Abstract
Background
We have developed a new hybrid warp-knit fabric for induction of in situ tissue regeneration that has shown appropriate antideterioration properties and expandability in preclinical studies. This study was performed to assess the clinical efficacy and safety of this fabric in the early postoperative period after congenital cardiac surgery.
Methods
The fabric comprises biodegradable (complete degradation period, 2-3 years) and nonbiodegradable yarns coated with cross-linked gelatin. A multicenter single-arm 3-year study was conducted. The primary end point for regulatory approval was the surgical success rate without death or reintervention related to fabric failure at 1 year after surgery. Secondary end points included the incidence of material-related secondary lesions.
Results
The fabric was implanted at 41 sites (pulmonary artery, n = 18; right ventricular outflow tract, n = 12; atrial septum, n = 7; and ventricular septum, n = 4) in 34 patients of median age 1 year 11 months (range, 4 months-58 years). The surgical success rate was 100%. There were no abnormal findings for the fabric on echocardiography and no serious adverse events in a median follow-up period of 40 months (range, 36-55 months), other than 3 stenotic lesions that were due to the surgical procedure. These results permitted regulatory approval for use of the fabric in Japan.
Conclusions
The new fabric promoting tissue self-organization through a novel technology showed acceptable efficacy and safety in the early postoperative period after congenital cardiac surgery.
Visual Abstract

In Short.
-
▪
A clinical trial was conducted with a new hybrid warp-knit fabric that induces tissue regeneration in congenital cardiac surgery.
-
▪
The fabric showed acceptable efficacy and safety in the early postoperative period up to 3 years.
-
▪
Autologous tissue induction with the new fabric is a promising alternative to conventional technology.
In surgical treatment of congenital heart disease (CHD), various materials that induce in situ tissue regeneration have been developed to reduce the risk of reoperation due to material deterioration. Among these materials, decellularized xenoproducts1,2 and electrospun microfibers with biodegradable polymers3,4 have emerged as scaffolds on which autologous tissue can be induced, but these products have not provided a definitive solution. As a new alternative, we have developed a synthetic hybrid fabric (SHF) and shown in a series of preclinical studies that SHF offers features of antidegeneration and stretch-expandability in induced autologous tissue.5,6 This prospective, multicenter, single-arm clinical study was conducted to examine the efficacy and safety of SHF in CHD surgery in humans.
Material and Methods
Ethics
This study was conducted in compliance with ethical principles based on the Declaration of Helsinki, the Pharmaceuticals and Medical Devices Law, and the Ministerial Ordinance on Good Clinical Practice for Medical Devices. The trial registration numbers of each institutional review board are as follows: Okayama University, D20192001; Tokyo Metropolitan Children's Medical Center, OFT-G1-301; National Cerebral and Cardiovascular Center, #1103; The University of Tokyo, 2019018-11Y; and the other 2 hospitals under the Central Review Board for the Pediatric Clinical Trials Network in Japan, NW2020103.
This study was registered in the Japan Registry of Clinical Trials (jRCT1080224691). The protocol was approved by each institutional review board.
Materials
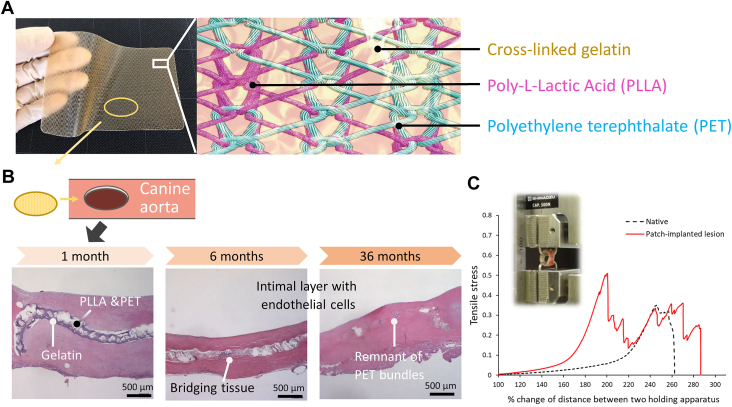
SHF is a warp-knit fabric consisting of biodegradable poly-L-lactic acid (PLLA) and nonbiodegradable polyethylene terephthalate yarns, coated with cross-linked gelatin derived from porcine skin5 (Figure 1A). SHF has been shown to have mechanical tolerance to surgical manipulation and expandability.5 An autologous wall was successfully restored by SHF in canine aorta and matured 2 to 3 years after implantation without chronic inflammation and calcification with complete degradation of PLLA5,6 (Figure 1B). The regenerated wall had stretch-expandability with sufficient mechanical strength6 (Figure 1C).
Figure 1.
