Abstract
To understand the function of cells such as neurons within an organism, it can be instrumental to inhibit cellular function, or to remove the cell (type) from the organism, and thus to observe the consequences on organismic and/or circuit function and animal behavior. A range of approaches and tools were developed and used over the past few decades that act either constitutively or acutely and reversibly, in systemic or local fashion. These approaches make use of either drugs or genetically encoded tools. Also, there are acutely acting inhibitory tools that require an exogenous trigger like light. Here, we give an overview of such methods developed and used in the nematode Caenorhabditis elegans.
Keywords: cell ablation, optogenetics, chemical ablation, channelrhodopsins, animal rhodopsins, photosensitizer, laser ablation, genetic ablation, hyperpolarization, synaptic vesicle release
Introduction
Eliminating neurons, and/or inducing the inhibition of their function, is a fundamental approach in the neurosciences, in order to understand the role of a neuron within the circuit(s) it is embedded in (Pei et al. 2008; Wiegert et al. 2017). This is a particularly fruitful and important approach in the nematode Caenorhabditis elegans, with its compact nervous system of a mere 302 neurons, where in many cases elimination of single neurons (or a class of neurons) has profound impact on circuit function and/or behavior (Qi et al. 2012; Lin et al. 2013b). Therefore, many approaches and (mostly genetically encoded) tools have been developed over the past 4 decades, to enable the inhibition of excitable cells. These range from pharmacological treatment to laser ablation, from expressing proteins inducing cell death, to photosensitizers allowing to eliminate individual cells or cell populations, to chemically or light-activated proteins that either hyperpolarize excitable cells, signal to inhibitory G protein pathways, or inhibit the release of neurotransmitter by destroying proteins required for synaptic transmission or by blocking synaptic vesicle (SV) mobility (Fig. 1a). Another approach is to (optogenetically) activate neurons that have inhibitory action (Liewald et al. 2008; Steuer Costa et al. 2019). The genetic encodability of such tools is particularly attractive, because it can be specific down to individual neurons, but also allows to address whole neuron classes as well as animal populations. The tools used for disruption or inhibition of (excitable) cell function can act at different spatial scales, affecting either the entire animal or parts of its body, down to individual cells or even synapses (the latter requires synapse-specific illumination and is possible, but not widely applied due to the technical difficulty of this approach; Fig. 1b). The tools described in this review are further characterized by the timescales of how fast (and tightly controllable) their use is in the onset, which can be submillisecond for microbial rhodopsin tools (Bergs et al. 2018), but also hours when it requires the expression of proteins (Fig. 1c). Last, the tools are characterized by the duration of their action as well as their reversibility: Some tools stop their action as soon as the stimulus is off, e.g. light, or can be very long lasting, e.g. if proteins need to be synthesized de novo to revert the manipulation. Other tools’ actions are permanent, e.g. if the cell is physically or genetically ablated. In the following sections, we briefly describe the tools and some of their applications. We did not attempt to be fully comprehensive, as these approaches cover a vast field of applications and some have been used for several decades.
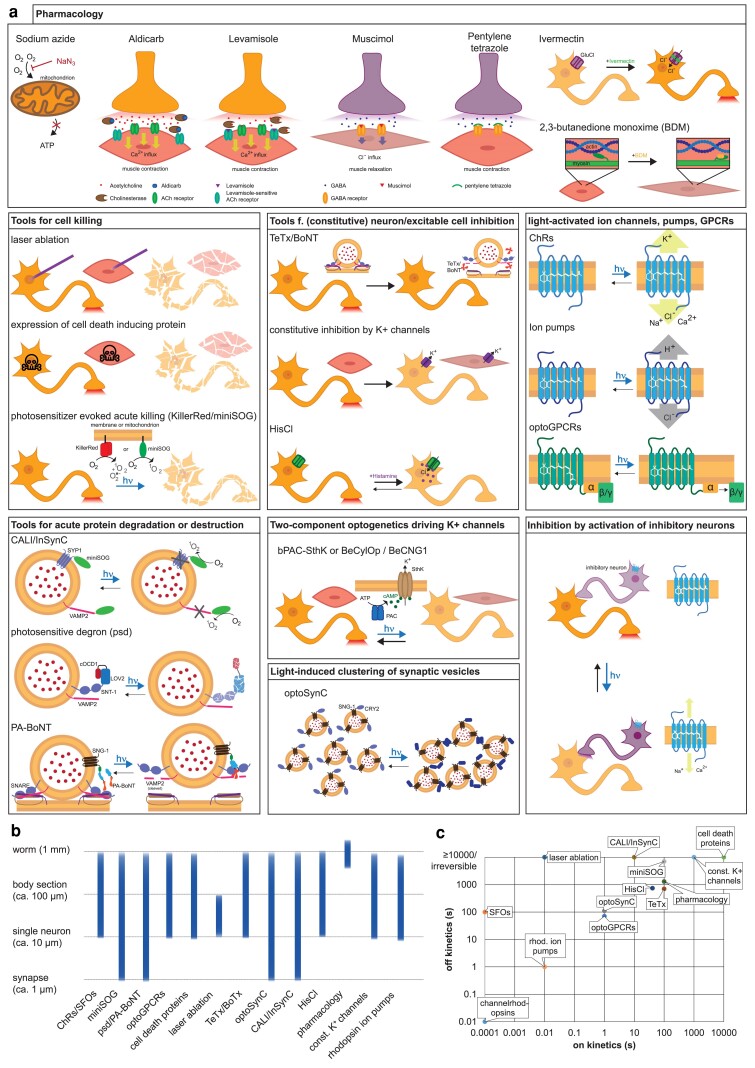
Fig. 1.
a) Pharmacological and other silencing approaches, physical ablation, and (optogenetic) tools for inhibition of excitable cells or cell ablation, covered in this review. b) Spatial scales at which the inhibitory tools and approaches can be used. c) Temporal scales for on and off times/recovery rate of the tools and approaches. The given times are only roughly indicated for comparability. Irreversible tools are shown at the top of the chart, for simplicity.
