Abstract
Background:
Impaired reactive responses to sudden environmental perturbations contribute to heightened fall-risk in healthy aging and neurologically impaired populations. Previous studies have demonstrated individual contributions of paretic and non-paretic sides to fall-risk in people with stroke with variable levels of motor impairment. However, the combined effect of aging and unilateral cortical lesion on reactive balance control is not clearly understood. We therefore aimed to examine age-related differences in reactive balance control and fall-risk during laboratory-induced gait-slips in people with comparable stroke-related motor impairments.
Methods:
Thirteen younger (45.61 ± 4.61 years) and thirteen older (71.92 ± 6.50 years) adults with similar stroke-related impairment (on Fugl-Meyer Lower Extremity Assessment) were exposed to one overground gait-slip under each limb (paretic and non-paretic). Center of mass state stability and slipping kinematics (slip displacement and velocity) were computed. Clinical balance and mobility were also assessed.
Results:
On non-paretic slips, older adults with chronic stroke demonstrated greater falls and lower center of mass stability (its position and velocity) at post-slip touchdown compared to younger adults with chronic stroke (p < 0.01). This was accompanied with a greater peak slip displacement and faster peak slip velocity (p < 0.01). However, there were no such group differences noted on the paretic slips (p > 0.01).
Conclusion:
Aging may have an independent, detrimental effect on reactive balance control in people with chronic stroke. Non-paretic deficits in controlling slip intensities (slip displacement and velocity) can accentuate fall-risk in older adults with chronic stroke. Further investigation is necessary to identify additional factors attributing to heightened fall-risk in older adults with chronic stroke.
Keywords: Gait slip, Reactive balance, Stroke, Stability, Older adults, Perturbation, Fall-risk
1. Introduction
More than 25% of older adults fall at least once annually [1], leading to complications like fear of falling [2], depression [3] and reduced physical activity [4]. Falls thus pose an enormous threat to the individual and the healthcare system. Increased susceptibility to falling with progressing age is multifactorial and possibly associated with sensorimotor, balance, and cognitive deteriorations [5,6]. Thus, identifying specificrisk factors in older adults is essential for designing effective fall-prevention strategies.
Reactive balance control is crucial to recover from balance losses following unexpected environmental disturbances (slips/trips) [7–9]. Successful recovery from such disturbances demands an intact reactive balance system with effective execution of in-place (ankle/hip strategies) [10] or compensatory (stepping/grasping) [11] responses depending on the perturbation magnitude to reestablish the center of mass stability relative to the base of support (BOS). The central nervous system via cortico-subcortical loops along with multisensory feedback (visual, vestibular, and somatosensory) is postulated to play a vital role in executing reactive balance responses [12,13]. Thus, age/pathology-related alterations in these substrates can result in impaired reactive balance control.
Several researchers have delivered sudden support-surface translations of varying magnitudes in a safe and controlled environment to assess compensatory stepping and fall-risk [14,15]. Studies have illustrated biomechanical differences in reactive balance possibly associated with age-related functional decline. Specifically, healthy older adults demonstrate delayed step-initiation time [16] and inability to modulate step length and/or trunk control with increasing perturbation magnitudes, thereby requiring multiple steps for balance recovery in standing/walking compared to young [17,18]. Such alterations contribute to higher fall-risk in healthy older adults.
Age-related physiological deficits coupled with stroke-related sensorimotor impairments result in about 70% of older adults with stroke falling at least once within the first six months and 50% falling repeatedly [19]. While researchers examined biomechanical factors associated with impaired reactive balance control in healthy aging, rarely studies have investigated the effect of aging and cortical lesion on fall-risk. A comparison of reactive balance following variable magnitude stance-slips indicated that older adults with stroke displayed lower center of mass stability and inability to modulate step length with increasing magnitudes compared to healthy counterparts [20]. Given the higher proportion of gait-slip falls in older adults with stroke, it is important to investigate task-specific biomechanical factors governing impaired reactive balance.
We examined age-related differences in reactive balance control and fall-risk in people with chronic stroke following a large-magnitude novel gait-slip. We hypothesized that when matched for motor impairment, older adults will demonstrate lower center of mass state stability and impaired slipping kinematics than younger adults with chronic stroke following non-paretic and paretic gait-slips. Further, we explored associations between clinical functional and reactive balance measures to identify age-specific contributors to higher fall-risk.
2. Methods
2.1. Participants
Twenty-six individuals from an ongoing study (R01HD088543) were included. Thirteen older (65–90 years) and thirteen younger adults (25–50 years) with chronic stroke (onset ≥ 6 months) with matched sensorimotor impairment (Fugl-Meyer Lower Extremity) and ability to ambulate independently with/without assistive device were included. Individuals were excluded if they demonstrated: low bone density (T score < −2 on heel ultrasound), cognitive impairment (≤ 26/30 on Montreal Cognitive Assessment), Aphasia (< 71/100 on Mississippi Aphasia Screening) and any pre-existing musculoskeletal/neurological disorders, uncontrolled cardiovascular disorders (e.g., Hypertension) or surgeries in < 6 months (e.g., Valvuloplasty). Participants were excluded if they complained of shortness of breath, pain (>3/10), pulse oxygen below 92% or could not achieve the age-specified distance on the Six-Minute Walk Test. Demographics and clinical measures (Tables 1–2) were assessed after participants provided written consent. This study was approved by the Institutional Review Board at the University of Illinois at Chicago.
Table 1.
