Abstract
The prevalence of obstructive sleep apnea (OSA) is on the rise, driven by various factors, including more sensitive diagnostic criteria, increased awareness, enhanced technology through at-home testing enabling easy and cost-effective diagnosis, and a growing incidence of comorbid conditions such as obesity. Treating symptomatic patients with OSA syndrome to enhance quality of life remains a cornerstone approach. However, there is a lack of consensus regarding treatment to improve cardiovascular disease (CVD) outcomes, particularly in light of overall negative results from several randomized controlled trials indicating no benefit of positive airway pressure therapy on primary and secondary CVD events. These randomized controlled trials were limited by suboptimal positive airway pressure adherence, use of composite CVD outcomes, and limited diversity and generalizability to sleep clinic patients. As such, this workshop assembled clinical experts, as well as researchers in basic and translational science, epidemiology, clinical trials, and population health, to discuss the current state and future research directions to guide personalized therapeutic strategies and future research directions in OSA. There was overall consensus among workshop participants that OSA represents a heterogeneous disease with variable endotypes and phenotypes and heterogeneous responses to treatment. Future research should prioritize using multimodal therapeutic approaches within innovative and adaptive trial designs, focusing on specific subgroups of patients with OSA hypothesized to benefit from a CVD perspective. Future work should also be inclusive of diverse populations and consider the life course of OSA to better comprehend treatment strategies that can address the disproportionate impact of OSA on racially minoritized groups. Furthermore, a more holistic approach to sleep must be adopted to include broader assessments of symptoms, sleep duration, and comorbid sleep and circadian disorders. Finally, it is imperative to establish a sleep research consortium dedicated to collecting raw data and biospecimens categorized by OSA subtypes. This will facilitate mechanistic determinations, foster collaborative research, and help bolster the pipeline of early-career researchers.
Keywords: obstructive sleep apnea, positive airway pressure therapy, cardiovascular disease outcomes, health equity
Contents
- Overview
- Research Deliverables
Introduction
Methods
- Workshop Report
- Section 1: Understanding Findings and Limitations of the Major RCTs of PAP for CVD Benefit
- Section 2: Identifying Patients with OSA at Increased Risk of CVD and Potential to Respond to PAP
- Section 3: Advancing the Science on the Treatment of OSA for CVD Benefit: Gaps and Future Directions
Conclusions
Overview
Evidence from observational studies demonstrates independent associations between obstructive sleep apnea (OSA) and cardiovascular disease (CVD) event outcomes (1–5). However, randomized controlled trial (RCT) data on the impact of positive airway pressure (PAP) therapy for OSA on composite CVD events have not demonstrated CVD benefit (6–10). As a result of this conflicting evidence between findings from observational and mechanistic studies versus RCTs, best practices for treating patients with OSA for CVD risk reduction remain controversial. Many clinicians continue to treat asymptomatic or minimally symptomatic patients across the OSA severity spectrum to reduce CVD risk. In contrast, others have shifted practice to treating only those with symptoms associated with OSA (11–13). It has become increasingly evident that OSA encompasses various subphenotypes, characterized by distinct predominant mechanistic traits linked to various outcomes and potentially responsive to differing interventions (14). It is therefore necessary to elucidate an evidence-based OSA management approach that carefully considers individual patient characteristics for clinical decision making. This includes accounting for OSA-related physiologic features, symptoms, comorbidities, lifestyle, social determinants, CVD risk-mitigating treatments, and individual CVD endpoints.
To further this effort, there is need for 1) the identification of OSA endotypes and phenotypes that confer an increased risk for CVD; 2) understanding the impact of OSA-specific interventions on CVD outcomes within these endotypic and phenotypic subgroups; and 3) developing a feasible strategy across a variety of clinical and research settings for identifying individuals with OSA at high risk for CVD who are likely to respond to treatment. Furthermore, as minoritized groups were not represented in the existing RCTs, future research endeavors must embrace diversity to achieve health equity. In response to these pressing gaps in our field, we organized this workshop and extended invitations to major stakeholders with expertise in OSA epidemiology, clinical trials, CVD, precision medicine, and health equity. The primary objective of this workshop was to review the current evidence regarding the role of OSA treatment for CVD health benefits and develop a strategic framework to generate the data needed to address existing scientific gaps and guide the field toward a precision medicine approach for CVD risk reduction in OSA. This American Thoracic Society (ATS) workshop panel included representation from various countries, and research and clinical backgrounds. We reviewed existing literature, synthesized expert opinions, and identified crucial research questions regarding OSA treatment for cardiovascular benefit.
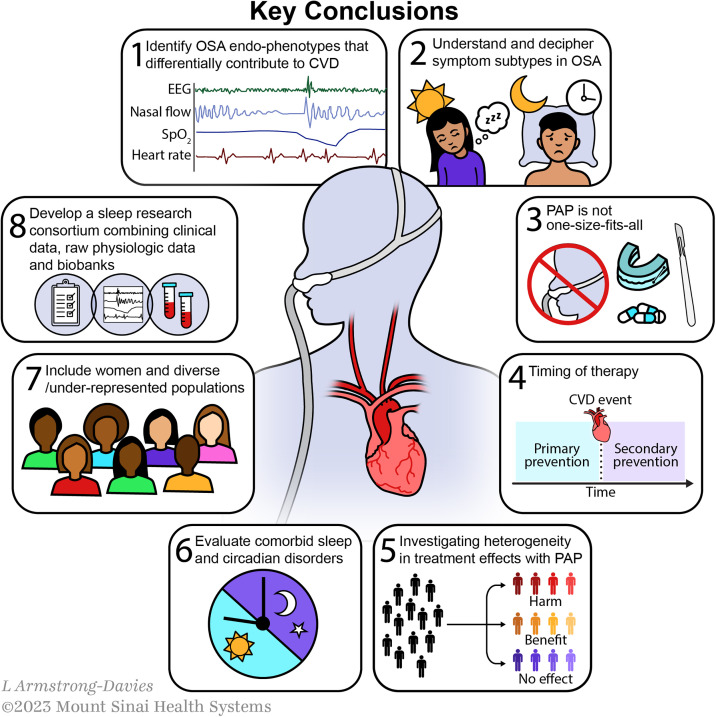
The workshop reports the following agreed-on key conclusions among the attendees and authors (Figure 1):
Identifying OSA endotypes and phenotypes that most strongly contribute to CVD and respond to intervention: Given the high rates of OSA (defined purely by apnea–hypopnea index [AHI]) (15) and the high likelihood for developing CVD with age (16), it is often the case that these two conditions are comorbid. It remains unclear which patients with OSA are at higher than expected risk of CVD and whether that risk is attributable to OSA-specific physiology. Identifying OSA subtypes and factors that contribute to CVD should be the focus in an era of precision medicine. Despite efforts to endotype and phenotype OSA on the basis of the anatomy and physiology of the upper airway, ventilatory control measures, event-related physiologic sequelae, clinical features, and biomarkers, there is uncertainty regarding which of these investigative approaches are 1) most feasible across diverse settings and 2) produce metrics that have sufficient reproducibility, discrimination, and predictive value for highest clinical and scientific impact.
Symptom subtypes in OSA, including patients with excessive daytime sleepiness (EDS) and insomnia symptoms: Future studies must consider enriching study cohorts with participants who have OSA-related symptoms such as EDS and insomnia symptoms in clinical trials. Including symptomatic patients in RCTs may be achieved safely and ethically through various strategies, such as rigorous informed consent procedures, external boards for close safety monitoring, adaptive trial designs, and active engagement of patients with OSA as advisers (17).
PAP is not one size fits all: The current evidence does not support the use of PAP for all patients with OSA, especially individuals with asymptomatic or minimally symptomatic OSA. Although several post hoc analyses suggest potential CVD benefits among those with putatively “adequate” PAP adherence (greater than four hours per night), a “healthy adherer” bias limits meaningful interpretation. Notably, however, there were workshop members who believed that this signal of benefit within those adherent to therapy should not be minimized. Moreover, caution must be exercised when extrapolating current RCT evidence to patients referred to sleep clinics. Existing trials primarily recruited patients with established CVD who presented to cardiology clinics or inpatient hospital units without significant daytime sleepiness.
Targeting OSA treatment for primary versus secondary CVD risk reduction: Although panelists agreed that the current literature does not support OSA treatment for secondary CVD prevention among all patients with elevated AHIs, there has been less research addressing the role of OSA treatment as a strategy for primary CVD risk reduction. This is due to the complexity and cost of conducting larger and longer trials required for such an investigation. A study by Barbé and colleagues (6) was one such primary prevention trial with negative findings, but the possibility remains that earlier intervention (before the development of irreversible damage to the vasculature and other organs) might reveal therapeutic benefit. Even so, the feasibility of initiating and maintaining lifelong OSA treatment in younger, healthier patients for primary CVD prevention is unclear and needs to be further examined. This goes together with the overarching need to better understand the natural history of OSA, including differences in the average age of onset across population subgroups, as well as the impact of these differences on disease severity and timing of therapy during the life course.
Investigating heterogeneity in treatment effects with PAP: Until recently, PAP had been considered a mostly benign intervention. However, the net neutral results from RCTs using PAP in OSA for CVD outcomes may be a result of balancing its favorable and unfavorable effects. There is evolving preclinical evidence to suggest that, especially at higher pressures, there may be a detrimental effect of PAP via alveolar stretch and lung injury, resulting in endothelial cell dysfunction and inflammation (18). Furthermore, eliminating the stimulus for ischemic preconditioning and coronary collateral formation (via intermittent hypoxemia) through PAP therapy may block a hypothesized teleological benefit of OSA among certain patient subgroups. These opinions were controversial and not shared by all workshop attendees; however, they should be considered in future research.
Incorporation of comorbid sleep disorders: Given that sleep and circadian traits (e.g., short and long sleep duration, sleep irregularity) have been independently associated with increased CVD mortality in observational studies (19, 20), future research should incorporate a more holistic consideration of sleep-related factors that may influence CVD risk among those with OSA. Comorbid sleep disorders, together with the sleep environment and neighborhood-level factors that disrupt sleep, have yet to be adequately assessed and quantified in the current OSA literature. Moreover, RCTs to date have not gauged the combined effects of OSA interventions and treatment of comorbid sleep disorders on CVD outcomes. It is important to note that PAP cannot rectify insufficient sleep duration and may occasionally result in more sleep disruption and awakenings, impairing sleep quality in some patients. The impact of these comorbidities and factors must be considered in future analyses of large datasets or clinical trials that test the effectiveness of OSA interventions on CVD risk. At a minimum, future trials in OSA should include robust objective measures of sleep duration and sleep–wake timing.
Inclusion of women and diverse populations: It is evident that there is a marked lack of inclusion of women, racially and ethnically diverse groups, and other marginalized communities, such as LGBTQ+ individuals, people with disabilities, and socioeconomically disadvantaged populations, in existing OSA literature, especially within RCTs investigating PAP for CVD outcomes. The inclusion of these groups is crucial, as observational evidence generally demonstrates earlier onset and more severe OSA at the time of clinical diagnosis, higher percentages of underdiagnosis, and lower rates of adequate therapy (including modifiable barriers to adherence) among racially minoritized groups (21, 22). In addition, these minoritized groups are at an increased risk of resistant hypertension and stroke/transient ischemic attack (23), two CVD outcomes strongly tied to OSA. However, observed disparities or differences across groups can be social, cultural, environmental, and/or behavioral. Understanding the cause of these disparities and differences across groups can illuminate mechanisms of pathogenesis and disease progression, which can subsequently inform interventions. As a field, we must better identify and include social determinants of health in future studies, as an understanding of these factors is critical to reduce the consequences of structural racism and discrimination and to promote health equity. Future research must develop, support, and evaluate the influence of system-level changes and create community partnerships to ensure that studies are developed and interventions are implemented by the community they serve.
Combine data collection efforts and develop a unified sleep research consortium: The National Sleep Research Resource (NSRR) has advanced the field of sleep research tremendously by organizing data from multiple sources (24, 25), providing access to investigators. Further efforts should be made to unify and combine prospective data collection, including clinical, physiologic, and biospecimen (biomarkers and genetic specimen for biobanking). This data collection and repository approach could support future evaluation of OSA endotyping and phenotyping for CVD risk reduction. In addition, such a unified consortium could establish common data elements and share protocols, allowing improved data harmonization and use. An in-depth review of the cost–benefit balance of this type of effort was not discussed as part of this workshop and requires careful attention in a future official ATS document. Ultimately, collaboration will further advance the field by leveraging local expertise and extending that experience more globally in an increasingly digital age.
Figure 1.
Key conclusions. CVD = cardiovascular disease; EEG = electroencephalography; OSA = obstructive sleep apnea; PAP = positive airway pressure; SpO2 = oxygen saturation as measured by pulse oximetry.
Research Deliverables
-
1.Tailored PAP trials
-
•Design RCTs enriched with patients with symptomatic OSA (i.e., those with fragmented sleep, poor quality sleep, nonrestorative sleep, and EDS) recruited from sleep clinics with both low and high CVD risk categories to discern which subgroups are most likely to derive benefit from treatment. For example, EDS with OSA has been associated with systemic inflammation, increased airway collapsibility, and increased hypoxic burden and thus may be a more PAP-responsive subtype. Subgroup analysis by age may be informative, given that elderly individuals with preexisting CVD may have limited CVD benefit from PAP.
-
•
-
2.
Inclusive and comprehensive trials
Design trials that accomplish the following aims:-
•Holistically address sleep by incorporating evaluations of insomnia, objective sleep duration, circadian rhythm disruption, and sleep patterns. Trials assessing the impact of interventions to improve insomnia, sleep opportunity, and sleep timing should be considered alongside treatment of patients with OSA.
-
•Prioritize the inclusion of diverse populations, with a focus on women and historically and persistently minoritized groups. This must be accompanied by the collection of data relating to social determinants of health to identify factors that may underlie such differences. In addition, the field must develop focused trials within minoritized populations. These goals can be achieved by addressing 1) system-level barriers to equitable access to clinical trials, 2) the use of health-promoting resources, 3) diversifying investigative research teams, and 4) partnering with community organizations and community advisory boards to ensure equitable representation and to better understand social determinants of health to recognize and combat structural biases.
-
•
-
3.
OSA endotype and phenotype standardization initiative with eventual application of machine learning and artificial intelligence approaches: Launch a comprehensive initiative that aims to reconcile and standardize the diverse methodologies used in endotyping and phenotyping OSA. This could involve creating a centralized database combining physiologic, biomarker, patient-reported, and other clinical data to identify subgroups and direct specific treatments more clearly by applying machine learning and artificial intelligence approaches, moving toward precision medicine. These larger collaborative datasets with multimodal data are necessary, as artificial intelligence methods require larger sample sizes for adequate model training, testing, and external validation. The ultimate goal from such an initiative would be to define and identify potentially hidden but scalable measures that predict CVD outcomes and treatment responses in patients with OSA.
-
4.
Comparative effectiveness of the broad spectrum of therapeutic approaches for CVD risk reduction: Initiate studies that explore alternative OSA-targeted therapeutic options beyond PAP (e.g., oral appliance therapy, positional therapy, surgical approaches, pharmacologic treatments), especially for younger, healthier patients and those without OSA-related symptoms, and compare these approaches with traditional PAP. This approach can also include exploring the effects and potential drawbacks of high-pressure PAP to understand its wider implications. Furthermore, the field must understand the comparative effectiveness of long-term medications used for CVD risk reduction (e.g., antihypertensives and lipid-lowering agent use) specifically for the reduction of CVD risks attributed to OSA as alternatives to OSA respiratory event/upper airway targeted treatments.
-
5.
Sleep research consortium and physiologic biobank: Recognizing the limitations of isolated national efforts in translating research into clinical practice, the expansion of the NSRR into a global consortium is proposed. This transformation would allow the use of larger, more diverse datasets, essential for applying advanced technologies such as artificial intelligence in sleep medicine and for comprehensively addressing the influence of genetics, culture, environment, and social determinants of health. This global collaboration would standardize research protocols, harmonize existing datasets, and reduce costs through economies of scale, potentially attracting significant international funding. Furthermore, this consortium would facilitate a unified approach to data, enabling organizations such as the ATS to convene researchers using a common language and datasets, reducing discrepancies in methodologies that often hinder progress at scientific meetings. By standardizing methods and datasets, the consortium aims to expedite scientific collaboration and accelerate the understanding and management of sleep disorders globally, leveraging the proven track record of the NSRR while significantly expanding its reach and impact.
Ensuring the success of these research deliverables will require that funding agencies prioritize these initiatives by releasing targeted funding announcement for tailored RCTs, encouraging a pipeline of early-career investigators to conduct this work, incentivizing collaborations for standardizing endotyping and phenotyping methodologies, and investigation of novel therapeutic avenues for OSA (Figure 2).
Figure 2.
