Abstract
Thymic carcinoma, a rare aggressive tumor, necessitates multidisciplinary approaches for optimal prognosis. The role of surgical interventions in stage IVb thymic carcinoma, as classified by the TNM and Masaoka-Koga staging systems, remains controversial; although some patients present with resectable disease, others do not. We report a case with supraclavicular metastasis and sternal invasion. Preoperative chemoradiotherapy, followed by extended thymectomy and thorough lymph node dissection, resulted in more than a decade of postoperative recurrence-free survival. Complete resection, including metastases, is essential for achieving optimal outcomes, underscoring the need for extensive lymph node dissection. Aggressive surgical procedure is recommended for suitably selected patients.
Thymic carcinoma is a rare malignant neoplasm with an incidence rate ranging between 0.07 and 0.38 per million person-years.1 Although complete resection of tumor is of paramount importance and provides good prognosis, multimodal treatment is reportedly important for advanced cases.2 Patients with advanced-stage TNM and Masaoka-Koga stage IVb disease are divided into 2 oncologically distinct groups by the likelihood of complete resection. We report a case of stage IVb thymic carcinoma with supraclavicular fossa lymph node metastasis and sternal invasion that was treated with preoperative chemoradiotherapy followed by extended thymectomy, combined resection and reconstruction of the sternum, and lymph node dissection from the left supraclavicular fossa to the neck with additional postoperative adjuvant chemotherapy, resulting in recurrence-free survival for >10 years after surgery.
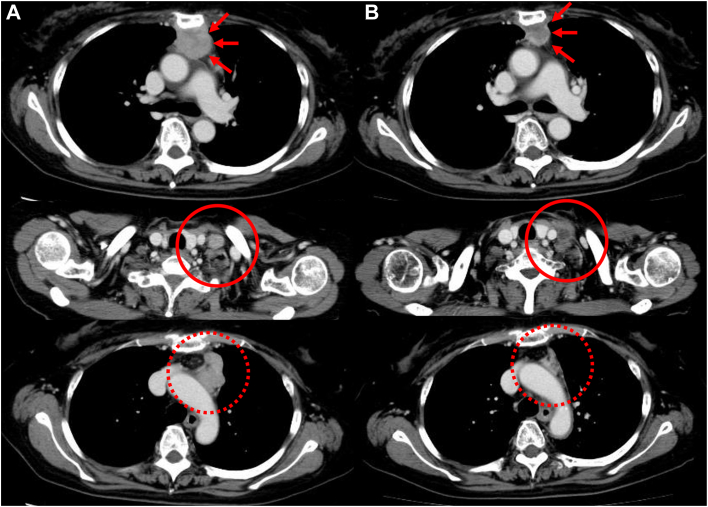
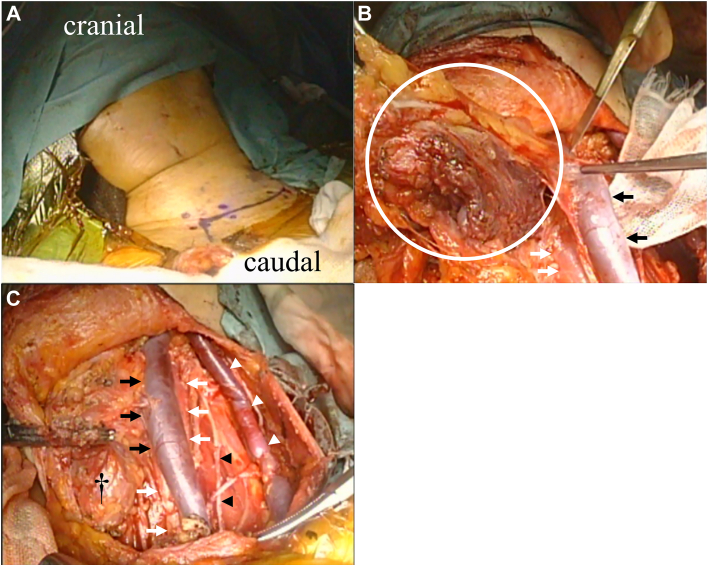
A 67-year-old woman presented with a chief complaint of intermittent anterior chest pain. Chest computed tomography and magnetic resonance imaging showed a 37-mm mass invading the sternal body. Biopsy revealed thymic carcinoma, cT3 N2 M0 stage IVb (Masaoka-Koga classification). After chemoradiotherapy (nedaplatin + docetaxel, 2 cycles, 40 Gy), the main tumor (21 mm) and lymph nodes shrank (Figure 1), and no new lesions appeared. Therefore, surgery was preferred and performed 2 months after chemoradiotherapy. After a median sternal incision, the sternum was transected at the level of the first and third intercostal spaces, and an enlarged thymectomy was performed in 1 lump with the tumor-infiltrated sternum. After mediastinal lymph node dissection, from the left cervical region, we performed a node dissection encompassing the bifurcation of the common carotid artery, the middle and lower internal jugular veins, and the posterior triangle lymph node group (level II-V; Figure 2).3 The defective sternum was repaired with polypropylene mesh. Postoperative pathologic examination revealed thymic squamous cell carcinoma with metastasis to the para-aortic arch and lymph nodes of the inferior internal jugular vein (pT3 N2 M0 stage IVb; Masaoka-Koga classification stage IVb). Postoperatively, ventilatory failure developed as a result of temporary phrenic nerve palsy and thoracic distraction, which improved after noninvasive positive pressure ventilation for several days. Two months after surgery, the patient underwent 2 cycles of nedaplatin + docetaxel as postoperative adjuvant chemotherapy. Computed tomography imaging was repeatedly performed every few months after completion of the last treatment cycle to confirm that the patient survived without recurrence for >10 years.
