Abstract
Objective:
To compare 2 omega-3 (n-3) preparations enriched with eicosapentaenoic acid (EPA) versus docosahexaenoic acid (DHA) as monotherapy for major depressive disorder (MDD) in a 2-site, placebo-controlled, randomized, double-blind clinical trial.
Method:
196 adults (53% female; mean [SD] age = 44.7 [13.4] years) with DSM-IV MDD and a baseline 17-item Hamilton Depression Rating Scale (HDRS-17) score ≥ 15 were randomized equally from May 18, 2006, to June 30, 2011, to 8 weeks of double-blind treatment with oral EPA-enriched n-3 1000 mg/d, DHA-enriched n-3 1,000 mg/d, or placebo.
Results:
154 subjects completed the study. Modified intent-to-treat (mITT) analysis (n = 177 subjects with ≥ 1 postbaseline visit; 59.3% female, mean [SD] age 45.8 [12.5] years) employed mixed-model repeated measures (MMRM). All 3 groups demonstrated statistically significant improvement in the HDRS-17 (primary outcome measure), 16-item Quick Inventory of Depressive Symptomatology–Self Report (QIDS-SR-16), and Clinical Global Improvement-Severity Scale (CGI-S) (P < .05), but neither n-3 preparation separated from placebo (P > .05). Response and remission rates were in the range of 40%–50% and 30%, respectively, for all treatments, with no significant differences between groups. One subject receiving EPA-enriched n-3 discontinued due to worsening depression, and 1 subject receiving placebo discontinued due to an unspecified “negative reaction” to pills.
Conclusions:
Neither EPA-enriched nor DHA-enriched n-3 was superior to placebo for the treatment of MDD.
Trial Registration:
ClinicalTrials.gov identifier: NCT00517036
Omega-3 (n-3) fatty acid supplementation is a popular treatment for mood disorders,1 yet recent systematic reviews and meta-analyses have questioned its putative antidepressant efficacy.2–5 There are more than 30 published clinical trials reporting varying degrees of efficacy for n-3 treatment of major depressive disorder (MDD)1–5 and for the depressive phase of bipolar disorder.6 Most of these studies investigated combination preparations of eicosapentaenoic acid (EPA) plus docosahexaenoic acid (DHA) or EPA alone, primarily as augmentation therapy in partial responders to standard antidepressants, while fewer studies have examined monotherapy.1–5 There are only 2 published studies of DHA monotherapy for MDD, one of which found no benefit of 2 g/d of DHA versus placebo,7 whereas an uncontrolled dose-finding study found an advantage for DHA 1 g/d compared to 2 g/d and 4 g/d.8
A recent meta-analysis4 suggested that preparations containing ≥ 60% EPA relative to DHA were more effective than those with a higher fraction of DHA. While the literature as a whole supports modest antidepressant efficacy for n-3 fatty acids, especially as adjuvant treatments, it remains unclear whether EPA is superior to DHA, particularly as monotherapy. To further characterize the “pure” antidepressant effects of n-3s, and given the relative paucity of studies of n-3 monotherapy, we carried out a randomized placebo-controlled, double-blind clinical trial of EPA-enriched versus DHA-enriched n-3 preparations for unmedicated adults with MDD. At the time of study design, we had preliminary positive data for both EPA and DHA in depressed patient samples.8–10 Thus, we postulated that EPA-enriched and DHA-enriched monotherapy would be more effective than placebo.
METHOD
Three hundred eighty-nine outpatients with MDD, ages 18–80, were recruited from May 18, 2006, to June 30, 2011, at Massachusetts General Hospital (MGH) and Cedars-Sinai Medical Center (CSMC) through advertisements and referrals from outpatient programs. Inclusion criteria were a diagnosis of MDD per the Structured Clinical Interview for DSM-IV Axis I Disorders–Patient Edition (SCID I/P),11 a Clinical Global Impressions-Severity of Illness scale (CGI-S)12 score ≥ 3, and a baseline 17-item Hamilton Depression Rating Scale (HDRS-17)13–15 score ≥ 15. The study was approved by institutional review boards at both sites. Prior to participation, all subjects signed a written informed consent form reviewed and discussed with a study physician.
Participants filled out the Food Processor 7.8 questionnaire (ESHA Research Inc, Salem, Oregon)16 for 3 consecutive days between screening and baseline visit to assess dietary n-3 polyunsaturated fatty acids (PUFAs). Data were analyzed at the baseline visit. Subjects with a mean n-3 daily intake of ≥ 3.0 g were excluded. The mean value of 3.0 g/d for the past 3 days prevented the exclusion of patients with occasional higher intakes of n-3. Subjects were asked not to significantly modify their diet during the study.
Subjects were excluded for the following reasons: pregnancy or women of childbearing potential who were not using a medically accepted means of contraception; suicidality or homicidality; serious or unstable medical illness; current or past history of organic mental disorders, substance use disorders, any psychotic disorders, and bipolar disorder; history of multiple adverse drug reactions or allergy to the study compounds; concurrent use of psychotropic medications, systematic corticosteroid or steroid antagonists, anticoagulants, or immunosuppressant agents; electroconvulsive therapy during the current episode; any trial of ≥ 6 weeks with citalopram 40 mg/d or equivalent antidepressant during the current episode (to select a less refractory sample that would be more likely to respond to treatment); history of use of 1 g/d of n-3 supplements; history of a bleeding disorder; psychotherapy; smoking > 10 cigarettes per day; vitamin E supplementation > 400 IU; menstruating individuals unable to have baseline and posttreatment blood drawn during the follicular phase; and individuals unable to refrain from nonsteroidal anti-inflammatory use for > 72 hours prior to blood work. Subjects with a Clinical Global Impressions-Improvement scale (CGI-I)12 score of 1 or 2 (ie, “much improved” or “very much improved”) during the baseline visit (1 week after the screen visit) were excluded from the study.
Eligible subjects were randomized equally to 1,000 mg/d of EPA-enriched mix, 1,000 mg/d of DHA-enriched mix, or placebo for 8 weeks. Doses were selected based on the literature’s support of about 1,000 mg/d for unipolar depression.4 The n-3 preparations and placebo were donated by Nordic Naturals (Watsonville, California). Randomization and treatment assignment were carried out by the research pharmacies of both institutions by standard allocation procedures and a fixed block size of 30 subjects (MGH) or a randomly permuted block size between 6 and 15 subjects (CSMC). Only blind treatment codes, coordinated between both site pharmacies, were noted on randomization lists provided to study staff. All study staff and participants remained blind to treatment assignment.