Role of funding agencies. 1) Tailored randomized controlled trials (RCTs): Prioritize and release targeted calls for RCTs to encourage the use of advanced trial designs, including enrichment and adaptive designs, phenotype-driven RCTs (with cardiovascular disease [CVD] risk defined by clinical criteria and/or biomarkers), and sufficient sample size and duration to allow subgroup analyses and to detect clinically important changes in hard outcomes. Cooperation between international funding sources and public and private sources would enable this initiative. 2) Supporting early-career investigators: Encourage training and support for investigators to conduct these clinical trials in our field, particularly those underrepresented. 3) Incentivize collaborations for standardizing endotyping and phenotyping methodologies: Funding bodies can initiate special grants or funding mechanisms that incentivize researchers to apply advanced statistical methods, including machine learning (ML) and artificial intelligence (AI) approaches, on existing data to systematically identify reproducible measures that have good discrimination/prediction, understand their utility for relevant populations and outcomes, and ensure that the code is open source, and made widely available. Furthermore, validating these measures within existing electronic health record data is a crucial step toward clinical translation, and providing funding for these efforts is essential. 4) Encourage investigation of novel therapeutic avenues for CVD risk reduction in obstructive sleep apnea (OSA): Release focused requests for proposals or requests for applications that push the envelope on alternative therapeutic avenues beyond PAP for OSA. The risks and benefits of PAP therapy compared with these alternatives and in conjunction with CVD risk reduction strategies (pharmacologic and behavioral) should be evaluated. Furthermore, such therapies should evaluate the effectiveness of therapy within the variety of OSA endotypes and phenotypes (identified in point 3) and across diverse patient groups, including women, low-income communities, and minoritized groups.
Introduction
OSA is characterized by repetitive collapse of the upper airway during sleep, exerting potentially deleterious cardiovascular effects through a combination of mechanisms, including intermittent hypoxia (IH), mechanical load, autonomic dysregulation, and inflammation, among others (26). Recent modeling data estimate the global burden of OSA at more than 400 million persons globally (using a defining criterion of AHI ≥ 15 events/h) (27). Continuous PAP therapy is the mainstay of OSA treatment, and current guidelines recommend therapy for all patients with OSA with sleep-related symptoms or elevated blood pressure (11, 12). These recommendations evolved over the past decade in response to several recent RCTs, which did not demonstrate a benefit of PAP therapy on composite CVD outcomes, including stroke and myocardial infarction (MI) in nonsleepy or mildly sleepy patients with moderate to severe OSA (Epworth Sleepiness Scale scores as high as 15 were included) (6–10). The first major study, published by Barbé and colleagues (6), showed no effect of PAP as a primary CVD prevention (including hypertension) strategy for nonsleepy patients with OSA. Subsequent studies, including Randomized Intervention with Continuous Positive Airway Pressure in CAD and OSA (RICCADSA) (8), Sleep Apnea Cardiovascular Endpoints Study (SAVE) (7), and Impact of Sleep Apnea Syndrome in the Evolution of Acute Coronary Syndrome (ISAACC) (9), evaluated the effect of PAP on secondary prevention of CVD outcomes. These studies also found that PAP did not affect composite cardiovascular outcomes among nonsleepy or mildly sleepy patients with moderate to severe OSA and histories of either coronary artery disease or acute coronary syndrome (ACS). Given these findings, in 2021, the Agency for Healthcare Research and Quality (AHRQ) performed a comprehensive review to address the effectiveness of PAP therapy (28) and concluded that the “published evidence mostly does not support that [continuous PAP] prescription affects long-term, clinically important outcomes.” In response, some experts criticized the AHRQ report, emphasizing that this conclusion does not reflect the totality of available evidence (29, 30) and that improvement of outcomes such as EDS and blood pressure after PAP were not considered. Neither were the barriers to conducting RCTs (e.g., PAP adherence, randomizing symptomatic patients) or the lack of adequate representation of women and racially minoritized groups in existing RCTs. Furthermore, the AHRQ report does not account for the burgeoning evidence on OSA heterogeneity with specific endotypes and phenotypes at heightened CVD risk and, more recently, the potential heterogeneity of treatment effects (HTE) with PAP therapy on CVD outcomes (31–35).
Methods
In light of these developments, the field has reached an important crossroads in the management of OSA, specifically, the need to leverage new developments in clinical phenotyping to maximize the cardiovascular benefit by personalizing therapy when most appropriate and to highlight the path ahead for future research in this area. A workshop proposal was submitted to the ATS Assembly on Sleep and Respiratory Neurobiology in August 2022 and funded in January 2023. Workshop participants were recruited from around the world to represent a diverse range of professional backgrounds and expertise; included were OSA and CVD content experts from various disciplines, including fundamental preclinical and translational research, clinical trials, epidemiology, data science, health outcomes, and diversity, equity, and inclusion research. All participants served as speakers, moderators, panelists, or specific content experts, contributing their expertise and unique perspectives.
These key stakeholders were tasked with reviewing and critically appraising the current state of the literature and addressing several key overarching objectives that were predetermined to guide the discussion:
Discuss and understand current opinions and limitations relating to existing evidence from RCTs of OSA treatment for composite CVD outcomes.
Conduct a focused discussion on phenotypes of OSA that predict CVD risk. Discuss evidence from large RCTs, post hoc analyses of RCTs, and other independent studies that have identified novel phenotypes of OSA with enhanced CVD risk and how this evidence can be applied effectively in clinical and research settings. We will also discuss strategies for future investigations to discover novel phenotypes.
Review critical gaps in the current evidence, emphasize new directions and methodology that can be adopted to advance cardiovascular risk prediction, and provide definitive evidence on whether OSA treatment results in CVD risk reduction in predefined OSA subgroups, with an emphasis on underrepresented populations that are disproportionately affected by OSA and not included in trials to date.
The live workshop meetings were held in two parts, with the first being a hybrid meeting during the ATS Annual International Conference in Washington, D.C., on May 23, 2023. A second virtual meeting took place on July 6, 2023. As outlined in Table 1, the agenda included lectures and discussions moderated by the co-chairs and guided by specific predetermined questions for each panelist. The co-chairs developed this document using notes and recordings from the live meetings. All participants could submit edits and revisions and ultimately approved the final workshop report. All participants’ conflicts of interest were submitted to and reviewed by the ATS Conflicts of Interest Department.
Table 1.
Workshop agenda (abridged)
| May 23, 2023: Hybrid in-person/virtual meeting at the ATS Annual International Conference |
| Lecture: Understanding existing literature: randomized controlled trials of OSA treatment on CVD outcomes: Vaishnavi Kundel |
| Moderated discussion (by co-chairs: Oren Cohen, Vaishnavi Kundel, Henry K. Yaggi, and Neomi A. Shah) |
| Panelists: Ferran Barbé, Yüksel Peker, Doug McEvoy, Susan Redline, Manuel Sánchez-de-la-Torre, Daniel J. Gottlieb, and T. Douglas Bradley |
| Lecture: OSA endotypes and phenotypes with increased CVD risk: Andrey Zinchuk and Ali Azarbarzin |
| Moderated discussion (by co-chairs: Oren Cohen, Vaishnavi Kundel, Henry K. Yaggi, and Neomi A. Shah) |
| Panelists: Andrey Zinchuk, Ali Azarbarzin, Atul Malhotra, Helena Schotland, David Gozal, Sanja Jelic, Alberto R. Ramos, Susan Redline, and Jennifer L. Martin |
| July 6, 2023: Virtual meeting |
| Lecture: Moving the needle on OSA treatment for CVD risk reduction in at risk groups: Sushmita Pamidi and Dayna A. Johnson |
| Moderated discussion (by co-chairs: Oren Cohen, Vaishnavi Kundel, Henry K. Yaggi, and Neomi A. Shah) |
| Panelists: Reena Mehra, Virend Somers, Camilla Hoyos, Chandra L. Jackson, Carmela Alcantara, Martha E. Billings, and Sanjay R. Patel |
Definition of abbreviations: ATS = American Thoracic Society; CVD = cardiovascular disease; OSA = obstructive sleep apnea.
Workshop Report
Section 1: Understanding Findings and Limitations of the Major RCTs of PAP for CVD Benefit
Summary of findings
Workshop attendees discussed current evidence for using PAP for cardiovascular benefit on the basis of four RCTs published over the past decade. These RCTs, Barbé and colleagues (6), RICCADSA (8), SAVE (7), and ISAACC (9), each showed no significant benefit of PAP in the primary analyses. Although post hoc subgroup analyses from two of the four trials demonstrated a benefit of PAP for CVD risk reduction among participants using PAP for >4 hours per night, and post hoc analysis from the SAVE trial showed a lower risk of stroke among those adherent to PAP, secondary subgroup analyses must be interpreted cautiously, as they are subject to potential healthy adherer bias and residual confounding, limiting the ability to draw causal inference from these results. Taken together, the current evidence does not support the use of PAP among asymptomatic patients with OSA to reduce CVD risk. However, there is an absence of prospective RCT data regarding CVD outcomes after treatment of OSA among symptomatic patients. Last, emerging secondary data analyses from these trials may support treatment in specific high-risk subgroups (33–35).
Explanation of findings.
This lack of demonstrable CVD benefit can be attributed to several factors:
Despite an abundance of data from observational epidemiology studies of OSA-related CVD risk, it remains possible that these studies have inflated the CVD risk of OSA because of residual confounding. Potential unmeasured confounders in these studies could include sedentary lifestyles, visceral adiposity, atherogenic diets, comorbid illness, other sleep disorders, short sleep duration, poor sleep quality, and other unknown factors. Most workshop participants believed that this was unlikely, as this would not explain the results of mechanistic studies. Another possibility may be that there is, in fact, no effect of PAP in improving CVD outcomes among patients with OSA and that quasiexperimental studies (comparing those on PAP with those not using PAP) may have overestimated the benefit of PAP in mitigating OSA-related CVD risk. Such quasiexperimental studies are also susceptible to the healthy adherer effect by comparing PAP adherers to nonadherers (36, 37). Although PAP may be effective in reducing CVD risk in isolation, its benefits may not provide further risk reduction beyond that provided by adjunctive therapies for CVD, which have been proved effective in CVD risk reduction. Most patients in secondary CVD prevention trials are already being actively treated with therapies to reduce CVD risk, which may also treat the downstream effects of OSA. For example, β-blockers may counteract sympathetic activation (38), antihypertensives may counteract the effects of OSA on 24-hour blood pressure (39), implantable pacemakers and defibrillators counteract life-threatening arrhythmias, and statins may improve endothelial dysfunction (40) and coronary artery disease–related events (41). In the setting of these therapies, PAP may provide only modest benefit, and trials with large samples would be required to ascertain any PAP-specific benefits.
PAP therapy might exhibit favorable and potentially deleterious effects across various patient subgroups, resulting in an overall net neutral influence on cardiovascular outcomes. For example, the expected benefits of PAP in reducing IH and arousals may be counteracted by mechanical effects of PAP, such as lung distention in a subset of patients, as suggested by results from recent studies (40, 42). In addition, among those with a preexisting burden of CVD, untreated OSA in a subgroup of patients may be cardioprotective by way of ischemic preconditioning, as suggested by prior research (43–46).
Another possible explanation is that the association between OSA and CVD is bidirectional. Schiza and colleagues (47) previously demonstrated that the prevalence of OSA was remarkably high in patients with ACS, but this high prevalence did not persist six months later. The ISAACC trial specifically enrolled participants during index hospitalization for ACS. It is possible some of those randomized to usual care did not have OSA at long-term follow-up. A preliminary report from the RICCADSA trial also supported a natural decline in the AHI among half the untreated participants and resolution of OSA among 11% (48). These studies highlight the notion that OSA in the setting of ACS may be transient and can improve with cardiovascular recovery after acute events. Perhaps focusing on this patient population in future RCTs is not advisable, as OSA is likely to improve over time in such patients without OSA-specific interventions.
Potential limitations.
Several limitations of these trials that may explain the null findings should be considered (Table 2):
-
•
Limited PAP adherence: A major limitation of the existing RCTs, often cited as the primary criticism, was the lack of adequate adherence to PAP therapy, with an average of 3–5 hours per night. This impairment in the “enactment fidelity” of existing clinical trials limits our ability to fully understand PAP’s efficacy in reducing cardiovascular outcomes (49). To complicate matters further, adherence to PAP is often confounded by a multitude of issues, including comorbid sleep disorders (e.g., insomnia, short sleep) not assessed in these trials, which themselves are associated with both low PAP use and an independent risk of cardiometabolic and CVD (50–55). In addition, the trials recruited patients with nonsleepy OSA, and rates of PAP use among asymptomatic patients are generally lower than those with OSA and symptoms of sleepiness (56). However, it is important to note that the benefit of behavioral interventions (e.g., motivational enhancement) to encourage PAP adherence has been demonstrated in clinical trials settings and could potentially be deployed in future trials (57).
-
•
PAP for secondary CVD prevention: Most RCTs focused on PAP for secondary prevention of CVD, recruiting patients with existing CVD from cardiology clinics or inpatient cardiac units with minimal sleepiness symptoms. This population is also often treated with other medical cotherapies that directly address CVD risk (e.g., statins, antihypertensives). Therefore, among patients with preexisting CVD and those without sleep difficulties, PAP may play a limited role in CVD risk reduction (58). Most workshop attendees (although not all) agreed that future studies should focus on recruiting patients from ambulatory sleep clinics who typically present with sleep difficulties to evaluate the role of OSA therapy and that benefit may be more pronounced for primary CVD prevention, assuming optimal use and sufficient duration of treatment.
-
•
Use of composite cardiovascular outcomes: The assessment of composite CVD outcomes after PAP helps improve our ability to detect a significant effect by reducing the required sample size to determine a treatment effect. However, OSA’s pathobiological mechanisms may differ for the various CVD outcomes measured (i.e., CVD mortality, hospitalization for ACS/MI, cardiac arrhythmia, heart failure, stroke, or transient ischemic attack). A recent meta-analysis using per protocol data (i.e., comparing control subjects with PAP-adherent participants) demonstrated a protective effect of PAP, particularly for cerebrovascular outcomes, but not for coronary outcomes and heart failure (59). In addition, these large RCTs did not assess certain CVD outcomes, such as atrial fibrillation. As such, composite events limit our ability to understand specific relationships and outcomes. Although not unanimous, most attendees agreed on the need to evaluate individual CVD outcomes in future research including mechanistic studies.
-
•Lack of generalizability
-
○Inclusion of women and racially minoritized populations: Fewer than 20% of the RCT populations were women. Furthermore, participants from racially and ethnically minoritized and underrepresented communities, which are disproportionately affected by OSA (21), were also not represented in these trials. As discussed in Section 3, future research must be intentional in its approach to promote diversity, equity, and inclusion among study participants and research teams, and account for social determinants of health.
-
○Inclusion of symptomatic patients with OSA: Exclusion criteria within each RCT limits generalizability to many patients encountered in a clinical setting, such as symptomatic patients and those with significant medical comorbidities. Among this excessively sleepy/symptomatic group, PAP has improved quality of life–related functional outcomes (60). Furthermore, sleepiness can predict PAP adherence, especially in combination with more severe disease (61). Therefore, excluding these groups limits the generalizability of these results to many patients seeking OSA care. Moreover, although men are more often believed to have “typical” OSA symptoms, presenting with EDS as the main symptom, women may present with different symptoms, such as fatigue and mood disruption (62). Future studies should make a concerted effort to include patients with all OSA-related symptoms, including those noted in the International Classification of Sleep Disorders – Third Edition, Text Revision (13) (i.e., sleepiness, nonrestorative sleep, fatigue, insomnia symptoms, and awakenings with breath holding, gasping, or choking), to improve generalizability in both men and women. A discussion on including symptomatic patients in research was the topic of an ATS workshop by Donovan and colleagues (17). That report described important considerations regarding this patient population, including equipoise, adequate informed consent processes, and monitoring for pathologic sleepiness.
-
○
-
•
PAP as “one size fits all”: Finally, workshop attendees discussed the heterogeneity within OSA and the possibility that a one-size-fits-all approach for PAP in OSA may be a major limiting factor resulting in the lack of a demonstrable CVD benefit. Recent exploration into endotypes and phenotypes of OSA suggests that some subgroups may be more susceptible to the protective effects of PAP treatment, whereas other subpopulations may be experiencing harm from PAP. For example, studies on pulse rate response (33) and hypoxic burden (34) in patients with OSA have shown that subgroups categorized by these metrics exhibit varying responses to PAP treatment. Other works have demonstrated the utility of machine learning in identifying such subgroups experiencing heterogeneous treatment effects using baseline predictors (35). Further identifying patients with increased CVD risk and heterogeneous treatment effects is addressed in the next section.
Table 2.