Figure 1.
Contrast-enhanced computed tomography image (A) at the time of consultation and (B) after preoperative chemoradiotherapy. The main tumor on the back of the sternum (arrows), supraclavicular fossa lymph node (circle), and para-aortic lymph node (dotted circle) have all shrunk.
Figure 2.
Cervical lymph node dissection. (A) A collar incision was made along the purple line, and upper, middle, and lower jugular group and posterior triangle group lymph node dissection (level II-V) was performed. (B) Lymph nodes (circle), left internal jugular vein (black arrows), and left common carotid artery (white arrows). (C) After dissection: left internal jugular vein (black arrows), left common carotid artery (white arrows), left external jugular vein (white arrowheads), left phrenic nerve (black arrowheads), and left lobe of the thyroid gland (†).
Comment
Thymic carcinoma is a rare but aggressive tumor, and multidisciplinary treatment is necessary to improve prognosis, particularly in advanced cases.2 Other factors associated with improved prognosis include complete resection and early Masaoka stage.4 However, some analyses only included up to Masaoka stage IVa,5 and some others recommended palliative treatment of stage IVb.6 Thus, the fact that treatment intervention is centered on surgery for stage IVb thymic carcinoma remains controversial. In this regard, Hishida and colleagues7 reported that there was no significant difference in postoperative survival between stage IVa and IVb groups. Furthermore, they revealed that the group that achieved complete resection, including metastatic lymph nodes and distant metastases, had survival results similar to those in the stage III group. Stage IVa is a disease that spreads into the thoracic cavity; it is oncologically impossible to resect tumors at this stage completely because of tumor dissemination into the thoracic cavity. There is no significant difference between the entire IVb group and the IVa group. Moreover, if complete excision is achieved, the outcomes in the IVb group may be more favorable than those in the IVa group. Therefore, it can be inferred that the IVb group is a heterogeneous mixture of patients. This mixed group includes patients with tumors that can be completely resected, even with taking metastatic sites into account, and patients with nonresectable tumors. This suggests that the subset of patients who cannot undergo complete excision might have an impact on the overall treatment outcomes in the IVb group. Specifically, cases of N2 M1a tumors that are manifested with seeding are difficult to undergo complete excision, leading to unsatisfactory treatment outcomes. However, even in cases classified as N2 M0 or those with distant metastasis, if they are identified as locoregional Nany M1b, they might achieve favorable outcomes through complete excision. Of these classifications, this case corresponded to N2 M0. With the addition of a multidisciplinary approach during the perioperative phase and comprehensive lymph node dissection involving the cervical region, we have secured more than a decade of postoperative recurrence-free survival. This suggests that even within the IVb classification, certain cases could achieve favorable prognosis.
