Abstract
Objectives
To compare disease-modifying therapy (DMT) use between people living with multiple sclerosis (pwMS) who resided in rural vs urban areas.
Methods
This retrospective cohort study used population-level individually linked administrative data to identify pwMS on April 1, 2019 (index date), in Alberta, Canada. DMT use was compared between pwMS who resided in rural vs urban areas during a 1-year postindex period. Structural equation modelling (SEM) and logistic regression (with 95% confidence intervals) were applied.
Results
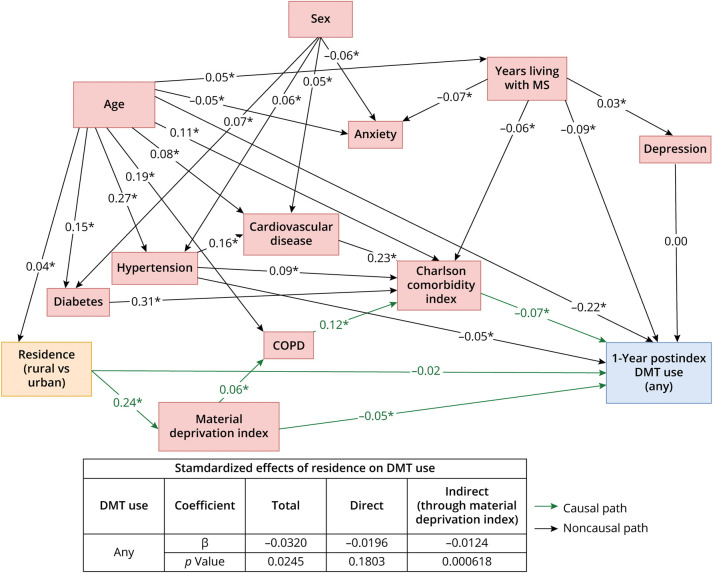
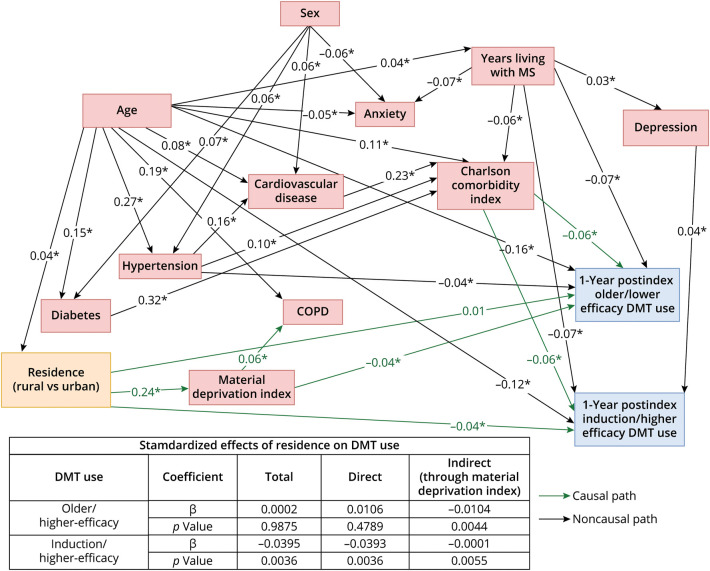
PwMS (n = 4,593) who resided in rural areas (vs urban) were 17% less likely to have received a DMT (odds ratio: 0.83 [0.69–0.99]; SEM total β: −0.032, p < 0.05), of which 39% of this disparity was explained by a lower socioeconomic status (SEM indirect β: −0.012 [p < 0.001]/total β: −0.032); 26% were less likely to have received an induction/higher efficacy therapy (odds ratio: 0.74 [0.57–0.95]), of which <1% of this disparity was explained by socioeconomic status (SEM indirect β: −0.0001 [p < 0.01]/total β: −0.040).
Discussion
PwMS residing in rural (vs urban) Alberta are less likely to receive any DMT, especially induction/higher-efficacy therapy; this inequality may be mediated by socioeconomic status and geography. Identifying and overcoming barriers to optimal clinical care in this patient population is needed.