Demographics characteristics of research participants with their respective Means and Standard deviations.
| Variables | Younger adults (n = 13) Mean (SD) |
Older adults (n = 13) Mean (SD) |
P Value |
|---|---|---|---|
| Age, y | 45.61 (4.01) | 71.92 (6.50) | < 0.001 |
| Age at stroke onset, y | 38.10 (4.00) | 55.00 (7.06) | < 0.001 |
| Sex, M/F | 8/5 | 7/6 | 0.71 |
| Height, m | 1.74 (0.08) | 1.58 (0.41) | 0.15 |
| Weight, kg | 85.79 (12.78) | 76.57 (23.90) | 0.44 |
| BMI, kg/m2 | 28.12 (3.35) | 26.67 (7.02) | 0.87 |
| Hemi-side, R/L | 4/9 | 6/7 | 0.10 |
| Chronicity, y | 7.52 (3.64) | 10.61 (7.30) | 0.12 |
| Type of stroke, H/I | 8/5 | 10/3 | 0.41 |
| AFO/No AFO | 8/5 | 9/4 | 0.69 |
Abbreviations: Type of stroke: H: Hemorrhagic; I: Ischemic; AFO: Ankle-foot orthosis;; y: years; M/F: Male/Female; m: meters; kg: kilogram; kg/m2: kilogram/meter squared; R/L: Right/Left.
Table 2.
Clinical functional measures (balance, gait, motor and cognitive function) of research participants with their respective Means, Standard deviations and Statistical significance Based on t-test results.
| Variables | Younger adults (n = 13) Mean (SD) |
Older adults (n = 13) Mean (SD) |
P Value |
|---|---|---|---|
| BBS (/56) | 50.46 (2.44) | 49.77 (4.20) | 0.62 |
| TUG (s) | 13.73 (5.71) | 15.09 (9.74) | 0.68 |
| 10MWT (m/s) | 1.00 (0.28) | 0.97 (0.29) | 0.99 |
| CMSA (Leg), (/7) | 5.15 (0.66) | 5.15 (0.53) | 1.00 |
| CMSA (Foot), (/7) | 4.08 (1.38) | 4.30 (0.91) | 0.48 |
| Fugl-Meyer (LE) (/86) | 71.54 (6.37) | 70.85 (7.37) | 0.81 |
| mRS (0–6) | 2.00 (0.68) | 1.69 (0.82) | 0.33 |
| MMSE (/30) | 28.85 (0.86) | 28.30 (0.82) | 0.13 |
Abbreviations: BBS: Berg Balance Scale; TUG: Timed up and go test; CMSA: Chedoke-McMaster Stroke Assessment scale; LE: Lower extremity; mRS: Modified Rankin Scale; MMSE: Mini-mental State Examination; s: Seconds, 10MWT: 10-Meter walk test; m/s: meter per second.
2.2. Experimental protocol
To protect against injuries from slipping, participants wore a safety harness that was attached to a load-cell mounted to an overhead trolley. A forward slip was induced by releasing low-friction, movable platforms embedded within the 8-meter walkway. Following three walking trials at the preferred speed, participants were instructed about the possible occurrence of slip-perturbation under either limb without warning about the timing/nature of the slip. Participants were instructed to do their best to recover balance and continue walking upon slipping. Upon detection of participant’s step by force plates (AMTI, Massachusetts, USA), a unilateral, forward slip was triggered with a computer-controlled release. The contralateral side was slipped after 5–8 walking trials. All participants experienced one forward slip (45 cm) under each limb in random order. ‘Paretic slip’ was the slip delivered to the paretic limb during walking where the non-paretic limb attempted to execute a step for balance recovery and vice versa for the ‘non-paretic slip’.
2.3. Data collection and analysis
An eight camera Qualisys system (Motion Analysis, California, USA) was used to capture body kinematics from a set of 30 reflective markers. 26 markers were placed on bony landmarks, 2 markers on moveable platforms, and 2 markers on the walkway. Ground reaction forces captured by force plates and load-cell data were collected at 600 Hz and synchronized with the kinematic data at 120 Hz.
3. Outcome measures
3.1. Primary outcomes
3.1.1. Fall/Recovery
The outcome of a slip was a fall if the peak load cell force upon slipping exceeded 30% of participant’s body-weight [21]. Otherwise, it was a recovery.
3.1.2. Recovery strategies
A recovery could be no or loss of balance based on the stepping response. As the perturbation (slip) was induced in the forward direction for both sides, the balance loss induced thereafter would be in the backward direction. A no loss of balance was identified when the contralateral limb landed anterior to the slipping limb, otherwise it was regarded as loss of balance. Strategies for loss of balance were either aborted or backward stepping. An aborted step was reloading of the stepping foot before its complete unloading, resulting in immediate touchdown. A backward step was complete unloading of the stepping foot after slipping, such that the heel landed posterior to the slipping limb.
3.1.3. Center of mass stability
Center of mass stability was the shortest distance between the center of mass state (its position and velocity relative to BOS) to the computational threshold against balance loss during slips [22]. Using the rear edge of BOS (the heel of slipping limb) as reference, center of mass state was used to calculate its instantaneous stability at post-slip touchdown. If the value was < 0, the center of mass state was below the threshold, indicating greater possibility of backward balance loss and vice versa.
3.1.4. Center of mass position and velocity
The center of mass position and velocity were calculated using a 12-segment body representation from the kinematic data [22]. The center of mass position and velocity were calculated in the anteroposterior direction, where the former was expressed relative to the rear edge of the BOS (i.e., slipping heel) and normalized by foot length; latter was expressed relative to the velocity of the BOS and normalized by the fraction of √g*h, where ‘g’ is acceleration of gravity and ‘h’ is body height. Both variables were assessed at the instance of post-slip touchdown, where values < 0 indicated a more posterior position/posteriorly-directed velocity with respect to the BOS.