Patients took 2 EPA-enriched capsules plus 2 identical placebo capsules, or 4 DHA-enriched capsules, or 4 placebo capsules, every morning. Per the randomization assignment, pill bottles contained EPA-enriched mix (ProEPAxtra: 530 mg EPA/137 mg DHA per soft gel [EPA:DHA = 4:1], plus 7% stearidonic acid [SDA, n-3], 1% heneicosapentaenoic acid [HPA, n-3], 1% docosapentaenoic acid [DPA, n-3], 1% eicosatetraenoic acid [ETA, n-3], 0.2% α-linolenic acid [ALA, n-3], 3% arachidonic acid [AA, n-6], 0.2% linoleic acid [LA, n-6], and 10%–11% unspecified fatty acids) or DHA-enriched mix (ProDHA: 225 mg DHA/45 mg EPA per soft gel [DHA:EPA = 5:1], plus 10% DPA, 2% HPA, 1% SDA, 1% ETA, 0.4% ALA, 1% AA, 0.5% LA, and 20% unspecified fatty acids), or placebo (980 mg soybean oil per cap; total 53.6% LA, 7.1% ALA, 0.1% myristic acid, 11% palmitic acid, 4% stearic acid, 0.2% palmitoleic acid, and 24% oleic acid). A double-dummy placebo design was used to maintain the blind, since DHA-enriched capsules differed in appearance from EPA-enriched capsules. Each patient took capsules from 2 bottles, with one containing either DHA-enriched or DHA-placebo and the other containing either EPA-enriched or EPA-placebo, depending on randomization. Adherence was determined by pill count from bottles returned at the next visit.
Subjects were evaluated every 2 weeks for 8 weeks. Clinical outcome measures at every study visit included HDRS-17 score (primary outcome measure), CGI-S and CGI-I scores, 16-item Quick Inventory of Depressive Symptomatology–Self-Report (QIDS-SR-16)17 scores, Well-Being Scale (WBS)18 scores, and Quality of Life Satisfaction Questionnaire (Q-LES-Q)19 scores.
Statistical Analyses
Descriptive statistics were obtained for the 3 treatment groups, based on a modified intent-to-treat (MITT) sample of 177 evaluable subjects with at least 1 post-baseline visit. Comparisons across treatment groups at baseline were made by analysis of variance (ANOVA) for continuous measures and χ2 tests for categorical variables.
Mixed-model repeated measures analysis (MMRM) was carried out to examine treatment group effect on changes from baseline to week 8 for measures of depressive severity, well-being, and quality of life. Models included subjects as a random effect and treatment group and study week as fixed effects. An auto-regressive covariance structure was used because it provided the best fit to the data. Site and baseline scores were included as covariates in all models. Other potential moderators of response (gender and presence/absence of comorbid anxiety disorder) were examined in relation to the primary outcome, but did not further contribute to prediction of change in HDRS-17 scores with treatment and so were not included as covariates. Treatment response was defined as an improvement of ≥ 50% in HDRS-17 score from baseline to study completion, and remission as a final HDRS-17 score ≤ 7. Comparisons in response and remission rates between treatment groups, based on HDRS-17 scores as well as CGI-S and CGI-I ratings by the end of treatment, were made using χ2 analysis.
Adverse effects (AEs) were measured using the Patient-Rated Inventory of Side Effects (PRISE).20 Because many subjects endorsed PRISE symptoms at baseline, the analysis focused on AEs that emerged or worsened during treatment. Comparisons between treatment groups were made using ANOVAs and χ2 tests.
All statistical analyses were carried out using SAS 8.2 software (2001; SAS Institute Inc, Cary, North Carolina). An α level of .05 was used to determine statistical significance. Analyses of primary and secondary measures of depression severity were carried out based on blind treatment codes.
RESULTS
We randomized 196 (53% female; mean [SD] age = 44.7 [13.4] years) of 389 screened patients. Nineteen subjects dropped out before completing at least 1 postbaseline visit, leaving 177 evaluable subjects (Figure 1).
Figure 1.
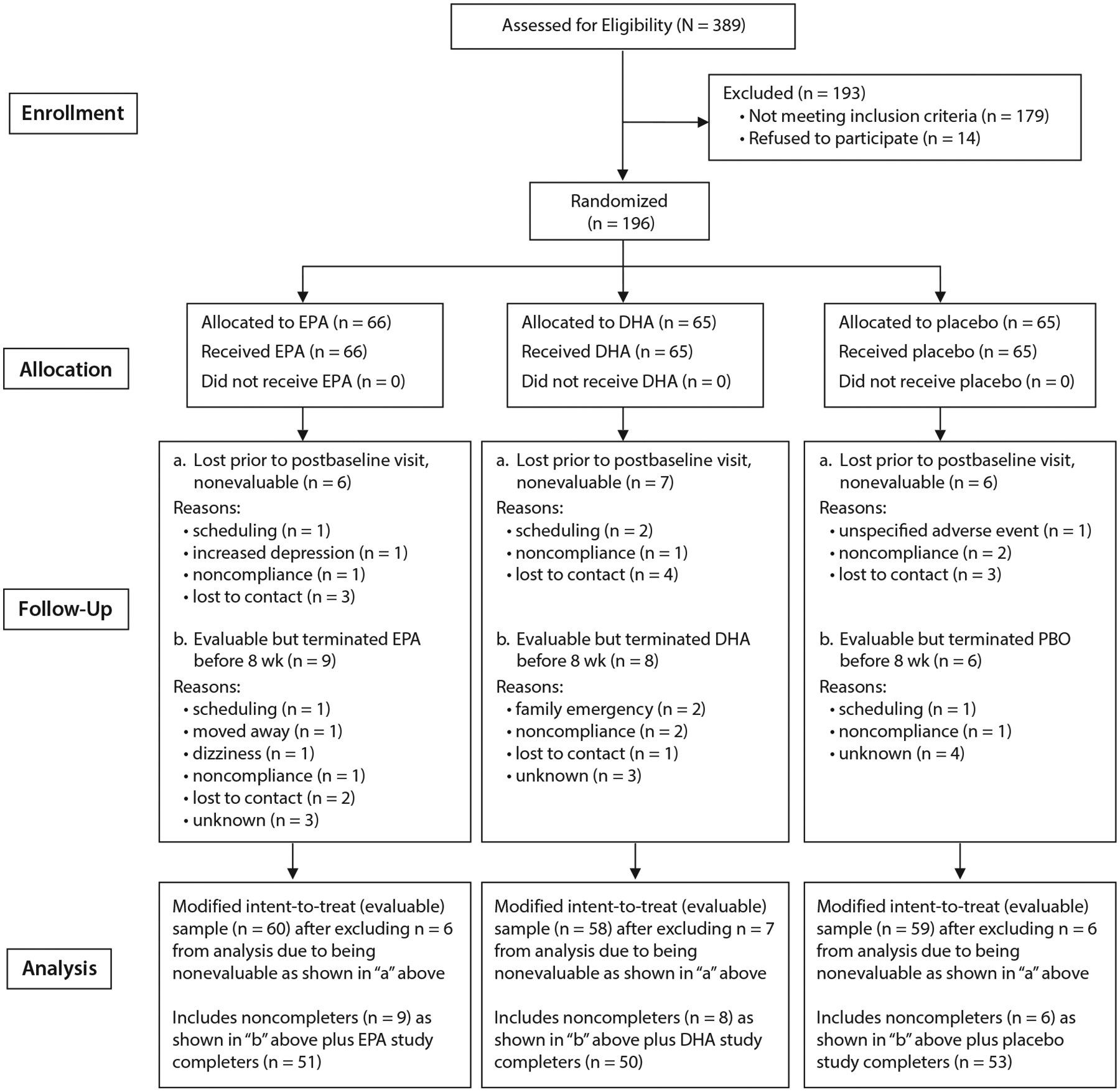
CONSORT Statement Flow Diagrama
aAbbreviations: DHA = docosahexaenoic acid, EPA = eicosapentaenoic acid.