Introduction
Disease-modifying therapy (DMT) is the current pharmacologic standard of care for treating people living with (relapsing-remitting) multiple sclerosis (pwMS).1 Early initiation of DMT after diagnosis produces long-term benefits compared with delayed treatment,2 and induction/higher-efficacy therapies have a greater and earlier effect on overall disease progression than older/lower-efficacy therapies.3 Given the proven efficacy of DMT, equitable access is of great importance for pwMS. Among the limited number of studies that have investigated potential inequalities in DMT use, lower socioeconomic status (SES) was consistently associated with lower DMT uptake.4 Recently, we found that rural (lower SES)/urban (higher SES) inequalities in DMT use may exist among pwMS in Alberta, Canada,5 supporting the need for further investigation. The objectives of this study were to compare DMT use and time to initiation of therapy between pwMS who resided in rural vs urban areas; SES was included as a potential mediator.
Methods
Ethics approval was received from the University of Alberta (Pro00115341); informed consent was waived. A retrospective, observational, population-based cohort study was conducted using administrative data from several databases (eTable 1). Eligibility criteria included those who (1) met a validated case definition for MS between 2008 and 2021,6 (2) had an MS incident date between 2008 and 2019, (3) had health insurance coverage, and (4) were alive on April 1, 2019 (index date) (eFigure 1).
Characteristics included urban (≥1,000 persons and ≥400 persons/km2)/rural (area remaining after urban delineation) residence (determined by second digit of postal code), age, sex, and SES (based on education, employment status, and average income derived from Canadian census data).7 Clinical characteristics included the number of years living with MS before the index date, a Charlson Comorbidity Index score (eTable 2), and comorbidities (eTable 3). DMT use (had ≥1 dispensation) during the 1-year postindex observation period was reported overall and by type (eTable 4). Time from the MS incidence date to DMT initiation was assessed between 2008 and 2021.
Statistical Analysis
Conceptual frameworks were developed (role of covariates in the relationship between geographical residence and DMT use) and tested using structural equation modelling (SEM) (eFigure 2).8 Informed by SEM, base logistic (odds ratio; OR), and Cox (hazard ratios: OR) regression models (with 95% CI) included the exposure (geographical residence), outcomes (DMT use; time to DMT initiation), and confounders of age, sex, the Charlson Comorbidity Index score, years living with MS (logistic model), and year of the MS incident date (Cox model); comorbidities were not included to avoid collinearity with the Charlson Comorbidity Index score. The exposure and covariates were measured on the index date for DMT use and on the MS incident date for time to DMT initiation. When statistical significance was observed (p < 0.05), an “explaining model” that included SES (mediator) was applied. Analyses were performed using SAS 9.4 software.
Data used in this study are available from Alberta Health Services and Alberta Health; data availability restrictions apply (e.g., qualified researcher). Data were used under license for this study and so are not publicly available.
Results
Cohort (n = 4,593; eFigure 3) characteristics are presented in eTable 5. During the observation period, 37.0% of pwMS who resided in rural areas and 41.8% of those who resided in urban areas received ≥1 DMT (eTable 6). Adjusting for confounders, pwMS who resided in rural areas (vs urban) were 17% less likely to receive a DMT (OR: 0.83 [0.69–0.99] [Table]; SEM total β: −0.032, p < 0.05 [Figure 1]), of which 39% of this disparity was explained by a lower SES (SEM indirect β: −0.012 [p < 0.001]/total β: −0.032 [Figure 1]); eTable 7 summarizes the full logistic regression model. Among the specific DMT categories, those who resided in rural areas (vs urban) were 26% less likely to have received an induction/higher-efficacy therapy (OR: 0.74 [0.57–0.95] [Table]; SEM total β: −0.040, p < 0.01 [Figure 2]), of which <1% of this disparity was explained by SES (SEM indirect β: −0.0001 [p < 0.01]/total β: −0.040 [Figure 2]). The likelihood of receiving an older/lower-efficacy therapy was not different between those who resided in rural vs urban areas (SEM total β: 0.0002, p = 0.99 [Figure 2]; OR: 1.01 [0.83–1.23] [Table]). Time to initiation of DMT was not different between those who resided in rural vs urban areas (HR: 0.89 [0.79, 1.01] DMT overall; 0.84 [0.69–1.02] infusion/higher-efficacy; 0.91 [0.80–1.03] older/lower-efficacy therapy) (Table). PwMS who had a more recent MS incident date initiated a DMT sooner (HR: 1.10 [1.08–1.12] DMT overall; 1.36 [1.32–1.40] induction/higher-efficacy; 1.06 [1.04–1.07] older/lower-efficacy therapy) (eTable 7, eFigure 4).