3.1.5. Slipping kinematics
Slip displacement and velocity were assessed using slider-marker trajectory since it is known that no relative motion occurs between the heel and the slipping plate marker [23]. Peak slip displacement was the maximum distance travelled by the slider-marker from slip onset to slipping foot lift off and peak slip velocity was the maximum value of the first order derivative of peak slip displacement.
3.2. Secondary measures
3.2.1. Dynamic stability
Berg Balance Scale (BBS) is a valid scale consisting of 14 tasks (4 points each, total 56 points) commonly used in people with chronic stroke to test dynamic balance.
3.2.2. Functional mobility
Timed Up and Go Test was used, which requires the participant to stand up from a chair, walk 3 m, turn around, walk back and sit “as quickly as possible.” For older adults with stroke, > 14 s is considered as high fall-risk.
3.2.3. Gait speed
10 Meter Walk Test (10MWT) was used, where participants walked at their comfortable speed for 10 m and the time was recorded.
3.2.4. Cognitive function
Mini-Mental State Examination (MMSE) was used, which is a 30-point assessment of global cognition including memory, attention, recall and calculation. A score of > 24/30 is cut-point for intact cognition.
3.2.5. Motor impairment
Chedoke McMaster Stroke Assessment (CMSA) - Foot, a 7-point scale examining the severity of motor impairment based on Brunnstorm Stages of recovery was used. A score of ≥ 4/7 is considered as low impairment. The Modified Rankin Scale (mRS) scale was also used, which is 7-point measure used to assess functional disability. A score of 0 is considered as no disability whereas 6 indicates death.
4. Statistical analysis
Generalized estimating equations (GEE) were used to determine the effect of age-group and/or slip-side (independent variables) on fall incidence and recovery strategies (aborted/backward stepping) (dependent variables). Chi-square tests were used for follow-up comparisons with Bonferroni corrections (α = 0.01). A 2 × 2 Analysis of covariance (ANCOVA) was used to test the effect of age-group and slip-side (independent variables) on center of mass stability, its position and velocity and slipping kinematics (dependent variables) with covariates (CMSA foot and mRS). Follow-up comparisons included Paired and Independent t-tests respectively for within- and between-group analyses with Bonferroni corrections (α = 0.01). Independent t-tests were used to examine differences in clinical measures (α = 0.05). Pearson’s correlation coefficient was used to test associations between clinical functional and reactive balance measures. All analyses were performed using IBM SPSS version 24.
5. Sample size justification
Sample size was estimated using G power software, version 9.7 from preliminary data (n = 10, 5/group), which yielded large effect sizes (Cohen’s d: 0.82–0.98) for primary outcomes. With expected effect sizes, an estimated sample of 12 participants per group was obtained to achieve 85% power. This sample size also followed the recommended statistical guidelines for clinical trials [24].
6. Results