Table 1 summarizes demographic characteristics and baseline clinical variables for the evaluable sample. No significant differences were found among the 3 treatment groups, except that the DHA-enriched group comprised significantly more employed subjects (P = .041). Over a quarter of subjects had a current secondary anxiety disorder (26.5%) or lifetime anxiety disorder diagnosis (31.2%), and 14.7% met criteria for dysthymia (data not shown).
Table 1.
Baseline Demographic and Clinical Characteristics, Overall and by Treatment Group of 177 Evaluable Subjects
| Characteristic | All Evaluable Subjects (N = 177) | EPA-Enriched (N = 60) | DHA-Enriched (N = 58) | Placebo (N = 59) | Significance | ||
|---|---|---|---|---|---|---|---|
| Demographicsa | χ2 | df | P | ||||
| Study site | 0.62 | 2 | .733 | ||||
| CSMC | 108 (61.0) | 38 (63.3) | 33 (56.9) | 37 (62.7) | |||
| MGH | 69 (39.0) | 22 (36.7) | 25 (43.1) | 22 (37.3) | |||
| Gender | 0.81 | 2 | .666 | ||||
| Male | 72 (40.7) | 22 (36.7) | 26 (44.8) | 24 (40.7) | |||
| Female | 105 (59.3) | 38 (63.3) | 32 (55.2) | 35 (59.3) | |||
| Race | 5.77b | 6 | .449 | ||||
| Caucasian | 120 (67.8) | 42 (70.0) | 38 (65.5) | 40 (67.8) | |||
| African American | 32 (18.1) | 13 (21.7) | 8 (13.8) | 11 (18.6) | |||
| Other | 15 (8.5) | 3 (5.0) | 6 (10.3) | 6 (10.2) | |||
| Prefer not to say | 10 (5.6) | 2 (3.3) | 6 (10.3) | 2 (3.4) | |||
| Ethnicityc | 0.19 | 2 | .911 | ||||
| Hispanic | 27 (15.8) | 10 (17.2) | 9 (15.8) | 8 (14.3) | |||
| Non-Hispanic | 144 (84.2) | 48 (82.8) | 48 (84.2) | 48 (85.7) | |||
| Educationc | 0.56 | 2 | .757 | ||||
| High school or less | 44 (26.0) | 17 (28.8) | 15 (26.3) | 12 (22.6) | |||
| Some college or more | 125 (74.0) | 42 (71.2) | 42 (73.7) | 41 (77.4) | |||
| Marital statusc | 1.19 | 4 | .879 | ||||
| Married/live together | 31 (19.5) | 11 (20.4) | 10 (19.2) | 10 (18.9) | |||
| Separated/widowed/divorced | 53 (33.3) | 19 (35.2) | 19 (36.5) | 15 (28.3) | |||
| Never married | 75 (47.2) | 24 (44.4) | 23 (44.2) | 28 (52.8) | |||
| Employment statusc | 13.15b | 6 | .041 | ||||
| Employed | 81 (47.6) | 26 (44.1) | 34 (58.6) | 21 (39.6) | |||
| Homemaker | 8 (4.7) | 3 (5.1) | 3 (5.2) | 2 (3.8) | |||
| Student | 11 (6.5) | 3 (5.1) | 0 (0.0) | 8 (15.1) | |||
| F | df | P | |||||
| Clinical Measuresd | F | df | P | ||||
| HDRS-17 score, mean (SD), [N] (range) | 19.5 (3.4), [177] (15–35) | 19.3 (3.8), [60] (15–35) | 19.8 (3.2), [58] (15–26) | 19.2 (3.1), [59] (15–28) | 0.62 | 2, 174 | .542 |
| QIDS-SR-16 score, mean (SD), [N] (range) | 13.2 (4.1), [173] (3–23) | 12.9 (3.9), [60] (3–23) | 13.3 (4.5), [58] (4–22) | 13.5 (3.8), [55] (6–19) | 0.30 | 2, 170 | .738 |
| CGI-S score, mean (SD), [N] (range) | 4.1 (0.6), [174] (3–6) | 4.2 (0.6), [59] (3–6) | 4.2 (0.7), [57] (3–6) | 4.0 (0.6), [58] (3–6) | 1.64 | 2, 171 | .198 |
| Q-LES-Q | |||||||
| % of max possible, items 1–14 | 43.5 (13.6) [171] | 43.5 (15.4) [58] | 41.6 (12.7) [56] | 45.3 (12.7) [57] | 1.07 | 2, 168 | .347 |
| % standardized around normse | −3.1 (1.2) [171] | −3.1 (1.3) [58] | −3.2 (1.1) [56] | −2.9 (1.1) [57] | 1.07 | 2, 168 | .347 |
| WBS score (standardized by gender)e | |||||||
| Environmental Mastery | −3.0 (1.2) [174] | −3.0 (1.2) [60] | −2.9 (1.3) [57] | −3.2 (1.1) [57] | 0.68 | 2, 171 | .508 |
| Self-Acceptance | −2.7 (1.2) [173] | −2.9 (1.1) [59] | −2.6 (1.1) [57] | −2.7 (1.3) [57] | 0.86 | 2, 170 | .425 |
| Purpose in Life | −1.9 (1.1) [174] | −1.9 (1.1) [60] | −2.0 (1.1) [57] | −1.9 (1.2) [57] | 0.27 | 2, 171 | .766 |
| Positive Relations with Others | −1.6 (1.3) [172] | −1.6 (1.3) [59] | −1.7 (1.2) [57] | −1.5 (1.3) [56] | 0.53 | 2, 169 | .588 |
| Personal Growth | −1.1 (1.2) [171] | −1.1 (1.2) [60] | −1.0 (1.1) [55] | −1.1 (1.3) [56] | 0.19 | 2, 168 | .828 |
| Autonomy | −0.7 (1.2) [174] | −0.7 (1.3) [60] | −0.8 (1.3) [57] | −0.7 (1.1) [57] | 0.13 | 2, 171 | .877 |
Demographic data shown as N (%) unless otherwise noted.
χ2 may not be valid because of the number of cells with expected count < 5.
Information is missing for some subjects.
Clinical measures data shown as mean (SD) [N] unless otherwise noted.
We have expressed these scores as standardized around group norms, much like a Z statistic, which explains the negative values observed.
Abbreviations: CGI-S = Clinical Global Impressions-Severity of Illness scale, CSMC = Cedars-Sinai Medical Center, DHA = docosahexaenoic acid, EPA = eicosapentaenoic acid, HDRS-17 = Hamilton Depression Rating Scale 17-item version, MGH = Massachusetts General Hospital, QIDS-SR-16 = 16-item Quick Inventory of Depressive Symptomatology–Self-Report, Q-LES-Q = Quality of Life Enjoyment and Satisfaction scale, WBS = Psychological Well-Being Scale.
