Abstract
A 57-year-old man with a known left main coronary artery aneurysm presented with acutely decompensated heart failure and ventricular tachycardia secondary to ST elevation myocardial infarction. Transthoracic echocardiography identified a left ventricular ejection fraction <20% and anterior/septal wall akinesis. Left-sided cardiac catheterization revealed left anterior descending coronary artery occlusion. After the patient was placed on extracorporeal membrane oxygenation, delayed left ventricular distention developed, requiring further surgical intervention. Because of immense size, a novel “no-touch” approach was taken to the left main coronary artery aneurysm; the patient concomitantly underwent ventricular tachycardia ablation and continuous-flow left ventricular assist device implantation.
Coronary artery aneurysm (CAA) is an uncommon clinical diagnosis with a variety of treatment modalities. Surgical treatment varies on the basis of the location and size of the aneurysm. Here, we report a novel technique for CAA treatment alongside the implementation of concomitant procedures in an acutely ill patient.
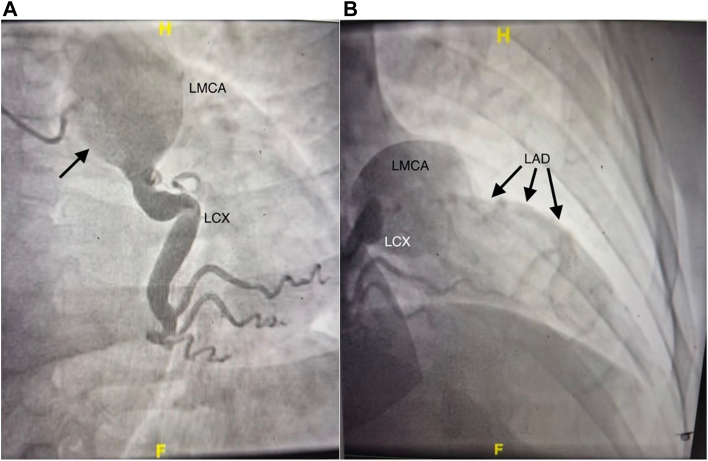
A 57-year-old man with a known left main (LM) CAA (Figure 1A) presented with acutely decompensated heart failure and ventricular tachycardia in the setting of ST elevation myocardial infarction. Transthoracic echocardiography identified a left ventricular (LV) ejection fraction <20%, anterior/septal wall akinesis, and LV end-diastolic dimension of 6.8 cm. Emergent left-sided cardiac catheterization revealed proximal left anterior descending (LAD) coronary artery occlusion but a widely patent left circumflex coronary artery (Figure 1B). However, given the duration of the patient’s symptoms and a likely thromboembolic cause, percutaneous revascularization was not attempted. The patient was managed pharmacologically and transferred to our institution.
Figure 1.
Angiography demonstrating (A) left main coronary artery (LMCA) aneurysm (arrow) and (B) left circumflex coronary artery (LCX) patency with concomitant left anterior descending coronary artery (LAD) occlusion (arrows).
On arrival, the patient had worsening shock and ventricular tachycardia, with pulmonary edema/acute hypoxemic respiratory failure, right ventricular (RV) dysfunction, and acute renal failure. He was taken to the operating room for venoarterial extracorporeal membrane oxygenation (ECMO), with possible additional left-sided cardiac venting; percutaneous right common femoral venous cannulation and open left axillary artery cannulation through a side graft were performed. The patient’s condition rapidly improved intraoperatively, with improved systemic arterial blood pressure, reduced pulmonary artery (PA) occlusion pressure, reduced PA pressure, reduced central venous pressure, and improved urine output. Consequently, left-sided venting was deferred. The patient improved for 3 days, but delayed LV distention developed acutely. Because of suspected LV thrombus, RV/PA venting was undertaken with a ProtekDuo (LivaNova) cannula, splicing it into the circuit inflow. The patient continued to improve and was liberated from mechanical ventilation. He was evaluated for advanced heart failure surgical therapy and ultimately deemed unsuitable for heart transplantation largely because of concerns about medication adherence. However, given possibly irreversible heart failure, durable mechanical circulatory support in the form of continuous-flow LV assist device (LVAD) implantation was offered. The LM CAA would be addressed concomitantly.