Table.
Comparison of DMT Use and Time to DMT Initiation Overall and by Type Among pwMS Who Resided in Rural vs Urban Areas
| DMT use during the 1-y postindex observation period (2019/2020) (odds ratio [95% CI]) | Time from MS incident date to DMT initiation between 2008 and 2021 (hazard ratio [95% CI]) | |||
| Base model | Explaining model | Base model | Explaining model | |
| Residence (rural vs urban) | ||||
| Overall | 0.83 (0.69–0.99) | 0.89 (0.74–1.07) | 0.89 (0.79–1.01) | N/A |
| Induction/higher-efficacy therapy | 0.74 (0.57–0.95) | 0.74 (0.57–0.97) | 0.84 (0.69–1.02) | N/A |
| Older/lower-efficacy therapy | 1.01 (0.83–1.23) | N/A | 0.91 (0.80–1.03) | N/A |
Abbreviations: DMT = disease-modifying therapy; N/A = not applicable; pwMS = people living with multiple sclerosis.
Bolded numbers indicate a statistical significance at p < 0.05. The base logistic and Cox models included the confounders of age, sex, the Charlson Comorbidity Index score, years living with MS (logistic model), and year of the MS incident date (Cox model); comorbidities were not included to avoid collinearity with the Charlson Comorbidity Index score. When statistical significance was observed between rural and urban residence, an additional model (‘explaining model’) added sociodemographic status (mediator). See eTable 7 for the full logistic and Cox models.
Figure 1. Final Structural Equation Model of the Relationship Between Geographical Residence (Rural vs Urban; Exposure) and DMT Use (Use vs None; Outcome).
Confounders included age (>60 vs ≤60 years), Charlson Comorbidity Index score (one score higher vs lower; continuous), comorbidities (living with a certain comorbidity vs not), and years living with MS (number of years since the MS incident date); socioeconomic status (material deprivation index score of 1 [most privileged] vs 2–5 [less privileged]) was a mediator. Model goodness-of-fit: χ2 = 118.0, degree of freedom (df) = 45, χ2/df ratio = 2.6, SRMR = 0.05, RMSEA = 0.02, CFI = 0.94, AGFI = 1.00. *Statistical significance at p < 0.05. COPD = chronic obstructive pulmonary disease; DMT = disease-modifying therapy; MS = multiple sclerosis.
Figure 2. Final Structural Equation Model of the Relationship Between Geographical Residence (Rural vs Urban; Exposure) and DMT Use by Type (Use vs None; Outcome).
Confounders included age (>60 vs ≤60 years), Charlson Comorbidity Index score (one score higher vs lower; continuous), comorbidities (living with a certain comorbidity vs not), and years living with MS (number of years since the MS incident date); socioeconomic status (material deprivation index score of 1 [most privileged] to 2–5 [less privileged]) was a mediator. Model goodness-of-fit: χ2 = 131.5, df = 53, χ2/df ratio = 2.5, SRMR = 0.05, RMSEA = 0.02, CFI = 0.94, AGFI = 0.99. *Statistical significance at p < 0.05. COPD = chronic obstructive pulmonary disease; DMT = disease-modifying therapy; MS = multiple sclerosis.
Discussion
SES was found to be a contributing factor in the observed rural/urban inequality in DMT use overall, which is in alignment with findings from other countries with universal health care that have shown pwMS who have a lower SES are less likely to be prescribed a DMT.9 A number of recommendations have been developed and successfully implemented across various geographical locations (rural/urban) to overcome barriers (e.g., medical model bias, patients who experienced prior stereotype/discrimination in clinical care, physicians own implicit bias, lack of knowledge about resources, unsureness of what actions to take, and perceived lack of time to address complex social needs) to adopting a social determinants (interconnected with SES) of health approach within the health care system10,11 and therefore may assist in providing equitable care management and optimal pharmacotherapy for pwMS.
