Abstract
Pleural extension of pseudomyxoma peritonei is rare, and treatment demands multidisciplinary care. Perioperative management during cytoreductive surgery and hyperthermic intrathoracic chemotherapy challenges anesthesiology and surgical teams in unique ways. Hemodynamic, arrhythmogenic, ventilatory, fluid balance, acid-base, and nephroprotection issues are important considerations. The use of cytoreductive surgery and hyperthermic intrathoracic chemotherapy for extraperitoneal pseudomyxoma peritonei is an innovative and potentially curative approach. Here, we describe our approach to managing these patients.
Pseudomyxoma peritonei (PMP) is a rare disease of the abdomen and pelvis characterized by mucinous tumor deposits. Pleural extension of PMP is extremely rare, occurring in 0.7% to 5% of cases.1,2 Pleural involvement is most commonly iatrogenic secondary to violation of the pleural space during abdominal surgery.3,4 However, thoracic extension can occur spontaneously, usually to the right side of the chest, by tumor migration across transdiaphragmatic lymphatic channels.5 Thoracic cytoreductive surgery (CRS) with extended pleurectomy/decortication with hyperthermic intrathoracic chemotherapy (HITHOC) is described in cases of pleural mesothelioma or metastatic thymoma.6,7 However, in the setting of PMP, experience is limited, with no standardization. The management of patients with PMP undergoing thoracic CRS and HITHOC presents unique challenges to the surgeons and anesthesiologists in the perioperative period.
Technique
Patient Evaluation
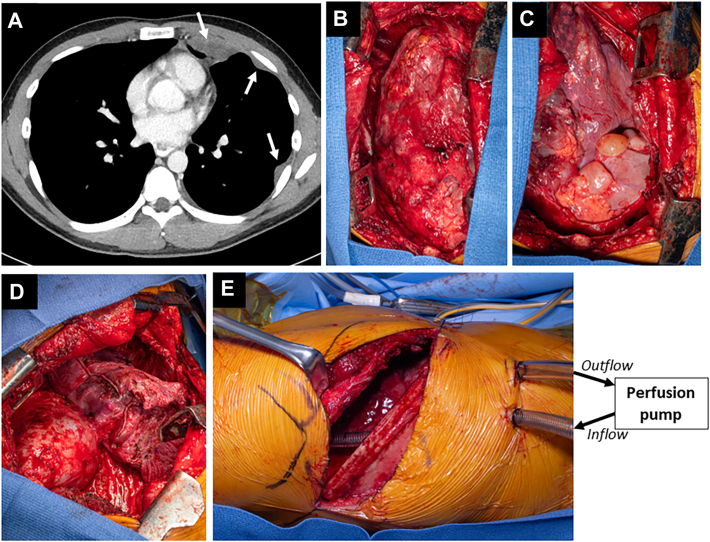
Patients need multidisciplinary evaluation by surgical oncologists and thoracic surgeons. Consideration of CRS to treat pleural extension of PMP should follow successful treatment of intra-abdominal disease. Combined abdominal and thoracic cytoreduction with intracavitary chemotherapy is discouraged. Timing depends on perceived cancer biology and the patient’s symptoms. Thoracic involvement can be addressed 3 months after abdominal CRS. Preoperative imaging consists of computed tomography of the chest to assess the burden of disease (Figure A). Positron emission tomography is of limited value and not routinely used. Pulmonary function tests are performed, and cardiac evaluation is obtained selectively. Prior operative reports need to be reviewed, with attention paid to subdiaphragmatic peritonectomy and the occurrence and repair of diaphragmatic injuries as this can predict involvement of diaphragm muscle by PMP with need for potential diaphragm resection.
Figure.
(A) Computed tomography imaging of disease burden. Arrows indicate metastatic pleural implants. (B-D) Intraoperative findings of disease burden during surgical resection. (E) Intraoperative perfusion setup for hyperthermic chemotherapy.