6.1. Primary outcomes
All individuals experienced balance loss on both sides upon novel gait-slips. GEE and ANCOVA showed no main effect of covariates (CMSA foot and mRS) on any of the outcome measures. There was a main effect of age-group (β = −2.49, p < 0.05) on fall incidence (Fig. 1a). Specifically, greater percentage of older adults fell compared to young during non-paretic slips (84.61% vs 38.46%, p < 0.01) (Fig. 1a). A main effect of slip-side was seen on recovery strategies (β = −2.49, p < 0.05) (Fig. 1b). Specifically, young (69.23%) and older (53.85%) adults exhibited a trend of more aborted stepping (p > 0.01) during non-paretic compared to paretic slips (Fig. 1b).
Fig. 1.
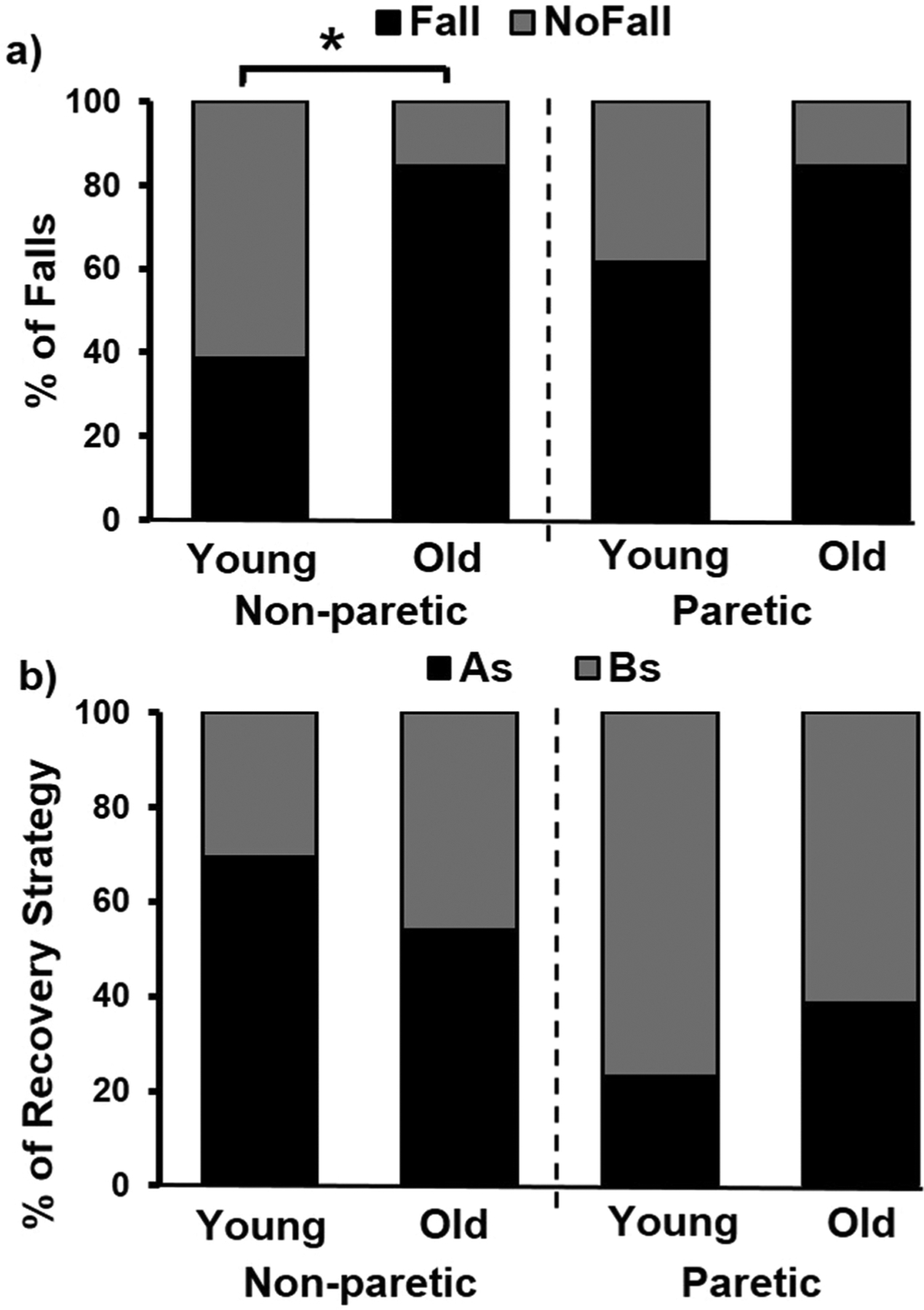
a) Percentage of falls in young and older adults with chronic stroke during novel slip delivered to the non-paretic and paretic limbs (slip distance: 45 cm) b) Percentage of recovery strategies including backward/recovery stepping and aborted stepping shown by young and older groups during novel slip delivered to the non-paretic and paretic limbs (slip distance: 45 cm). In the figure, “non-paretic” refers to slip delivered to the non-paretic limb and “paretic” refers to the slip delivered to the paretic limb. Abbreviations: BS: Backward step, AS: Aborted step. Significant differences are indicated with * p < 0.01.
The ANCOVA showed age-group × slip-side interaction [F (1, 46) = 7.16, p < 0.05] on post-slip center of mass stability. Specifically, the older group displayed lower stability compared to young only during non-paretic slips (p < 0.01) (Fig. 2a). Between the two sides, only the younger group exhibited lower stability during paretic compared to non-paretic slips (p < 0.01). The ANCOVA showed a main effect of age-group on post-slip center of mass position [F (1, 46) = 5.14, p < 0.05]. Post-hoc analysis revealed that older group displayed a more posterior position compared to younger group only during non-paretic slips (p < 0.01) (Fig. 2b). Further, there was an age-group × slip-side interaction [F (1, 46) = 7.09, p < 0.05] on post-slip center of mass velocity. Post-hoc analysis showed that older group demonstrated a more posteriorly directed velocity compared to young only during non-paretic slips (p < 0.01) (Fig. 2c). Between the sides, only younger group demonstrated a more posteriorly directed velocity during paretic compared to non-paretic slips (p < 0.01) (Fig. 2c).
Fig. 2.
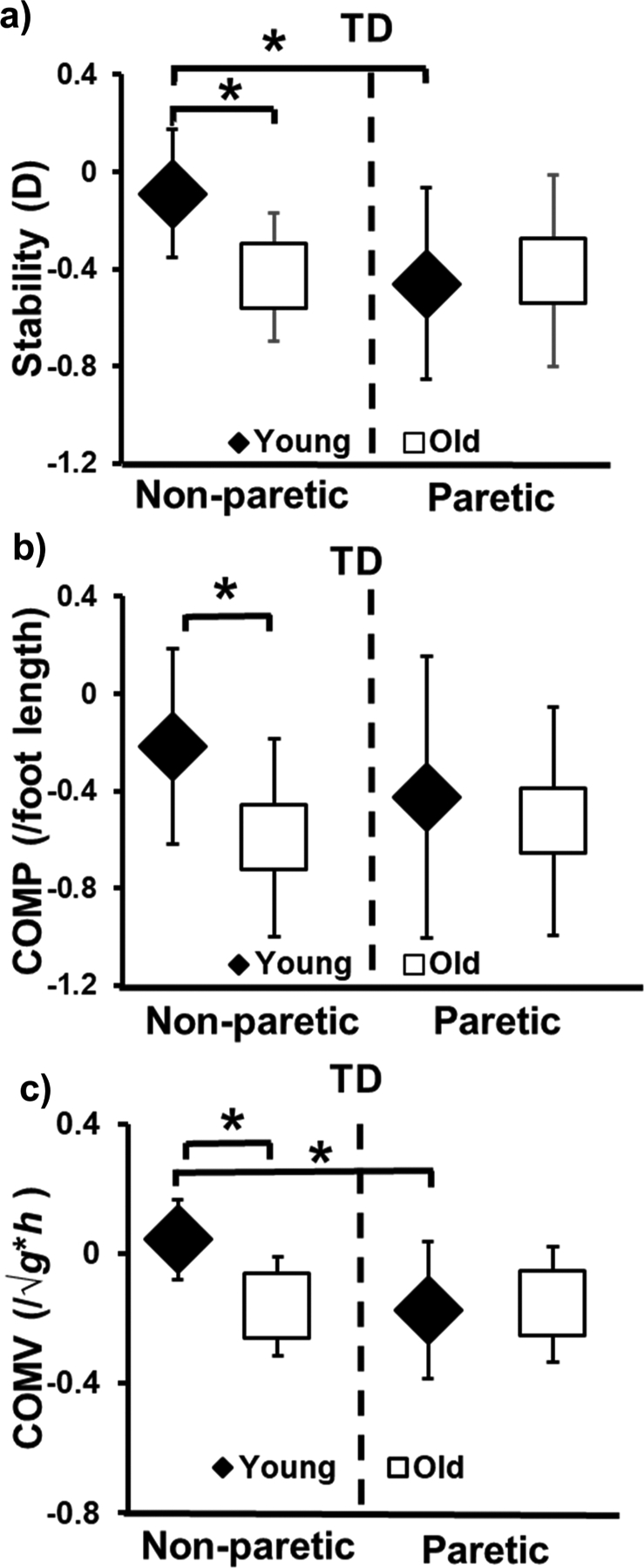
a) Comparison of means (± standard deviation) of stability (D=dimensionless) at post-slip touchdown; b) Comparison of means (± standard deviation) of COM position (COMP/foot length) at post-slip touchdown and c) Comparison of means (± standard deviation) of COM velocity (COMV/l/√g*h) at post-slip touchdown that are plotted for younger and older adults with chronic stroke during novel slip delivered to the paretic and non-paretic limbs. In the figure, “non-paretic” refers to slip delivered to the non-paretic limb, and “paretic” refers to the slip delivered to the paretic limb. COM stability > 0 indicates a lesser probability of backward loss of balance. COM position < 0 indicates more posterior position of COM with respect to BOS and COM position > 0 indicates more anterior position of COM with respect to BOS. COM velocity < 0 indicates greater posteriorly directed velocity of COM with respect to BOS and COM velocity > 0 indicates greater anteriorly directed velocity of COM with respect to BOS. COM: Center of mass. Significant differences are indicated with * p < 0.01.