Pharmacology
The most straightforward possibility to silence neuronal activity or locomotion is through the use of drugs. Often, these act in a reversible way, if the animal is removed from the drug source, though prolonged exposure to some drugs will cause cellular damage or even death. Sodium azide (NaN3) causes rapid paralysis due to its in inhibitory effect on cellular respiration. This causes a quick depletion of cellular energy equivalents (mostly adenosine triphosphate, ATP) and thus cessation of muscular activity, as well as synaptic transmission, that requires ATP for transmitter loading of SVs as well as for SV recycling. The neuromuscular junction can be used as a target for immobilizing drugs, mostly by activating nicotinic acetylcholine receptors (nAChRs) or inhibition of ACh degradation. These drugs, levamisole [a levamisole receptor agonist (Lewis et al. 1980a, 1980b)] and aldicarb [inhibiting ACh esterase (Miller et al. 1996)], cause or lead to excessive stimulation of nAChRs and thus evoke muscle hypercontraction. A light-activated version of ACh, AzoCholine, has also been used to enhance cholinergic function, though this does not lead to full paralysis on its own; yet, AzoCholine can enhance the effects of aldicarb in a light-dependent manner (Damijonaitis et al. 2015). Also, the inhibitory side of the neuromuscular junction (NMJ) can be used to induce paralysis, e.g. the GABAA receptor agonist muscimol (flaccid paralysis) or the gamma-aminobutyric acid (GABA) antagonist pentylenetetrazole (PTZ; causing overexcitation of muscles; Schaeffer and Bergstrom 1988; Avery and Horvitz 1990; Abraham et al. 2011). A very strong form of rapid paralysis is induced by ivermectin, which is agonist for glutamate-gated chloride channels (GluCls), residing on (cholinergic) motor neurons (Avery and Horvitz 1990; Cully et al. 1994; Arena et al. 1995; Dent et al. 1997; Hibbs and Gouaux 2011). Also, muscle can be inhibited by direct drug action, likely on the actin-myosin contractile apparatus, by using butanedione monoxime (BDM) (2,3-BDM; Miriam Goodman and Martin Chalfie, personal communication; see also Emtage et al. 2004 and Bounoutas et al. 2009).
Laser ablation
Cell ablation is used to understand the role of cells in a specific biological context. Cells can be ablated by intense light illumination, provided the light wavelength is absorbed by the tissue. Laser light is focused to a spot of the dimensions of a cell nucleus, where it causes heating and pressure increase. Light energy is typically provided in a pulsed fashion (3–70 ns duration, at <20 Hz), such that heating remains local and does only damage the cell of interest and not cells in the vicinity (Bargmann and Avery 1995). The first approaches for cell ablation utilized visible laser light (ca. 440 nm, directly absorbed, but possibly also by causing 2-photon excitation), or UV laser irradiation of the nucleus of the respective cell, which leads to cell death within comparably short time periods, and thus the loss of the function of the respective cell. This approach is quite powerful and has been widely used over the decades, since its first establishment in the late 1970s and early 1980s (White and Horvitz 1979; Sulston and White 1980). Considerations of how to set up a laser ablation system, and experimental parameters, are well summarized in 2 methods book chapters, for further reading (Bargmann and Avery 1995; Fang-Yen et al. 2012). Often, pulsed nitrogen-pumped tunable dye lasers, producing microjoule-level UV pulses with nanosecond durations, are used. These rather specialized lasers may require specific microscope setups and are rather expensive. Alternatively, femtosecond laser pulses of ca. 50 nJ, in the near-infrared, for multiphoton absorption, can be used at 1 kHz or at up to 80 MHz (Chung and Mazur 2009; Fang-Yen et al. 2012). Laser ablation has been used on various neuron types in Caenorhabditis elegans to identify cells involved in neuronal regulation of development (Bargmann and Horvitz 1991b) or behaviors such as chemotaxis (Bargmann and Horvitz 1991a; Bargmann et al. 1993), thermotaxis (Mori and Ohshima 1995), and locomotion (Gray et al. 2005). However, there are limitations to this approach: (1) It requires practice and experience to master, and it is of low throughput; (2) it does not allow to eliminate genetically defined cell populations, unless they are just a few; and (3) it does not allow to generate populations of animals lacking the cell of interest.
Genetically encoded cell death
Because of the limitations of laser ablation studies for high throughput, other approaches were chosen and/or developed. Proteins like caspases, or constitutively active ion channels, can lead to cell death or necrosis and thus cell loss. Consequently, genetic cell ablation methods have become widely used. Cells can be targeted for elimination by expressing cytotoxic proteins using cell-specific promoters. Several such proteins were established, including caspases, and split versions thereof, for combinatorial expression (CED-3 and human caspase 3; Chelur and Chalfie 2007; Bendesky et al. 2011; Glauser et al. 2011; Ikeda et al. 2020; Atanas et al. 2023; Chandra et al. 2023), CSP-1 (Denning et al. 2013), mammalian caspase 1 (also known as interleukin 1 beta convertase, ICE; Zheng et al. 1999; Kim et al. 2009; Bendesky et al. 2011; Oranth et al. 2018), or apoptosis regulators such as EGL-1 (Conradt and Horvitz 1998; Chang et al. 2006; Rauthan et al. 2007), a member of the BH3-only protein family.
Dominant alleles of mec-4 and deg-1, which encode the hyperactive degenerin/ENaC family of epithelial sodium channels, lead to the necrotic death of specific sets of neurons (Chalfie and Wolinsky 1990; Driscoll and Chalfie 1991; Bianchi et al. 2004). Ectopic expression of the dominant allele mec-4(d) can disrupt the function of various cells (Harbinder et al. 1997; Shinkai et al. 2011; Teo et al. 2022). The ability of these dominant alleles to induce cellular degeneration depends on mec-6 (Chalfie and Wolinsky 1990; Driscoll and Chalfie 1991; Harbinder et al. 1997), which encodes a putative chaperone for MEC-4 (Chen et al. 2016a).