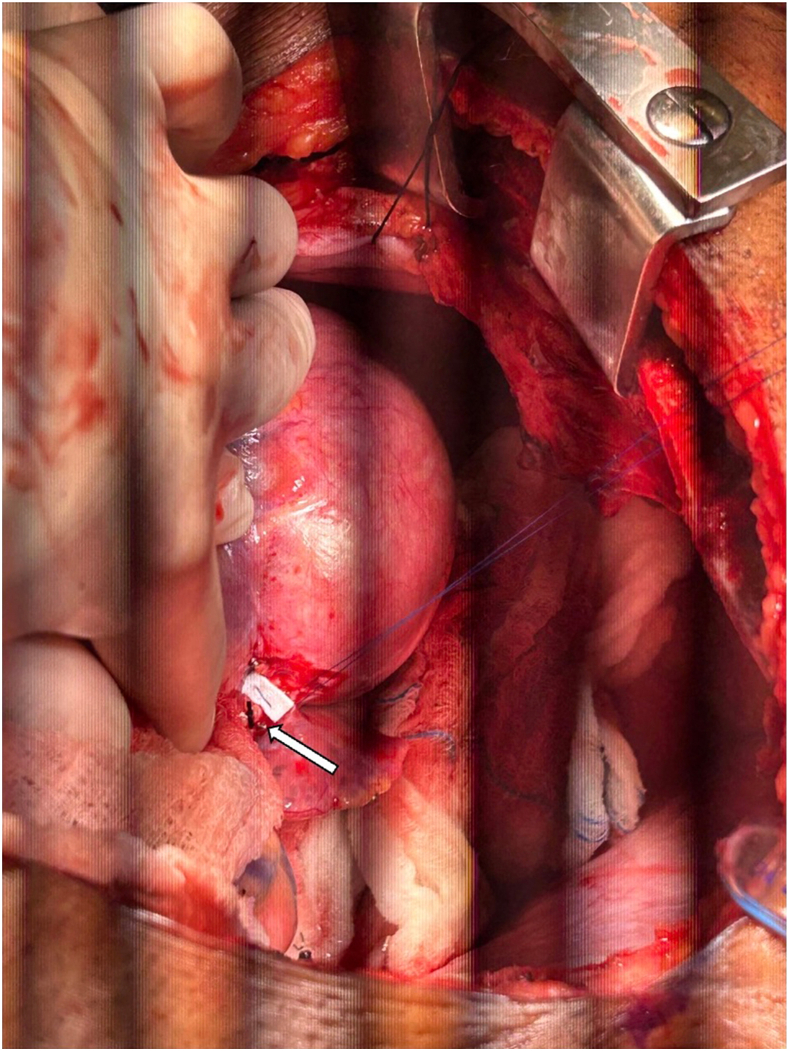
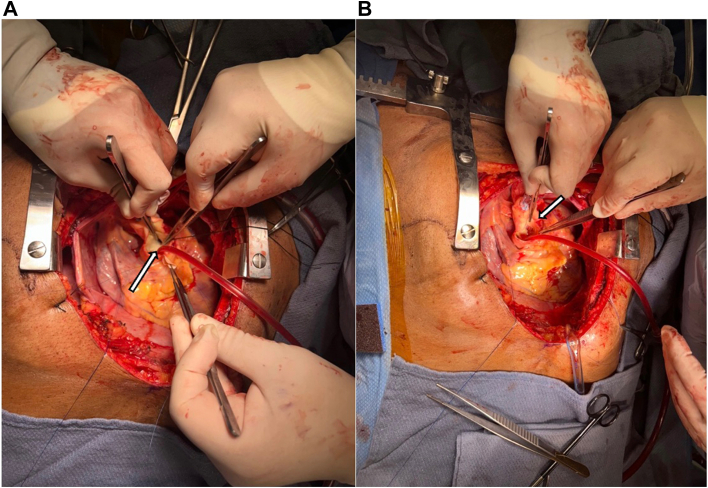
The patient was taken to the operating room, and median sternotomy was performed; the right great saphenous vein was harvested endoscopically for use in coronary artery bypass grafting (CABG). Venoarterial ECMO was converted to full cardiopulmonary bypass with PA venting. Mild systemic hypothermia to 32 °C was initiated, and cardiac arrest was induced by aortic cross-clamping and antegrade administration of cold del Nido cardioplegia. The left circumflex coronary artery was dissected out at its origin from the CAA (Figure 2) and ligated with sutures, as was the LAD coronary artery. This established distal control of the CAA. Next, a transverse aortotomy was created 1.5 cm distal to the sinutubular junction, and the ostium of the LM coronary artery was visualized. The LM coronary artery ostium was closed with a bovine pericardial patch (Figure 3), establishing proximal control of the CAA. The aortotomy was closed. Next, CABG distal anastomoses were performed to the second obtuse marginal (OM2) and mid-LAD coronary arteries, using the saphenous vein grafts (SVGs). Of note, occlusive organized thrombus was extracted from the proximal LAD coronary artery before creation of the distal anastomosis. Systemic rewarming was initiated, and the aortic cross-clamp was released.
Figure 2.
Left circumflex coronary artery dissection at coronary artery aneurysm origin (arrow).
Figure 3.
(A) Left main coronary artery aneurysm at the ostium (arrow) and (B) left main ostial patch closure (arrow).
Next, attention was paid to HeartMate 3 (Abbott Cardiovascular) LVAD implantation. The LV apex was noted to be aneurysmal. The sewing ring–LV apex suture line was created, followed by apical coring. No intracavitary thrombus was noted. Through the apical coring site, endocardial ventricular tachycardia cryoablation was performed at 4 sites: anterior, lateral, posterior, and medial (septal). The LVAD inflow cannula was engaged into the coring site and secured. Under partial-occlusion aortic clamping, the outflow graft anastomosis was performed, as was a CABG proximal anastomosis for the graft to OM2. Finally, a side-to-end SVG (for OM2)–SVG (for LAD coronary artery) anastomosis was performed.