ANCOVA showed age-group × slip-side interaction [F (1, 46) = 51.70, p < 0.05] on peak slip displacement. Older group displayed higher peak slip displacement compared to young only during non-paretic slips (p < 0.01) (Fig. 3a). Between the sides, younger group demonstrated higher peak slip displacement during paretic (p < 0.01) than non-paretic slips. Lastly, ANCOVA showed age-group × slip-side interaction [F (1, 46) = 15.35, p < 0.05] on peak slip velocity. Older group demonstrated higher peak slip velocity only during non-paretic slips compared to young (p < 0.01) (Fig. 3b). Further, both groups (p < 0.01) demonstrated higher peak slip velocity during paretic compared to non-paretic slips.
Fig. 3.
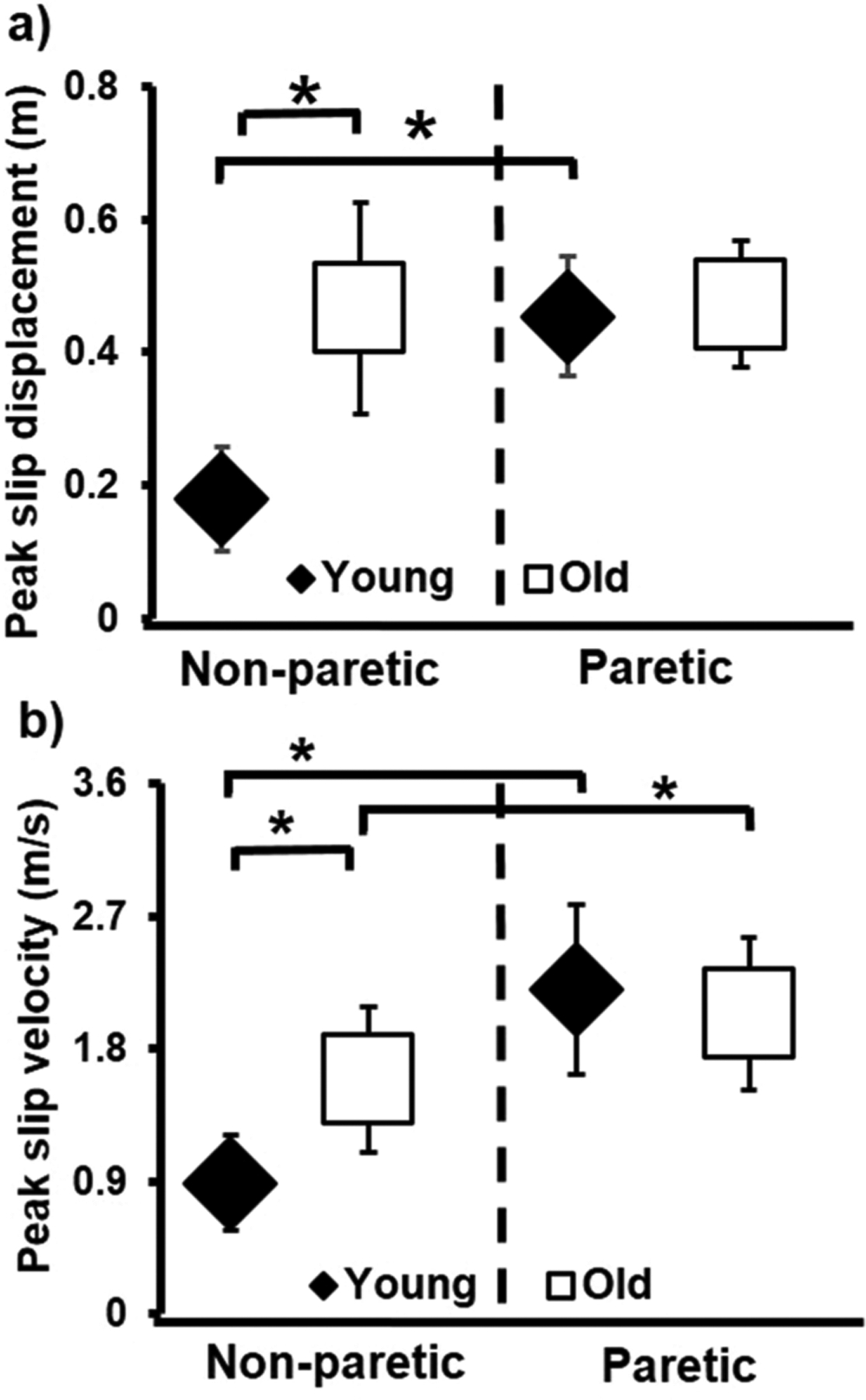
Comparison of means (± standard deviation) of a) Peak slip displacement and b) Peak slip velocity that are plotted for younger adults and older adults with chronic stroke during novel slip delivered to the non-paretic and paretic limbs. In the figure, “non-paretic” refers to slip delivered to the non-paretic limb, and “paretic” refers to the slip delivered to the paretic limb. Slip displacement < 0 indicates greater posterior displacement of BOS and slip displacement > 0 indicates greater anterior displacement of BOS. Slip velocity < 0 indicates greater posterior velocity of BOS slipping heel marker and slip velocity > 0 indicates greater anterior velocity of BOS. Significant differences are indicated with * p < 0.01.
6.2. Secondary clinical measures
There were no significant age-group differences in any clinical measures and Pearson’s correlation showed no significant correlation between clinical and reactive balance measures in both groups (p > 0.05) (Table 2).
7. Discussion
We investigated the effect of aging on reactive balance control and fall-risk in people with chronic stroke. Results showed that the older group demonstrated greater falls and lower post-slip center of mass stability accompanied by higher slip intensity compared to the younger group only during non-paretic but not paretic slips.
All participants experienced a balance loss on both side-slips followed by a backward/aborted step for recovery. Consistent with previous studies in people with stroke [25,26], both young and older adults showed a greater proportion of aborted stepping during non-paretic compared to paretic slips. While a backward stepping response could be more beneficial and the preferred choice in healthy young [27,28], current study groups executed a greater proportion of aborted stepping. Moreover, younger adults exhibited a trend of greater aborted stepping than older on non-paretic slips. Although aborted stepping could increase the risk of