Eighty-seven percent of 177 evaluable subjects completed the study. Completers were more likely to be employed and to be from the CSMC site, but there were no other significant differences (data not shown).
Table 2 summarizes change from baseline to week 8 for evaluable subjects (59.3% female; mean [SD] age = 45.8 [12.5] years), per MMRM analysis. All 3 groups experienced statistically significant improvement in the HDRS-17, QIDS-SR-16, CGI-S, Q-LES-Q, and the 6 WBS scales. Treatment groups did not differ significantly in terms of 8-week change on any scale, and effect sizes between group pairs did not suggest a meaningful advantage for any treatment.
Table 2.
Change From Baseline to Treatment Week 8 for 177 Evaluable Subjects Based on Mixed-Model Repeated Measures Analysisa
| Standardized Effect Size at Week 8d | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Assessment Scale | Change at Treatment Week 8,b LS Mean (SE) [N] | Significance of Treatment-by-Time Interaction | EPA-Enriched vs Placebo | DHA-Enriched vs Placebo | EPA-Enriched vs DHA-Enriched | ||||||
| EPA-Enriched | DHA-Enriched | Placebo | F | df c | P | ||||||
| Depression | |||||||||||
| HDRS-17 | −10.34 (0.62) [60] | −9.26 (0.62) [58] | −9.49 (0.61) [59] | 0.23 | 2, 687 | .794 | −0.179 | +0.049 | −0.228 | ||
| QIDS-SR-16 | −5.01 (0.47) [60] | −4.79 (0.47) [58] | −5.54 (0.47) [56] | 0.39 | 2, 715 | .676 | +0.148 | +0.211 | −0.061 | ||
| CGI-S | −1.46 (0.11) [59] | −1.33 (0.11) [57] | −1.41 (0.11) [58] | 0.07 | 2, 682 | .937 | −0.061 | +0.090 | −0.151 | ||
| Q-LES-Q % of max possible, items 1–14 | +11.05 (1.40) [59] | +10.89 (1.39) [57] | +11.13 (1.38) [59] | 0.01 | 2, 733 | .990 | −0.007 | −0.023 | +0.015 | ||
| WBS (standardized by gender) | |||||||||||
| Environmental mastery | +0.68 (0.11) [60] | +0.58 (0.11) [57] | +0.78 (0.11) [57] | 0.17 | 2, 724 | .842 | −0.119 | −0.241 | +0.119 | ||
| Self-acceptance | +0.53 (0.11) [59] | +0.69 (0.11) [57] | +0.49 (0.11) [57] | 0.81 | 2, 730 | .444 | +0.048 | +0.241 | −0.191 | ||
| Purpose in life | +0.32 (0.11) [60] | +0.54 (0.11) [57] | +0.65 (0.11) [57] | 0.86 | 2, 717 | .425 | −0.392 | −0.132 | −0.261 | ||
| Positive relations with others | +0.31 (0.10) [59] | +0.59 (0.10) [57] | +0.49 (0.10) [56] | 0.58 | 2, 718 | .562 | −0.237 | +0.133 | −0.368 | ||
| Personal growth | +0.59 (0.10) [60] | +0.33 (0.10) [55] | +0.37 (0.10) [56] | 1.22 | 2, 711 | .295 | +0.289 | −0.054 | +0.343 | ||
| Autonomy | +0.32 (0.09) [60] | +0.21 (0.09) [57] | +0.49 (0.09) [57] | 0.70 | 2, 702 | .498 | −0.247 | −0.412 | +0.160 | ||
The assessments were administered at baseline and at 2-week intervals during the 8-week study. MMRM analyses were performed on change from baseline to week 8 for these measures. A full-model MMRM was performed testing the significance of effects of treatment, visit, and treatment-by-visit interaction, as well as the covariates of site, baseline score, and baseline score–by-visit interaction.
Change at 8 weeks is significantly different from zero for each treatment group on every assessment in the table, at P ≤ .001 with 2 exceptions: EPA-enriched group on WBS Purpose in Life (P = .004) and DHA-enriched group on WBS Autonomy (P = .018).
Degrees of freedom are determined using the Satterthwaite approximation method.
By Cohen d effect size = (difference between LS mean change)/pooled SD for each pair of treatments (SD per group computed from SE of LS mean from MMRM). For depression scales, a negative effect size indicates that the first group improved more than the second one (has a lower negative LS-mean change). For Q-LES-Q and WBS scores, a positive effect size indicates that the first group improved more than the second one (has a higher positive LS-mean change).
Abbreviations: CGI-S = Clinical Global Impressions-Severity of Illness scale, DHA = docosahexaenoic acid, EPA = eicosapentaenoic acid, HDRS-17 = Hamilton Depression Rating Scale 17-item version, LS = least squares, MMRM = mixed-model repeated measures, QIDS-SR-16 = 16-item Quick Inventory of Depressive Symptomatology–Self-Report, Q-LES-Q = Quality of Life Enjoyment and Satisfaction scale, WBS = Psychological Well-Being Scale.
In Table 3, response and remission rates on HDRS-17 or CGI did not differ for evaluable subjects in the 3 treatment arms. Response rates per HDRS-17 scores were between 40% and 50% for each treatment, and remission rates were approximately 30% for each group.
Table 3.
Binary Measures of Outcome by Last Treatment Visit for 177 Evaluable Subjects
| Binary Measure of Outcome | Treatment Group, N (%) [total N] | Significance | ||||
|---|---|---|---|---|---|---|
| EPA-Enriched | DHA-Enriched | Placebo | χ2 | df | P | |
| HDRS-17 | ||||||
| Remitter (total score ≤ 7) | 20 (33.3) [60] | 16 (27.6) [58] | 19 (32.2) [59] | 0.508 | 2 | .776 |
| Responder (decrease ≥ 50% from baseline) | 26 (43.3) [60] | 26 (44.8) [58] | 28 (47.5) [59] | 0.209 | 2 | .901 |
| CGI-S | ||||||
| Normal or only borderline mentally ill (value 1 or 2 on 7-point scale) | 22 (37.3) [59] | 18 (31.6) [57] | 25 (43.1) [58] | 1.632 | 2 | .442 |
| CGI-I | ||||||
| Very much or much improved (value 1 or 2 on 7-point scale) | 34 (57.6) [59] | 28 (49.1) [57] | 31 (53.4) [58] | 0.843 | 2 | .656 |
Abbreviations: CGI-I = Clinical Global Impressions-Improvement scale, CGI-S = Clinical Global Impressions-Severity of Illness scale, DHA = docosahexaenoic acid, EPA = eicosapentaenoic acid, HDRS-17 = 17-item Hamilton Depression Rating Scale.
The 74 subjects with a baseline HDRS-17 score ≥ 20 had a more robust clinical improvement than those with a baseline HDRS-17 score < 20, but at either severity level the 3 treatment groups did not differ significantly on any continuous or dichotomous outcomes (Table 4). Among subjects with moderate-to-severe baseline depression (HDRS-17 score ≥ 20), the treatment effect size was −0.40 for EPA-enriched preparation compared to DHA-enriched and placebo, but near zero for DHA-enriched vs placebo. All treatment effect sizes were negligible among subjects with moderate baseline depression (HDRS-17 score = 15–19).