PEEL-1 is a native C. elegans sperm-derived toxin that is normally counteracted in the embryo by its antidote, ZEEL-1 (Seidel et al. 2008). Ectopic expression of PEEL-1 can also be used to induce cell ablation (Seidel et al. 2011; Frøkjær-Jensen et al. 2012; Chen et al. 2016b)
Acutely induced cell death
Caspases and other cell ablation proteins may act as soon as they become expressed, which is only ill-controllable, and the lack of cells during development of an animal can induce compensatory/homeostatic plasticity mechanisms. Therefore, there is a need for tools that enable acute destruction of cells in a short period of time, yet still retain the benefit of genetic encodability to systemically address cell populations, as well as entire animal populations. To this end, photosensitizers based on light-absorbing proteins have been developed. These generate, in response to blue light, reactive oxygen species (ROS) that act in a destructive way on any oxidizable (amino acid) residues in the vicinity of the photosensitizer chromophore. This can occur through methionine oxidation, or by inducing cross-linking, and depending on subcellular localization, will damage nearby protein and can even induce cell death (Jacobson et al. 2008). Such tools include the green fluorescent miniature singlet oxygen generator (miniSOG); the red fluorescent KillerRed, which produces superoxide anion radicals; and SuperNova. Here, we discuss their development and their applications in C. elegans.
KillerRed
KillerRed, a derivative of the hydrozoan chromoprotein anm2CP, which is a homolog of green fluorescent protein (GFP), was initially developed for light-induced cell ablation of bacteria and eukaryotic cells, as well as for targeted protein inactivation (Bulina et al. 2006b). Subsequent adaptations enabled the use of KillerRed for effective neuronal inactivation in C. elegans, which can be induced by a 5 min illumination period, leading to morphological changes appearing after hours and persisting for several days (Kobayashi et al. 2013; Williams et al. 2013). Since its introduction, KillerRed-induced neural dysfunction has been employed in numerous studies (Lee et al. 2014; Luo et al. 2014; Berendzen et al. 2016; Shao et al. 2016; Young et al. 2019; Pohl et al. 2023), highlighting its utility in neurobiological research. However, it is noteworthy that certain types of neurons, including AWB, AFD, and PDE, exhibit resistance to KillerRed-induced cell inactivation (Williams et al. 2013). Additionally, when KillerRed is expressed in mechanosensory neurons, normal mechanosensation is disrupted even without light exposure (Takemoto et al. 2013). Several variants of KillerRed have been developed. KillerOrange, which is activated by both blue and green light, has been developed and tested in vitro (Sarkisyan et al. 2015), offering greater flexibility when concurrent manipulation of multiple proteins or cell types is required, due to the broader color spectrum. Since KillerRed tends to form homodimers, which might interfere with the normal function of the fusion proteins (Bulina et al. 2006a), monomeric variants called SuperNova, SuperNova Green, and SuperNova2 have been established in bacterial and cultured cell systems (Takemoto et al. 2013; Riani et al. 2018; Gorbachev et al. 2020). SuperNova exhibits proper localization with targeted proteins, which increases its potential to disrupt synaptic proteins or specific neurotransmitter receptors for functional analysis.
miniSOG
The miniSOG is a green fluorescent flavoprotein, which was engineered from the Arabidopsis phototropin 2 (Shu et al. 2011). While KillerRed is relatively large (237 amino acids) and requires dimerization, miniSOG only consists of 106 amino acids and is acting as a monomer. miniSOG was originally developed for use in correlative light and electron microscopy (Shu et al. 2011), by inducing the formation of an electron-dense polymeric precipitate from diaminobenzidine. As miniSOG produces a sufficient amount of ROS, specifically singlet oxygen, upon blue light illumination, it could also be used for light-induced cell ablation. By targeting miniSOG to the outer mitochondrial membrane, the production of ROS upon illumination results in cell degeneration and death (Qi et al. 2012). Since its development, mito-miniSOG was used in different ablation experiments in C. elegans, such as the study of a salt sensory circuit (Leinwand and Chalasani 2013) or identification of VAV-1 acting in signaling in the sensory and interneuron ALA (Fry et al. 2014). To cause cell ablation, mito-miniSOG requires 0.5–1.5 h blue light illumination, which can be limiting for its use in C. elegans as this organism can be killed by extended blue light exposure (Edwards et al. 2008). Additionally, if mito-miniSOG is highly overexpressed, it might have deleterious effects in absence of blue light, as ROS generation can be leaky under ambient light (Qi et al. 2012). Development of a (plasma-)membrane-targeted miniSOG allowed highly efficient ablation of multiple cell types, including neurons, muscles, and the epidermis (Xu and Chisholm 2016). Therefore, a mutant version of miniSOG (Q103L), showing enhanced ROS generation in vitro (Westberg et al. 2015) and enhanced cell killing efficiency in vivo, was targeted to cell membranes by using membrane targeting signals such as the pleckstrin homology (PH) domain from rat PLC-δ (Audhya et al. 2005) or the predicted myristoylation signal sequence from C. elegans NCS-2, myr (Xu and Chisholm 2016). miniSOG (Q103L), targeted to membranes, yielded a 10-fold increased efficiency compared to mito-miniSOG for cell ablation (Xu and Chisholm 2016). Besides identifying a role of the epidermis in locomotion (Xu and Chisholm 2016), this miniSOG variant was used to ablate AS motor neurons, thereby disrupting locomotion patterns (Tolstenkov et al. 2018).