Various combinations of perioperative chemotherapy and radiotherapy have been reported, depending on whether they are used before or after surgery and in combination. As this case was treated >10 years ago, chemotherapy was administered before and after surgery with a nedaplatin-based cytotoxic anticancer drug, as in squamous cell lung cancer.8 In the future, molecular-targeted agents and immune checkpoint inhibitors may be used according to individual tissues. In addition, the establishment of more evidence-based treatment methods is needed. Aggressive surgical procedure is recommended for suitably selected patients.
Acknowledgments
Funding Sources
The authors have no funding sources to disclose.
Disclosures
The authors have no conflicts of interest to disclose.
Patient Consent
Obtained.
References
- 1.Roden A.C., Ahmad U., Cardillo G., et al. Thymic carcinomas—a concise multidisciplinary update on recent developments from the Thymic Carcinoma Working Group of the International Thymic Malignancy Interest Group. J Thorac Oncol. 2022;17:637–650. doi: 10.1016/j.jtho.2022.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shintani Y., Inoue M., Kawamura T., Funaki S., Minami M., Okumura M. Multimodality treatment for advanced thymic carcinoma: outcomes of induction therapy followed by surgical resection in 16 cases at a single institution. Gen Thorac Cardiovasc Surg. 2015;63:159–163. doi: 10.1007/s11748-014-0486-7. [DOI] [PubMed] [Google Scholar]
- 3.Robbins K.T., Clayman G., Levine P.A., et al. Neck dissection classification update: revisions proposed by the American Head and Neck Society and the American Academy of Otolaryngology–Head and Neck Surgery. Arch Otolaryngol Head Neck Surg. 2002;128:751–758. doi: 10.1001/archotol.128.7.751. [DOI] [PubMed] [Google Scholar]
- 4.Ahmad U., Yao X., Detterbeck F., et al. Thymic carcinoma outcomes and prognosis: results of an international analysis. J Thorac Cardiovasc Surg. 2015;149:95–100. doi: 10.1016/j.jtcvs.2014.09.124. 101.e1-e2. [DOI] [PubMed] [Google Scholar]
- 5.Modh A., Rimner A., Allen P.K., et al. Treatment modalities and outcomes in patients with advanced invasive thymoma or thymic carcinoma: a retrospective multicenter study. Am J Clin Oncol. 2016;39:120–125. doi: 10.1097/COC.0000000000000024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tosi D., Damarco F., Franzi S., Mohamed S., Palleschi A., Mendogni P. Outcomes of extended surgical resections for locally advanced thymic malignancies: a narrative review. Gland Surg. 2022;11:611–621. doi: 10.21037/gs-21-642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hishida T., Nomura S., Yano M., et al. Long-term outcome and prognostic factors of surgically treated thymic carcinoma: results of 306 cases from a Japanese Nationwide Database Study. Eur J Cardiothorac Surg. 2016;49:835–841. doi: 10.1093/ejcts/ezv239. [DOI] [PubMed] [Google Scholar]
- 8.Shukuya T., Yamanaka T., Seto T., et al. Nedaplatin plus docetaxel versus cisplatin plus docetaxel for advanced or relapsed squamous cell carcinoma of the lung (WJOG5208L): a randomised, open-label, phase 3 trial. Lancet Oncol. 2015;16:1630–1638. doi: 10.1016/S1470-2045(15)00305-8. [DOI] [PubMed] [Google Scholar]