Limitations of RCTs of PAP for CVD risk reduction among nonsleepy to mildly sleepy patients with OSA and future directions
| Limitation | Consideration | Future Directions |
|---|---|---|
| PAP adherence | Low adherence to therapy biases treatment effects toward the null. Post hoc analysis by adherence is confounded by the healthy adherer effect. | Develop adherence optimization strategies and multimodal treatment algorithms that incorporate any available therapy to achieve a balance between ability to eliminate obstructive respiratory events and tolerability of treatment. |
| PAP for secondary CVD prevention | In the setting of preexisting comorbid disease and disease-modifying treatments (e.g., statins, antihypertensives, β-blockers), elimination of OSA may or may not exert a significant effect. | Understand the effects of existing cardiovascular medical therapies on OSA-associated physiologic responses and cardiovascular risk. Continue to focus on PAP for improvement of quality of life and functional outcomes. |
| Composite cardiovascular outcomes | Physiologic responses to OSA-associated respiratory events are variable and may be linked to differential CVD outcomes. Composite events are helpful in RCTs to meet sample size requirements but detract from the scientific understanding of OSA and its relationship to specific CVD subtypes (e.g., coronary heart disease, heart failure, stroke, arrhythmia). | Examine the relationship between OSA and individual CVD events. Although this will require larger sample sizes, modern statistical techniques may help reduce the requisite sample size. Consider integrating objective sleep measurements into larger CVD trials to assess the impact of sleep on CVD outcomes, in accordance with the American Heart Association’s Life’s Essential 8 requirement. |
| Lack of generalizability | Participants were recruited from non–sleep clinic settings, but these data are being applied most commonly to patients seeking sleep medicine consultation. Furthermore, studies did not adequately represent the diverse racial and ethnic U.S. population and had limited inclusion of women. | Direct future trials to recruit patients presenting to sleep medicine clinics, potentially including symptomatic patients. Note that this was discussed in a prior ATS workshop report (17). In addition, future trials must be more inclusive in terms of race, ethnicity, and gender. |
| Heterogeneity within OSA | Current understanding suggests that OSA is an umbrella diagnosis that represents a variety of subtypes. These have been described by studies exploring OSA endotypes and phenotypes. Post hoc data suggest that there are subgroups that exhibit heterogeneous effects of PAP therapy. | Develop better classification schemes for OSA and prospective testing of OSA treatment among these subgroups to evaluate effect on CVD outcomes. Embracing machine learning and artificial intelligence methods in our field is crucial, as their application to existing sleep data will open up new avenues for precision medicine research and personalized treatment for CVD risk reduction in OSA. This includes investigating potential adverse impact of PAP or excessive PAP pressures in OSA subgroups. |
Definition of abbreviations: ATS = American Thoracic Society; CVD = cardiovascular disease; OSA = obstructive sleep apnea; PAP = positive airway pressure; RCT = randomized controlled trial.
In summary, the workshop attendees agreed that the next most important step was identifying those at the highest risk for CVD from OSA and targeting these patients for OSA treatment within an RCT. A definitive conclusion from these trials is that asymptomatic patients with elevated AHIs recruited outside of the sleep clinic setting do not, on average, experience secondary CVD benefits from assignment to receive PAP.
Section 2: Identifying Patients with OSA at Increased Risk of CVD and Potential to Respond to PAP
OSA is a heterogeneous disorder. Using the AHI alone to infer the risk of CVD and identify which patients benefit from therapy does not capture this heterogeneity, potentially resulting in neutral results in prior RCTs. Although some studies have shown an increase in cumulative mortality with increasing OSA severity by AHI (63), emerging evidence demonstrates that physiologic OSA-related factors other than the AHI can predict composite CVD outcomes (31, 64) and differentiate responses to PAP (33, 34). The AHI does not effectively capture physiologic disturbances that occur with respiratory events. For any given AHI, there is also wide variability in respiratory event duration and depth, burden of oxygen desaturation, pulse rate changes, and cortical activity (26). Such variability may differentially contribute to the mechanisms linking OSA to CVD, including autonomic and endothelial dysfunction, inflammation, and metabolic dysregulation (32). As a result, current RCTs of PAP for CVD risk reduction might have been underpowered because of the inclusion of all patients with moderate to severe OSA, determined solely by AHI. This dilution of risk and HTE within RCTs was discussed in a critical care perspective review by Iwashyna and colleagues (65). By integrating additional factors, we can enhance our understanding of treatment responses and outcomes. The workshop attendees discussed several recently identified OSA subgroups extrapolated from observational studies and RCT data that may be at increased risk of CVD. These at-risk endotypes and phenotypes can be identified by 1) physiologic responses, 2) symptom subtypes and comorbid sleep disorders, and 3) advanced statistical modeling approaches for the enrichment of future trials for targeted therapy, as discussed below.
Features of polysomnography (PSG) and physiologic responses that predict CVD risk include the following:
-
•
PSG-based physiologic signals: Ventilatory burden, hypoxic burden, and arousal burden are three physiologic consequences of respiratory events in OSA (66). The ventilatory burden is the area under the ventilation signals related to respiratory events. Similarly, the hypoxic burden is defined as the area under the desaturation curves. Arousal burden has been described as a normalized duration of arousal. In recent work, OSA-related hypoxic burden strongly predicted CVD and all-cause mortality (unlike the AHI) in two separate cohorts (67). Another study demonstrated that both hypoxic and ventilatory burden were significantly associated with incident CVD (68). Parekh and colleagues (69) developed an automated ventilatory burden measure to effectively assess OSA severity as an alternative to the AHI, which was found to be predictive of all-cause and CVD mortality in three separate cohorts. On the other hand, arousal burden has not been shown to predict CVD outcomes (68). Although a high arousal index is a marker of sleep fragmentation, a low arousal index is associated with higher risk of cerebral white matter disease (70) and atrial fibrillation (71). Across three cohorts, the inclusion of arousals in the definition of AHI weakened associations with CVD incidence (72). Moreover, in this study, a higher baseline AHI was associated with a reduction of arousal index over time. The authors suggested that there may be adaptation of untreated OSA resulting in reduced arousability, challenging the conventional assumption that arousal data improves risk stratification.
-
•
Pulse-rate response (ΔHR) and pulse wave amplitude drops (PWADs): Sympathetic surges in response to OSA respiratory events were initially described by Somers and colleagues (73) decades ago. OSA activates the sympathetic nervous system through IH, hypercapnia, and arousals. It is plausible that OSA-specific ΔHR encapsulates key aspects of the autonomic response to respiratory events, making it a potentially useful prognostic marker for OSA-related CVD risk. Recent work by Azarbarzin and colleagues (74) showed a U-shaped relationship between ΔHR and CVD mortality, whereby those with low and high ΔHR have increased CVD mortality. An advantage to ΔHR response is the ease of assessment, especially with lower tech wearables. Importantly, it does not create the same potential issue of racial bias and inequity that currently surrounds oximetry (75). However, the variations in normal ΔHR on the basis of age or specific demographics and comorbidities are unknown, as is the influence of negative chronotropic medications such as β-blockers and PAP, which may also affect this metric (76). Another measure of autonomic responsiveness and vascular reactivity is PWADs. This metric quantifies drops in the amplitude of the pulse oximetry–based photoplethysmography signal, where a low number of PWADs per hour of sleep among patients with OSA has been associated with higher CVD risk (77). Enhancing our comprehension of metrics such as ΔHR, PWADs, and other innovative indicators of sympathetic activity in OSA can contribute to our knowledge about autonomic balance and various CVD consequences.
-
•
OSA respiratory event duration: Yet another predictor of mortality is short respiratory event duration (78), which predicts all-cause mortality in both men and women. Event duration also correlates with arousal threshold (79). Individuals with shorter respiratory events may be predisposed to augmented autonomic nervous system responses because of more frequent awakenings and sympathetic surges, potentially increasing the likelihood of adverse health outcomes. Both women and Black adults have shorter respiratory event duration than White men, suggesting both the value of this marker in understudied groups and the ramifications and underlying mechanisms associated with this marker of CVD risk. Given the correlation between event duration and low arousal threshold and the association between low arousal threshold and poorer PAP adherence, individuals with shorter events may be better suited to non-PAP treatments. Additional research disentangling phenotypes on the basis of these physiologic measures will be important as the field enters an era of precision medicine in OSA.
-
•
Rapid eye movement (REM)–related OSA: Last, REM-specific OSA may be a key factor in predicting OSA-related CVD risk. OSA events during REM sleep are typically more frequent and are associated with greater oxygen desaturation than events during non-REM sleep. REM sleep is also associated with hemodynamic variability, increased sympathetic activity, and myocardial demand. Mokhlesi and colleagues (80) demonstrated that REM OSA is longitudinally associated with 24% higher odds of hypertension. Aurora and colleagues (81) reported that severe OSA occurring primarily during REM sleep is associated with a higher incidence of composite cardiovascular events, but only among those with prevalent CVD.
Features of PSG physiologic responses that predict PAP response include the following:
-
•Several factors noted above may affect treatment responsiveness, particularly hypoxic burden, ΔHR, and OSA event duration.
-
○For example, in the ISAACC study, those with high hypoxic burden had a reduction in CVD events with PAP (34). Zinchuk and colleagues (82) also reported that patients with severe OSA and marked hypoxemia exhibited an increased risk of incident CVD or death and may benefit from PAP, whereas those who have severe OSA without significant hypoxemia exhibited a lower rate of PAP adherence without a significant reduction in CVD or death with PAP use. As such, assessing the burden of physiologic responses to OSA could provide valuable insights for tailoring therapeutic approaches.
-
○Using data from the RICCADSA study, Azarbarzin and colleagues (33) demonstrated that those with higher ΔHR treated with PAP exhibited CVD risk reduction with PAP in contrast to those with lower ΔHR who had no response.
-
○Another post hoc analysis of the ISAACC RCT applying machine learning recently identified short average OSA event duration as a predictor of improved CVD outcomes among participants randomized to PAP compared with usual care (35).
-
○
These novel physiologic measures should be considered for incorporation for patient selection in future prospective RCTs to determine the CVD response to PAP among subgroups. However, it is vital that these physiology-based OSA subtypes be shown in observational studies to predict PAP response before carrying them forward to designing future RCTs. Although some of these phenotypes may be markers of underlying disease and physiology unrelated to a specific OSA subtype (e.g., it is possible that high pulse rate responses may reflect cardiac reserve), others may reflect a specific OSA endotype (e.g., event duration or hypoxic burden which may reflect a high arousal threshold and increased collapsibility). Furthermore, determining how to collect these novel signals in a clinical setting and leveraging the signals for a precision medicine strategy to mitigate CVD risk is a pressing challenge. In addition, the algorithms used to generate these metrics must be made publicly available. Efforts made through ongoing research to define clinically relevant thresholds for each will be required before they are ready for integration into clinical practice. Last, it is imperative to consider potential undiscovered physiologic measures or “hidden features.” For instance, numerous variables—including social determinants of health, obesity, gender, PAP pressure, hours of PAP use, machine type, and others—might play an unidentified role in this complex equation. The task is to continue to explore existing datasets for new features to understand how they all intertwine to predict CVD response to continuous PAP.
Subtypes based on symptoms, comorbidities, demographics, and genetics include the following:
-
•
Symptom subtypes: Patients with OSA can be classified into different symptom-based subtypes. Mazzotti and colleagues (83) identified four OSA symptom subtypes, labeled minimally symptomatic, moderately sleepy, excessively sleepy, and disturbed sleep. The excessively sleepy subtype was associated with a threefold increased risk of prevalent heart failure and an increased risk of incident CVD. This is precisely the symptom subtype that has been excluded from previous RCTs assessing PAP treatment of OSA for CVD outcomes. Similar findings were reported by Labarca and colleagues (84), with a significant association between an excessively sleepy subtype and increased cardiovascular mortality in a Hispanic population from South America. EDS is associated with inflammatory biomarkers, including higher C-reactive protein concentrations (85, 86), and these findings, including those of Mendelian randomization studies (87), suggest that OSA with EDS is a more inflammatory phenotype and that genetic risk for inflammation is associated with OSA–EDS. The prevalence of this symptom phenotype was very low in clinical trials to date, as only those with nonsleepy or minimally sleepy OSA (Epworth Sleepiness Scale scores ≤15) were recruited. On the other hand, however, after applying an inverse probability of treatment weighting (a propensity score method that mimics randomization), the RICCADSA investigators found no significant difference in adverse cardiovascular outcomes among patients with versus without EDS when untreated or nonadherent to treatment (88). In fact, PAP use, at least 4 hours per night, was associated with reduced cardiovascular outcomes in participants without EDS. These findings challenge the notion that sleepiness is a marker of favorable cardiovascular outcomes in patients treated with PAP. They also highlight the importance of measuring sleep duration, timing, and quality in addition to PAP adherence to capture non-OSA etiologies that contribute to EDS and are not fixed by PAP alone. Reliable methods to identify such patients are needed for prospective testing of this subtype’s implications for CVD and PAP response.
-
•
Comorbid sleep disorders and sleep/circadian traits: Another OSA phenotype that has recently gained attention is patients with OSA and comorbid sleep disorders and variable sleep and circadian traits, including short sleep duration and irregular sleep schedules. In addition to OSA, short sleep duration, sleep irregularity, and comorbid insomnia and sleep apnea have independently been associated with increased CVD mortality in observational studies (5, 20, 54, 89–92). Furthermore, OSA is believed to be a risk factor for the development of insomnia (93). However, RCTs studying OSA have not systematically measured total sleep time or insomnia diagnoses. The impact of OSA treatment on the overall duration and quality of sleep remains unexplored, and there is an absence of treatment strategies that integrate the management of both coexisting OSA and insomnia. In addition, insomnia often limits adherence to PAP, and therefore, insomnia treatment is an imperative part of PAP adherence interventions (94). This represents an important OSA phenotype that may be at increased CVD risk. This requires additional exploration, particularly among patients with sleepy versus nonsleepy comorbid insomnia and sleep apnea. Furthermore, exclusion criteria that limit patients with comorbid sleep diagnoses may be perpetuating biases within our RCTs as rates of insomnia are particularly high among women (95) and minoritized groups (96).
-
•Demographics
-
○Sex: An important factor when considering these new subgroups of OSA is the exploration of sex-specific differences between and within subgroups and the role of sex in response to OSA events. For example, women generally have a lower OSA event–related arousal threshold (97), less airway collapsibility, and shorter OSA event duration (98) and are more likely to have insomnia (99). These endotypic and phenotypic features may manifest with an elevated ΔHR to obstructive events, a marker or mediator of increased CVD, especially in women (74). May and colleagues (100) found differential sex-specific responses to PAP therapy among circulating inflammatory biomarkers, setting the stage for future validation studies and biochemical pathway elucidation to inform sex-specific personalized treatment approaches. Several observational studies have demonstrated important sex differences in the relationship between OSA and CVD, often with women demonstrating significantly greater risk (1, 101–103). However, women constituted less than 20% of the previous prospective RCTs. The inclusion of women must be prioritized before releasing definitive statements regarding the effect of OSA treatment on outcomes for all patients.
-
○Age of onset: Cross-sectional studies show that OSA prevalence increases approximately 17-fold between young adulthood and 60–65 years of age, with a possible plateau with advanced age (104, 105). However, OSA may differ by age both with regard to disease mechanisms (obesity and male sex are stronger risk factors in middle compared with older age) (104) and in association with CVD risk (generally stronger associations with CVD outcomes in individuals less than 65 years of age compared with older individuals) (2). There are scant longitudinal data regarding longitudinal trajectories across the life course. However, Black children and young adults have more severe OSA than their White counterparts (105, 106), suggesting that an earlier age of disease onset and potential for greater cumulative burden among minoritized people may contribute to CVD disparities. To better understand these findings the field must incorporate a life-course approach to the study of OSA. For example, Spilsbury and colleagues found that after adjusting for race, adverse neighborhood conditions, which are known to differ by race and disproportionately burden minoritized groups, were significantly associated with OSA (107). Collecting long-term longitudinal data and incorporating social and structural determinants of health will be necessary to fully understand the effect of disease duration on outcomes and the optimal timing of therapy. The observed racial and ethnic disparity in disease incidence and prevalence highlights the need to better understand the broader determinants of healthcare inequities to avoid misleading conclusions. These investigations are paramount to promote a more accurate and comprehensive understanding of health disparities as well as social and environmental factors that precipitate and perpetuate the earlier age of onset and increased severity of OSA among minoritized people.
-
○Race, ethnicity and social determinants of health: The interplay of race, ethnicity, social (e.g., socioeconomics), and environment-level (e.g., neighborhood) factors on sleep health, OSA, and CVD should be included in assessments (108). Previous research has demonstrated that racially minoritized groups generally have a higher prevalence and severity of OSA and CVD compared with their White counterparts in the United States (109). Notably, disparities are also evident in underdiagnosis of OSA (96) and PAP treatment adherence (110), with minoritized populations generally having lower adherence compared with their White counterparts (22, 111). These differences and vulnerability to disease are rooted in preventable and multifactorial social factors, including racism and other forms of discrimination, contextual factors/sleep environment, differential access to culturally appropriate healthcare (112), and shift work and limited sleep opportunities (109, 113). In particular, the neighborhood and its environment are key contributors to these disparities, with pollution concentrations, heat intensity, and community violence due to lower socioeconomic circumstances negatively affecting sleep quality, insomnia symptoms, sleep opportunity, and OSA (109, 114). As such, addressing disparities linked to residential racial segregation and adverse social and environmental factors is crucial for understanding and improving the CVD outcomes associated with OSA.