split-falls (unilateral slip causing “split” of the slipping and trailing limb resulting in both limbs wide apart) [28], such a trend might be a preferred strategy to reestablish stability and “ride” out the effect of perturbation [26]. Arguably, aborted stepping could have been the only option due to the inability of paretic limb to execute a backward step. However, a previous study showed the intact ability of the paretic limb to execute a backward step [29]. Hence, greater proportion of aborted stepping seen here was more likely a “choice strategy” to maximize stability by minimizing mobility until the perturbation effect was abated. Further, aborted stepping enabled younger group to eliminate the risk of accurately placing their foot in the optimal landing zone [30]. Aborted stepping could have also helped reestablish stability by increasing double stance time.
It is known that post-slip stability is not only associated with recovery strategies but also affected by slipping intensity, such that greater slip displacement and faster slip velocity could lower post-slip stability [31,32]. Similarly, the older group in this study showed greater slip displacement and faster slip velocity than the young during non-paretic slips, indicating aging could reduce the ability to control slipping intensity. Consequently, the paretic stepping in the older group resulted in lower post-slip stability and a greater percentage of falls compared to the young. Previous studies have also shown that compared to young, healthy older adults exhibited faster heel contact velocity during unperturbed walking [33,34], which could lead to higher intensity upon unexpected slipping. Thus, our findings suggest that aging with chronic stroke affects slip intensity resulting in failure to maintain stability.
There were no age-group differences in reactive balance outcomes during paretic slips, suggesting that stroke-related impairment may play a dominant role during paretic slips compared to aging. Consistently, studies have postulated that level of impairment influences motor adaptation and fall-risk in people with chronic stroke [25,35]. Inclusion of young and older adults with similar sensorimotor impairments could have eliminated the paretic reactive balance differences and highlighted the non-paretic age-related differences. It could be postulated that factors like physical activity, fall history, and community participation might contribute to governing paretic reactive balance. Furthermore, factors like muscle strength of slipping limb could also affect reactive balance during non-paretic slip. Previous studies have established that loss of lean muscle tissue and reduced cross-sectional area in thigh muscles weaken the isometric knee strength and increase difficulty in carrying out functional tasks in healthy older adults [36,37], and isometric hip extensor strength [38] could be a fall-risk predictor in people with stroke. Thus, bilateral muscular weakness, impaired non-paretic ability to control slipping kinematics and paretic deficits in reactive stepping could contribute to higher fall-risk in older adults.