Table 4.
HDRS-17 Score Change, and Remission and Response Rates During Treatment for Groups Defined by Baseline Depressive Severity Level
| Baseline Depressive Severity Level | Change in HDRS-17 Score From Baseline to Treatment Week 8,a LS Mean (SE) [N] | Significance of Treatment-by-Time Interactionc | ||||
|---|---|---|---|---|---|---|
| EPA-Enrichedb | DHA-Enrichedb | Placebob | F | df d | P | |
| Moderate (HDRS-17 = 15–19) | −7.89 (0.65) [37] | −7.46 (0.74) [28] | −7.89 (0.63) [38] | 0.28 | 2, 481 | .755 |
| Moderate-Severe (HDRS-17 = 20–35) | −14.05 (1.01) [23] | −12.12 (0.88) [30] | −12.15 (1.04) [21] | 0.65 | 2, 281 | .523 |
| Remission and Response Rates by Last Treatment Visit, N (%) | Significance of Treatment | |||||
| EPA-Enriched | DHA-Enriched | Placebo | χ2 | df | P | |
| Moderate (HDRS-17 = 15–19) | ||||||
| Remissione | 12 (32.4) | 8 (28.6) | 13 (34.2) | 0.239 | 2 | .887 |
| Responsef | 14 (37.8) | 11 (39.3) | 16 (42.1) | 0.147 | 2 | .929 |
| Moderate-severe (HDRS-17 = 20–35) | ||||||
| Remissione | 8 (34.8) | 8 (26.7) | 6 (28.6) | 0.429 | 2 | .807 |
| Responsef | 12 (52.2) | 15 (50.0) | 12 (57.1) | 0.257 | 2 | .880 |
The HDRS-17 was administered at baseline and at 2-week intervals during the 8-week study. A full-model MMRM analysis was performed on change from baseline to week 8, testing the significance of effects of treatment, visit, and treatment-by-visit interaction, as well as the covariates of site, baseline score, and baseline score–by-visit interaction.
Change at 8 weeks is significantly different from zero for all treatment groups and baseline severity levels, at P ≤ .001.
Standardized treatment effect size between pairs of treatment groups for subjects is as follows: a negative effect size indicates that the first group improved more than the second one (has a lower negative LS mean change): moderate baseline severity: EPA-enriched vs placebo = −0.001; DHA-enriched vs placebo = +0.110; EPA-enriched vs DHA-enriched = −0.109; severe/very severe baseline severity: EPA-enriched vs placebo = −0.395; DHA-enriched vs placebo = + 0.005; EPA-enriched vs DHA-enriched = −0.398.
Degrees of freedom were determined using the Satterthwaite approximation method.
Remission is defined as a HDRS-17 score ≤ 7.
Response is defined as ≥ 50% decrease in HDRS-17 score from baseline.
Abbreviations: DHA = docosahexaenoic acid, EPA = eicosapentaenoic acid, HDRS-17 = 17-item Hamilton Depression Rating Scale, LS = least squares, MMRM = mixed-model repeated measures.
There were 45 subjects with comorbid anxiety disorders, and improvement in their HDRS-17 scores and response and remission rates were lower than for subjects without anxiety (Supplementary eTable 1). Although the overall treatment-by-time effect was not significant within either group, those with a comorbid anxiety disorder had a medium treatment effect size for HDRS-17 improvement by week 8 (−0.43 and −0.47 for EPA-enriched and DHA-enriched preparations, respectively, vs placebo; F162 = 0.72, P = .489); these were in contrast to corresponding effect sizes of −0.21 and +0.18 among those without comorbid anxiety (F575 = 0.09, P = .916).
Among evaluable subjects, 108 were from CSMC and 69 from MGH. Although the completion rate at CSMC was significantly higher than at MGH (93.5% vs 76.8%; χ21 = 10.39, P = .001), response and remission rates on the primary outcome measure (HDRS-17) did not differ significantly between sites (data not shown).
Tolerability data were available for the safety sample of 173 subjects. Between 20% and 30% of subjects across the 3 groups endorsed some baseline PRISE physical or depressive symptoms. No significant differences across treatment groups were observed for the number of AEs emerging or worsening, or emerging or worsening to a distressing level. Of the 21 physical symptoms assessed, only 2 were significantly different by treatment group (constipation: 13.3% for EPA-enriched, 14.3% for DHA-enriched, and 0.0% for placebo; P = .010; and tremors: 1.7% for EPA-enriched, 8.9% for DHA-enriched, and 0% for placebo; P = .020). One EPA-enriched group subject discontinued due to worsening depression, and 1 placebo patient discontinued due to an unspecified “negative reaction to pills” (Figure 1).
DISCUSSION
Although both n-3 preparations were well tolerated, neither demonstrated an advantage over placebo treatment for MDD. All 3 treatment arms experienced a 9- to 10-point improvement in HDRS-17 scores and failed to differ significantly on measures of well-being or quality of life or secondary measures of depression symptoms including response and remission rates. Response rates for the n-3 preparations were somewhat lower than the known efficacy rates of established antidepressants.21 Remission rates were only 10%–15% lower than response rates and were closer to what is usually expected in depression trials.22
The lack of separation from placebo was unexpected, although recent meta-analyses suggest only modest effect size for improvement with n-3 preparations.23 Augmentation, rather than monotherapy, may be preferable for n-3s, since 2 compounds with complementary mechanisms may work synergistically. In a recent report, 12 weeks of adjunctive 1 g/d of EPA produced significantly greater reductions in HDRS-17 scores than 1 g/d of DHA in antidepressant-treated subjects with MDD.24 Likewise, EPA augmentation of citalopram was significantly more efficacious than placebo augmentation in MDD.10 Finally, an EPA-plus-fluoxetine combination was significantly more effective for MDD than either therapy alone.25 These reports agree with a meta-analysis suggesting that EPA may be more beneficial as an augmenting agent than as monotherapy.3
Depression severity may have influenced outcomes; our sample was moderately depressed (mean [SD] baseline HDRS score = 19.5 [3.4]; range, 15–35). Interestingly, subjects with greater depressive severity had a more robust improvement with EPA-enriched treatment. This may suggest that more severely ill patients should be the focus of future studies.
Lespérance and colleagues26 found that patients without comorbid anxiety fared significantly better with combined EPA and DHA treatment compared to placebo. We (conversely) found greater improvement with a moderately large effect size among patients with comorbid anxiety for both n-3 groups versus placebo. Over a dozen human reports of n-3 fatty acids show anxiolytic effects in substance abusers,27,28 women with premenstrual syndrome,29 depressed adults with comorbid anxiety,30 post–myocardial infarction patients,31 Japanese with posttraumatic stress disorder,32–37 and young healthy students,38,39 although not in perimenopausal and postmenopausal women40 or in older patients.41,42 Further investigation of n-3 effects on anxiety is warranted.