Tools for silencing of excitable cell activity and synaptic transmission
Neurons and muscles are excitable cells and responsible for the action sequences that drive behavior or other organismic activities, like gut movements or pharyngeal pumping. Therefore, inhibition of such cells can be used to reduce or even abolish the behavior driven by the respective cell. Many tools have been developed over the past decades that enable excitable cell inhibition. This includes tools for constitutive inhibition which allows to silence cells on a permanent basis, as soon as they begin expressing the respective protein.
Constitutively open potassium channels
Since potassium ions have a steep downhill concentration gradient from the cytosol to the extracellular medium, expressing a constitutively open K+ channel will allow positive charge to constantly leak from the cell and thus hyperpolarize its membrane potential. In excitable cells, this typically causes inactivation. This class of protein tools comprises several potassium channels of the 2-pore domain potassium (TWK) channel family. The first mutations discovered that led to a constitutively open TWK channel were twk-18(gf), the constitutive allele e1913, encoding the mutation G165D, and the temperature-sensitive allele cn110 (M280I), which leads to complete paralysis above 25°C (Hosono et al. 1985; Reiner et al. 1995; Kunkel et al. 2000). The mutant protein was used as a selection marker for single-copy transgene insertions using MosSCI (Frokjaer-Jensen et al. 2008) but has also been used to study neuronal/circuit function in the locomotion nervous system in order to silence premotor interneurons (Kawano et al. 2011) and to study the sleep neuron RIS (Koutsoumparis et al. 2022). The latter work also used a second TWK channel g.o.f. mutation, i.e. egl-23(gf), and both proteins were further used in Busack and Bringmann (2023) for the same purpose. Recently, the TWK channel g.o.f. mutant, twk40(gf), was used to study the premotor interneuron AVA (Meng et al. 2024).
Tetanus toxin/botulinum neurotoxin
A conventional method for inducing long-term disruption of synaptic transmission involves genetic expression or direct application of Clostridium botulinum or tetanus neurotoxins (Brooks et al. 1957; Molgo et al. 1990; Sweeney et al. 1995). These potent inhibitors of synaptic transmission are metalloproteases that target SNAREs, i.e. proteins required for SV fusion. Tetanus toxins (TeTx), which are zinc-dependent endoproteases, and several clostridial toxins cleave synaptobrevin at unique sites, via their catalytic light chains (Schiavo et al. 1992; Blasi et al. 1993; Arata et al. 1997). While TeTx may lack the temporal precision of chemogenetic or optogenetic approaches, it can effectively silence neurons over extended periods, especially those projecting primarily to a single downstream target (with collateral effects on other neurons). However, these toxins also target other cellular counterparts of synaptobrevin, such as cellubrevin, which are ubiquitously expressed (McMahon et al. 1993). Notably, TeTx has been observed to impede cell membrane repair in fibroblasts and Xenopus oocytes, implying that molecules akin to synaptobrevin play roles in regulating additional fusion events (Steinhardt et al. 1994). Thus, the action of TeTx implicates that the protease cleavage of other targets contributes to the transmission abnormalities observed in toxin-treated cells.
In C. elegans, TeTx from the tdc-1 promoter was used to inhibit RIM and RIC neurons, resulting in defects similar to those observed in tdc-1 mutants (Sordillo and Bargmann 2021). In another study, TeTx was used to block DCV release in AVK neurons, partially mitigating increases in speed and body bending upon AVK photoactivation in flp-1 mutants (Aoki et al. 2023). Additionally, TeTx was used to analyze the synaptic output of RMG, by utilizing the Cre/Lox system to express the light chain (LC) of TeTx, which revealed distributed synaptic outputs for aggregation with contributions from both RMG and ASK/ASJ neurons (Macosko et al. 2009).
Botulinum neurotoxin (BoNT) is a lethal toxin responsible for causing botulism, a condition characterized by paralysis resulting from food-borne contamination (Davletov et al. 2005). BoNTs consist of a ∼50 kDa LC and a ∼100 kDa heavy chain (HC), linked by a disulfide bridge (Montecucco and Schiavo 1994). The LC serves as the catalytic domain, while the HC facilitates the delivery of BoNT into neurons. BoNTs are primarily known for their ability to block synaptic transmission at the NMJ (Montecucco and Schiavo 1994). Specifically, BoNT/A targets the C-terminal 9 amino acids of synaptosome-associated protein of 25 kDa (SNAP-25). The resulting truncated SNAP-25 may persist to interact with plasma membrane syntaxin, thereby disrupting the normal process of vesicle fusion and leading to potent toxicity.
While regulated promoters or recombinase systems enable some level of temporal control over toxin expression (Yamamoto et al. 2003; Nakashiba et al. 2008; Airan et al. 2009), achieving rapid and localized control remains challenging at present. However, a photoactivated version of BoNT (PA-BoNT) has been generated and used to silence motor neurons (see PA-BoNT).
InSynC/chromophore-assisted light inactivation
The ability to generate ROS via miniSOG has further been taken advantage of for developing an optogenetic tool for neuronal silencing. The chromophore-assisted light inactivation (CALI) of synaptic proteins was developed by using miniSOG and targeting it to the presynaptic terminal using proteins such as synaptobrevin (VAMP2) or synaptophysin (SYP1). This allows inactivating the SNARE complex following blue light illumination (Ache and Young 2005). This approach was also used in C. elegans to target neurons via synaptobrevin or synaptotagmin (Lin et al. 2013b; Hermann et al. 2015). Using CALI as a neuronal silencer, however, comes with the same disadvantages as miniSOG used for cell ablation: The generation of damaging radicals may have off-target effects, and it is not known whether CALI has long-lasting effects on synaptic strength. As SNARE proteins are damaged by ROS generation, recovery from the inhibiting effect requires de novo synthesis of targeted proteins.