Intraoperative Anesthesia Considerations
Preoperative briefing between surgical oncology, thoracic surgery, anesthesiology, and perfusion teams is critical to discuss the conduct of the operation and to anticipate needs. Patients receive a preinduction thoracic epidural catheter for analgesia. After induction of general anesthesia, a left-sided double-lumen endotracheal tube is placed for single-lung ventilation. Standard American Society of Anesthesiologists monitors, arterial access, and large-bore central vascular access for intraoperative hemodynamic monitoring are recommended. Core body temperature is monitored by esophageal and bladder probes. Blood availability should be checked. Patients are positioned in a lateral decubitus position, and the chest is prepared widely, allowing access from the spine to the costal margin.
Because CRS and HITHOC involve significant insensible fluid losses from extensive pleurectomy and a substantial inflammatory response with third-space losses due to the heated chemotherapy, intraoperative fluid balance is imperative. Maintaining intravascular volume for the delivery of oxygen to end organs and providing nephroprotection while ensuring adequate urine output are critical components of perioperative management. Fluid administration can be liberal as the possibility of an extrapleural pneumonectomy is negligible in these cases. Renal protection is critical with the use of nephrotoxic chemotherapy. Amifostine and sodium thiosulfate have been used for nephroprotection during HITHOC for other pathologic processes.8
Fluid shifts and inflammatory effects may cause significant acid-base derangements that require prompt intervention. A baseline arterial blood gas determination is made to ensure optimal metabolic status. Subsequent intraoperative laboratory draws consist of an arterial blood gas analysis with hemoglobin, lactate, and electrolytes at 20-minute intervals with initiation of HITHOC and concluding with incision closure. Derangements are corrected aggressively with fluid resuscitation, intravenous calcium, and sodium bicarbonate.
Surgical Technique
Thoracic CRS consists of pleurectomy/decortication. Diaphragm invasion can be encountered if there were diaphragm injuries during prior abdominal CRS. As in operations for mesothelioma, an extended posterolateral thoracotomy with an anterior component to the costal margin is used. Resection of the sixth rib is not needed in cases of pleural extension of PMP. The extrapleural plane is developed, separating the parietal pleura from the intercostal muscles and endothoracic fascia (Figure B). Hemostasis of the chest wall with an energy device is important to limit blood loss. Dissection of the mediastinal pleura is done meticulously using “peanut” sponges or a right-angle clamp to gently mobilize mediastinal structures. Anteriorly, the mediastinal pleura is mobilized away from the pericardium. Pericardial involvement is unlikely because of the noninvasive nature of PMP. The bulk of disease can then be found on the diaphragm as it corresponds to the dependent region (Figure C). Free-flowing mucinous material is removed. The diaphragmatic pleura can be stripped at this point. Deeper involvement of the diaphragm might require partial or complete resection. The diaphragm can be reconstructed with a 2-mm polytetrafluoroethylene patch. The mobilized pleura is then resected around the lung hilum. The visceral pleura and lung are carefully inspected. Larger deposits or confluent nodules might be amenable to wedge resections. Smaller visceral pleural implants can be resected with a needle-tip electrocautery. Mediastinal lymph node dissection is not necessary. After maximal cytoreduction, ideally achieving macroscopic complete resection (Figure D), HITHOC is delivered.
HITHOC
An apical inflow cannula (24F) and a basal/diaphragmatic outflow cannula (36F) are placed inside the chest through separate incisions (Figure E) and secured with U stitches to minimize leakage. Watertight temporary closure of the thoracotomy is achieved with a simple running heavy monofilament suture. HITHOC is delivered with mitomycin C with or without cisplatin for 60 minutes at 42.5 °C with target flow of 2 L/min by a centrifugal pump such as the SCPC System (LivaNova) with an attached CardioQuip heater (CardioQuip LLC). If cisplatin is used, patients receive a sodium thiosulfate bolus and a 12-hour maintenance infusion for nephroprotection. At the initiation of HITHOC, increases in intrathoracic pressure and decreases in chest compliance can result in decreased preload and increased airway pressures, respectively. Furthermore, reduction in venous return paired with direct compression of mediastinal structures can lead to reductions in cardiac output.6 It is important to monitor hemodynamic or ventilatory changes that could result from pressurization of the chest during HITHOC. Arrhythmias during HITHOC have been described but have not been part of our experience. Interruption of HITHOC intraoperatively due to hemodynamic or ventilatory derangements is infrequent, but open communication between team members is key to address any issues.