Geographical barriers may have also contributed the observed inequality in DMT use in this study, such as experiencing greater difficulty in accessing MS-related care (where DMT access is facilitated) due to a lack of specialized health care providers within rural areas and an inability to travel the distance needed to access care.12,13 In addition, a lack of access to infusion clinics may have been a barrier to receiving induction/higher-efficacy therapy in rural areas in this study, which has been previously reported for other chronic conditions.14 Collectively, improvements in access to fully resourced MS specialist neurologists (such as increasing telemedicine access) and increased local health care infrastructure with associated human resources including infusion clinics (whose need will likely continue to rise because practice is shifting toward the early use of induction/higher-efficacy therapies3) for pwMS who reside in rural areas may support equitable DMT use.
This study has several important strengths including the large size, population-based cohort design, and analytical approach. However, this study is also subject to a number of limitations. As this study did not use medical records, there is a potential for misclassification of the cohort or measures; to address this, validated case definitions were used when available. The drug database provides information on medication dispensations and therefore may not represent actual medication uptake.
Conclusion
Findings from this population-based study identified improvements in earlier DMT initiation after the MS incident date over time (between 2008 and 2021), along with disparities in DMT use (observed during a 1-year period; 2019/2020). PwMS who resided in rural Alberta were less likely to receive a DMT, especially induction/higher-efficacy therapy, compared with those who resided in urban areas that may be mediated by SES and geography; this may be generalized to similar rural areas of industrialized countries whose residents have a lower SES (vs urban) and/or geographical organization of health care infrastructure and resources. Identifying and overcoming barriers to optimal clinical care in this patient population is needed, along with implementation and assessment of solutions to confirm their efficacy such as strategies that address social determinants of health, and improved access to specialized MS-related care management and infrastructure in rural areas.
Acknowledgment
The authors thank the participants of this study. The authors also thank Phuong Uyen Nguyen who assisted with creating the tables and figures, and supplementary material. This study is based in part on anonymized raw data from Alberta Health and Alberta Health Services, which was provided by the Alberta Strategy for Patient Oriented Research (SPOR) Unit housed within Alberta Health Services. The interpretation and conclusions contained herein are those of the researchers and do not necessarily represent the views or opinions of the Government of Alberta or Alberta Health Services. Scott Klarenbach was supported by the Kidney Health Research Chair and the Division of Nephrology at the University of Alberta.
Author Contributions
E.F. Balcom: drafting/revision of the manuscript for content, including medical writing for content; study concept or design; analysis or interpretation of data. J.A. Mccombe: drafting/revision of the manuscript for content, including medical writing for content; study concept or design; analysis or interpretation of data. M.P. Kate: drafting/revision of the manuscript for content, including medical writing for content. K. Vu: drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design; analysis or interpretation of data. K.J.B. Martins: drafting/revision of the manuscript for content, including medical writing for content; study concept or design; analysis or interpretation of data. S. Aponte-Hao: drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design; analysis or interpretation of data. L. Richer: drafting/revision of the manuscript for content, including medical writing for content; study concept or design; analysis or interpretation of data. T. Williamson: drafting/revision of the manuscript for content, including medical writing for content; study concept or design; analysis or interpretation of data. S.W. Klarenbach: drafting/revision of the manuscript for content, including medical writing for content; study concept or design; analysis or interpretation of data. P.S. Smyth: drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design; analysis or interpretation of data.
Study Funding
This research study was funded by the Prairies Economic Development Canada Fund; funds were held at the University of Alberta by LR. The funders had no role in the study design, analysis, interpretation of the data, drafting of the manuscript, or in the decision to submit for publication.
Disclosure
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this manuscript: K. Vu, K.J.B. Martins, S. Aponte-Hao, L. Richer, T. Williamson, and S.W. Klarenbach are members of the Alberta Real World Evidence Consortium (ARWEC) and/or the Alberta Drug and Therapeutic Evaluation Consortium (ADTEC); these entities (composed of individuals from the University of Alberta, University of Calgary, and Institutes of Health Economics) conduct research including investigator-initiated industry-funded studies (ARWEC) and government-funded studies (ADTEC). E.F. Balcom, J.A. Mccombe, M.P. Kate, and P.S. Smyth declare no competing interests. All authors of this study had complete autonomy over the content and submission of the manuscript, as well as the design and execution of the study. Go to Neurology.org/N for full disclosures.