-
○Obesity: Obesity is highly comorbid with OSA, and the growing obesity epidemic in the United States (115) has resulted in an increased occurrence of obesity-related outcomes. Excess body weight is estimated to be responsible for more than half of moderate to severe OSA and more than a third of mild OSA (116, 117). A recent meta-analysis assessed the relationship between weight reduction and improvement in OSA severity (combining results from 27 studies investigating bariatric surgery, lifestyle intervention, and pharmacotherapy for weight loss in OSA) (118). The results suggest that a 20% reduction in body mass index (BMI) yields a 57% reduction in OSA severity (by AHI). As such, weight loss interventions including comprehensive lifestyle intervention programs, as well as antiobesity pharmacotherapy and bariatric surgery, are associated with significant improvements in OSA severity and cardiometabolic comorbidities and are recommended in the routine management of patients with OSA (119). Importantly, GIP (glucose-dependent insulinotropic polypeptide) and GLP1 (glucagon-like peptide 1) agonist pharmacotherapy, initially developed for type 2 diabetes, has recently gained approval from the Food and Drug Administration for the management of obesity. Moreover, these medications have demonstrated significant reductions in major adverse cardiovascular events (120). For example, in an RCT among patients with preexisting CVD and obesity without diabetes, weekly semaglutide was superior to placebo in reducing the incidence of death from cardiovascular causes, nonfatal acute MI, and nonfatal stroke (121). It is therefore imperative for future research to prioritize targeted weight-loss strategies among overweight and obese patients with OSA and investigate the therapeutic effectiveness of these drugs for CVD risk reduction in OSA by targeting an underlying etiology (122).
-
○Genetic factors: The understanding of genetics of OSA is less developed than other complex disorders, but it is clear that OSA has a strong familial basis, with some traits (event duration) more heritable than the AHI (123). A heterogeneous basis for OSA is supported by variation in the reported genetic associations for OSA by both sex and REM/non-REM state (124–126). Although identifying genetic loci for OSA would benefit from improved OSA phenotyping, likewise, a better understanding of the genetic heterogeneity in OSA would likely inform the understanding of pathways that mediate OSA-related CVD risk and treatment responses. For example, genetic risk for inflammation was shown to predict an OSA subtype associated with sleepiness (86) (an endophenotype characterized by increased collapsibility, hypoxemia, and CVD risk). Genetic correlations between OSA-related and cardiometabolic traits were reported to be stronger for women than men in a large Hispanic U.S. population (127), suggesting biological factors that mediate sex differences in OSA-related CVD.
-
○
Advanced analytical approaches
Advanced analytical approaches and machine learning techniques, such as unsupervised clustering methods, supervised risk prediction models, and HTE analyses, may be used in sleep research to uncover relevant features (128). For example, to identify the aforementioned symptom subtypes, Mazzotti and colleagues (83) used latent class analysis to organize predominantly symptom features, shedding light on how factors coalesce to inform important symptom-based OSA phenotypes at risk of incident CVD. Zinchuk and colleagues (31) applied a similar approach to determining polysomnographic phenotypes that heighten cardiovascular risk. Xu and colleagues (129) used latent class analysis and supervised feature selection tools to identify multidimensional subgroups using demographics, comorbidities, and PSG measures. PAP was most protective among a specific cluster of younger, obese patients with high AHIs and sleep time with oxygen saturation < 90%. Cohen and colleagues (35) used a form of HTE analysis (model-based recursive partitioning) to identify subgroups within a training set of the ISAACC study with differential responses to PAP on the basis of OSA event duration and hyperlipidemia status. This study suggests that instead of relying on a single risk factor, a combination of risk factors and physiologic sleep signals may help identify high-risk and treatment-responsive subgroups and should be an important consideration in future research. Unsupervised analytical methods such as clustering and latent class analysis can elucidate patterns, while supervised learning tasks assist in identification of important features in predictive modeling. Moreover, other analyses of HTE and causal inference techniques based on treatment response–driven subgroup selection offer innovative and robust means to extract valuable insights from existing data, aiding in personalized interventions and improving health outcomes (65, 130–132). As with discerning the utility of any predictive metric, validation in independent cohorts is necessary. Other unique challenges to such analyses include 1) the need for large sample sizes and 2) the lack of insight into causality and potential amplification of biases, resulting in difficulty disentangling the effects of each component that makes up various subgroups. Despite these challenges, using advanced analytic methodology will help uncover novel directions for future trials by enhanced predictive modeling for treatment-responsive subgroups in patients with OSA.
Potentially detrimental effects of PAP
Reversal of ischemic preconditioning: All previous studies identifying OSA endotypes and phenotypes have assumed that PAP treatment would result in either neutral or beneficial impacts on CVD outcomes. However, there has been little discussion on the potential teleological benefits of OSA. How did OSA develop in our population (i.e., does it confer survival benefits among certain subgroups)? A body of literature may answer these questions by assessing the effects of ischemic preconditioning in OSA. Human studies have demonstrated that among patients with acute MI, those with OSA have an augmented coronary collateral circulation compared with those without OSA (44). Moreover, sleep apnea has also been associated with increased proliferation and angiogenic capacity of endothelial progenitor cells during acute MI (133) and elevated plasma VEGF concentrations, which are suppressed by treatment of intermittent hypoxemia with PAP (134). Furthermore, multiple studies have shown that a higher AHI is associated with lower peak troponin concentrations during acute nonfatal MI (43, 135). These studies may suggest answers to possible benefits of OSA, demonstrating potentially positive effects of ischemic preconditioning in OSA (43, 46), an effect that PAP would prevent or reverse.
Biomarker assessments and risk scores: Recent work casts doubt on the assumption that PAP itself is entirely a benign intervention. These works explore the effects of OSA and PAP on Ang-II (angiopoietin-2), an endothelial growth factor released from pulmonary endothelial cells. Ang-II modulates a vascular inflammatory response to lung injury (136) and is associated with adverse CVD outcomes (137). Intermittent hypoxemia promotes degranulation of Weibel-Palade bodies, which release Ang-II (138). It stands to reason that PAP, which eliminates the hypoxemic burden, would decrease Ang-II. Contrary to this theory, however, a recent study demonstrated that there was an increase in circulating concentrations of Ang-II after PAP (40, 42). Interestingly, a follow-up study in patients with recent coronary revascularization and nonsleepy OSA showed that Ang-II remained elevated in the PAP group. Higher concentrations of Ang-II and higher degrees of PAP were associated with greater mortality and worse cardiovascular outcomes. In contrast, Ang-II declined and VEGF-A remained elevated in the usual-care group (18). Such a response attributed to PAP may vary across populations and subgroups and add to the HTE in PAP, requiring further investigation.
Adverse socioeconomic consequences: One must also take into consideration the potential adverse consequences of “overprescribing” PAP to all patients with OSA and subgroups that may not derive direct benefit. The associated costs, operational inconvenience, time commitment, disruption of sleep, and bureaucratic complexities of insurance claims may collectively constitute a burdensome undertaking for numerous patients and sleep providers. In addition, the broader concerns surrounding recent PAP recall events and the potential harm arising from off-gassing of particulate matter further accentuate the need for a comprehensive evaluation of PAP prescription practices, limited to groups that would derive the most benefit (139, 140).
In summary, it is imperative to understand the measures and outcomes tied to OSA from a pathophysiological standpoint to move the needle for CVD risk. We must also grasp how disease progression affects the optimal timing of therapeutic interventions. Ultimately, OSA and CVD are both highly prevalent conditions and may often coexist in subgroups without being directly related. Identifying indicators that signify a true link between OSA and CVD is essential. Enriching the data collected in RCTs and observational studies with detailed physiologic measures, symptoms, comorbidities, and novel biomarkers and analyzing such data with robust machine learning and artificial intelligence techniques will be crucial for directing the next wave of research in OSA and CVD to tailor OSA therapies to reduce CVD burden.
Section 3: Advancing the Science on the Treatment of OSA for CVD Benefit: Gaps and Future Directions
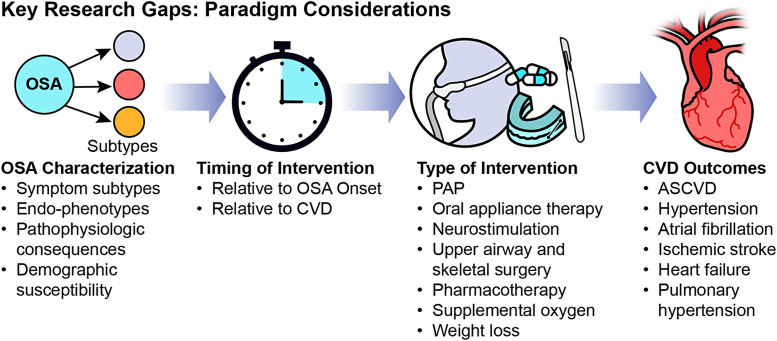
The key research gaps can be summarized within four arenas (Figure 3): OSA characterization, timing of intervention, type of intervention, and CVD outcome. Below, we outline key components to incorporate in future research:
Figure 3.
Key research gaps. ASCVD = atherosclerotic cardiovascular disease; CVD = cardiovascular disease; OSA = obstructive sleep apnea; PAP = positive airway pressure.
OSA endotypes and phenotypes and HTE
Given the emerging data on the various OSA endotypes and phenotypes, these innovative metrics must be incorporated into the design and data collection of forthcoming OSA trials. A prudent next step entails the inclusion of participants characterized by these metrics that may acquire the most benefit from treatment for CVD risk reduction. This approach includes those with increased hypoxic burden (33), ΔHR (74), shorter OSA respiratory event duration (35, 78), and an excessive sleepiness phenotype (83) willing to engage in long-term use of PAP. Although there are potential ethical considerations tied to withholding treatment from symptomatic patients, in light of 1) cumulative evidence showing no benefit of PAP from RCTs including those with at least mild sleepiness (SAVE trial) and 2) emerging evidence on the potential adverse effects linked to PAP (18, 40, 42), there is equipoise in withholding the prescription of PAP even among this group. A robust data safety monitoring board, close oversight and clear rules for early termination of trials in those with symptoms will help alleviate concerns for equipoise. Furthermore, any theoretical harms of PAP are balanced against its potentially small relative effect size on CVD outcomes, especially compared with other interventions such as statins, antidiabetic agents, smoking cessation, and antihypertensives. The field must understand the effects of each of these on OSA pathophysiology and how they work in concert with OSA treatment. Another important consideration of subgroup-focused trials (beyond symptoms) is the holistic approach to sleep health in patients with OSA. Additional measures such as actigraphy and insomnia metrics to assess for comorbid sleep disorders and treatment with behavioral interventions should be an integral part of trial protocols. Although this introduces another layer of complexity to trial design, it may ultimately enable a more precise and comprehensive approach to sleep care by including all dimensions of sleep health. Last, in light of emerging data, it is equally crucial to discern subsets of patients with OSA for whom PAP might yield harm and explore the concept of ischemic preconditioning—or potentially identify those for whom PAP could lead to unfavorable outcomes using machine learning and advanced multiomics analyses (141–143).
Cardiovascular outcomes and primary versus secondary prevention
To date, RCTs have relied on composite CVD events. Although the field aims to differentiate the influence of OSA on distinct cardiovascular outcomes such as stroke and MI, composite endpoints are most feasible because of statistical power. However, ultimately, they amalgamate a broader spectrum of CVD risks. We must balance our intention to assess the impacts of PAP (our most effective therapy) and patient-centered outcomes. Emerging statistical techniques such as differentially weighting individual components within a composite outcome (144) might strike this equilibrium by assigning varied importance to each endpoint type within an RCT. This adjustment could reduce the required sample size and enhance patient centricity, as it considers the perceived severity of each outcome.
For the use of PAP in the setting of existing CVD as secondary disease prevention, the field needs to better understand the effect of adjunctive therapies of CVD (e.g., concomitant use of statins, antihypertensives, and β-blockers) among patients with OSA. Some of these therapies might already target specific OSA-related pathophysiologic effects (such as ΔHR), thereby mitigating the influence of OSA on CVD by reducing endothelial damage, elevated blood pressure, and sympathetic activation (76). Furthermore, applying PAP after the development of CVD may be too late to affect recurrent CVD events. As other cardiovascular therapies such as antihypertensive agents (145), β-blockers, and statins (146) are already quite effective in secondary disease prevention, PAP may have only a marginal effect, if any. It is also plausible that this effect could vary on the basis of the specific CVD process, as exemplified by atrial fibrillation, where treatment efficacy could be meaningful to reduce disease progression (147) but not in later stages. Exploring existing longitudinal studies can offer insights into the natural progression of OSA across different age groups and its repercussions on CVD, thereby aiding in identifying the optimal timing for interventions. This involves determining the point at which the pathophysiological consequences of OSA become irreversible.
In addition, future cardiovascular outcome trials are encouraged to capture basic information about sleep duration as well as presence or absence of a diagnosis of OSA, at a minimum. Given that cardiovascular organizations such as the American Heart Association consider sleep as one of Life’s Essential 8 measures to improve cardiovascular health (148), there is momentum for this sort of change. Such information becomes even more important as drugs that lead to marked weight reduction, such as GLP-1 agonists and dual incretins, gain widespread use as cardiovascular risk reducing agents that can also favorably affect conditions such as OSA.
Improving generalizability
Increasing diversity and inclusion of minoritized groups.
Historically and persistently minoritized groups exhibit a heightened prevalence of OSA and experience more severe forms of disease and underdiagnoses (21). Furthermore, disparities in disease management follow-up and other social factors contribute to lower PAP adherence (22). Many of the same factors that result in these sleep health disparities (e.g., discrimination, limited access to health care, neighborhood-level features) (107, 149) have also been shown to increase the risk of CVD, type 2 diabetes, obesity, and stroke. It is therefore imperative to include racially minoritized groups in future studies and to account for social determinants of health throughout the research process (e.g., study design, data collection, analysis, interpretation), as they are linked to both exposure and outcomes. An illustration of this issue was provided by Jean-Louis colleagues (150), demonstrating the use of tailored sleep health education that engages the community and reflects the notion that interventions need to originate within a specific community to resonate effectively. Our studies must be designed collaboratively with interventions aligned with the community’s needs, reaching participants in their homes and communities to establish an inclusive research environment. Neglecting proper screening and disregarding social determinants of health can inadvertently propagate bias in research.
PAP alternatives and multimodal therapies
Several non-PAP therapies are available or on the horizon for the management of OSA (151). Mandibular advancement devices (MADs) offer a noninvasive option for those who struggle with PAP, especially in cases of mild to moderate OSA. Although PAP therapy has better efficacy in treating OSA, MADs have a better adherence profile compared with PAP (152), with the most favorable response in younger patients with lower BMIs (153). In addition, MADs have also been shown to be noninferior to PAP in reducing CVD risk factors such as 24-hour mean arterial blood pressure in patients with hypertension (154). Surgical approaches for modifications of the upper airway soft tissue, or hypoglossal nerve stimulation, a surgical procedure that increases pharyngeal dilator muscle tone, may also be appropriate for certain patients with symptomatic OSA who are intolerant of PAP therapy, typically most suitable in patients with a lower BMIs (155). Pharmacologic therapies targeting upper airway dilator muscles to increase muscle tone (156), increase ventilatory drive, or raise arousal threshold are also being studied (151). The efficacy and success of these alternative therapies in the treatment of patients with OSA can be variable and is dependent on appropriate patient selection. In addition, the impact of these alternative therapies on CVD risk reduction in OSA has not yet been studied.
Given the significant overlap between OSA and obesity, the new GLP-1/GIP medications, recently approved by the Food and Drug Administration for obesity, will be instrumental in the management of obesity-related OSA. Notably, a recent phase III RCT evaluated the efficacy of tirzepatide (a dual GLP-1/GIP agonist) in patients with moderate to severe OSA and obesity (157). This study by Malhotra and colleagues comprised two trials, one with patients unwilling or unable to use PAP therapy and the other with patients who were stable on PAP therapy for at least 3 months. At 1 year, there was a 16–17% reduction in body weight, resulting in a 48–56% reduction in AHI (a decrease of 20–24 events/h) compared with placebo. In addition, there were significant improvements in systolic blood pressure, C-reactive protein, hypoxic burden, and patient-reported outcomes (improvements in sleep disturbance and sleep-related impairment). The impact of this drug on CVD events and long-term efficacy outcomes in OSA are not yet known.
Last, the Adaptive Servo Ventilation on Survival and Hospital Admissions in Heart Failure (ADVENT-HF) trial (158) recently evaluated the effects of an alternative PAP-based therapy, specifically peak flow–triggered adaptive servoventilation (ASVPF), on CVD outcomes among patients with heart failure with reduced ejection fraction and sleep-disordered breathing, 73% of whom had OSA. In this study, Bradley et al. found that ASVPF had several benefits not previously described with PAP. Participants randomized to ASVPF experienced small but statistically significant improvements in quality-of-life measures and sleepiness, as well as changes in sleep architecture with increases in slow-wave sleep and REM sleep and reductions in arousal frequency and stage N1 sleep. However, as with other PAP trials, this therapy did not result in reductions in CVD events or mortality.
These alternatives to traditional PAP represent the beginning of a new era in OSA therapy, one that is increasingly moving toward personalized and multimodal treatment strategies to achieve the best patient-centered outcomes. Future research should incorporate and compare multiple therapeutic options using novel trial designs to understand the impact of these treatments on CVD outcomes among patients with OSA.
Trial designs and collaborative research
Adaptive trial designs.