Our study has several limitations. We based our original power estimates on 100 subjects randomized to each treatment arm and 80% completion, with a 30% placebo response rate and a 50% response rate for the n-3 preparations. The target sample had an expected 80% power to detect an effect size > 0.40 between treatment group pairs. However, a masked interim analysis indicated no treatment group difference, and continuing recruitment would unnecessarily expose subjects to unproven interventions. Our sample is large enough to provide a statement about lack of efficacy of n-3 monotherapies for outpatients with MDD. Although placebo response rates in antidepressant trials are substantive,43 we found that complementary and alternative medicine MDD trials were more likely to have lower placebo response rates than standard antidepressant trials,44 but this was not the case here. The 3-arm design with 2 active treatment arms may have contributed to the high placebo response rate (47.5%) and could have impeded signal detection. Other large 3-armed studies of natural products have also failed due to high placebo response rates.45,46 Studies with a > 40% placebo response rate are unlikely to show a statistically significant effect of the active agent,47 and placebo response rates increase when there is a greater likelihood of receiving active treatment.48,49 We did not measure subject expectancy and credibility of treatment effect, but placebo response rates may have been influenced by subject expectancy and enthusiasm as well as the benign treatment side effect profiles. The unusually high completion rate (87%, based on 177 evaluable patients) suggests a strong therapeutic alliance.
Our results differ from epidemiologic studies that examine populations that consume more foods rich in n-3 fatty acids than foods rich in n-6 fatty acids,50 because our subjects were allowed to consume their usual n-6 rich diet. Thus, our intervention sought to modify the n-3/n-6 ratio rather than increase n-3 intake while decreasing n-6 intake.
The n-3 preparations were not pure, but rather “enriched” for one n-3 or the other, and contained other fatty acids. The ProEPAxtra contained a ratio of EPA:DHA = 4:1, and the ProDHA had a ratio of DHA:EPA = 5:1. The placebo contained primarily linoleic and linolenic acids and other fatty acids. The complexity of the preparations may raise concerns about unintended biological effects. For example, oleic and linoleic acid have anti-inflammatory effects in rats,51 and this could potentially have an impact on antidepressant effects, given the link between inflammation and depression. However, the lack of “purity” of our EPA and DHA preparations should not have an impact on the efficacy findings, particularly of EPA, given that Sublette at al4 supported preparations of at least 60% EPA as the most effective. Likewise, because n-6 fatty acids are predominant in the industrialized world’s diet, and the Food Processor Questionnaire excluded subjects with robust dietary n-3 intake, the n-6 content of the placebo is not likely to have clinically relevant effects over and above any from dietary n-6.
In summary, in the first head-to-head comparison of EPA- versus DHA-enriched monotherapy for MDD, a heterogenous sample of outpatients improved equally both on n-3 preparations and on placebo. Consequently it is impossible to rule out placebo effects as the reason for improvement from n-3. Because MDD patients typically exhibit deficits in EPA and DHA compared with healthy controls,52 examination of plasma lipids is being planned to provide insight into degree of absorption and saturation after 8 weeks of treatment. Analyses are in progress to examine inflammatory biomarkers as potential moderators or mediators of treatment response. These results may clarify whether certain subsets of depressed individuals may be better candidates for n-3 treatment.
Supplementary Material
Clinical Points.
In this clinical trial involving treatment of major depressive disorder (MDD), about 40% of patients receiving EPA-enriched n-3 or DHA-enriched n-3 experienced improvement in their symptoms of depression.
However, response rates for placebo were also high (close to 50%), and there was no significant separation between either of the n-3 fatty acid preparations versus placebo.
On the basis of this study, it is not possible to rule out placebo effects as a contributor to the clinical antidepressant effectiveness of n-3 fatty acids.
Potential conflicts of interest:
Dr Mischoulon has received research support from the Bowman Family Foundation, FisherWallace, Nordic Naturals, Methylation Sciences, Inc (MSI), and PharmoRx. He has received honoraria for speaking from Pamlab and the Massachusetts General Hospital Psychiatry Academy. He has received royalties from Lippincott Williams & Wilkins for the book Natural Medications for Psychiatric Disorders: Considering the Alternatives. Dr Nierenberg has served as a consultant to: Appliance Computing Inc (Mindsite), Brain Cells, Inc, Brandeis University, Bristol-Myers Squibb, Clintara, Dianippon Sumitomo (now Sunovion), Eli Lilly and Company, EpiQ, Forest, Novartis, Pamlab, PGx Health, Shire, Schering-Plough, Sunovion, Takeda Pharmaceuticals, Teva, and Targacept. He has consulted through the MGH Clinical Trials Network and Institute (CTNI): Astra Zeneca, Brain Cells, Inc, Dianippon Sumitomo/Sepracor, Johnson & Johnson, Labopharm, Merck, Methylation Science, Novartis, PGx Health, Shire, Schering-Plough, Targacept, and Takeda/Lundbeck Pharmaceuticals. He has received honoraria or travel expenses including CME activities from APSARD, Belvoir Publishing, Boston Center for the Arts, University of Texas Southwestern Dallas, Hillside Hospital, American Drug Utilization Review, American Society for Clinical Psychopharmacology, Bayamon Region Psychiatric Society (San Juan, Puerto Rico), Baystate Medical Center, Canadian Psychiatric Association, Columbia University, Douglas Hospital/McGill University, IMEDEX, International Society for Bipolar Disorders, Israel Society for Biological Psychiatry, John Hopkins University, MJ Consulting, New York State, Massachusetts Association of College Counselors, Medscape, MBL Publishing, Physicians Postgraduate Press, Ryan Licht Sang Foundation, Slack Publishing, SUNY Buffalo, University of Florida, University of Miami, University of Wisconsin, University of Pisa, and SciMed. He is a presenter for the Massachusetts General Hospital Psychiatry Academy (MGHPA). The education programs conducted by the MGHPA were supported through Independent Medical Education (IME) grants from the following pharmaceutical companies: in 2008, by Astra Zeneca, Eli Lilly, and Janssen Pharmaceuticals; in 2009, by Astra Zeneca, Eli Lilly, and Bristol-Myers Squibb. He has served on no speaker bureaus or boards since 2003. He owns stock options in Appliance Computing, Inc, and Brain Cells, Inc. Additional income is possible from Infomedic.com depending on overall revenues of the company but no revenue has been received to date. Through MGH, he is named for copyrights to the Clinical Positive Affect Scale and the MGH Structured Clinical Interview for the Montgomery-Asberg Depression Rating Scale exclusively licensed to the MGH Clinical Trials Network and Institute (CTNI). He has received grant/research support through MGH from AHRQ, Cephalon, Forest, Mylan, National Institute of Mental Health (NIMH), Pamlab, Pfizer Pharmaceuticals, Takeda, Elan, and Shire. Dr Schettler works part-time both as Senior Research Associate in the Department of Psychiatry and Behavioral Sciences at the Emory University School of Medicine, Atlanta, Georgia; as well as Principal Statistician in the Department of Psychiatry of the School of Medicine at the University of California, San Diego. She has no other direct or indirect affiliations or financial interests in connection with the contents of this article. Dr Rapaport has provided consulting services to PAX, Inc (unpaid). Dr Kinkead, Ms Fehling, and Mr Martinson report no conflicts of interest.