References
- 1.Dutta R, Trapp BD. Relapsing and progressive forms of multiple sclerosis: insights from pathology. Curr Opin Neurol. 2014;27(3):271-278. doi: 10.1097/WCO.0000000000000094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chalmer TA, Baggesen LM, Nørgaard M, Koch-Henriksen N, Magyari M, Sorensen PS, Danish Multiple Sclerosis Group. Early versus later treatment start in multiple sclerosis: a register-based cohort study. Eur J Neurol. 2018;25(10):1262-e1110. doi: 10.1111/ene.13692 [DOI] [PubMed] [Google Scholar]
- 3.Spelman T, Magyari M, Piehl F, et al. Treatment escalation vs immediate initiation of highly effective treatment for patients with relapsing-remitting multiple sclerosis: data from 2 different national strategies. JAMA Neurol. 2021;78(10):1197-1204. doi: 10.1001/jamaneurol.2021.2738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roddam H, Rog D, Janssen J, et al. Inequalities in access to health and social care among adults with multiple sclerosis: a scoping review of the literature. Mult Scler Relat Disord. 2019;28:290-304. doi: 10.1016/j.msard.2018.12.043 [DOI] [PubMed] [Google Scholar]
- 5.Balcom E, McCombe J, Mahesh K, et al. Geographical variation in medication and health resource use in multiple sclerosis. Can J Neurol Sci. 2024:1-9. doi: 10.1017/cjn.2024.54 [DOI] [PubMed] [Google Scholar]
- 6.Widdifield J, Ivers NM, Young J, et al. Development and validation of an administrative data algorithm to estimate the disease burden and epidemiology of multiple sclerosis in Ontario, Canada. Mult Scler. 2015;21(8):1045-1054. doi: 10.1177/1352458514556303 [DOI] [PubMed] [Google Scholar]
- 7.Pampalon R, Hamel D, Gamache P, Raymond G. A deprivation index for health planning in Canada. Chronic Dis Can. 2009;29(4):178-191. doi: 10.24095/hpcdp.29.4.05 [DOI] [PubMed] [Google Scholar]
- 8.Danner D, Hagemann D, Fiedler K. Mediation analysis with structural equation models: combining theory, design, and statistics. Eur J Social Psychol. 2015;45(4):460-481. doi: 10.1002/ejsp.2106 [DOI] [Google Scholar]
- 9.Das J, Rog DJ, Middleton R, Rodgers JW, Fry R, Nicholas R. The association between deprivation and the access to disease modifying therapies for multiple sclerosis: an England wide community-based study in the UK MS Register. Mult Scler Relat Disord. 2022;57:103474. doi: 10.1016/j.msard.2021.103474 [DOI] [PubMed] [Google Scholar]
- 10.Andermann A, CLEAR Collaboration. Taking action on the social determinants of health in clinical practice: a framework for health professionals. CMAJ. 2016;188(17-18):E474-E483. doi: 10.1503/cmaj.160177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Browne AJ, Varcoe C, Ford-Gilboe M, Wathen CN, EQUIP Research Team. EQUIP Healthcare: an overview of a multi-component intervention to enhance equity-oriented care in primary health care settings. Int J Equity Health. 2015;14:152. doi: 10.1186/s12939-015-0271-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buchanan RJ, Stuifbergen A, Chakravorty BJ, Wang S, Zhu L, Kim M. Urban/rural differences in access and barriers to health care for people with multiple sclerosis. J Health Hum Serv Adm. 2006;29(3):360-375. doi: 10.1177/107937390602900301 [DOI] [PubMed] [Google Scholar]
- 13.Buchanan RJ, Wang S, Stuifbergen A, Chakravorty BJ, Zhu L, Kim M. Urban/rural differences in the use of physician services by people with multiple sclerosis. NeuroRehabilitation. 2006;21(3):177-187. doi: 10.3233/nre-2006-21301 [DOI] [PubMed] [Google Scholar]
- 14.Rohatinsky N, Boyd I, Dickson A, et al. Perspectives of health care use and access to care for individuals living with inflammatory bowel disease in rural Canada. Rural Remote Health. 2021;21(2):6358. doi: 10.22605/RRH6358 [DOI] [PubMed] [Google Scholar]