Structure of synthetic hybrid fabric and summary of the in vivo preclinical study. (A) Gross appearance and structural illustration. (B) Representative histologic findings over time for the restored aortic wall over the implanted synthetic hybrid fabric in a canine model (hematoxylin and eosin staining). (C) Representative tensile stress: percentage extension relationship of the excised restored canine aortic wall at 4 years after implantation in comparison with the native aortic wall.
Study Design
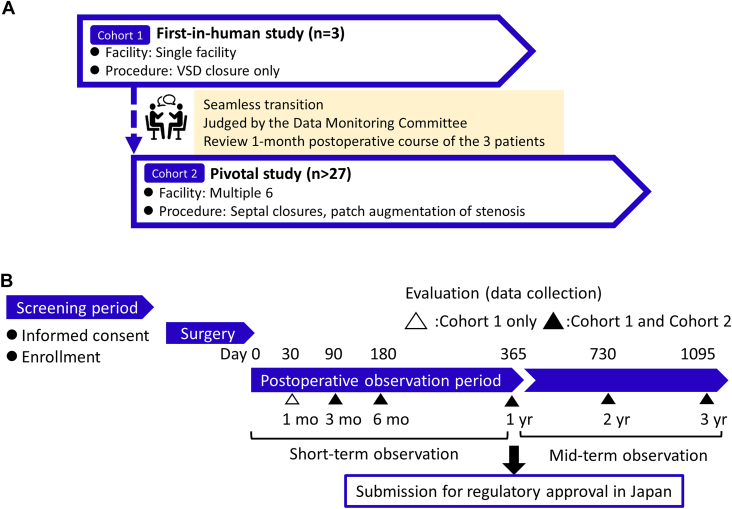
The study protocol used a tandem style, starting with a first-in-human study (cohort 1) followed by a pivotal study (cohort 2; Figure 2A; Supplemental Table 1). The observation period was set for at least 3 years after surgery based on the PLLA degradation period found in preclinical studies5,6 (Figure 2B). The following end points at 1 year after surgery were used for application for regulatory approval in Japan.
Figure 2.
Study design. (A) Study cohort. (B) Data collection timeline. (VSD, ventricular septal defect.)
End Points
The primary end point was defined as the surgical success rate of no death or reintervention due to SHF failure. The secondary end points were defined as abnormal findings detected on echocardiography (Supplemental Table 2) and adverse events (AEs). Definitions of AE severity and serious AEs are given in Supplemental Tables 3 and 4, respectively. The protocol was determined through in-depth consultation with the Pharmaceutical and Medical Devices Agency, which is the Japanese regulatory authority.
Patients
The inclusion and exclusion criteria are listed in Supplemental Table 5. Cohort 1 was limited to patients undergoing ventricular septal defect closure. At least 60% of all patients were planned to receive SHF implantation in the right ventricular outflow tract (RVOT) or pulmonary artery (PA).
Surgical Procedures
SHF was implanted by standard surgical techniques. When multiple patch implantation was required, SHF was placed in the RVOT and PA, and an existing product was used for septal defect closure. Use of SHF for grafts and valve leaflets was strictly prohibited.
Statistics
With reference to a prospective epidemiologic survey of tetralogy of Fallot,7 28 patients were required for enrollment based on a power of 80% and a 1-sided significance level of 5%. The surgical success rate and its 2-sided 90% CI and 95% CI were calculated by the Clopper-Pearson method for all participants. For the secondary end point, descriptive statistics were calculated for each SHF implant. All data were processed and analyzed with SAS 9.4 software (SAS Institute).
Results
The study period was from May 23, 2019, to December 22, 2023. A total of 34 participants were enrolled and completed at least 3-year postoperative follow-up, with a median follow-up period of 40 months (range, 36-55 months).