Funding/support:
The study was funded by grant 5R01MH74085 from the National Institutes of Health (NIH) to Drs Mischoulon and Rapaport. EPA-enriched and DHA-enriched preparations and matching placebos were kindly provided by NordicNaturals.
Role of the sponsors:
The sponsors had no further role in the study design, collection, analysis and interpretation of data, writing of the report, and the decision to submit the paper for publication. This manuscript reflects the views of the authors and may not reflect the opinions or views of all the study investigators or the NIH.
Footnotes
Drug names: citalopram (Celexa and others), fluoxetine (Prozac and others).
Supplementary material: Available at PSYCHIATRIST.COM.
Contributor Information
David Mischoulon, Depression Clinical and Research Program, Massachusetts General Hospital, Harvard Medical School, Boston.
Andrew A. Nierenberg, Depression Clinical and Research Program, Massachusetts General Hospital, Harvard Medical School, Boston.
Pamela J. Schettler, Department of Psychiatry and Behavioral Sciences, Emory University, Atlanta, Georgia.
Becky L. Kinkead, Department of Psychiatry and Behavioral Sciences, Emory University, Atlanta, Georgia.
Kiki Fehling, Depression Clinical and Research Program, Massachusetts General Hospital, Harvard Medical School, Boston.
Max A. Martinson, Depression Clinical and Research Program, Massachusetts General Hospital, Harvard Medical School, Boston.
Mark Hyman Rapaport, Department of Psychiatry and Behavioral Sciences, Emory University, Atlanta, Georgia.
REFERENCES
- 1.Freeman MP, Hibbeln JR, Wisner KL, et al. Omega-3 fatty acids: evidence basis for treatment and future research in psychiatry. J Clin Psychiatry. 2006;67(12):1954–1967. [DOI] [PubMed] [Google Scholar]
- 2.Lin PY, Su KP. A meta-analytic review of double-blind, placebo-controlled trials of antidepressant efficacy of omega-3 fatty acids. J Clin Psychiatry. 2007;68(7):1056–1061. [DOI] [PubMed] [Google Scholar]
- 3.Appleton KM, Rogers PJ, Ness AR. Updated systematic review and meta-analysis of the effects of n-3 long-chain polyunsaturated fatty acids on depressed mood. Am J Clin Nutr. 2010;91(3):757–770. [DOI] [PubMed] [Google Scholar]
- 4.Sublette ME, Ellis SP, Geant AL, et al. Meta-analysis of the effects of eicosapentaenoic acid (EPA) in clinical trials in depression. J Clin Psychiatry. 2011;72(12):1577–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bloch MH, Hannestad J. Omega-3 fatty acids for the treatment of depression: systematic review and meta-analysis. Mol Psychiatry. 2012;17(12): 1272–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sarris J, Mischoulon D, Schweitzer I. Omega-3 for bipolar disorder: meta-analyses of use in mania and bipolar depression. J Clin Psychiatry. 2012;73(1):81–86. [DOI] [PubMed] [Google Scholar]
- 7.Marangell LB, Martinez JM, Zboyan HA, et al. A double-blind, placebo-controlled study of the omega-3 fatty acid docosahexaenoic acid in the treatment of major depression. Am J Psychiatry. 2003;160(5):996–998. [DOI] [PubMed] [Google Scholar]
- 8.Mischoulon D, Best-Popescu C, Laposata M, et al. A double-blind dose-finding pilot study of docosahexaenoic acid (DHA) for major depressive disorder. Eur Neuropsychopharmacol. 2008;18(9):639–645. [DOI] [PubMed] [Google Scholar]
- 9.Mischoulon D, Papakostas GI, Dording CM, et al. A double-blind randomized controlled trial of ethyl-eicosapentaenoate (EPA-E) for major depressive disorder. J Clin Psychiatry. 2009;70(12):1636–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gertsik L, Poland RE, Bresee C, et al. Omega-3 fatty acid augmentation of citalopram treatment for patients with major depressive disorder. J Clin Psychopharmacol. 2012;32(1):61–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.First MB, Spitzer RL, Gibbon M, et al. Structured Clinical Interview for DSM-IV Axis I Disorders–Patient Edition (SCID-I/P, Version 2.0). New York, NY: Biometrics Research Department, New York State Psychiatric Institute; 1995. [Google Scholar]
- 12.Guy W ECDEU Assessment Manual for Psychopharmacology, revised. US Department of Health, Education, and Welfare publication (ADM) 76–338. Rockville, MD: National Institute of Mental Health: 1976. [Google Scholar]
- 13.Hamilton M A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23(1):56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hamilton M Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol. 1967;6(4):278–296. [DOI] [PubMed] [Google Scholar]
- 15.Williams JB. A structured interview guide for the Hamilton Depression Rating Scale. Arch Gen Psychiatry. 1988;45(8):742–747. [DOI] [PubMed] [Google Scholar]
- 16.Block G, Wakimoto P, Jensen C, et al. Validation of a food frequency questionnaire for Hispanics. Prev Chronic Dis. 2006;3(3):A77. [PMC free article] [PubMed] [Google Scholar]
- 17.Rush AJ, Trivedi MH, Ibrahim HM, et al. The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol Psychiatry. 2003;54(5):573–583. [DOI] [PubMed] [Google Scholar]
- 18.Ryff CD, Keyes CL. The structure of psychological well-being revisited. J Pers Soc Psychol. 1995;69(4):719–727. [DOI] [PubMed] [Google Scholar]
- 19.Endicott J, Nee J, Harrison W, et al. Quality of life enjoyment and satisfaction questionnaire (Q-LES-Q): a new measure. Psychopharmacol Bull. 1993;29(2):321–326. [PubMed] [Google Scholar]
- 20.Patient Rated Inventory of Side Effects (PRISE). http://www.edc.gsph.pitt.edu/stard/public/assessment_forms.html?y. Accessed August 8, 2013.
