Abstract
Anomalous hepatic venous connection without other cardiac anomalies is a rare condition but does not have high clinical significance. We describe a patient in whom a portion of the left hepatic vein was directly connected to the left atrium, without intracardiac or extracardiac anomalies. A left-to-right shunt with tricuspid regurgitation and atrial fibrillation occurred from right atrial volume overload. Shunt ligation with tricuspid valvuloplasty and a maze procedure were performed as a treatment. This report is meaningful because we describe a case of increased preload caused by a chronic left atrium–hepatic vein shunt that resulted in right-sided heart failure.
Anatomic shunts can cause venous admixture, which may result in hypoxemia. Anomalous hepatic venous connection, without other cardiac anomalies, is a rare example of an anatomic shunt. However, this condition does not exhibit high clinical significance because blood still drains into the right side of the heart through the superior vena cava or inferior vena cava (IVC).1 In most reports, partial anomalous hepatic venous drainage typically involves drainage into the right atrium (RA). The clinical significance of partial anomalous hepatic venous drainage is also moderate, except for situations requiring cannulation during cardiac surgery. We report the case of an adult patient, without other intracardiac or extracardiac anomalies, in whom a portion of the hepatic vein (HV) was directly connected to the left atrium (LA).
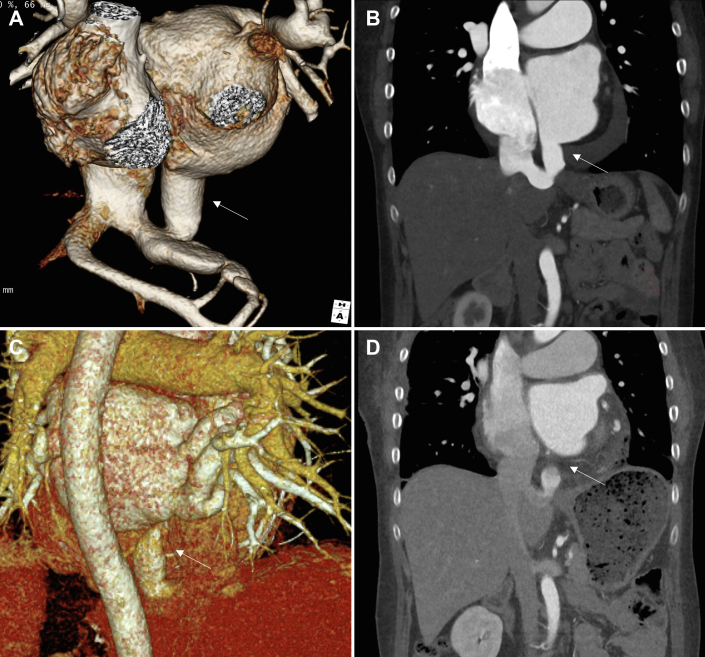
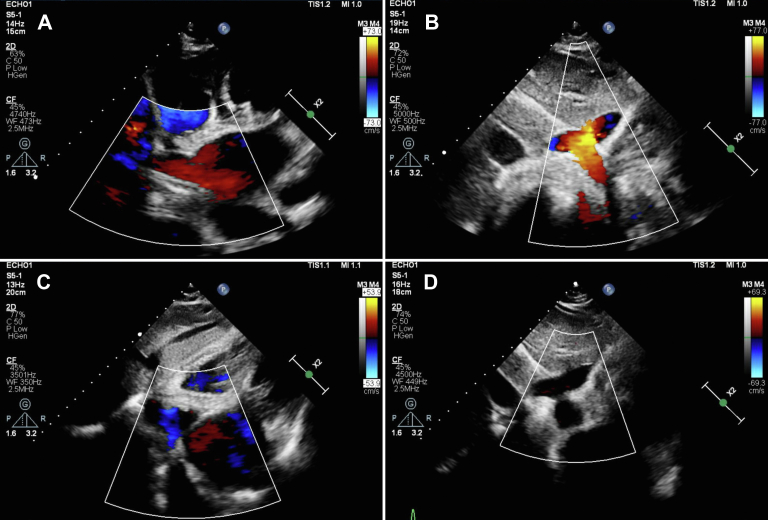
A 58-year-old woman with no specific adverse medical history visited a clinic with dyspnea, cough, and leg edema that arose 3 weeks before visiting the clinic. The patient was referred to a tertiary medical center after cardiomegaly (shown on chest radiography) and atrial fibrillation (AF; shown on electrocardiography) were observed. Echocardiography revealed an enlarged LA (54 mm), moderate pulmonary hypertension (right ventricle [RV]–RA pressure gradient of 43.6 mm Hg), and a shunt draining from the LA into the HV (Figures 1A, 1B, 3A, 3B). Grade 3 tricuspid regurgitation (TR) was also observed (TR jet area, 12 cm2; proximal isovelocity surface area radius, 6.7 mm; effective regurgitant orifice area, 26.5 mm2). A roughly 6-mm left-to-right shunt through the patent foramen ovale (PFO) was also observed, but left ventricular function was preserved (with an ejection fraction of 65%). Although dyspnea on exertion was present, we did not perform preoperative cardiac catheterization because the noninvasive preoperative evaluation (echocardiography and computed tomography) clearly showed that the symptoms were due to heart failure caused by the left-to-right shunt. The patient underwent surgical treatment (Figure 2), including LA-to-HV shunt internal closure