Photosensitive degron
To overcome the disadvantages of miniSOG-enabled synaptic silencing, a photosensitive degron (psd) was developed (Hermann et al. 2015). The psd comprises 2 domains: a photo-switchable LOV2 domain, which can undergo light-dependent conformational changes (Kennis et al. 2004), and a C-terminal degradation sequence derived from mouse ornithine decarboxylase (cODC). This sequence contains a Cys-Ala motif which is recognized by the proteasome (Takeuchi et al. 2008). Fused to a protein of interest (POI), such as synaptotagmin (SNT-1) for neuronal targeting, allows the degradation of the POI upon blue light illumination and thereby inhibition of synaptic transmission. The resulting silencing effect of psd is comparable to inactivation via miniSOG but enables more accurate targeting without off-target effects. However, expression of the degron-tagged POI in the presence of the endogenous protein is not sufficient to eliminate POI function, which is why one needs to work with a rescuing transgene in a mutant background lacking the endogenous protein. Alternatively, the psd must be introduced into the endogenous locus (Hermann et al. 2015; Jánosi et al. 2024).
PA-BoNT
To achieve the rapid and localized control of C. botulinum neurotoxin (BoNT), a photoactivatable form of BoNT serotype B (BoNT/B) LC protease was engineered (Liu et al. 2019). To this end, the Avena sativa AsLOV2-derived improved light-inducible dimerization system iLID (Guntas et al. 2015) was used for generation of a light-dependent reconstitution of BoNT/B, which was split into N- and C-terminal fragments. This resulting PA-BoNT could cleave significant fractions of VAMP2, yet some remaining uncleaved VAMP2 still enabled fusion of SVs. Thus, a variant of PA-BoNT was engineered that was directly targeted to SVs, vPA-BoNT. This was achieved by fusion of the C-terminal BoNT fragment to the C-terminus of synaptogyrin (SNG-1) and coexpressing it with the N-terminal BoNT fragment fused to iLID (Liu et al. 2019). Photoactivation of vPA-BoNT resulted in specific cleavage of VAMP2, at a spatial and temporal resolution comparable to the psd. Although PA-BoNT does not require constant illumination for long-term silencing, its recovery requires the de novo synthesis of SNAREs.
OptoSynC
To supplement the optogenetic toolbox with a light-activated neuronal silencing tool that allows for temporally and spatially precise silencing with a fast recovery time, optoSynC (optogenetic synaptic vesicle clustering) was developed. OptoSynC comprises 2 primary constituents: the C. elegans SV protein synaptogyrin SNG-1 and the Arabidopsis thaliana cryptochrome 2 (CRY2). While CRY2 serves as the photoactivatable element that enhances its capacity to form homo-oligomers after photoactivation, SNG-1 directs the tool to the SV membrane. Due to the abundant presence of SNG-1 in the SV membrane, fusing CRY2 to it enhances the likelihood of efficient SV clustering, due to increased avidity. Moreover, SNG-1 is dispensable for neuronal activity or synaptogenesis (Abraham et al. 2006). Since SNG-1 is not involved in the fusion process, optoSynC operates mechanistically differently from other optogenetic silencers, i.e. by achieving clustering of SVs in the reserve and recycling pools. Light-induced silencing of synaptic transmission using optoSynC was shown in different neuron classes and down to single neurons (Vettkotter et al. 2022). Formation of CRY2 homo-oligomers was facilitated by using CRY2olig(535) (Taslimi et al. 2016), a truncated variant with reduced dark activity [this term refers to background activity of some optogenetic tools that can occur already without illumination, e.g. for light-activated enzymes like bPAC; see Indirectly gating potassium channels with light (2-component optogenetics)]. Additionally, the E490G mutation enhances oligomerization (Taslimi et al. 2014). OptoSynC is highly light sensitive, has fast onset (only few seconds), and recovers within minutes.
Indirectly gating potassium channels with light (2-component optogenetics)
The use of constitutively active K+ channels for silencing prompted the idea that this approach could also be used to generate regulatable tools, i.e. if K+ channels could be acutely gated by the experimenter. One approach that was explored is the use of cyclic nucleotide–gated K+ channels, e.g. the cAMP-gated K+ channel SthK from Spirochaeta thermophila, or the cGMP-gated K+ channel BeCNG1 from Blastocladiella emersonii. These channels could be combined with light-activated adenylyl and guanylyl cyclases, respectively. Such proteins, like Euglena or Beggiatoa photoactivated adenylyl cyclase (EuPAC or bPAC, respectively) or the engineered IlaC, as well as Blastocladiella or Catenaria nucleotidyl cyclase opsins (CyclOps) have been well established in C. elegans (Weissenberger et al. 2011; Ryu et al. 2014; Gao et al. 2015; Steuer Costa et al. 2017; Henss et al. 2022). The combination with SthK or BeCNG1 demonstrated light-activated K+-mediated inhibition; however, these tools so far were either not very powerful: BeCNG1, its activation by BeCyclOp, both expressed in body wall muscle (BWM), caused a moderate body length increase of ca. 2% due to reduction of the muscle tone (note, the strongest effects evoked by Cl− or K+ conducting (channel)rhodopsins are ca. 8% body length increase; Zhang et al. 2007; Bergs et al. 2018). Or, the effects were too strong, since SthK, expressed in cholinergic motor neurons or in muscles, already responded to endogenous levels of cAMP, or background cAMP generated by bPAC in the dark; nonetheless, photoactivation of bPAC could evoke additional effects (Henss et al. 2022).