- 21.Insel TR, Wang PS. The STAR*D trial: revealing the need for better treatments. Psychiatr Serv. 2009;60(11):1466–1467. [DOI] [PubMed] [Google Scholar]
- 22.Gaynes BN, Rush AJ, Trivedi MH, et al. Primary versus specialty care outcomes for depressed outpatients managed with measurement-based care: results from STAR*D. J Gen Intern Med. 2008;23(5):551–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fournier JC, DeRubeis RJ, Hollon SD, et al. Antidepressant drug effects and depression severity: a patient-level meta-analysis. JAMA. 2010;303(1):47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mozaffari-Khosravi H, Yassini-Ardakani M, Karamati M, et al. Eicosapentaenoic acid versus docosahexaenoic acid in mild-to-moderate depression: a randomized, double-blind, placebo-controlled trial. Eur Neuropsychopharmacol. 2013;23(7):636–644. [DOI] [PubMed] [Google Scholar]
- 25.Jazayeri S, Tehrani-Doost M, Keshavarz SA, et al. Comparison of therapeutic effects of omega-3 fatty acid eicosapentaenoic acid and fluoxetine, separately and in combination, in major depressive disorder. Aust N Z J Psychiatry. 2008;42(3):192–198. [DOI] [PubMed] [Google Scholar]
- 26.Lespérance F, Frasure-Smith N, St-André E, et al. The efficacy of omega-3 supplementation for major depression: a randomized controlled trial. J Clin Psychiatry. 2011;72(8):1054–1062. [DOI] [PubMed] [Google Scholar]
- 27.Buydens-Branchey L, Branchey M. n-3 polyunsaturated fatty acids decrease anxiety feelings in a population of substance abusers. J Clin Psychopharmacol. 2006;26(6):661–665. [DOI] [PubMed] [Google Scholar]
- 28.Barbadoro P, Annino I, Ponzio E, et al. Fish oil supplementation reduces cortisol basal levels and perceived stress: a randomized, placebo-controlled trial in abstinent alcoholics. Mol Nutr Food Res. 2013;57(6):1110–1114. [DOI] [PubMed] [Google Scholar]
- 29.Sohrabi N, Kashanian M, Ghafoori SS, et al. Evaluation of the effect of omega-3 fatty acids in the treatment of premenstrual syndrome: “a pilot trial”. Complement Ther Med. 2013;21(3):141–146. [DOI] [PubMed] [Google Scholar]
- 30.Liu JJ, Galfalvy HC, Cooper TB, et al. Omega-3 polyunsaturated fatty acid (PUFA) status in major depressive disorder with comorbid anxiety disorders. J Clin Psychiatry. 2013;74(7):732–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haberka M, Mizia-Stec K, Mizia M, et al. Effects of n-3 polyunsaturated fatty acids on depressive symptoms, anxiety and emotional state in patients with acute myocardial infarction. Pharmacol Rep. 2013;65(1):59–68. [DOI] [PubMed] [Google Scholar]
- 32.Matsuoka Y, Nishi D, Yonemoto N, et al. Omega-3 fatty acids for secondary prevention of posttraumatic stress disorder after accidental injury: an open-label pilot study. J Clin Psychopharmacol. 2010;30(2):217–219. [DOI] [PubMed] [Google Scholar]
- 33.Matsuoka Y Clearance of fear memory from the hippocampus through neurogenesis by omega-3 fatty acids: a novel preventive strategy for posttraumatic stress disorder? Biopsychosoc Med. 2011;5(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matsuoka Y, Nishi D, Yonemoto N, et al. Potential role of brain-derived neurotrophic factor in omega-3 Fatty Acid supplementation to prevent posttraumatic distress after accidental injury: an open-label pilot study. Psychother Psychosom. 2011;80(5):310–312. [DOI] [PubMed] [Google Scholar]
- 35.Matsuoka Y, Nishi D, Nakaya N, et al. Attenuating posttraumatic distress with omega-3 polyunsaturated fatty acids among disaster medical assistance team members after the Great East Japan Earthquake: the APOP randomized controlled trial. BMC Psychiatry. 2011;11(1):132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matsumura K, Noguchi H, Nishi D, et al. The effect of omega-3 fatty acids on psychophysiological assessment for the secondary prevention of posttraumatic stress disorder: an open-label pilot study. Glob J Health Sci. 2011;4(1):3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Matsuoka Y, Nishi D, Yonemoto N, et al. Tachikawa project for prevention of posttraumatic stress disorder with polyunsaturated fatty acid (TPOP): study protocol for a randomized controlled trial. BMC Psychiatry. 2013;13(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yehuda S, Rabinovitz S, Mostofsky DI. Mixture of essential fatty acids lowers test anxiety. Nutr Neurosci. 2005;8(4):265–267. [DOI] [PubMed] [Google Scholar]
- 39.Kiecolt-Glaser JK, Belury MA, Andridge R, et al. Omega-3 supplementation lowers inflammation and anxiety in medical students: a randomized controlled trial. Brain Behav Immun. 2011;25(8):1725–1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cohen LS, Joffe H, Guthrie KA, et al. Efficacy of omega-3 for vasomotor symptoms treatment: a randomized controlled trial. Menopause. 2014;21(4):347–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van de Rest O, Geleijnse JM, Kok FJ, et al. Effect of fish-oil supplementation on mental well-being in older subjects: a randomized, double-blind, placebo-controlled trial. Am J Clin Nutr. 2008;88(3):706–713. [DOI] [PubMed] [Google Scholar]
- 42.Jadoon A, Chiu CC, McDermott L, et al. Associations of polyunsaturated fatty acids with residual depression or anxiety in older people with major depression. J Affect Disord. 2012;136(3):918–925. [DOI] [PubMed] [Google Scholar]
- 43.Walsh BT, Seidman SN, Sysko R, et al. Placebo response in studies of major depression: variable, substantial, and growing. JAMA. 2002;287(14):1840–1847. [DOI] [PubMed] [Google Scholar]
- 44.Freeman MP, Mischoulon D, Tedeschini E, et al. Complementary and alternative medicine for major depressive disorder: a meta-analysis of patient characteristics, placebo-response rates, and treatment outcomes relative to standard antidepressants. J Clin Psychiatry. 2010;71(6):682–688. [DOI] [PubMed] [Google Scholar]
- 45.Hypericum Depression Trial Study Group. Effect of Hypericum perforatum (St John’s wort) in major depressive disorder: a randomized controlled trial. JAMA. 2002;287(14):1807–1814. [DOI] [PubMed] [Google Scholar]
- 46.Mischoulon D, Price LH, Carpenter LL, et al. A double-blind, randomized, placebo-controlled clinical trial of S-adenosyl-l-methionine (SAMe) versus escitalopram in major depressive disorder. J Clin Psychiatry. 2014;75(4):370–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Iovieno N, Papakostas GI. Correlation between different levels of placebo response rate and clinical trial outcome in major depressive disorder: a meta-analysis. J Clin Psychiatry. 2012;73(10):1300–1306. [DOI] [PubMed] [Google Scholar]
- 48.Khan A, Kolts RL, Thase ME, et al. Research design features and patient characteristics associated with the outcome of antidepressant clinical trials. Am J Psychiatry. 2004;161(11):2045–2049. [DOI] [PubMed] [Google Scholar]
- 49.Papakostas GI, Fava M. Does the probability of receiving placebo influence clinical trial outcome? A meta-regression of double-blind, randomized clinical trials in MDD. Eur Neuropsychopharmacol. 2009;19(1):34–40. [DOI] [PubMed] [Google Scholar]
- 50.Chowdhury R, Stevens S, Gorman D, et al. Association between fish consumption, long chain omega 3 fatty acids, and risk of cerebrovascular disease: systematic review and meta-analysis. BMJ. 2012;345:e6698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Magdalon J, Vinolo MA, Rodrigues HG, et al. Oral administration of oleic or linoleic acids modulates the production of inflammatory mediators by rat macrophages. Lipids. 2012;47(8):803–812. [DOI] [PubMed] [Google Scholar]
- 52.Lin PY, Huang SY, Su KP. A meta-analytic review of polyunsaturated fatty acid compositions in patients with depression. Biol Psychiatry. 2010;68(2):140–147. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