Postoperative Care
Extubation is performed at the conclusion of the procedure in the operating room, and patients are admitted to the intensive care unit for extended monitoring. We do not employ forced diuresis to maintain a certain rate of urine output. Avoidance of nephrotoxic agents (eg, nonsteroidal anti-inflammatory drugs) is universally enforced. A thoracic epidural catheter for analgesia is paramount. Patients are mobilized early and encouraged to participate in respiratory physiotherapy.
Comment
Pleural extension of PMP is rare. The principles of management of this disease in the abdomen can also be applied in cases of pleural extension by means of thoracic CRS, usually in the form of a pleurectomy/decortication, and HITHOC. Multidisciplinary evaluation and participation are key for this procedure to be performed safely. Given the lack of alternative effective treatment options, this strategy should be considered in patients with pleural extension of PMP.
Acknowledgments
Funding Sources
The authors have no funding sources to disclose.
Disclosures
Luis F. Tapias reports a relationship with AtriCure Inc that includes: consulting or advisory; with Intuitive Surgical Inc that includes: consulting or advisory; and with AstraZeneca Pharmaceuticals LP that includes: consulting or advisory. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Patient Consent
Obtained.
References
- 1.Doyle M.P., Villanueva C.I., Davies M.P., et al. Cytoreductive surgery and heated intrathoracic chemotherapy for thoracic extension of pseudomyxoma peritonei. Integr Cancer Sci Ther. 2016;3:504–508. [Google Scholar]
- 2.Pestieau S.R., Esquivel J., Sugarbaker P.H. Pleural extension of mucinous tumor in patients with pseudomyxoma peritonei syndrome. Ann Surg Oncol. 2000;7:199–203. doi: 10.1007/BF02523654. [DOI] [PubMed] [Google Scholar]
- 3.Zoetmulder F.A., Sugarbaker P.H. Patterns of failure following treatment of pseudomyxoma peritonei of appendiceal origin. Eur J Cancer. 1996;32A:1727–1733. doi: 10.1016/0959-8049(96)00178-5. [DOI] [PubMed] [Google Scholar]
- 4.Lee B.Y., Kim H.S., Lee S.H., et al. Pseudomyxoma peritonei: extraperitoneal spread to the pleural cavity and lung. J Thorac Imaging. 2004;19:123–126. doi: 10.1097/00005382-200404000-00013. [DOI] [PubMed] [Google Scholar]
- 5.Aygun C., Demir H., Senturk O. Differential diagnosis of hepatic hydrothorax by 99mTc sulfur colloid peritoneal scintigraphy: two cases. Gastroenterol Res. 2009;2:248–252. doi: 10.4021/gr2009.08.1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kerscher C., Ried M., Hofmann H.S., Graf B.M., Zausig Y.A. Anaesthetic management of cytoreductive surgery followed by hyperthermic intrathoracic chemotherapy perfusion. J Cardiothorac Surg. 2014;9:1–8. doi: 10.1186/1749-8090-9-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ried M., Kovács J., Markowiak T., et al. Hyperthermic intrathoracic chemotherapy (HITOC) after cytoreductive surgery for pleural malignancies—a retrospective, multicentre study. Cancers (Basel) 2021;13:4580. doi: 10.3390/cancers13184580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Markowiak T., Kerner N., Neu R., et al. Adequate nephroprotection reduces renal complications after hyperthermic intrathoracic chemotherapy. J Surg Oncol. 2019;120:1220–1226. doi: 10.1002/jso.25726. [DOI] [PubMed] [Google Scholar]