Patient Background
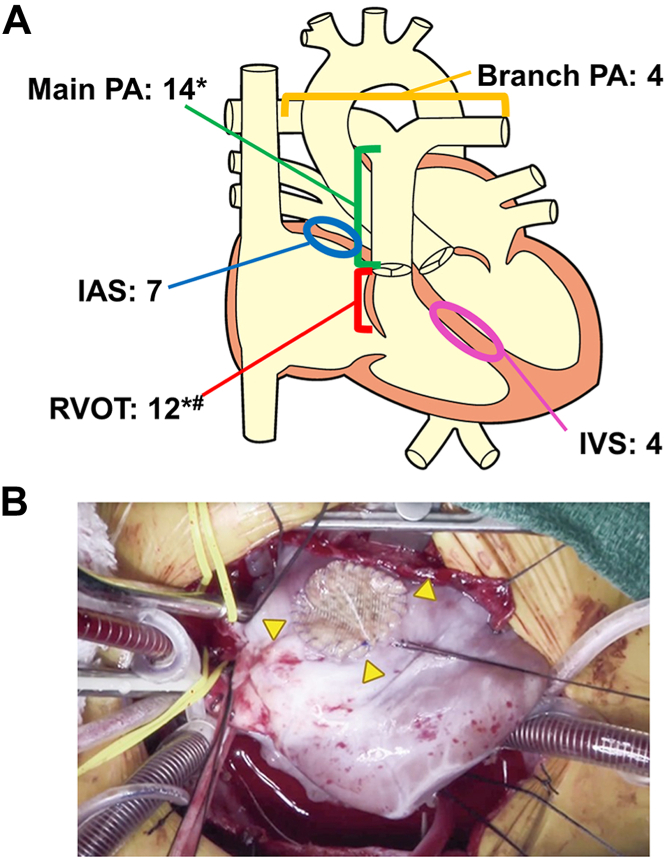
Patient background, surgeries, and prior surgeries are shown in Supplemental Tables 6 to 8, respectively. A diagram of the SHF implant site is shown in Figure 3A. SHF was directly sutured to other material in 11 patients (Supplemental Table 7). A representative intraoperative photograph of the implanted SHF is shown in Figure 3B (Video).
Figure 3.
Synthetic hybrid fabric (SHF) implantation. (A) Anatomic diagram of the SHF implantation site (41 sites in 34 patients). *Pulmonary artery (PA) + right ventricular outflow tract (RVOT), 5; #transannular patch, 6. (B) Representative photograph of an SHF implant in the right ventricular outflow tract (arrowheads). (IAS, intra-arterial septum; IVS, intraventricular septum.)
Surgical Mortality and AEs
There was no surgical death during the study period. Of the serious AEs summarized in Supplemental Table 9, 1 reexploration was required for hemostasis after redo surgery on postoperative day 1 because of bleeding due to lung injury caused by adhesion release. A mediastinal hematoma without cardiovascular compression was found on postoperative day 4 in another redo surgery but regressed during the natural course. Two other serious AEs of native PA stenosis occurred by 1 year postoperatively. One was mild stenosis distally adjacent to the SHF transannular patch in an infant who underwent tetralogy of Fallot repair. The other stenosis occurred between an SHF patch implanted in the left PA and a graft anastomosed to the right PA in a boy who underwent completion of total cavopulmonary connection. The stenosis was released by balloon dilation, surgical patch augmentation, and subsequently another balloon dilation. An asymptomatic stenosis at the junction of 2 SHF patches implanted in the main PA and left PA was incidentally found at 2 years after implantation in another boy who underwent pulmonary valve replacement. The stenosis was successfully released by balloon dilation. All of these AEs were determined to be moderate and not associated with SHF.
Primary End Point
The surgical success rate at 1 year after the operation was 100% (34/34 patients; 90% CI, 91.6%-100%; 95% CI, 89.7%-100%). Because the lower limit exceeded the predefined threshold, the primary end point was successfully achieved.
Secondary End Point
Abnormal findings on echocardiography of the implanted SHF are shown in Supplemental Table 10. Whereas no abnormality was detected by 1 year postoperatively, 2 cases had minor thickening of the SHF implant without hemodynamic disturbance in the atrial septum or PA at 1 to 3 years postoperatively. Because both end points were achieved at 1 year after implantation, regulatory approval was granted for use of SHF in Japan in July 2023 (approval No.: 30500BZX00169000).
Comment
This clinical study had 2 major findings. First, SHF was successfully implanted with acceptable efficacy and safety at several anatomic sites in routinely performed CHD surgery. Second, no abnormal findings directly related to the implanted SHF were detected on echocardiography by 3 years postoperatively.
Commercially available decellularized xenomaterials are currently derived from bovine pericardium (CardioCel; LeMaitre Vascular Inc) or porcine intestinal submucosa (CorMatrix; CorMatrix Inc). Contrary to preclinical expectations for these materials, reintervention was required in 2.8% to 62% of patients during 1 to 70 months after surgery,8, 9, 10 with particular caution advised for use in the RVOT, PA, and aorta.8, 9, 10 Excised specimens showed thickening of the materials with chronic inflammatory infiltration and no sign of autologous tissue replacement.1,2,9,10 Given the better reintervention-free rate of SHF in the PA or RVOT, these results suggest that xenomaterials, with or without decellularization, are not suitable for autologous tissue induction.