Histamine-gated chloride channel
Histamine is used as a neurotransmitter in many animal species; however, it is not found in C. elegans (Chase and Koelle 2007; Hobert 2013). Histamine receptors in insects, such as the Drosophila histamine-gated chloride channel HisCl1/2, function as ligand-gated chloride channels (Gisselmann et al. 2002; Liu and Wilson 2013). Because histamine evokes no endogenous effects in C. elegans, these characteristics render HisCl1/2 an exemplary tool for orthogonal neural inhibition, allowing for the selective silencing of neurons by hyperpolarization, without disrupting overall neural function. Pokala et al. (2014) demonstrated that these channels could be expressed in C. elegans neurons and that administration of exogenous histamine effectively inhibited activity of neurons expressing HisCl1/2. Various applications of the histamine-HisCl1 system facilitated studies on synaptic transmission through silencing neurons (Nelson et al. 2014; Hoerndli et al. 2015; Ghosh et al. 2016; Michelassi et al. 2017; Lopez-Cruz et al. 2019; Choi et al. 2021; Setty et al. 2022; Huang et al. 2023) or muscle cells (Ravi et al. 2018; Molina-García et al. 2020; Zhan et al. 2023). Compared to optogenetic silencers such as NpHR (Zhang et al. 2007; Bergs et al. 2018), this system provides significant convenience for prolonged neural inhibition, because it requires the presence of a chemical, not light. Yet, compared to optogenetic approaches, the HisCl1 system has limitations in assays requiring precise temporal control of neural (in)activity. Recently, HisCl1 has also been applied as a negative selection marker to facilitate generation of single-copy transgene insertions (Abiusi et al. 2017; El Mouridi et al. 2021; Mueller et al. 2023).
Targeted protein degradation via the auxin-inducible degron
Genetically encoded tools for targeted protein degradation (TPD) exploit endogenous protein degradation machineries, such as the ubiquitin–proteasome system, or the lysosome pathway, allowing researchers to exogenously induce protein degradation in a spatiotemporal manner. If this is applied to proteins of the synaptic transmission machinery, or ion channels required for neuronal depolarization, it may allow to silence neuronal function. This has been shown in some cases, targeting the UNC-31 protein, required for dense core vesicle fusion, or the UNC-7 gap junction subunit (Liu et al. 2017; Cornell et al. 2022). In this section, we discuss the development of auxin-inducible degron (AID), and its applications in exploring the function of synaptic proteins in C. elegans. For more comprehensive discussions on other TPD tools, readers are referred to previous reviews (Nance and Frøkjær-Jensen 2019; Zhang et al. 2022).
Auxins, a class of plant growth factors, are used in plants to regulate gene expression posttranslationally, by inducing the interaction of a degradation substrate, exposing the “auxin inducible degron” sequence, and the F-Box protein TIR1, eventually leading to protein degradation (Teale et al. 2006). In 2 pioneer studies (Nishimura et al. 2009; Kanke et al. 2011), the AID system was successfully transplanted from plants into cultured vertebrate cell lines and yeast. To this end, TIR1 needs to be expressed in the system of choice, and the AID target sequence needs to be introduced into the target as well. Zhang et al. (2015) employed the AID system in C. elegans, demonstrating rapid and reversible depletion of AID-targeted proteins. Limitations of the AID system, sometimes observed across various organisms, are leaky degradation, independent of the presence of auxin, and inefficient depletion (Natsume et al. 2016; Kerk et al. 2017; Daniel et al. 2018; Schiksnis et al. 2020). Additionally, auxins have been reported to induce undesired effects in worms, such as enhanced resistance to endoplasmic reticulum (ER) stress and increased lifespan (Bhoi et al. 2021; Loose and Ghazi 2021). Some approaches for refinement have been reported in mammalian cells, aiming to reduce basal degradation and to accelerate depletion (Li et al. 2019; Sathyan et al. 2019). Further improvements in C. elegans, as in the AID2 system, employed AtTIR1(F79G), the mini IAA7 degron, and 5-phenyl-indole-3-acetic acid (5-Ph-IAA) to reduce auxin-independent degradation and to enhance the loss-of-function phenotype of AID-targeted proteins (Uchida et al. 2018; Yesbolatova et al. 2020; Hills-Muckey et al. 2021; Negishi et al. 2022; Sepers et al. 2022). Xiao et al. (2023) established an expandable FLP-ON::TIR1 system, addressing the challenge that cell type–specific expression of TIR1 often results in less efficient protein degradation. The AID system was used to investigate loss-of-function phenotypes of various C. elegans proteins, including neuronal ones (Kerk et al. 2017; Liu et al. 2017; Yu et al. 2017; Shen et al. 2018; Zhang et al. 2018; Cornell et al. 2022; Stojanovski et al. 2023; Turner et al. 2023; Stefanakis et al. 2024).
Rhodopsin-based, light-controlled silencing
Microbial opsins are integral membrane proteins with 7 transmembrane helices found in a subset of archaea, bacteria, and even lower eukaryotes like algae. Acting as light-driven ion channels and pumps, these proteins either provide perception of electromagnetic radiation, which allows the organisms to adapt to their environment through phototaxis, or build up ion gradients to convert light energy into a chemical equivalent (Oesterhelt and Tittor 1989; Béjà et al. 2000). Absorption of photons triggers isomerization of the cofactor retinal and a conformational change of the protein, leading to either channel opening and passive ion transport or induction of pumping activity—a mechanism common to all microbial rhodopsins (Nagel et al. 2002).