Contrary to our expectation, no correlations were found between reactive and clinical measures (e.g., BBS, 10MWT), which might be due to potential ceiling effects. Previous studies have indicated that clinical measures like BBS failed to distinguish slow and fast walkers [39] and predict falls in people with chronic stroke [39]. Additionally, these clinical measures might be related to volitional but not reactive balance control. Further, there were no group differences in any of the clinical functional measures (Table 2). Thus, our results suggest that clinical assessments that do not specifically examine reactive balance components possibly cannot identify age-related differences in fall-risk in people with chronic stroke.
7.1. Study limitations and strengths
This study had some limitations. Firstly, this study had a small sample, hence results could be validated with a larger clinical trial. Secondly, there were significant group differences in age at the time of stroke (Table 1). However, it is known that age at the time of stroke onset has a small effect on functional recovery [40]. We did not extract information regarding the location/severity of lesion at the time of stroke. It is possible that such circumstances could have affected participant’s initial recovery. However, these factors might not be as relevant as the current capability of participants in chronic phases of recovery, which was accounted for by examining present sensorimotor, cognitive, and functional status. However, our findings cannot be generalized to acute/subacute phases. Lastly, while volitional measures were assessed, clinical reactive measures (e.g., Mini-BESTest) were not included.
This study has some strengths. Firstly, both groups were matched for stroke-related impairment and clinical measures like balance, gait, sensorimotor and cognitive function were taken into account. Thus, the group differences in reactive balance outcomes are postulated to be associated with aging alone. Secondly, all participants were exposed to novel slips on the paretic and non-paretic side in random order to reduce any transfer effect.
8. Conclusion
We highlighted age-related differences in fall-risk in people with chronic stroke, such that older adults exhibited greater falls, worse center of mass post-slip stability and poor slipping kinematics during non-paretic slips compared to younger adults. While age might have affected reactive balance control on the non-paretic side, level of impairment might play a predominant role in governing paretic reactive balance control. Exploring the mechanisms and supplementary factors responsible for this age-related decline can help formulate effective interventions for fall-prevention in people with chronic stroke.
Acknowledgements
The authors would like to thank Riddhi Panchal, MS, PT, Lakshmi Kannan, MS, PT, Rachana Gangwani, MS, PT, and Gonzalo Varas Diaz, MS, PT for assistance with data collection. This study was funded by National Institute of Health (NIH) [R01HD088543-01A1] awarded to Dr. Tanvi Bhatt.
Footnotes
Conflict of interest
None declared.
References
- [1].Bergen G, Stevens MR, Burns ER, Falls and fall injuries among adults aged >/=65 Years - United States, 2014, MMWR Morb. Mortal. Wkly Rep 65 (37) (2016) 993–998. [DOI] [PubMed] [Google Scholar]
- [2].Iaboni A, Flint AJ, The complex interplay of depression and falls in older adults: a clinical review, Am. J. Geriatr. Psychiatry 21 (5) (2013) 484–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Lavedan A, et al. , Fear of falling in community-dwelling older adults: a cause of falls, a consequence, or both? PLoS One 13 (3) (2018), e0194967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Sherrington C, et al. , Evidence on physical activity and falls prevention for people aged 65+ years: systematic review to inform the WHO guidelines on physical activity and sedentary behaviour, Int J. Behav. Nutr. Phys. Act 17 (1) (2020) 144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Laurence BD, Michel L, The fall in older adults: physical and cognitive problems, Curr. Aging Sci 10 (3) (2017) 185–200. [DOI] [PubMed] [Google Scholar]
- [6].McCrum C, et al. , A systematic review of gait perturbation paradigms for improving reactive stepping responses and falls risk among healthy older adults, Eur. Rev. Aging Phys. Act 14 (2017) 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Mansfield A, et al. , Does perturbation-based balance training prevent falls? systematic review and meta-analysis of preliminary randomized controlled trials, Phys. Ther 95 (5) (2015) 700–709. [DOI] [PubMed] [Google Scholar]
- [8].Pai YC, et al. , Adaptability to perturbation as a predictor of future falls: a preliminary prospective study, J. Geriatr. Phys. Ther 33 (2) (2010) 50–55. [PMC free article] [PubMed] [Google Scholar]
- [9].Weerdesteyn V, et al. , Falls in individuals with stroke, J. Rehabil. Res Dev 45 (8) (2008) 1195–1213. [PubMed] [Google Scholar]
- [10].Horak FB, Nashner LM, Central programming of postural movements: adaptation to altered support-surface configurations, J. Neurophysiol 55 (6) (1986) 1369–1381. [DOI] [PubMed] [Google Scholar]
- [11].Maki BE, McIlroy WE, The role of limb movements in maintaining upright stance: the “change-in-support” strategy, Phys. Ther 77 (5) (1997) 488–507. [DOI] [PubMed] [Google Scholar]
- [12].Bastian AJ, Understanding sensorimotor adaptation and learning for rehabilitation, Curr. Opin. Neurol 21 (6) (2008) 628–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Bolton DA, The role of the cerebral cortex in postural responses to externally induced perturbations, Neurosci. Biobehav Rev 57 (2015) 142–155. [DOI] [PubMed] [Google Scholar]