Adaptive trial designs offer a means to determine the optimal treatment choice from available alternatives for individual patients on the basis of unique participant characteristics. For example, biomarker-driven adaptive designs might use the physiology-based biomarkers outlined previously, multiomics profiling, or patient-reported measures (i.e., EDS) to tailor treatments for each patient. This notion demands expanding the validation of biomarkers predicting responses to OSA treatment or establishing a causal link between OSA and CVD. This concept encompasses a variety of design types, such as basket trials, biomarker adaptive trials, covariate-adjusted response adaptive trials, population enrichment trials (prioritizing the inclusion of women and racially minoritized groups), and umbrella trials (159). Adaptive trials can also increase efficiency, reduce cost, and test multiple therapies (160, 161). Nonetheless, these trials can be intricate, necessitating a collaborative team-science approach that involves close consultation with statisticians, data scientists, and bioinformatics experts, as statistical inference in such trials can be complex. In addition, each design comes with its limitations and potential biases, particularly those involving unblinding and midtrial treatment reallocation. However, they contribute valuable insights into treatment effects within specific subgroups and have found utility in diverse fields (e.g., oncology [162, 163], and coronavirus disease [COVID-19] research [164, 165]). These novel trial designs represent a new frontier in the field of OSA with treatment algorithms (including medications) based on various OSA endotypes and phenotypes.
Collaborative research consortiums in the sleep medicine setting.
To enhance the applicability of our findings to the broader sleep community, it is imperative to shift OSA trials from cardiology settings to sleep medicine clinics. Furthermore, to facilitate progress, the field can draw lessons from other disciplines and establish a collaborative research consortium akin to the Acute Respiratory Distress Syndrome Network (ARDSNet) and the Clinical Trials Network for the Prevention and Early Treatment of Acute Lung Injury (PETAL Network) in critical care research. This approach would enable local experts to apply advanced data processing and analysis techniques remotely, thereby propelling the field forward, similar to strides made with the creation of the NSRR and collaborative efforts of the lead authors from the SAVE, RICCADSA, ISAACC, and ADVENT-HF trials. Aligned with current national imperatives to share research data, investigators should be encouraged to deposit data in repositories such as the NSRR, enabling both pooling of data for additional power and generalizability and encouraging novel questions to be addressed by the international community. Furthermore, collaborative efforts to establish large and diverse prospective multicenter trials are needed to increase the generalizability of results.
Finally, future complex trial designs must involve collaboration with all stakeholders, including patients with OSA, to conduct trials effectively. Although the concept of the healthy adherer effect has been considered in those who use PAP, it is equally vital to understand PAP acceptance and acknowledgment among those who refrain from therapy because of mistrust in the healthcare system. Comparative effectiveness (166) and pragmatic RCT (167) designs present an opportunity to safely incorporate these often excluded populations into future trials.
Conclusions
There was consensus among the workshop participants that OSA is not a homogeneous disease. It manifests as a multifaceted interplay of risk factors, encompassing endotypic, behavioral, environmental, and genetic elements. This complexity is further compounded by phenotypic variability in individual physiologic responses to OSA-related respiratory events, such as autonomic responses and hypoxic burden. This has the potential to yield differing cardiovascular repercussions to OSA-specific treatments. This lack of specificity in defining OSA subtypes is compounded by a lack of diversity in existing RCTs. The aforementioned null trials include a lower proportion of women and have not been conducted among racially minoritized populations disproportionately made more vulnerable to CVD. This collective perspective underscores the limitations of our current trials in effectively understanding the effects of OSA treatment on CVD outcomes. Consequently, it is impossible to draw firm conclusions about the presence or absence of cardiovascular benefits for most individuals presenting to sleep medicine clinics given the RCTs to date. The path forward necessitates augmented sample sizes, diverse groups of participants with various clinical symptoms, standardized endotyping and phenotyping approaches, robust support for recruitment and adherence, and modern study designs accounting for interactions between OSA and other sleep conditions. Finally, establishing a comprehensive taxonomy of sleep apnea that seamlessly integrates social determinants of health, endotypic and phenotypic variations, cardiovascular risk profiles, and treatment response is imperative. Such a taxonomy would yield a more meaningful and impactful categorization of OSA, thereby paving the way for future RCTs to evaluate interventions from a patient-centered perspective.
Acknowledgments
This official workshop report was prepared by an ad hoc subcommittee of the ATS Assembly on Sleep and Respiratory Neurobiology.
Members of the subcommittee are as follows:
Oren Cohen, M.D. (Co-Chair)1*‡
Vaishnavi Kundel, M.D., M.S. (Co-Chair)1*‡§
Henry K. Yaggi, M.D., M.P.H. (Co-Chair)4,6*‡
Neomi A. Shah, M.D., M.P.H., M.Sc. (Co-Chair)1*‡
Carmela Alcantara, Ph.D.7‡ǁ¶
Ali Azarbarzin, Ph.D.8‡§ǁ¶
Ferran Barbé, Ph.D.9,10‡ǁ¶
Deepak L. Bhatt, M.D., M.P.H., M.B.A.2‡¶
Martha E. Billings, M.D.11‡ǁ¶
T. Douglas Bradley, M.D.12,13‡ǁ¶
Daniel J. Gottlieb, M.D., M.P.H.8,14‡ǁ¶
David Gozal, M.D., M.B.A., Ph.D. (Hon.)15‡ǁ¶
Camilla M. Hoyos, Ph.D.16,17,18‡ǁ¶
Chandra L. Jackson, Ph.D., M.S.19‡ǁ¶
Sanja Jelic, M.D.20‡ǁ¶
Dayna A. Johnson, Ph.D., M.P.H., M.S.W., M.S.21द
Atul Malhotra, M.D.22‡ǁ¶
Jennifer L. Martin, Ph.D.23,24‡ǁ¶
Reena Mehra, M.D., M.S.11‡ǁ¶
Doug McEvoy, M.D., M.B. B.S., F.R.A.C.P.25‡ǁ¶
Sushmita Pamidi, M.D.26द
Sanjay R. Patel, M.D., M.S.27‡ǁ¶
Yüksel Peker, M.D.8,27,28,29‡ǁ¶
Alberto R. Ramos, M.D., M.S., F.A.A.N., F.A.A.S.M.30‡ǁ¶
Susan Redline, M.D., M.P.H.8,31‡ǁ¶
Manuel Sánchez-de-la-Torre, Ph.D.32,33‡ǁ¶
Helena Schotland, M.D.1‡ǁ¶
Virend K. Somers, M.D., Ph.D.34‡ǁ¶
Mayte Suárez-Fariñas, Ph.D.3‡¶
Andrey Zinchuk, M.D., M.H.S.5‡§ǁ¶
*Moderator.
‡Author.
§Speaker.
ǁPanelist.
¶Participant.
1Division of Pulmonary, Critical Care, and Sleep Medicine, Department of Medicine, 2Mount Sinai Fuster Heart Hospital, and 3Center for Biostatistics, Department of Population Health Science and Policy, Icahn School of Medicine at Mount Sinai, New York, New York; 4Section of Pulmonary, Critical Care and Sleep Medicine and 5Department of Internal Medicine, Yale School of Medicine, New Haven, Connecticut; 6Clinical Epidemiology Research Center, Connecticut Department of Veterans Affairs, West Haven, Connecticut; 7School of Social Work, Columbia University, New York, New York; 8Division of Sleep and Circadian Disorders, Brigham and Women’s Hospital, Harvard Medical School, Boston, Massachusetts; 9Translational Research in Respiratory Medicine, University Hospital Arnau de Vilanova and Santa Maria, IRBLleida, Lleida, Spain; 10Centro de Investigación Biomédica en Red de Enfermedades Respiratorias, Institute of Health Carlos III, Madrid, Spain; 11Division of Pulmonary, Critical Care, and Sleep Medicine, Department of Medicine, School of Medicine, University of Washington, Seattle, Washington; 12University Health Network Toronto Rehabilitation Institute, Toronto, Ontario, Canada; 13Department of Medicine, Toronto General Hospital, Toronto, Ontario, Canada; 14Department of Medicine, VA Boston Healthcare System, Boston, Massachusetts; 15Joan C. Edwards School of Medicine, Marshall University, Huntington, West Virgina; 16Department of Health Science, Faculty of Medicine and Human Health Sciences, and 17NHMRC Center of Research Excellence to Optimize Sleep in Brain Ageing and Neurodegeneration, Macquarie University, Sydney, New South Wales, Australia; 18Centre for Sleep and Chronobiology, Woolcock Institute of Medical Research, Glebe, New South Wales, Australia; 19Epidemiology Branch, Social and Environmental Determinants of Health Equity, National Institute of Environmental Health Sciences, National Institutes of Health, Research Triangle Park, North Carolina; 20Division of Pulmonary, Allergy, and Critical Care Medicine, Columbia University Medical Center, New York, New York; 21Department of Epidemiology, Rollins School of Public Health, Emory University, Atlanta, Georgia; 22Division of Pulmonary, Critical Care, Sleep Medicine, and Physiology, University of California, San Diego, La Jolla, California; 23Geriatric Research, Education, and Clinical Center, VA Greater Los Angeles Healthcare System, Los Angeles, California; 24David Geffen School of Medicine, University of California, Los Angeles, Los Angeles, California; 25Adelaide Institute for Sleep Health, Flinders Health and Medical Research Institute, Sleep Health, Flinders University, Adelaide, South Australia, Australia; 26Research Institute of the McGill University Health Center, McGill University, Montreal, Quebec, Canada; 27Division of Pulmonary, Allergy, Critical Care, and Sleep Medicine, University of Pittsburgh, Pittsburgh, Pennsylvania; 28Department of Pulmonary Medicine, Koc University School of Medicine, Istanbul, Turkey; 29Department of Molecular and Clinical Medicine, Institute of Medicine, Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden; 30Sleep Disorders Program, Department of Neurology, Miller School of Medicine, University of Miami, Miami, Florida; 31T.H. Chan School of Public Health, Harvard University, Boston, Massachusetts; 32Group of Precision Medicine in Chronic Diseases, Hospital Nacional de Parapléjicos, IDISCAM, Centro de Investigación Biomédica en Red de Enfermedades Respiratorias, Madrid, Spain; 33Department of Nursing, Physiotherapy, and Occupational Therapy, Faculty of Physiotherapy and Nursing, University of Castilla–La Mancha, Toledo, Spain; and 34Department of Cardiovascular Medicine, Mayo Clinic, Rochester, Minnesota
Footnotes
This official Workshop Report of the American Thoracic Society was approved September 2024
Subcommittee Disclosures: O.C. served as a consultant for Zoll Respicardia and received research support from Stony Wold-Herbert Foundation.V.K. served as a consultant for Zoll Respicardia; received research support from AASM Foundation and NIH/NHLBI; served as a speaker for Academy of Continued Healthcare Learning; and received travel support from Eli Lilly and Company.Y.P. received research support from ResMed Foundation.D.M. received royalties from Phillips.M.S.T. served as a consultant for Philips; received research support from Instituto de Salud Carlos III; and served as a speaker and received travel support from Jazz Pharmaceuticals.D.J.G. served on advisory boards for Apnimed, Signifier Medical Technologies, and Wesper; served as a consultant for Powell Mansfield, Signifier Medical Technologies, and Wesper; and received honoraria from ProSomnus and SleepRes.T.D.B. received research support from Philips and received honoraria for speaking.A.Z. served as a consultant for Restful Robotics and received research support from NHLBI and ResMed Foundation.A.A. served as a consultant for Apnimed, Cerebra, Eli Lilly and Company, Inspire Medical Systems, Somnifix, and Zoll Respicardia; received honoraria from British Royal Society of Medicine, Philips Respironics, and ProSomnus; holds issued patent for endo-phenotyping and risk stratifying obstructive sleep apnea and non-transitory computer readable medium and apparatus for arousal intensity scoring; and received research support from American Academy of Sleep Medicine, American Heart Association, NIH, Philips Respironics, Somnifix.A.M. served as a consultant for Eli Lilly and Company, Livanova, Powell Mansfield, and Zoll Respicardia and received research support from NIH and Resmed.H.S. received royalties from Obstructive Sleep Apnea Knowledge and Attitudes.S.J. served on an advisory board for NovaResp; received honoraria from American Academy of Dental Medicine; and received research support from NIH.A.R.R. served on advisory boards for Chest and NIH/NHLBI; served as a consultant for Neurogeneces, One Care Media, Surgical Monitoring Associates, and Zoll Respicardia; is an employee of NHLBI and University of Miami; received honoraria from Medical Education Speakers Network; and received research support from Axsome, NIH, and National Institutes of Aging.J.L.M. served on an advisory board for Agency for Healthcare Research and Quality; served in a leadership role for American Academy of Sleep Medicine and Greater Los Angeles VA Research Education Foundation; received royalties from UpToDate; received research support from NIH/NHLBI and the U.S Department of Veterans Affairs; and received travel support from American Academy of Sleep Medicine.S.P. received research support from Canadian Institutes of Health Research and Fonds de Recherche du Quebec and received travel support from Canadian Institutes of Health Research.D.A.J. served as a consultant for Idorsia and received research support from NIH.R.M. served as chair and speaker for American Academy of Sleep Medicine; served as a contributing writer for American Board of Internal Medicine and UpToDate; served as an investigator for NIH; received research support from grant R21HL170206; and received royalties from UpToDate.V.K.S. served on an advisory board for Sleep Number; served as a consultant for Apnimed, Axome, Eli Lilly and Company, Huxley Medical, Jazz Pharmaceuticals, Know Labs, Resmed Corp, and Zoll Respicardia; served on a data safety and monitoring board for NIH; received equipment from Medtronic; received research support from NIH; and served as a speaker for Cardiometabolic Health Conference.C.M.H. served on an advisory board for Australasian Sleep Association; and received research support from ABM, BOD Australia, Fischer Paykel, Itamar Medical, Lowenstein, Sante Group, and Somnomed.M.E.B. served in a leadership role for Washington, Hawaii, Alaska Thoracic Society. D.L.B. served on advisory boards for Acesion Pharma, Assistance Publique-Hôpitaux de Paris, AngioWave, Baim Institute, Bayer, Boehringer Ingelheim, Boston Scientific, Cardax, CellProthera, Cereno Scientific, Cleveland Clinic, Contego Medical, Duke Clinical Research Institute, Elsevier Practice Update Cardiology, Janssen, Level Ex, Mayo Clinic, Medscape Cardiology, Merck, Mount Sinai School of Medicine, MyoKardia, NirvaMed, Novartis, Novo Nordisk, PhaseBio, PLx Pharma, Regado Biosciences, Population Health Research Institute, and Stasys; served as a consultant for Broadview Ventures, GlaxoSmithKline, Hims, McKinsey, SFJ, and Youngene; provided expert panel testimony for Arnold and Porter Law Firm; served in leadership roles for Abbott, American College of Cardiology, American Heart Association Quality Oversight Committee, AngioWave, Biotronik, Boston Scientific, Boston VA Research Institute, Bristol Myers Squibb, Clinical Cardiology, Contego Medical, CSI, DRS.LINQ, FlowCo, High Enroll, NCDR-ACTION Registry Steering Committee, Philips SpectraWAVE, Society of Cardiovascular Patient Care, Svelte, Takeda, TobeSoft, AHA NYC Chapter, Vascular Solutions, and VA CART Research and Publications Committee; received honoraria from American College of Cardiology, Baim Institute for Clinical Research, Belvoir Publications, Boston Scientific, Cleveland Clinic, Duke Clinical Research Institute, Mayo Clinic, Mount Sinai School of Medicine, Novartis, Population Health Research Institute, Rutgers University, Canadian Medical and Surgical Knowledge Translation Research Group, Cowen and Company, HMP Global, Journal of the American College of Cardiology, K2P, Level Ex, Medtelligence/ReachMD, MJH Life Sciences, Oakstone CME, Piper Sandler, Population Health Research Institute, Slack Publications, WebMD, Wiley, Society of Cardiovascular Patient Care, and CSL Behring; holds pending patent for Sotagliflozin; received research support from 89Bio, Abbott, Acesion Pharma, Afimmune, Aker Biomarine, Alnylam, Amarin, American College of Cardiology Foundation, Amgen, AstraZeneca, Bayer, Beren, Boehringer Ingelheim, Boston Scientific, Bristol Myers Squibb, Cardax, CellProthera, Cereno Scientific, Chiesi, Cincor, Cleerly, Contego Medical, CSL Behring, Eisai, Eli Lilly and Company, Ethicon, Faraday Pharmaceuticals, Ferring Pharmaceuticals, Forest Laboratories, Fractyl, Garmin, HLS Therapeutics, Idorsia, Ironwood, Ischemix, Janssen, Javelin, Lexicon, Medtronic, Merck, Moderna, MyoKardia, NirvaMed, Novartis, Novo Nordisk, Owkin, Pfizer Inc, PhaseBio, PLx Pharma, Recardio, Regeneron, Reid Hoffman Foundation, Roche, Sanofi, Stasys, Synaptic, The Medicines Company, Youngene, 89bio, Otsuka, and Alnylam; received royalties from Elsevier; holds stock in AngioWave, Bristol Myers Squibb, DRS.LINQ, and High Enroll; and received travel support from American College of Cardiology, American Heart Association, and Society of Cardiovascular Patient Care.S.R.P. served as a consultant for Apnimed, Bayer, Eli Lilly and Company, NovaResp, Philips Respironics, and Powell Mansfield. S.R.P is an employee of University of Pittsburgh; and has served in a leadership role for Breathe Pennsylvania; received research support from Philips Respironics and Sommetrics; and received travel support from Signifier Medical. S.R. served on advisory boards for Apnimed and NHLBI; served as a consultant for Eli Lilly; served in leadership roles for Alliance of Sleep Apnea Partners and National Sleep Foundation; received research support from NIH; and received travel support from Eli Lilly and Company.N.A.S. served on advisory boards for Bresotec and DSMB; served as a consultant for Respicardia; served on a data safety and monitoring board for Wayne State University; received honoraria from Hello Sunrise, Med Learning Group, HealthConnect, and Respicardia; holds stock in Abbott; and received research and travel support from American Academy of Sleep Medicine and NIH/NHBLI.F.B., M.S.F., D.G., C.L.J., C.A., and H.K.Y. reported no commercial or relevant non-commercial interests from ineligible companies.