through the LA, PFO closure, MC3 (Edwards Lifesciences) tricuspid annuloplasty (28 mm), and biatrial maze operation (using cryoablation, with LA appendage preservation) through a right anterior mini-thoracotomy using SoloAssist (Stryker). Surgical findings showed severe atrial wall thinning, for both atria, and tricuspid valve annulus dilation. On postoperative day (POD) 2, the patient was transferred to the general ward; on POD 3, LA-HV shunt connection was not observed on computed tomography (Figures 1C, 1D). Remnant LA-HV shunt flow was not observed on echocardiography, which was performed on POD 4 (Figures 3C, 3D). With mild TR (TR jet area, 6 cm2; proximal isovelocity surface area radius, 3.1 mm; effective regurgitant orifice area, 5.08 mm2), RV contractility was reduced and resting pulmonary hypertension was observed (pressure gradient between RV-RA, 64 mm Hg), although subjective symptoms of the patient had improved. After maze surgery, the conversion to sinus rhythm was observed in the heart. After her discharge from the hospital on POD 8, the patient was followed up on an outpatient basis; sildenafil and diuretics were prescribed to manage her TR and to reduce RV contractility.
Figure 1.
Preoperative and postoperative computed tomography of the patient’s heart and great vessels. (A, B) Preoperative computed tomography images of the patient’s heart, (A) 3-dimensional reconstruction and (B) sagittal view. The arrow indicates the hepatic vein connected to the left atrium. (C, D) Postoperative computed tomography images of the patient’s heart, (C) 3-dimensional reconstruction and (D) sagittal view. The arrow indicates the disconnect of the hepatic vein from the left atrium after shunt ligation.
Figure 3.
Preoperative and postoperative echocardiography. (A, B) A shunt draining into the hepatic vein from the left atrium, observed on preoperative color Doppler echocardiography. (C, D) After shunt ligation, no remnant shunt was found.
Figure 2.
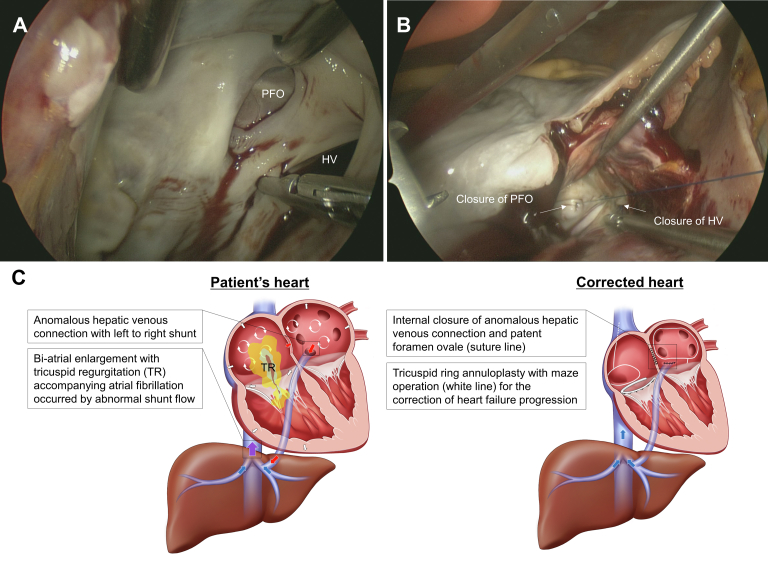
Picture of the operative field of the shunt closure and schematic illustration of the patient’s heart and surgically corrected heart. (A) Hepatic vein (HV) connected to the left atrium and patent foramen ovale (PFO), confirmed by left atriotomy through the Waterston's groove after cardiopulmonary bypass. (B) Confirming that no remnant shunt exists, after temporarily stopping cardiopulmonary bypass with HV and PFO closure. (C) Blue arrows show normal hepatic venous drainage to inferior vena cava. Red arrows indicate left-to-right shunts through anomalous hepatic venous shunt and PFO. Purple arrow indicates mixed venous blood flow of HV and hepatic venous shunt of left atrium. White arrows indicate biatrial and right ventricular enlargement by left-to-right shunt. White circulating arrows express atrial fibrillation of patient’s heart.