Electrospun nanofibers using supramolecular biodegradable polymer (Xeltis BV) have been tested in clinical trials as an extracardiac conduit with or without valve leaflets.3,4 Although there was no foreign body reaction, patchy absorption was found in excised samples, suggesting loss of mechanical strength due to an insufficient and unpredictable balance between polymer absorption and autologous tissue replacement, leading to valve prolapse or perforation in 5 of 18 patients (28%).4 A direct comparison cannot be made because of the different uses, but SHF showed well-maintained physical properties with no signs of structural damage on echocardiography, even after complete PLLA absorption. This suggests that the remaining nonabsorbable polyethylene terephthalate frames and the regenerated autologous tissue retained strength. Thus, combination of bioabsorbable materials and fabrication techniques is crucial in successful in situ tissue restoration after CHD surgery.
Limitations
There are several limitations in the study, including the small number of participants and the lack of implantation of SHF in the aorta. Therefore, the safety of SHF has not been fully demonstrated. Second, clinical tissue specimens were not observed to verify the features of SHF found in the preclinical studies. A 5-year postmarketing surveillance including use in the aorta is scheduled to validate the results of this study.
Conclusion
Additional long-term follow-up is required, but this 3-year clinical study showed that the novel SHF has considerable potential for use as a surgical material in CHD surgery.
Acknowledgments
The Supplemental Material can be viewed in the online version of this article [https://doi.org/10.1016/j.atssr.2024.04.020] on http://www.annalsthoracicsurgery.org.
The authors wish to thank Mr Yusei Hamada (Implantable Medical Device Development Department, Teijin Ltd) for clinical data collection.
Funding Sources
This study was sponsored by the Japan Agency for Medical Research and Development (grant: JP19he1302009) and Teijin Limited (Tokyo, Japan).
Disclosures
Shingo Kasahara reports a relationship with Teijin Ltd that includes: speaking and lecture fees. Shintaro Nemoto reports a relationship with Teijin Ltd that includes: consulting or advisory and speaking and lecture fees. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Presented at the Sixtieth Annual Meeting of The Society of Thoracic Surgeons, San Antonio, TX, Jan 27-29, 2024.
Supplementary Data
References
- 1.Nordmeyer S., Kretzschmar J., Murin P., et al. ADAPT-treated pericardium for aortic valve reconstruction in congenital heart disease: histological analysis of a series of human explants. Eur J Cardiothorac Surg. 2019;56:1170–1177. doi: 10.1093/ejcts/ezz228. [DOI] [PubMed] [Google Scholar]
- 2.Mosala Nezhad Z., Baldin P., Poncelet A., EI Khoury G. Calcific degeneration of CorMatrix 4 years after bicuspidization of unicuspid aortic valve. Ann Thorac Surg. 2017;104:e431–e433. doi: 10.1016/j.athoracsur.2017.07.040. [DOI] [PubMed] [Google Scholar]
- 3.Bockeria L.A., Svanidze O., Kim A., et al. Total cavopulmonary connection with a new bioabsorbable vascular graft: first clinical experience. J Thorac Cardiovasc Surg. 2017;153:1542–1550. doi: 10.1016/j.jtcvs.2016.11.071. [DOI] [PubMed] [Google Scholar]
- 4.Morales D.L., Herrington C., Bacha E.A., et al. A novel restorative pulmonary valve conduit: early outcomes of two clinical trials. Front Cardiovasc Med. 2021;7 doi: 10.3389/fcvm.2020.583360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nemoto S., Konishi H., Shimada R., et al. In situ tissue regeneration using a warp-knitted fabric in the canine aorta and inferior vena cava. Eur J Cardiothorac Surg. 2018;54:318–327. doi: 10.1093/ejcts/ezy045. [DOI] [PubMed] [Google Scholar]
- 6.Nemoto S., Konishi H., Suzuki T., et al. Long-term viability and extensibility of an in situ regenerated canine aortic wall using hybrid warp-knitted fabric. Interact Cardiovasc Thorac Surg. 2021;33:165–172. doi: 10.1093/icvts/ivab064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tetralogy of Fallot for Life (TOF-Life) https://clinicaltrials.gov/ct2/show/study/NCT02968264 Accessed May 28, 2018.
- 8.Bell D., Betts K., Justo R., et al. Multicenter experience with 500 CardioCel implants used for the repair of congenital heart defects. Ann Thorac Surg. 2019;108:1883–1888. doi: 10.1016/j.athoracsur.2019.04.085. [DOI] [PubMed] [Google Scholar]
- 9.Woo J.S., Fishbein M.C., Reemtsen B. Histologic examination of decellularized porcine intestinal submucosa extracellular matrix (CorMatrix) in pediatric congenital heart surgery. Cardiovasc Pathol. 2016;25:12–17. doi: 10.1016/j.carpath.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 10.Hibino N., McConnell P., Shinoka T., Malik M., Galantowicz M. Preliminary experience in the use of an extracellular matrix (CorMatrix) as a tube graft: word of caution. Semin Thorac Cardiovasc Surg. 2015;27:288–295. doi: 10.1053/j.semtcvs.2015.08.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.