References
- 1. Campos-Rodriguez F, Martinez-Garcia MA, de la Cruz-Moron I, Almeida-Gonzalez C, Catalan-Serra P, Montserrat JM. Cardiovascular mortality in women with obstructive sleep apnea with or without continuous positive airway pressure treatment: a cohort study. Ann Intern Med . 2012;156:115–122. doi: 10.7326/0003-4819-156-2-201201170-00006. [DOI] [PubMed] [Google Scholar]
- 2. Gottlieb DJ, Yenokyan G, Newman AB, O’Connor GT, Punjabi NM, Quan SF, et al. A prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: the Sleep Heart Health Study. Circulation . 2010;122:352–360. doi: 10.1161/CIRCULATIONAHA.109.901801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shah NA, Yaggi HK, Concato J, Mohsenin V. Obstructive sleep apnea as a risk factor for coronary events or cardiovascular death. Sleep Breath . 2010;14:131–136. doi: 10.1007/s11325-009-0298-7. [DOI] [PubMed] [Google Scholar]
- 4. Peker Y, Carlson J, Hedner J. Increased incidence of coronary artery disease in sleep apnoea: a long-term follow-up. Eur Respir J . 2006;28:596–602. doi: 10.1183/09031936.06.00107805. [DOI] [PubMed] [Google Scholar]
- 5. Marin JM, Carrizo SJ, Vicente E, Agusti AGN. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet . 2005;365:1046–1053. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 6. Barbé F, Durán-Cantolla J, Sánchez-de-la-Torre M, Martínez-Alonso M, Carmona C, Barceló A, et al. Spanish Sleep and Breathing Network Effect of continuous positive airway pressure on the incidence of hypertension and cardiovascular events in nonsleepy patients with obstructive sleep apnea: a randomized controlled trial. JAMA . 2012;307:2161–2168. doi: 10.1001/jama.2012.4366. [DOI] [PubMed] [Google Scholar]
- 7. McEvoy RD, Antic NA, Heeley E, Luo Y, Ou Q, Zhang X, et al. SAVE Investigators and Coordinators CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med . 2016;375:919–931. doi: 10.1056/NEJMoa1606599. [DOI] [PubMed] [Google Scholar]
- 8. Peker Y, Glantz H, Eulenburg C, Wegscheider K, Herlitz J, Thunström E. Effect of positive airway pressure on cardiovascular outcomes in coronary artery disease patients with nonsleepy obstructive sleep apnea: the RICCADSA randomized controlled trial. Am J Respir Crit Care Med . 2016;194:613–620. doi: 10.1164/rccm.201601-0088OC. [DOI] [PubMed] [Google Scholar]
- 9. Sánchez-de-la-Torre M, Sánchez-de-la-Torre A, Bertran S, Abad J, Duran-Cantolla J, Cabriada V, et al. Spanish Sleep Network Effect of obstructive sleep apnoea and its treatment with continuous positive airway pressure on the prevalence of cardiovascular events in patients with acute coronary syndrome (ISAACC study): a randomised controlled trial. Lancet Respir Med . 2020;8:359–367. doi: 10.1016/S2213-2600(19)30271-1. [DOI] [PubMed] [Google Scholar]
- 10. Sánchez-de-la-Torre M, Gracia-Lavedan E, Benitez ID, Sánchez-de-la-Torre A, Moncusí-Moix A, Torres G, et al. Adherence to CPAP treatment and the risk of recurrent cardiovascular events: a meta-analysis. JAMA . 2023;330:1255–1265. doi: 10.1001/jama.2023.17465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Patil SP, Ayappa IA, Caples SM, Kimoff RJ, Patel SR, Harrod CG. Treatment of adult obstructive sleep apnea with positive airway pressure: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med . 2019;15:335–343. doi: 10.5664/jcsm.7640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Epstein LJ, Kristo D, Strollo PJ, Jr, Friedman N, Malhotra A, Patil SP, et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med . 2009;5:263–276. [PMC free article] [PubMed] [Google Scholar]
- 13.American Academy of Sleep Medicine. Darien, IL: American Academy of Sleep Medicine; 2014. International Classification of Sleep Disorders—Third Edition (ICSD-3)https://learn.aasm.org/Users/LearningActivity/LearningActivityDetail.aspx?LearningActivityID=%2fgqQVDMQIT%2fEDy86PWgqgQ%3d%3d [Google Scholar]
- 14. Redline S, Azarbarzin A, Peker Y. Obstructive sleep apnoea heterogeneity and cardiovascular disease. Nat Rev Cardiol . 2023;20:560–573. doi: 10.1038/s41569-023-00846-6. [DOI] [PubMed] [Google Scholar]
- 15. Heinzer R, Vat S, Marques-Vidal P, Marti-Soler H, Andries D, Tobback N, et al. Prevalence of sleep-disordered breathing in the general population: the HypnoLaus study. Lancet Respir Med . 2015;3:310–318. doi: 10.1016/S2213-2600(15)00043-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vaduganathan M, Mensah GA, Turco JV, Fuster V, Roth GA. The global burden of cardiovascular diseases and risk: a compass for future health. J Am Coll Cardiol . 2022;80:2361–2371. doi: 10.1016/j.jacc.2022.11.005. [DOI] [PubMed] [Google Scholar]
- 17. Donovan LM, Hoyos CM, Kimoff RJ, Morrell MJ, Bosch NA, Chooljian DM, et al. Strategies to assess the effect of continuous positive airway pressure on long-term clinically important outcomes among patients with symptomatic obstructive sleep apnea: an official American Thoracic Society workshop report. Ann Am Thorac Soc . 2023;20:931–943. doi: 10.1513/AnnalsATS.202303-258ST. [DOI] [PubMed] [Google Scholar]
- 18. Peker Y, Celik Y, Behboudi A, Redline S, Lyu J, Wei Y, et al. CPAP may promote an endothelial inflammatory milieu in sleep apnoea after coronary revascularization. EBioMedicine . 2024;101:105015. doi: 10.1016/j.ebiom.2024.105015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Huang T, Mariani S, Redline S. Sleep irregularity and risk of cardiovascular events: the Multi-Ethnic Study of Atherosclerosis. J Am Coll Cardiol . 2020;75:991–999. doi: 10.1016/j.jacc.2019.12.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Full KM, Huang T, Shah NA, Allison MA, Michos ED, Duprez DA, et al. Sleep irregularity and subclinical markers of cardiovascular disease: the Multi-Ethnic Study of Atherosclerosis. J Am Heart Assoc . 2023;12:e027361. doi: 10.1161/JAHA.122.027361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Thornton JD, Dudley KA, Saeed GJ, Schuster ST, Schell A, Spilsbury JC, et al. Differences in symptoms and severity of obstructive sleep apnea between Black and White patients. Ann Am Thorac Soc . 2022;19:272–278. doi: 10.1513/AnnalsATS.202012-1483OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. May AM, Patel SR, Yamauchi M, Verma TK, Weaver TE, Chai-Coetzer CL, et al. Moving toward equitable care for sleep apnea in the United States: positive airway pressure adherence thresholds: an official American Thoracic Society policy statement. Am J Respir Crit Care Med . 2023;207:244–254. doi: 10.1164/rccm.202210-1846ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee Heart disease and stroke statistics—2015 update. Circulation . 2015;131:e29–e39. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 24. Dean DA, Goldberger AL, Mueller R, Kim M, Rueschman M, Mobley D, et al. Scaling up scientific discovery in sleep medicine: the National Sleep Research Resource. Sleep . 2016;39:1151–1164. doi: 10.5665/sleep.5774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhang G-Q, Cui L, Mueller R, Tao S, Kim M, Rueschman M, et al. The National Sleep Research Resource: towards a sleep data commons. J Am Med Inform Assoc . 2018;25:1351–1358. doi: 10.1093/jamia/ocy064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Javaheri S, Barbe F, Campos-Rodriguez F, Dempsey JA, Khayat R, Javaheri S, et al. Sleep apnea: types, mechanisms, and clinical cardiovascular consequences. J Am Coll Cardiol . 2017;69:841–858. doi: 10.1016/j.jacc.2016.11.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Benjafield AV, Ayas NT, Eastwood PR, Heinzer R, Ip MSM, Morrell MJ, et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: a literature-based analysis. Lancet Respir Med . 2019;7:687–698. doi: 10.1016/S2213-2600(19)30198-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Agency for Healthcare Research and Quality. Rockville, MD: Agency for Healthcare Research and Quality; 2021. Draft technology assessment—continuous positive airway pressure treatment for obstructive sleep apnea.https://www.ahrq.gov/sites/default/files/wysiwyg/research/findings/ta/drafts-for-review/sleep-apnea-draftreport.pdf [Google Scholar]
- 29.Patil SP, Kimoff RJ, Gay PC, Pack AI, Johnson K. Darien, IL: American Academy of Sleep Medicine; 2021. Response to AHRQ draft CPAP technology assessment.https://aasm.org/wp-content/uploads/2021/04/ahrq-cpap-sleep-apnea-report-comment-letter.pdf [Google Scholar]
- 30. Patil SP, Billings ME, Bourjeily G, Collop NA, Gottlieb DJ, Johnson KG, et al. Long-term health outcomes for patients with obstructive sleep apnea: placing the Agency for Healthcare Research and Quality report in context—a multi-society commentary. J Clin Sleep Med . 2023;20:135–149. doi: 10.5664/jcsm.10832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zinchuk AV, Jeon S, Koo BB, Yan X, Bravata DM, Qin L, et al. Polysomnographic phenotypes and their cardiovascular implications in obstructive sleep apnoea. Thorax . 2018;73:472–480. doi: 10.1136/thoraxjnl-2017-210431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zinchuk A, Yaggi HK. Phenotypic subtypes of OSA: a challenge and opportunity for precision medicine. Chest . 2020;157:403–420. doi: 10.1016/j.chest.2019.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Azarbarzin A, Zinchuk A, Wellman A, Labarca G, Vena D, Gell L, et al. Cardiovascular benefit of continuous positive airway pressure in adults with coronary artery disease and obstructive sleep apnea without excessive sleepiness. Am J Respir Crit Care Med . 2022;206:767–774. doi: 10.1164/rccm.202111-2608OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pinilla L, Esmaeili N, Labarca G, Martinez-Garcia MÁ, Torres G, Gracia-Lavedan E, et al. Hypoxic burden to guide CPAP treatment allocation in patients with obstructive sleep apnoea: a post-hoc study of the ISAACC trial. Eur Respir J . 2023;62:2300828. doi: 10.1183/13993003.00828-2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cohen O, Sánchez-de-la-Torre M, Al-Taie Z, Khan S, Kundel V, Kovacic JC, et al. Heterogeneous effects of CPAP in non-sleepy OSA on CVD outcomes: post-hoc machine learning analysis of the ISAACC trial (ECSACT study) Ann Am Thorac Soc . 2024;21:1074–1084. doi: 10.1513/AnnalsATS.202309-799OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Simpson SH, Eurich DT, Majumdar SR, Padwal RS, Tsuyuki RT, Varney J, et al. A meta-analysis of the association between adherence to drug therapy and mortality. BMJ . 2006;333:15. doi: 10.1136/bmj.38875.675486.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Granger BB, Swedberg K, Ekman I, Granger CB, Olofsson B, McMurray JJV, et al. CHARM Investigators Adherence to candesartan and placebo and outcomes in chronic heart failure in the CHARM programme: double-blind, randomised, controlled clinical trial. Lancet . 2005;366:2005–2011. doi: 10.1016/S0140-6736(05)67760-4. [DOI] [PubMed] [Google Scholar]
- 38. Florea VG, Cohn JN. The autonomic nervous system and heart failure. Circ Res . 2014;114:1815–1826. doi: 10.1161/CIRCRESAHA.114.302589. [DOI] [PubMed] [Google Scholar]
- 39. Pépin JL, Tamisier R, Barone-Rochette G, Launois SH, Lévy P, Baguet JP. Comparison of continuous positive airway pressure and valsartan in hypertensive patients with sleep apnea. Am J Respir Crit Care Med . 2010;182:954–960. doi: 10.1164/rccm.200912-1803OC. [DOI] [PubMed] [Google Scholar]
- 40. Shah R, Patel N, Emin M, Celik Y, Jimenez A, Gao S, et al. Statins restore endothelial protection against complement activity in obstructive sleep apnea: a randomized clinical trial. Ann Am Thorac Soc . 2023;20:1029–1037. doi: 10.1513/AnnalsATS.202209-761OC. [DOI] [PubMed] [Google Scholar]
- 41. Oesterle A, Laufs U, Liao JK. Pleiotropic effects of statins on the cardiovascular system. Circ Res . 2017;120:229–243. doi: 10.1161/CIRCRESAHA.116.308537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gottlieb DJ, Lederer DJ, Kim JS, Tracy RP, Gao S, Redline S, et al. Effect of positive airway pressure therapy of obstructive sleep apnea on circulating angiopoietin-2. Sleep Med . 2022;96:119–121. doi: 10.1016/j.sleep.2022.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Shah N, Redline S, Yaggi HK, Wu R, Zhao CG, Ostfeld R, et al. Obstructive sleep apnea and acute myocardial infarction severity: ischemic preconditioning? Sleep Breath . 2013;17:819–826. doi: 10.1007/s11325-012-0770-7. [DOI] [PubMed] [Google Scholar]
- 44. Steiner S, Schueller PO, Schulze V, Strauer BE. Occurrence of coronary collateral vessels in patients with sleep apnea and total coronary occlusion. Chest . 2010;137:516–520. doi: 10.1378/chest.09-1136. [DOI] [PubMed] [Google Scholar]
- 45. Summerer V, Arzt M, Fox H, Oldenburg O, Zeman F, Debl K, et al. Occurrence of coronary collaterals in acute myocardial infarction and sleep apnea. J Am Heart Assoc . 2021;10:e020340. doi: 10.1161/JAHA.120.020340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lavie L, Lavie P. Ischemic preconditioning as a possible explanation for the age decline relative mortality in sleep apnea. Med Hypotheses . 2006;66:1069–1073. doi: 10.1016/j.mehy.2005.10.033. [DOI] [PubMed] [Google Scholar]
- 47. Schiza SE, Simantirakis E, Bouloukaki I, Mermigkis C, Kallergis EM, Chrysostomakis S, et al. Sleep disordered breathing in patients with acute coronary syndromes. J Clin Sleep Med . 2012;8:21–26. doi: 10.5664/jcsm.1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Peker Y, Saygin M, Thunström E, Strollo PJ., Jr Natural course of untreated obstructive sleep apnoea in patients with coronary artery disease in the RICCADSA trial. Eur Respir J . 2021;58:OA4388. [Google Scholar]
- 49. Bellg AJ, Borrelli B, Resnick B, Hecht J, Minicucci DS, Ory M, et al. Treatment Fidelity Workgroup of the NIH Behavior Change Consortium Enhancing treatment fidelity in health behavior change studies: best practices and recommendations from the NIH Behavior Change Consortium. Health Psychol . 2004;23:443–451. doi: 10.1037/0278-6133.23.5.443. [DOI] [PubMed] [Google Scholar]
- 50. Javaheri S, Redline S. Insomnia and risk of cardiovascular disease. Chest . 2017;152:435–444. doi: 10.1016/j.chest.2017.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lechat B, Appleton S, Melaku YA, Hansen K, McEvoy RD, Adams R, et al. Comorbid insomnia and sleep apnoea is associated with all-cause mortality. Eur Respir J . 2022;60:2101958. doi: 10.1183/13993003.01958-2021. [DOI] [PubMed] [Google Scholar]
- 52. Vgontzas AN, Liao D, Bixler EO, Chrousos GP, Vela-Bueno A. Insomnia with objective short sleep duration is associated with a high risk for hypertension. Sleep . 2009;32:491–497. doi: 10.1093/sleep/32.4.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lechat B, Loffler KA, Wallace DM, Reynolds A, Appleton SL, Scott H, et al. All-cause mortality in people with co-occurring insomnia symptoms and sleep apnea: analysis of the Wisconsin Sleep Cohort. Nat Sci Sleep . 2022;14:1817–1828. doi: 10.2147/NSS.S379252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Bertisch SM, Pollock BD, Mittleman MA, Buysse DJ, Bazzano LA, Gottlieb DJ, et al. Insomnia with objective short sleep duration and risk of incident cardiovascular disease and all-cause mortality: Sleep Heart Health Study. Sleep . 2018;41:zsy047. doi: 10.1093/sleep/zsy047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sweetman A, Lechat B, Appleton S, Reynolds A, Adams R, Melaku YA. Association of co-morbid insomnia and sleep apnoea symptoms with all-cause mortality: analysis of the NHANES 2005–2008 data. Sleep Epidemiology . 2022;2:100043. [Google Scholar]