Weaning and separation from cardiopulmonary bypass were performed. LVAD support was initiated, and the ProtekDuo cannula was converted for RV assistance and ECMO. The right common femoral vein and left axillary artery were decannulated, and closure was performed.
The patient required mediastinal reexploration for bleeding on postoperative day 0. The site of LAD coronary artery ligation was bleeding due to suture cutting through the arterial wall; a single reinforcing ligation suture rendered satisfactory hemostasis. Closure was performed once again, and the patient was returned to the intensive care unit in satisfactory condition. Postoperatively, the patient suffered an embolic right middle cerebral artery stroke, manifested with left upper extremity paresis. The remainder of his postoperative course was satisfactory, achieving RV assist device decannulation and liberation from mechanical ventilation after tracheostomy placement. He was discharged in good condition to a rehabilitation facility on postoperative day 28, decannulated from tracheostomy and with improving left upper extremity function. He continues to be in good condition.
Comment
CAAs are rare, with a reported incidence of 0.5% to 5.3%; causes are most commonly atherosclerosis, vasculitides including Takayasu arteritis and Kawasaki disease, and congenital disease.1 As aneurysm is defined by an arterial diameter ≥1.5 times the upper limit of predicted normal; most CAAs are <1.0 cm in maximal diameter. Massive aneurysms such as this are far less common. The natural history governing treatment is largely predicted by the presence of associated in situ occlusive disease and distal embolization arising from intra-aneurysm sac thrombus, which may cause acute myocardial infarction, as in this case.
Consequently, the most important criteria for operative treatment relate to acute myocardial ischemia or infarction from distal embolization and primary indications for management of chronic occlusive disease.2 Other reported criteria include massive aneurysm (≥4 times normal diameter), rapid expansion, LM CAA, and mechanical complications (compression, fistula formation, rupture). In this case, several criteria were satisfied.3
Operative techniques for CAA generally involve resection and interposition grafting or ligation and bypass revascularization.2 However, depending on CAA location and size, these approaches involve aneurysm manipulation, which could increase the risk of acute distal embolization. Moreover, LM CAA may require transection of the main PA or even the ascending thoracic aorta. For proximal CAAs such as of the LM coronary artery, our technique of proximal control through transaortic ostial closure avoids both aneurysm manipulation and extensive reconstruction of other structures. With respect to CAA operative treatment and concomitant continuous-flow LVAD implantation, this has recently been reported by another group.4
Furthermore, our reasoning for performing these operations concomitantly was because this patient was receiving dual antiarrhythmic medication infusions for ventricular tachycardia preoperatively. The principal concern of treating the aneurysm alone without bypass revascularization was that this would result in essentially total LV infarction, with exacerbated ventricular arrhythmias. It is also possible that mechanical complications of myocardial infarction could ensue. Whereas LV recovery certainly was not anticipated or expected, revascularization may provide some benefit with respect to the possibility of recovery. The patient was on full temporary mechanical circulatory support preoperatively, with venoarterial ECMO plus a ProtekDuo cannula being used as a PA vent, which was then converted intraoperatively for use in a standard percutaneous RV assist device. Therefore, we thought that the operative risks were mildly mitigated. Simply treating the aneurysm and performing continuous-flow LVAD implantation was possible; the malignant arrhythmia risk of excluding the entire left coronary arterial system outweighed this in our calculus.
In conclusion, this case describes a novel approach to the treatment of CAA, with concomitant myocardial revascularization and continuous-flow LVAD implantation. This “no-touch” operative approach is well suited to the management of ostial/proximal CAAs. Concomitant myocardial revascularization, when appropriate, may preserve or even improve LV systolic function, possibly achieving bridging to LV recovery in patients such as ours.
Acknowledgments
Funding Sources
The authors have no funding sources to disclose.
Disclosures
The authors have no conflicts of interest to disclose.
Patient Consent
Obtained.
References
- 1.Abou Sherif S., Ozden Tok O., Taşköylü Ö., Goktekin O., Kilic I.D. Coronary artery aneurysms: a review of the epidemiology, pathophysiology, diagnosis, and treatment. Front Cardiovasc Med. 2017;4:24. doi: 10.3389/fcvm.2017.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kawsara A., Núñez Gil I.J., Alqahtani F., Moreland J., Rihal C.S., Alkhouli M. Management of coronary artery aneurysms. JACC Cardiovasc Interv. 2018;11:1211–1223. doi: 10.1016/j.jcin.2018.02.041. [DOI] [PubMed] [Google Scholar]
- 3.Singh S.K., Goyal T., Sethi R., et al. Surgical treatment for coronary artery aneurysm: a single-centre experience. Interact Cardiovasc Thorac Surg. 2013;17:632–636. doi: 10.1093/icvts/ivt282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kunioka S., Tadokoro N., Fujita T., Fukushima S. Successful exclusion of left main trunk coronary artery aneurysm and concomitant HeartMate 3 implantation in a patient with a history of infective endocarditis: a case report. Eur Heart J Case Rep. 2023;7:ytad080. doi: 10.1093/ehjcr/ytad080. [DOI] [PMC free article] [PubMed] [Google Scholar]