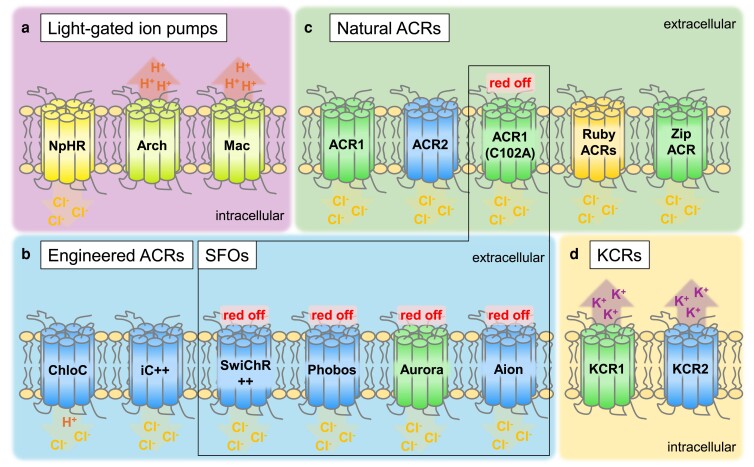
Halorhodopsin
In optogenetics, the most used rhodopsin-based tool, channelrhodopsin-2 (ChR2), a depolarizing nonselective cation channel, is complemented by a growing repertoire of optogenetic silencers. Here, halorhodopsin (NpHR) from the archaeon Natronomonas pharaonis is one of the most frequently used light-driven ion pumps for hyperpolarization, which—when stimulated by yellow light—pumps chloride ions into the cell cytoplasm (Fig. 2a) (Han and Boyden 2007; Zhang et al. 2007). Just as ChR2 enables activation, halorhodopsins provide silencing of excitable cells, like neurons, following brief light stimuli with high spatiotemporal precision. Being spectrally distinct to ChR2 (absorption maximum at 470 nm as opposed to 590 nm for NpHR), multiplexing with NpHR is possible, providing independent access to both de- and hyperpolarization in the same cell. In contrast to channelrhodopsins, where absorption of 1 single photon allows the passage of many ions, for NpHR, only 1 ion is moved per photon (and photocycle), as is generally the case for light-driven ion pumps. Hence, plasma membrane localization is crucial to achieve robust light-induced effects. The initial accumulation tendency of NpHR in the ER was overcome for mammalian cells by adding ER export motifs resulting in the improved version eNpHR2.0, while limitations in membrane localization were countered by addition of Golgi export and membrane trafficking signals, then termed eNpHR3.0 (Gradinaru et al. 2008, 2010). C. elegans was the first model organism in which NpHR was established in vivo and in which triggered behavioral outcomes were characterized (Zhang et al. 2007). Here, muscle activity was probed in swimming and body length analysis assays, when NpHR was expressed from the myo-3 promoter (BWMs), or indirectly, when expressed in cholinergic motor neurons, from the unc-17 promoter. When stimulated by light, activation of NpHR immediately arrested swimming behavior and resulted in relaxation of muscles leading to increased body length of the animals by up to 7.5%. Patch-clamp recordings in BWMs of dissected animals revealed light-evoked outward currents of 265 pA. As mentioned above, combined expression of NpHR and ChR2 enabled to counteract ChR2-driven body contraction by activation of NpHR with yellow light. Further studies using NpHR in C. elegans demonstrated, among other effects, a significant decrease in the release of SVs upon light-induced hyperpolarization of cholinergic motor neurons (Liu et al. 2009). Furthermore, using NpHR allowed to remote-control temperature-seeking behavior in a bidirectional manner together with ChR2 (Kuhara et al. 2011) and helped to investigate the gentle touch circuit (Husson et al. 2012).
Fig. 2.
Classes of rhodopsin-based optogenetic silencers. a) Light-driven ion pumps. b) eACRs and step function opsins for long-lasting photoinhibition. c) Natural anion channelrhodopsins. d) KCRs. Tools are color-coded according to their absorption maximum. Ions conducted or pumped, and direction based on electrochemical gradient, or pump vectoriality, are indicated.
However, as the trafficking motifs mentioned above are not conserved, usage of improved NpHR versions did not overcome aggregation issues in the worm (Husson et al. 2012), which narrows the applicability of NpHR to cells with strong promoters. Thus, subsequent C. elegans studies established the light-activatable proton pumps Mac from Leptosphaeria maculans and archaerhodopsin (Arch) from Halorubrum sodomense as alternatives for optical silencing, where hyperpolarization is achieved via an extrusion of protons (Chow et al. 2010). The 2 inhibitory tools, maximally absorbing at 550 nm (Mac) and 566 nm (Arch), respectively, show improved plasma membrane expression and slightly higher photocurrents compared to NpHR (Husson et al. 2012). This way, it was possible to investigate the nociceptive ASH circuit, where Mac and Arch were used to interfere with downstream signaling while upstream neurons were stimulated by ChR2. An improved variant of Arch, ArchT, was also implemented in C. elegans (Okazaki and Takagi 2013; Busack et al. 2020; Maluck et al. 2020).
Engineered anion channelrhodopsins
Since active transport restricts the utility of rhodopsin-based hyperpolarizing ion pumps, for which continuous and intense illumination is required, a lot of effort was devoted to invert the ion selectivity of ChR2 and turn it into a hyperpolarizing channel (engineered anion channelrhodopsins, eACRs; Fig. 2b). Substitution of Glu-90 in the central selectivity filter and near the retinal Schiff base led to a hyperpolarizing Cl−-conducting variant (ChloC) (Wietek et al. 2014). Additional point mutations within the proton pathway resulted in the variants iChloC (improved ChloC) and iC++ and eliminated the residual proton conductance of ChloC (Wietek et al. 2015; Berndt et al. 2016). Further introduction of point mutations within the so-called DC-gate improved conductivity and operational light sensitivity, as the closing kinetics of the channel were significantly decelerated (step function opsins). These attempts resulted in the slow-cycling variants SwiChR++ (Berndt et al. 2016), the color-tuned eACRs Aurora and Phobos based on red light–activatable ReaChR (Lin et al. 2013a) and the aforementioned iC++, respectively (Wietek et al. 2017). Most recently Aion with a closing time constant of around 15 min was engineered, based on Phobos (Rodriguez-Rozada et al. 2022). All these tools enable silencing without the need for continuous illumination and, moreover, allow to trigger channel closure by way of a second illumination pulse of longer wavelength.