- 56. Pien GW, Ye L, Keenan BT, Maislin G, Björnsdóttir E, Arnardottir ES, et al. Changing faces of obstructive sleep apnea: treatment effects by cluster designation in the Icelandic Sleep Apnea Cohort. Sleep . 2018;41:1–13. doi: 10.1093/sleep/zsx201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Drager LF, Malhotra A, Yan Y, Pépin J-L, Armitstead JP, Woehrle H, et al. medXcloud Group Adherence with positive airway pressure therapy for obstructive sleep apnea in developing vs. developed countries: a big data study. J Clin Sleep Med . 2021;17:703–709. doi: 10.5664/jcsm.9008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Zapater A, Sánchez-de-la-Torre M, Benítez ID, Targa A, Bertran S, Torres G, et al. Spanish Sleep Network The effect of sleep apnea on cardiovascular events in different acute coronary syndrome phenotypes. Am J Respir Crit Care Med . 2020;202:1698–1706. doi: 10.1164/rccm.202004-1127OC. [DOI] [PubMed] [Google Scholar]
- 59. Javaheri S, Peker Y, Yaggi HK, Bassetti CLA. Obstructive sleep apnea and stroke: the mechanisms, the randomized trials, and the road ahead. Sleep Med Rev . 2022;61:101568. doi: 10.1016/j.smrv.2021.101568. [DOI] [PubMed] [Google Scholar]
- 60. Weaver TE, Mancini C, Maislin G, Cater J, Staley B, Landis JR, et al. Continuous positive airway pressure treatment of sleepy patients with milder obstructive sleep apnea: results of the CPAP Apnea Trial North American Program (CATNAP) randomized clinical trial. Am J Respir Crit Care Med . 2012;186:677–683. doi: 10.1164/rccm.201202-0200OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. McArdle N, Devereux G, Heidarnejad H, Engleman HM, Mackay TW, Douglas NJ. Long-term use of CPAP therapy for sleep apnea/hypopnea syndrome. Am J Respir Crit Care Med . 1999;159:1108–1114. doi: 10.1164/ajrccm.159.4.9807111. [DOI] [PubMed] [Google Scholar]
- 62. Martınez-Garcıa MA, Labarca G. Obstructive sleep apnea in women: scientific evidence is urgently needed. J Clin Sleep Med . 2022;18:1–2. doi: 10.5664/jcsm.9684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Martínez-García M-A, Campos-Rodríguez F, Catalán-Serra P, Soler-Cataluña J-J, Almeida-Gonzalez C, De la Cruz Morón I, et al. Cardiovascular mortality in obstructive sleep apnea in the elderly: role of long-term continuous positive airway pressure treatment. Am J Respir Crit Care Med . 2012;186:909–916. doi: 10.1164/rccm.201203-0448OC. [DOI] [PubMed] [Google Scholar]
- 64. Kendzerska T, Gershon AS, Hawker G, Leung RS, Tomlinson G. Obstructive sleep apnea and risk of cardiovascular events and all-cause mortality: a decade-long historical cohort study. PLoS Med . 2014;11:e1001599. doi: 10.1371/journal.pmed.1001599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Iwashyna TJ, Burke JF, Sussman JB, Prescott HC, Hayward RA, Angus DC. Implications of heterogeneity of treatment effect for reporting and analysis of randomized trials in critical care. Am J Respir Crit Care Med . 2015;192:1045–1051. doi: 10.1164/rccm.201411-2125CP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Azarbarzin A, Labarca G, Kwon Y, Wellman A. Physiological consequences of upper airway obstruction in sleep apnea. Chest . 2024 doi: 10.1016/j.chest.2024.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Azarbarzin A, Sands SA, Stone KL, Taranto-Montemurro L, Messineo L, Terrill PI, et al. The hypoxic burden of sleep apnoea predicts cardiovascular disease-related mortality: the Osteoporotic Fractures in Men Study and the Sleep Heart Health Study. Eur Heart J . 2019;40:1149–1157. doi: 10.1093/eurheartj/ehy624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Labarca G, Vena D, Hu W-H, Esmaeili N, Gell L, Yang HC, et al. Sleep apnea physiological burdens and cardiovascular morbidity and mortality. Am J Respir Crit Care Med . 2023;208:802–813. doi: 10.1164/rccm.202209-1808OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Parekh A, Kam K, Wickramaratne S, Tolbert TM, Varga A, Osorio R, et al. Ventilatory burden as a measure of obstructive sleep apnea severity is predictive of cardiovascular and all-cause mortality. Am J Respir Crit Care Med . 2023;208:1216–1226. doi: 10.1164/rccm.202301-0109OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Ding J, Nieto FJ, Beauchamp NJ, Harris TB, Robbins JA, Hetmanski JB, et al. Sleep-disordered breathing and white matter disease in the brainstem in older adults. Sleep . 2004;27:474–479. doi: 10.1093/sleep/27.3.474. [DOI] [PubMed] [Google Scholar]
- 71. Kwon Y, Gharib SA, Biggs ML, Jacobs DR, Alonso A, Duprez D, et al. Association of sleep characteristics with atrial fibrillation: the Multi-Ethnic Study of Atherosclerosis. Thorax . 2015;70:873–879. doi: 10.1136/thoraxjnl-2014-206655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Azarbarzin A, Sands SA, Han S, Sofer T, Labarca G, Stone KL, et al. Relevance of cortical arousals for risk stratification in sleep apnea: a 3 cohort analysis. J Clin Sleep Med . 2023;19:1475–1484. doi: 10.5664/jcsm.10598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest . 1995;96:1897–1904. doi: 10.1172/JCI118235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Azarbarzin A, Sands SA, Younes M, Taranto-Montemurro L, Sofer T, Vena D, et al. The sleep apnea-specific pulse-rate response predicts cardiovascular morbidity and mortality. Am J Respir Crit Care Med . 2021;203:1546–1555. doi: 10.1164/rccm.202010-3900OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Valbuena VSM, Merchant RM, Hough CL. Racial and ethnic bias in pulse oximetry and clinical outcomes. JAMA Intern Med . 2022;182:699–700. doi: 10.1001/jamainternmed.2022.1903. [DOI] [PubMed] [Google Scholar]
- 76. Pamidi S, Shah N. Continuous positive airway pressure and cardiovascular risk reduction in patients without excessive sleepiness importance of the pulse rate response. Am J Respir Crit Care Med . 2022;206:666–667. doi: 10.1164/rccm.202206-1050ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Solelhac G, Sánchez-de-la-Torre M, Blanchard M, Berger M, Hirotsu C, Imler T, et al. Pulse wave amplitude drops index: a biomarker of cardiovascular risk in obstructive sleep apnea. Am J Respir Crit Care Med . 2023;207:1620–1632. doi: 10.1164/rccm.202206-1223OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Butler MP, Emch JT, Rueschman M, Sands SA, Shea SA, Wellman A, et al. Apnea-hypopnea event duration predicts mortality in men and women in the Sleep Heart Health Study. Am J Respir Crit Care Med . 2019;199:903–912. doi: 10.1164/rccm.201804-0758OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Sands SA, Terrill PI, Edwards BA, Taranto Montemurro L, Azarbarzin A, Marques M, et al. Quantifying the arousal threshold using polysomnography in obstructive sleep apnea. Sleep . 2018;41:zsx183. doi: 10.1093/sleep/zsx183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Mokhlesi B, Finn LA, Hagen EW, Young T, Hla KM, Van Cauter E, et al. Obstructive sleep apnea during REM sleep and hypertension: results of the Wisconsin Sleep Cohort. Am J Respir Crit Care Med . 2014;190:1158–1167. doi: 10.1164/rccm.201406-1136OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Nisha Aurora R, Crainiceanu C, Gottlieb DJ, Kim JS, Punjabi NM. Obstructive sleep apnea during REM sleep and cardiovascular disease. Am J Respir Crit Care Med . 2018;197:653–660. doi: 10.1164/rccm.201706-1112OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Zinchuk AV, Gentry MJ, Concato J, Yaggi HK. Phenotypes in obstructive sleep apnea: a definition, examples and evolution of approaches. Sleep Med Rev . 2017;35:113–123. doi: 10.1016/j.smrv.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Mazzotti DR, Keenan BT, Lim DC, Gottlieb DJ, Kim J, Pack AI. Symptom subtypes of obstructive sleep apnea predict incidence of cardiovascular outcomes. Am J Respir Crit Care Med . 2019;200:493–506. doi: 10.1164/rccm.201808-1509OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Labarca G, Dreyse J, Salas C, Letelier F, Jorquera J. A validation study of four different cluster analyses of OSA and the incidence of cardiovascular mortality in a Hispanic population. Chest . 2021;160:2266–2274. doi: 10.1016/j.chest.2021.06.047. [DOI] [PubMed] [Google Scholar]
- 85. Huang T, Goodman M, Li X, Sands SA, Li J, Stampfer MJ, et al. C-reactive protein and risk of OSA in four US cohorts. Chest . 2021;159:2439–2448. doi: 10.1016/j.chest.2021.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Huang T, Goodman M, Wang H, Sofer T, Tworoger SS, Stampfer MJ, et al. Genetic predisposition to elevated C-reactive protein and risk of obstructive sleep apnea. Am J Respir Crit Care Med . 2024;209:329–331. doi: 10.1164/rccm.202307-1159LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Goodman MO, Dashti HS, Lane JM, Windred DP, Burns A, Jones SE, et al. Causal association between subtypes of excessive daytime sleepiness and risk of cardiovascular diseases. J Am Heart Assoc . 2023;12:e030568. doi: 10.1161/JAHA.122.030568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Eulenburg C, Celik Y, Redline S, Thunström E, Glantz H, Strollo PJ, et al. Cardiovascular outcomes in adults with coronary artery disease and obstructive sleep apnea with versus without excessive daytime sleepiness in the RICCADSA clinical trial. Ann Am Thorac Soc . 2023;20:1048–1056. doi: 10.1513/AnnalsATS.202208-676OC. [DOI] [PubMed] [Google Scholar]
- 89. Fernandez-Mendoza J, He F, Vgontzas AN, Liao D, Bixler EO. Interplay of objective sleep duration and cardiovascular and cerebrovascular diseases on cause-specific mortality. J Am Heart Assoc . 2019;8:e013043. doi: 10.1161/JAHA.119.013043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Sofi F, Cesari F, Casini A, Macchi C, Abbate R, Gensini GF. Insomnia and risk of cardiovascular disease: a meta-analysis. Eur J Prev Cardiol . 2014;21:57–64. doi: 10.1177/2047487312460020. [DOI] [PubMed] [Google Scholar]
- 91. Domínguez F, Fuster V, Fernández-Alvira JM, Fernández-Friera L, López-Melgar B, Blanco-Rojo R, et al. Association of sleep duration and quality with subclinical atherosclerosis. J Am Coll Cardiol . 2019;73:134–144. doi: 10.1016/j.jacc.2018.10.060. [DOI] [PubMed] [Google Scholar]
- 92. Gottlieb DJ, Bhatt DL. More evidence that we could all use a good night’s sleep. J Am Coll Cardiol . 2019;73:145–147. doi: 10.1016/j.jacc.2018.11.019. [DOI] [PubMed] [Google Scholar]
- 93. Benetó A, Gomez-Siurana E, Rubio-Sanchez P. Comorbidity between sleep apnea and insomnia. Sleep Med Rev . 2009;13:287–293. doi: 10.1016/j.smrv.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 94. Alessi CA, Fung CH, Dzierzewski JM, Fiorentino L, Stepnowsky C, Rodriguez Tapia JC, et al. Randomized controlled trial of an integrated approach to treating insomnia and improving the use of positive airway pressure therapy in veterans with comorbid insomnia disorder and obstructive sleep apnea. Sleep . 2021;44:zsaa235. doi: 10.1093/sleep/zsaa235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Martin JL, Schweizer CA, Hughes JM, Fung CH, Dzierzewski JM, Washington DL, et al. Estimated prevalence of insomnia among women veterans: results of a postal survey. Womens Health Issues . 2017;27:366–373. doi: 10.1016/j.whi.2016.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Chen X, Wang R, Zee P, Lutsey PL, Javaheri S, Alcántara C, et al. Racial/ethnic differences in sleep disturbances: the Multi-Ethnic Study of Atherosclerosis (MESA) Sleep . 2015;38:877–888. doi: 10.5665/sleep.4732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Won CHJ, Reid M, Sofer T, Azarbarzin A, Purcell S, White D, et al. Sex differences in obstructive sleep apnea phenotypes, the Multi-Ethnic Study of Atherosclerosis. Sleep . 2020;43:1–8. doi: 10.1093/sleep/zsz274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Sands SA, Alex RM, Mann D, Vena D, Terrill PI, Gell LK, et al. Pathophysiology underlying demographic and obesity determinants of sleep apnea severity. Ann Am Thorac Soc . 2023;20:440–449. doi: 10.1513/AnnalsATS.202203-271OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Zhang B, Wing YK. Sex differences in insomnia: a meta-analysis. Sleep . 2006;29:85–93. doi: 10.1093/sleep/29.1.85. [DOI] [PubMed] [Google Scholar]
- 100. May AM, Wang L, Strohl KP, Walia H, Hazen SL, Mehra R. Sex-specific differential responses of circulating biomarkers in obstructive sleep apnea treatment: a post hoc analysis of a randomized controlled trial. Ann Am Thorac Soc . 2020;17:605–613. doi: 10.1513/AnnalsATS.201908-593OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Campos-Rodriguez F, Martinez-Garcia MA, Reyes-Nuñez N, Caballero-Martinez I, Catalan-Serra P, Almeida-Gonzalez CV. Role of sleep apnea and continuous positive airway pressure therapy in the incidence of stroke or coronary heart disease in women. Am J Respir Crit Care Med . 2014;189:1544–1550. doi: 10.1164/rccm.201311-2012OC. [DOI] [PubMed] [Google Scholar]
- 102. Nestor Z, Siddharth S, Baba RY, Shah N. The influence of sex on the sleep-cardiovascular disease relationship: a review. Curr Epidemiol Rep . 2015;2:257–262. [Google Scholar]
- 103. Freitas LS, Drager LF. Gender and cardiovascular impact of obstructive sleep apnea: work in progress! J Thorac Dis . 2017;9:3579–3582. doi: 10.21037/jtd.2017.09.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Young T, Shahar E, Nieto FJ, Redline S, Newman AB, Gottlieb DJ, et al. Sleep Heart Health Study Research Group Predictors of sleep-disordered breathing in community-dwelling adults: the Sleep Heart Health Study. Arch Intern Med . 2002;162:893–900. doi: 10.1001/archinte.162.8.893. [DOI] [PubMed] [Google Scholar]
- 105. Redline S, Schluchter MD, Larkin EK, Tishler PV. Predictors of longitudinal change in sleep-disordered breathing in a nonclinic population. Sleep . 2003;26:703–709. doi: 10.1093/sleep/26.6.703. [DOI] [PubMed] [Google Scholar]
- 106. Gueye-Ndiaye S, Williamson AA, Redline S. Disparities in sleep-disordered breathing: upstream risk factors, mechanisms, and implications. Clin Chest Med . 2023;44:585–603. doi: 10.1016/j.ccm.2023.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Spilsbury JC, Storfer-Isser A, Kirchner HL, Nelson L, Rosen CL, Drotar D, et al. Neighborhood disadvantage as a risk factor for pediatric obstructive sleep apnea. J Pediatr . 2006;149:342–347. doi: 10.1016/j.jpeds.2006.04.061. [DOI] [PubMed] [Google Scholar]
- 108. Jackson CL, Redline S, Emmons KM. Sleep as a potential fundamental contributor to disparities in cardiovascular health. Annu Rev Public Health . 2015;36:417–440. doi: 10.1146/annurev-publhealth-031914-122838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Johnson DA, Ohanele C, Alcántara C, Jackson CL. The need for social and environmental determinants of health research to understand and intervene on racial/ethnic disparities in obstructive sleep apnea. Clin Chest Med . 2022;43:199–216. doi: 10.1016/j.ccm.2022.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. May AM, Billings ME. Racial differences in positive airway pressure adherence in the treatment of sleep apnea. Sleep Med Clin . 2022;17:543–550. doi: 10.1016/j.jsmc.2022.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Jean-Louis G, von Gizycki H, Zizi F, Dharawat A, Lazar JM, Brown CD. Evaluation of sleep apnea in a sample of Black patients. J Clin Sleep Med . 2008;4:421–425. [PMC free article] [PubMed] [Google Scholar]