Comment
In adults, only 5 cases of HV-LA connection without other intracardiac or extracardiac anomalies have been reported. In 4 of the reported cases, the shunt direction was right to left, with intrahepatic IVC interruption (with azygos extension to the superior vena cava).2, 3, 4, 5 As in our case, during examination, in 1 case it was accidentally discovered that part of the HV was connected to the LA with a hypoplastic left pulmonary artery, despite normal IVC. No mention of shunt direction was described in this case, however.6
This is a rarely reported adult case of surgical intervention for LA-HV connection without cardiac or great vessel connection anomaly (Figure 1, Figure 2). Thus far, all reported HV-LA connection cases for which surgical intervention was required were accompanied by IVC interruption, and the direction of the shunts was typically from the HV to the LA. Therefore, the main symptom of these patients was dyspnea due to hypoxemia.
In this case, a left-to-right shunt occurred because LA pressure was higher than HV pressure; some HVs drained normally into the IVC despite the systemic vein’s being connected to the LA. Because of this, TR occurred from RA volume overload and enlargement. As RA enlargement continued, AF occurred, creating a vicious circle in which RA enlargement became more severe,7 a process that might be accelerated by the accompanying PFO (Figure 2C). We did not perform preoperative diagnostic cardiac catheterization because the updated guidelines recommend selective diagnostic catheterization only when noninvasive imaging cannot be performed or when it is impossible to check a specific lesion in sufficient detail (class 1A). Moreover, the RV fractional area change value (normal value, >35%) was 37% and the tricuspid annular systolic velocity value (normal value, >10.5 cm/s) was 13.7 cm/s; therefore, there was no RV dysfunction. To treat progressive but reversible heart failure, the fundamental cause of RA volume overload was eliminated by ligation of the HV-LA connection. The PFO, which served as an additional shunt, was also blocked. TR and AF caused by RA enlargement were solved by valvuloplasty and a maze operation (Figure 2C).
This report is meaningful in that we describe an increase in preload caused by a persistent, abnormal left-to-right shunt that resulted in RA enlargement, TR, AF, and pulmonary hypertension.8 In partial HV-LA connection that is found incidentally,6 without any symptoms, shunt ligation should be considered if left-to-right shunt is present. Because the byproducts of this shunt (such as RA enlargement, TR, AF, and pulmonary hypertension) can lead to events such as heart failure, early prevention measures could decrease the risk of future adverse events in patients with anomalous hepatic venous connection. The importance of prompt treatment is highlighted in this case report.
Acknowledgments
Funding Sources
The authors have no funding sources to disclose.
Disclosures
The authors have no conflicts of interest to disclose.
Patient Consent
Obtained.
References
- 1.Anderson R.H., Spicer D.E., Hlavacek A.M., Cook A.C., Backer C.L. Cambridge University Press; 2013. Wilcox's Surgical Anatomy of the Heart. [Google Scholar]
- 2.Brochard P., Lejonc J., Loisance D., Nitenberg A. A rare cause of cyanosis and polycythemia: anomalous systemic venous connections without associated intracardiac malformations: the blue milkman story. Eur Heart J. 1981;2:227–233. doi: 10.1093/oxfordjournals.eurheartj.a061198. [DOI] [PubMed] [Google Scholar]
- 3.Kloppenburg G.T., Post M.C., Mager H.J., Schepens M.A. Rerouting anomalous hepatic venous connection to the left atrium. Ann Thorac Surg. 2010;90:638–640. doi: 10.1016/j.athoracsur.2010.01.080. [DOI] [PubMed] [Google Scholar]
- 4.Kirsch J., Araoz P.A., Breen J.F., Chareonthaitawee P. Isolated total anomalous connection of the hepatic veins to the left atrium. J Cardiovasc Comput Tomogr. 2007;1:55–57. doi: 10.1016/j.jcct.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 5.Stoller J.K., Hoffman R.M., White R.D., Mee R.B. Anomalous hepatic venous drainage into the left atrium: an unusual cause of hypoxemia. Respir Care. 2003;48:58–62. [PubMed] [Google Scholar]
- 6.Song B.G., Park S.J., Park J.R., Park S.W., Choi Y.H., Yang J.H. Anomalous hepatic venous drainage into the left atrium and inferior vena cava. Clin Cardiol. 2010;33:E121. doi: 10.1002/clc.20696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen Y.C., Voskoboinik A., La Gerche A., Marwick T.H., McMullen J.R. Prevention of pathological atrial remodeling and atrial fibrillation: JACC state-of-the-art review. J Am Coll Cardiol. 2021;77:2846–2864. doi: 10.1016/j.jacc.2021.04.012. [DOI] [PubMed] [Google Scholar]
- 8.Burkett D.A. Common left-to-right shunts. Pediatr Clin. 2020;67:821–842. doi: 10.1016/j.pcl.2020.06.007. [DOI] [PubMed] [Google Scholar]