatory modes. J Biomed Sci. 2020;27(1):87. doi: 10.1186/s12929-020-00679-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bai X., Jiang Y. Key factors in mTOR regulation. Cell Mol Life Sci. 2010;67(2):239–253. doi: 10.1007/s00018-009-0163-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kim J., Guan K.L. Amino acid signaling in TOR activation. Annu Rev Biochem. 2011;80:1001–1032. doi: 10.1146/annurev-biochem-062209-094414. [DOI] [PubMed] [Google Scholar]
- 88.Proud C.G. Ras, PI3-kinase and mTOR signaling in cardiac hypertrophy. Cardiovasc Res. 2004;63(3):403–413. doi: 10.1016/j.cardiores.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 89.Sciarretta S., Forte M., Frati G., Sadoshima J. New insights into the role of mTOR signaling in the cardiovascular system. Circ Res. 2018;122(3):489–505. doi: 10.1161/CIRCRESAHA.117.311147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nagatomo Y., Carabello B.A., Hamawaki M., Nemoto S., Matsuo T., McDermott P.J. Translational mechanisms accelerate the rate of protein synthesis during canine pressure-overload hypertrophy. Am J Physiol Heart Circ Physiol. 1999;277(6):H2176–H2184. doi: 10.1152/ajpheart.1999.277.6.H2176. [DOI] [PubMed] [Google Scholar]
- 91.Morgan H.E., Beinlich C.J. Contributions of increased efficiency and capacity of protein synthesis to rapid cardiac growth. Mol Cell Biol. 1997;176(1–2):145–151. [PubMed] [Google Scholar]
- 92.Brandenburger Y., Jenkins A., Autelitano D.J., Hannan R.D. Increased expression of UBF is a critical determinant for rRNA synthesis and hypertrophic growth of cardiac myocytes. FASEB J. 2001;15:2051–2053. doi: 10.1096/fj.01-0853fje. [DOI] [PubMed] [Google Scholar]
- 93.Hannan R.D., Jenkins A., Jenkins A.K., Brandenburger Y. Cardiac hypertrophy: a matter of translation. Clin Exp Pharmacol Physiol. 2003;30(8):517–527. doi: 10.1046/j.1440-1681.2003.03873.x. [DOI] [PubMed] [Google Scholar]
- 94.Hannan R.D., Stefanovsky V., Taylor L., Moss T., Rothblum L.I. Overexpression of the transcription factor UBF1 is sufficient to increase ribosomal DNA transcription in neonatal cardiomyocytes: implications for cardiac hypertrophy. PNAS. 1996;93(16):8750–8755. doi: 10.1073/pnas.93.16.8750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rohini A., Agrawal N., Koyani C.N., Singh R. Molecular targets and regulators of cardiac hypertrophy. Pharmacol Res. 2010;61(4):269–280. doi: 10.1016/j.phrs.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 96.Psychogios N., Hau D.D., Peng J., Guo A.C., Mandal R., Bouatra S., et al. The human serum metabolome. PloS One. 2011;6(2) doi: 10.1371/journal.pone.0016957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gardner D.K. Informa Healthcare; 2007. In vitro fertilization: a practical approach. [Google Scholar]
- 98.Vajta G., Rienzi L., Cobo A., Yovich J. Embryo culture: can we perform better than nature? Reprod Biomed Online. 2010;20(4):453–469. doi: 10.1016/j.rbmo.2009.12.018. [DOI] [PubMed] [Google Scholar]
- 99.Dunlevy L.P.E., Burren K.A., Chitty L.S., Copp A.J., Greene N.D.E. Excess methionine suppresses the methylation cycle and inhibits neural tube closure in mouse embryos. FEBS Lett. 2006;580(11):2803–2807. doi: 10.1016/j.febslet.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 100.Kwong W.Y., Adamiak S.J., Gwynn A., Singh R., Sinclair K.D. Endogenous folates and single-carbon metabolism in the ovarian follicle, oocyte and pre-implantation embryo. Reproduction. 2010;139(4):705–715. doi: 10.1530/REP-09-0517. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data supporting the results are presented in the manuscript.