- [14].Tokuno CD, et al. , Age-related changes in postural responses revealed by support-surface translations with a long acceleration-deceleration interval, Clin. Neurophysiol 121 (1) (2010) 109–117. [DOI] [PubMed] [Google Scholar]
- [15].Wang S, et al. , Slip-induced fall-risk assessment based on regular gait pattern in older adults, J. Biomech 96 (2019), 109334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Tseng SC, Stanhope SJ, Morton SM, Impaired reactive stepping adjustments in older adults, J. Gerontol. A Biol. Sci. Med Sci 64 (7) (2009) 807–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Maki BE, McIlroy WE, Control of rapid limb movements for balance recovery: age-related changes and implications for fall prevention, Age Ageing 35 (Suppl 2) (2006) ii12–ii18. [DOI] [PubMed] [Google Scholar]
- [18].Mansfield A, et al. , A perturbation-based balance training program for older adults: study protocol for a randomised controlled trial, BMC Geriatr. 7 (2007) 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Mackintosh SF, Goldie P, Hill K, Falls incidence and factors associated with falling in older, community-dwelling, chronic stroke survivors (> 1 year after stroke) and matched controls, Aging Clin. Exp. Res 17 (2005) 74–81. [DOI] [PubMed] [Google Scholar]
- [20].Patel PJ, Bhatt T, Does aging with a cortical lesion increase fall-risk: Examining effect of age versus stroke on intensity modulation of reactive balance responses from slip-like perturbations, Neuroscience 333 (2016) 252–263. [DOI] [PubMed] [Google Scholar]
- [21].Yang F, Pai Y-C, Automatic recognition of falls in gait-slip training: harness load cell based criteria, J. Biomech 44 (12) (2011) 2243–2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Pai YC, Iqbal K, Simulated movement termination for balance recovery: can movement strategies be sought to maintain stability in the presence of slipping or forced sliding? J. Biomech 32 (8) (1999) 779–786. [DOI] [PubMed] [Google Scholar]
- [23].Bhatt T, Wening JD, Pai YC, Adaptive control of gait stability in reducing slip-related backward loss of balance, Exp. Brain Res 170 (1) (2006) 61–73. [DOI] [PubMed] [Google Scholar]
- [24].Julious SA, Sample size of 12 per group rule of thumb for a pilot study, Pharm. Stat.: J. Appl. Stat. Pharm. Ind 4 (4) (2005) 287–291. [Google Scholar]
- [25].Dusane S, et al. , Does stroke-induced sensorimotor impairment and perturbation intensity affect gait-slip outcomes? J. Biomech 118 (2021), 110255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Kajrolkar T, et al. , Dynamic stability and compensatory stepping responses during anterior gait-slip perturbations in people with chronic hemiparetic stroke, J. Biomech 47 (11) (2014) 2751–2758. [DOI] [PubMed] [Google Scholar]
- [27].Bhatt T, Wening JD, Pai YC, Influence of gait speed on stability: recovery from anterior slips and compensatory stepping, Gait Posture 21 (2) (2005) 146–156. [DOI] [PubMed] [Google Scholar]
- [28].Yang F, et al. , Two types of slip-induced falls among community dwelling older adults, J. Biomech 45 (7) (2012) 1259–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Kajrolkar T, Bhatt T, Falls-risk post-stroke: examining contributions from paretic versus non paretic limbs to unexpected forward gait slips, J. Biomech 49 (13) (2016) 2702–2708. [DOI] [PubMed] [Google Scholar]
- [30].Wang S, Pai YC, Bhatt T, Is there an optimal recovery step landing zone against slip-induced backward falls during walking? Ann. Biomed. Eng 48 (6) (2020) 1768–1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Bhatt T, et al. , Adaptation and generalization to opposing perturbations in walking, Neuroscience 246 (2013) 435–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Bhatt T, Wening J, Pai Y-C, Adaptive control of gait stability in reducing slip-related backward loss of balance, Exp. Brain Res 170 (1) (2006) 61–73. [DOI] [PubMed] [Google Scholar]
- [33].Karst GM, et al. , Reliability of foot trajectory measures within and between testing sessions, J. Gerontol. A Biol. Sci. Med Sci 54 (7) (1999) M343–M347. [DOI] [PubMed] [Google Scholar]
- [34].Mills PM, Barrett RS, Swing phase mechanics of healthy young and elderly men, Hum. Mov. Sci 20 (4–5) (2001) 427–446. [DOI] [PubMed] [Google Scholar]
- [35].Bhatt T, Dusane S, Patel P, Does severity of motor impairment affect reactive adaptation and fall-risk in chronic stroke survivors? J. Neuroeng. Rehabil 16 (1) (2019) 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Tracy BL and Enoka RM, Older adults are less steady during submaximal isometric contractions with the knee extensor muscles. J Appl Physiol (1985), 2002. 92(3): p. 1004–12. [DOI] [PubMed] [Google Scholar]
- [37].Landers KA, et al. , The interrelationship among muscle mass, strength, and the ability to perform physical tasks of daily living in younger and older women, J. Gerontol. A Biol. Sci. Med Sci 56 (10) (2001) B443–B448. [DOI] [PubMed] [Google Scholar]
- [38].Gangwani R, et al. , Slip-fall predictors in community-dwelling, ambulatory stroke survivors: a cross-sectional study, J. Neurol. Phys. Ther 44 (4) (2020) 248–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Madhavan S, Bishnoi A, Comparison of the mini-balance evaluations systems test with the berg balance scale in relationship to walking speed and motor recovery post stroke, Top. Stroke Rehabil 24 (8) (2017) 579–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Kugler C, et al. , Does age influence early recovery from ischemic stroke? J. Neurol 250 (6) (2003) 676–681. [DOI] [PubMed] [Google Scholar]