- 112. Singh R, Juarez PD, Redline S, Jackson CL. Shortage of sleep medicine specialists in federally qualified health centers: an illustrative example of differential access to care. J Clin Sleep Med . 2023;19:1849–1850. doi: 10.5664/jcsm.10688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Johnson DA, Jackson CL, Williams NJ, Alcántara C. Are sleep patterns influenced by race/ethnicity—a marker of relative advantage or disadvantage? Evidence to date. Nat Sci Sleep . 2019;11:79–95. doi: 10.2147/NSS.S169312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Billings ME, Hale L, Johnson DA. Physical and social environment relationship with sleep health and disorders. Chest . 2020;157:1304–1312. doi: 10.1016/j.chest.2019.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Ward ZJ, Bleich SN, Cradock AL, Barrett JL, Giles CM, Flax C, et al. Projected US state-level prevalence of adult obesity and severe obesity. N Engl J Med . 2019;381:2440–2450. doi: 10.1056/NEJMsa1909301. [DOI] [PubMed] [Google Scholar]
- 116. Peppard PE, Young T, Palta M, Dempsey J, Skatrud J. Longitudinal study of moderate weight change and sleep-disordered breathing. JAMA . 2000;284:3015–3021. doi: 10.1001/jama.284.23.3015. [DOI] [PubMed] [Google Scholar]
- 117. Young T, Peppard PE, Taheri S. Excess weight and sleep-disordered breathing. J Appl Physiol (1985) . 2005;99:1592–1599. doi: 10.1152/japplphysiol.00587.2005. [DOI] [PubMed] [Google Scholar]
- 118. Malhotra A, Heilmann CR, Banerjee KK, Dunn JP, Bunck MC, Bednarik J. Weight reduction and the impact on apnea-hypopnea index: a systematic meta-analysis. Sleep Med . 2024;121:26–31. doi: 10.1016/j.sleep.2024.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Hudgel DW, Patel SR, Ahasic AM, Bartlett SJ, Bessesen DH, Coaker MA, et al. American Thoracic Society Assembly on Sleep and Respiratory Neurobiology The role of weight management in the treatment of adult obstructive sleep apnea: an official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med . 2018;198:e70–e87. doi: 10.1164/rccm.201807-1326ST. [DOI] [PubMed] [Google Scholar]
- 120. Michos ED, Lopez-Jimenez F, Gulati M. Role of glucagon-like peptide-1 receptor agonists in achieving weight loss and improving cardiovascular outcomes in people with overweight and obesity. J Am Heart Assoc . 2023;12:e029282. doi: 10.1161/JAHA.122.029282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Lincoff AM, Brown-Frandsen K, Colhoun HM, Deanfield J, Emerson SS, Esbjerg S, et al. SELECT Trial Investigators Semaglutide and cardiovascular outcomes in obesity without diabetes. N Engl J Med . 2023;389:2221–2232. doi: 10.1056/NEJMoa2307563. [DOI] [PubMed] [Google Scholar]
- 122. Malhotra A, Bednarik J, Curtis A, Dunn J, Weaver T, Grunstein R, et al. 0567 Tirzepatide for the treatment of OSA: rationale and design of the SURMOUNT-OSA phase 3 trial. Sleep . 2023;46:A249–A250. [Google Scholar]
- 123. Liang J, Cade BE, Wang H, Chen H, Gleason KJ, Larkin EK, et al. Comparison of heritability estimation and linkage analysis for multiple traits using principal component analyses. Genet Epidemiol . 2016;40:222–232. doi: 10.1002/gepi.21957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Cade BE, Chen H, Stilp AM, Louie T, Ancoli-Israel S, Arens R, et al. Associations of variants in the hexokinase 1 and interleukin 18 receptor regions with oxyhemoglobin saturation during sleep. PLoS Genet . 2019;15:e1007739. doi: 10.1371/journal.pgen.1007739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Cade BE, Lee J, Sofer T, Wang H, Zhang M, Chen H, et al. TOPMed Sleep Working Group Whole-genome association analyses of sleep-disordered breathing phenotypes in the NHLBI TOPMed program. Genome Med . 2021;13:136. doi: 10.1186/s13073-021-00917-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Chen H, Cade BE, Gleason KJ, Bjonnes AC, Stilp AM, Sofer T, et al. Multiethnic meta-analysis identifies RAI1 as a possible obstructive sleep apnea-related quantitative trait locus in men. Am J Respir Cell Mol Biol . 2018;58:391–401. doi: 10.1165/rcmb.2017-0237OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Zhang Y, Elgart M, Kurniansyah N, Spitzer BW, Wang H, Kim D, et al. Genetic determinants of cardiometabolic and pulmonary phenotypes and obstructive sleep apnoea in HCHS/SOL. EBioMedicine . 2022;84:104288. doi: 10.1016/j.ebiom.2022.104288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Cohen O, Kundel V, Robson P, Al-Taie Z, Suárez-Fariñas M, Shah NA. Achieving better understanding of obstructive sleep apnea treatment effects on cardiovascular disease outcomes through machine learning approaches: a narrative review. J Clin Med . 2024;13:1415. doi: 10.3390/jcm13051415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Xu PH, Fong DYT, Lui MMS, Lam DCL, Ip MSM. Cardiovascular outcomes in obstructive sleep apnoea and implications of clinical phenotyping on effect of CPAP treatment. Thorax . 2023;78:76–84. doi: 10.1136/thoraxjnl-2021-217714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Cui Y, Kosorok MR, Sverdrup E, Wager S, Zhu R. Estimating heterogeneous treatment effects with right-censored data via causal survival forests. J R Stat Soc Series B Stat Methodol . 2020;85:179–211. [Google Scholar]
- 131. Kravitz RL, Duan N, Braslow J. Evidence-based medicine, heterogeneity of treatment effects, and the trouble with averages. Milbank Q . 2004;82:661–687. doi: 10.1111/j.0887-378X.2004.00327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Yeh RW, Kramer DB. Decision tools to improve personalized care in cardiovascular disease: moving the art of medicine toward science. Circulation . 2017;135:1097–1100. doi: 10.1161/CIRCULATIONAHA.116.024247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Berger S, Aronson D, Lavie P, Lavie L. Endothelial progenitor cells in acute myocardial infarction and sleep-disordered breathing. Am J Respir Crit Care Med . 2013;187:90–98. doi: 10.1164/rccm.201206-1144OC. [DOI] [PubMed] [Google Scholar]
- 134. Lavie L, Kraiczi H, Hefetz A, Ghandour H, Perelman A, Hedner J, et al. Plasma vascular endothelial growth factor in sleep apnea syndrome: effects of nasal continuous positive air pressure treatment. Am J Respir Crit Care Med . 2002;165:1624–1628. doi: 10.1164/rccm.20110-040OC. [DOI] [PubMed] [Google Scholar]
- 135. Sánchez-de-la-Torre A, Soler X, Barbé F, Florés M, Maisel A, Malhotra A, et al. Spanish Sleep Network Cardiac troponin values in patients with acute coronary syndrome and sleep apnea: a pilot study. Chest . 2018;153:329–338. doi: 10.1016/j.chest.2017.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Vassiliou AG, Kotanidou A, Dimopoulou I, Orfanos SE. Endothelial damage in acute respiratory distress syndrome. Int J Mol Sci . 2020;21:1–31. doi: 10.3390/ijms21228793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Lieb W, Zachariah JP, Xanthakis V, Safa R, Chen M-H, Sullivan LM, et al. Clinical and genetic correlates of circulating angiopoietin-2 and soluble Tie-2 in the community. Circ Cardiovasc Genet . 2010;3:300–306. doi: 10.1161/CIRCGENETICS.109.914556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Emin M, Wang G, Castagna F, Rodriguez-Lopez J, Wahab R, Wang J, et al. Increased internalization of complement inhibitor CD59 may contribute to endothelial inflammation in obstructive sleep apnea. Sci Transl Med . 2016;8:320ra1. doi: 10.1126/scitranslmed.aad0634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Lowe RA. Recall of Philips respironics ventilators and CPAP machines—breakdowns in medical device surveillance. JAMA Intern Med . 2023;183:495–496. doi: 10.1001/jamainternmed.2023.0066. [DOI] [PubMed] [Google Scholar]
- 140. Robbins R, Epstein LJ, Pavlova MK, Iyer J, Batool-Anwar S, Bertisch SM, et al. Quantifying the impact of the Philips recall on patients with sleep apnea and clinicians. J Clin Sleep Med . 2023;19:1677–1683. doi: 10.5664/jcsm.10652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Zapater A, Gracia-Lavedan E, Torres G, Mínguez O, Pascual L, Cortijo A, et al. Spanish Sleep Network Proteomic profiling for prediction of recurrent cardiovascular event in patients with acute coronary syndrome and obstructive sleep apnea: a post-hoc analysis from the ISAACC study. Biomed Pharmacother . 2023;158:114125. doi: 10.1016/j.biopha.2022.114125. [DOI] [PubMed] [Google Scholar]
- 142. Cohen O, Kaufman AE, Choi H, Khan S, Robson PM, Suárez-Fariñas M, et al. Pharyngeal inflammation on positron emission tomography/magnetic resonance imaging before and after obstructive sleep apnea treatment. Ann Am Thorac Soc . 2023;20:574–583. doi: 10.1513/AnnalsATS.202207-594OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Kundel V, Cohen O, Khan S, Patel M, Kim-Schulze S, Kovacic J, et al. Advanced proteomics and cluster analysis for identifying novel obstructive sleep apnea subtypes before and after continuous positive airway pressure therapy. Ann Am Thorac Soc . 2023;20:1038–1047. doi: 10.1513/AnnalsATS.202210-897OC. [DOI] [PubMed] [Google Scholar]
- 144. Armstrong PW, Westerhout CM. Composite end points in clinical research: a time for reappraisal. Circulation . 2017;135:2299–2307. doi: 10.1161/CIRCULATIONAHA.117.026229. [DOI] [PubMed] [Google Scholar]
- 145. Gottlieb DJ, Punjabi NM, Mehra R, Patel SR, Quan SF, Babineau DC, et al. CPAP versus oxygen in obstructive sleep apnea. N Engl J Med . 2014;370:2276–2285. doi: 10.1056/NEJMoa1306766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Virani SS, Smith SC, Stone NJ, Grundy SM. Secondary prevention for atherosclerotic cardiovascular disease: comparing recent US and European guidelines on dyslipidemia. Circulation . 2020;141:1121–1123. doi: 10.1161/CIRCULATIONAHA.119.044282. [DOI] [PubMed] [Google Scholar]
- 147. Holmqvist F, Guan N, Zhu Z, Kowey PR, Allen LA, Fonarow GC, et al. ORBIT-AF Investigators Impact of obstructive sleep apnea and continuous positive airway pressure therapy on outcomes in patients with atrial fibrillation—results from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF) Am Heart J . 2015;169:647–654.e2. doi: 10.1016/j.ahj.2014.12.024. [DOI] [PubMed] [Google Scholar]
- 148. St-Onge M-P, Grandner MA, Brown D, Conroy MB, Jean-Louis G, Coons M, et al. American Heart Association Obesity, Behavior Change, Diabetes, and Nutrition Committees of the Council on Lifestyle and Cardiometabolic Health; Council on Cardiovascular Disease in the Young; Council on Clinical Cardiology; and Stroke Council Sleep duration and quality: impact on lifestyle behaviors and cardiometabolic health: a scientific statement from the American Heart Association. Circulation . 2016;134:e367–e386. doi: 10.1161/CIR.0000000000000444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Wang R, Dong Y, Weng J, Kontos EZ, Chervin RD, Rosen CL, et al. Associations among Neighborhood, Race, and Sleep Apnea Severity in Children. A Six-City Analysis. Ann Am Thorac Soc . 2017;14:76–84. doi: 10.1513/AnnalsATS.201609-662OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Jean-Louis G, Robbins R, Williams NJ, Allegrante JP, Rapoport DM, Cohall A, et al. Tailored Approach to Sleep Health Education (TASHE): a randomized controlled trial of a web-based application. J Clin Sleep Med . 2020;16:1331–1341. doi: 10.5664/jcsm.8510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Gottlieb DJ, Punjabi NM. Diagnosis and management of obstructive sleep apnea: a review. JAMA . 2020;323:1389–1400. doi: 10.1001/jama.2020.3514. [DOI] [PubMed] [Google Scholar]
- 152. Phillips CL, Grunstein RR, Darendeliler MA, Mihailidou AS, Srinivasan VK, Yee BJ, et al. Health outcomes of continuous positive airway pressure versus oral appliance treatment for obstructive sleep apnea: a randomized controlled trial. Am J Respir Crit Care Med . 2013;187:879–887. doi: 10.1164/rccm.201212-2223OC. [DOI] [PubMed] [Google Scholar]
- 153. Ramar K, Dort LC, Katz SG, Lettieri CJ, Harrod CG, Thomas SM, et al. Clinical practice guideline for the treatment of obstructive sleep apnea and snoring with oral appliance therapy: an update for 2015. J Clin Sleep Med . 2015;11:773–827. doi: 10.5664/jcsm.4858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Ou Y-H, Colpani JT, Cheong CS, Loke W, Thant AT, Shih E’C, et al. Mandibular advancement vs CPAP for blood pressure reduction in patients with obstructive sleep apnea. J Am Coll Cardiol . 2024;83:1760–1772. doi: 10.1016/j.jacc.2024.03.359. [DOI] [PubMed] [Google Scholar]
- 155. Strollo PJ, Soose RJ, Maurer JT, de Vries N, Cornelius J, Froymovich O, et al. STAR Trial Group Upper-airway stimulation for obstructive sleep apnea. N Engl J Med . 2014;370:139–149. doi: 10.1056/NEJMoa1308659. [DOI] [PubMed] [Google Scholar]
- 156. Schweitzer PK, Taranto-Montemurro L, Ojile JM, Thein SG, Drake CL, Rosenberg R, et al. The combination of aroxybutynin and atomoxetine in the treatment of obstructive sleep apnea (MARIPOSA): a randomized controlled trial. Am J Respir Crit Care Med . 2023;208:1316–1327. doi: 10.1164/rccm.202306-1036OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Malhotra A, Grunstein RR, Fietze I, Weaver TE, Redline S, Azarbarzin A, et al. for the SURMOUNT-OSA Investigators Tirzepatide for the treatment of obstructive sleep apnea and obesity. N Engl J Med . 2024;391:1193–1205. doi: 10.1056/NEJMoa2404881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Bradley TD, Logan AG, Lorenzi Filho G, Kimoff RJ, Durán Cantolla J, Arzt M, et al. ADVENT-HF Investigators Adaptive servo-ventilation for sleep-disordered breathing in patients with heart failure with reduced ejection fraction (ADVENT-HF): a multicentre, multinational, parallel-group, open-label, phase 3 randomised controlled trial. Lancet Respir Med . 2024;12:153–166. doi: 10.1016/S2213-2600(23)00374-0. [DOI] [PubMed] [Google Scholar]
- 159. Burnett T, Mozgunov P, Pallmann P, Villar SS, Wheeler GM, Jaki T. Adding flexibility to clinical trial designs: an example-based guide to the practical use of adaptive designs. BMC Med . 2020;18:352. doi: 10.1186/s12916-020-01808-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Bhatt DL, Mehta C. Adaptive designs for clinical trials. N Engl J Med . 2016;375:65–74. doi: 10.1056/NEJMra1510061. [DOI] [PubMed] [Google Scholar]
- 161. Pallmann P, Bedding AW, Choodari-Oskooei B, Dimairo M, Flight L, Hampson LV, et al. Adaptive designs in clinical trials: why use them, and how to run and report them. BMC Med . 2018;16 doi: 10.1186/s12916-018-1017-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Mistry P, Dunn JA, Marshall A. A literature review of applied adaptive design methodology within the field of oncology in randomised controlled trials and a proposed extension to the CONSORT guidelines. BMC Med Res Methodol . 2017;17:108–109. doi: 10.1186/s12874-017-0393-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Bothwell LE, Avorn J, Khan NF, Kesselheim AS. Adaptive design clinical trials: a review of the literature and ClinicalTrials.gov. BMJ Open . 2018;8:e018320. doi: 10.1136/bmjopen-2017-018320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Branch-Elliman W, Elwy AR, Monach P. Bringing new meaning to the term “adaptive trial”: challenges of conducting clinical research during the coronavirus disease 2019 pandemic and implications for implementation science. Open Forum Infect Dis . 2020;7:ofaa490. doi: 10.1093/ofid/ofaa490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Calfee CS, Liu KD, Asare AL, Beitler JR, Berger PA, III, Coleman MH, et al. The I-SPY. COVID Consortium Clinical trial design during and beyond the pandemic: the I-SPY COVID trial. Nat Med . 2022;28:9–11. doi: 10.1038/s41591-021-01617-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Fiore LD, Lavori PW. Integrating randomized comparative effectiveness research with patient care. N Engl J Med . 2016;374:2152–2158. doi: 10.1056/NEJMra1510057. [DOI] [PubMed] [Google Scholar]
- 167. Loudon K, Treweek S, Sullivan F, Donnan P, Thorpe KE, Zwarenstein M. The PRECIS-2 tool: designing trials that are fit for purpose. BMJ . 2015;350:h2147. doi: 10.1136/bmj.h2147. [DOI] [PubMed] [Google Scholar]