Natural anion channelrhodopsins
Inspired by the determinants of Cl− conductance as found during molecular engineering of existing ChRs, researchers performed BLAST searches of known genomes. This revealed naturally occurring light-activatable Cl−-conducting ChRs in the cryptophyte Guillardia theta that were then termed anion channelrhodopsins (ACRs, Fig. 2c). Optimized by evolution, the first 2 described tools—GtACR1 and GtACR2—proved to be superior to both eACRs and depolarizing ChR2 in terms of steady state current amplitudes (Mahn et al. 2018). While ACR1 exhibits larger plateau currents at an absorption maximum of 515 nm, ACR2 demonstrated faster kinetics, maximally absorbing at 470 nm. Due to their exceptional high light sensitivity and superior conductance, ACRs finally constitute equal rank to the most powerful depolarizing tools in the inhibitory range for optogenetic applications. Further screening studies identified 2 additional ACRs with fast kinetics, namely, ZipACR (from Proteomonas sulcata) and RapACR (from Rhodomonas salina), that showed even higher photocurrents, enabling up to 100 Hz spike suppression (Govorunova et al. 2017, 2018). Furthermore, RubyACRs (from Labyrinthulea) were identified: These are spectrally red-shifted with absorption maxima at 590–610 nm (Govorunova et al. 2020). ACRs have been proven to function as potent neural inhibitors in various model animals (Mauss et al. 2017; Mohamed et al. 2017; Mohammad et al. 2017; Mahn et al. 2018).
In C. elegans, ACR1, ACR2, and ZipACR, as well as the slow-closing (step function) variant ACR1(C102A), were first introduced into BWMs and characterized in a comparative study using behavioral readout (Bergs et al. 2018). In body length analyses, upon light stimulation, ACR-expressing animals demonstrated exceptional effects at low light intensities that could be repetitively induced with high stability. Patch-clamp recordings revealed large peak photocurrents of up to 1530 pA (ACR2)—5–10 times larger compared to NpHR. Further C. elegans studies applied ACRs to neurons of locomotory and behavioral regulatory circuits (Tolstenkov et al. 2018; Xu et al. 2018; Aoki et al. 2023) or tested their applicability for long-term inhibition (Yamanashi et al. 2019). In addition to that, ACR2 was used in a tandem configuration together with the red light–activatable depolarizer Chrimson (termed BiPOLES) and combined with the genetically encoded voltage indicator QuasAr2 to generate an optogenetic voltage clamp in live worms (Bergs et al. 2023).
Potassium channelrhodopsins
Although ACRs are highly effective and reversible silencers of excitable cells (Govorunova et al. 2015; Mohammad et al. 2017), they have certain limitations, especially in cells (e.g. cardiomyocytes) or subcellular compartments (e.g. axons) with high intracellular chloride concentrations, where they may cause depolarization (Mahn et al. 2016; Malyshev et al. 2017; Ott et al. 2024). Moreover, upon prolonged gating of their artificial chloride conductance, they trigger unwanted and long-lasting downstream effects, putatively due to the exhaustion of the endogenous Cl− gradients and the time it takes the cell to recover them (Bergs et al. 2018).
Since repolarization of membrane potentials generally happens through efflux of K+ via voltage-gated potassium channels (Hodgkin and Huxley 1952; Johnstone et al. 1997; Jan and Jan 2012), inhibition of depolarization may occur by artificial manipulation of K+ currents. To achieve this, major efforts have attempted to generate K+-selective light-gated channels for example by fusion of light-reactive elements such as the LOV2 domain or coexpression of K+ channels with light-absorbing activators (Alberio et al. 2018; Bernal Sierra et al. 2018) [see Indirectly gating potassium channels with light (2-component optogenetics) for a discussion of the use of the SthK channel in C. elegans]. Engineered K+-selective light-gated channels have major drawbacks, namely, poor surface expression (Cosentino et al. 2015), slow kinetics, or unwanted activation of cellular signaling pathways (Beck et al. 2018; Henss et al. 2022). Therefore, the recent discovery of natural light-gated channels with a high selectivity of K+ over Na+ proved a significant improvement for precise inhibition of excitable cells (Govorunova et al. 2022) (Fig. 2d). These K+-selective channelrhodopsins [potassium channelrhodopsins (KCRs)] have been shown to efficiently inhibit action potentials upon illumination in cultured mammalian neurons and cardiomyocytes.
Structural analysis of the novel K+ channel signature motif in pore-lining amino acid residues of the Hyphochytrium catenoides KCRs (HcKCR1/2) led to the discovery of improved variants with even higher K+-selectivity by either phylogenetic analysis (WiChR; Vierock et al. 2022) or point mutation (Tajima et al. 2023). A recent study has also shown the capabilities of KCRs in small model organisms in which they enabled silencing of cells with high intracellular chloride in which ACRs were ineffective (Ott et al. 2024). Yet the study only briefly assessed their use in C. elegans. Here, KCRs were expressed pan-neuronally and not selectively in stimulatory cholinergic or inhibitory GABAergic neurons in which measurement of body length could indicate de- or hyperpolarization (Liewald et al. 2008). The observed impact on crawling speed may also arise from depolarization and thus hypercontraction of muscle cells (Tolstenkov et al. 2018). Nevertheless, the results obtained from Drosophila melanogaster promise rapid and reversible inhibition of excitable cells in invertebrates (Ott et al. 2024). While KCRs seem to be auspicious tools for many applications, the residual Na+ conductivity is substantial. The KCR with the currently highest relative permeability ratio of K+ over Na+ of 80 (WiChR; for stationary photocurrents) still conducts significantly more Na+ than mammalian voltage–gated potassium channels (PK/PNa ∼100–1000; LeMasurier et al. 2001; Mironenko et al. 2021). This may pose a problem in some cases and must be remembered when using these tools, especially when applying continuous stimulation, working with a highly negative membrane potential or in conditions with high external Na+ (Govorunova et al. 2022; Vierock et al. 2022).
Inhibition via light-activated G protein-coupled receptors (animal rhodopsins) with Gi/o coupling specificity
Reversible regulation of cellular activity can also be achieved through G protein–coupled receptors (GPCRs). Different light-reactive, inhibitory GPCRs have been previously expressed and characterized in neurons of C. elegans. One of the most commonly used inhibitory GPCRs is bovine rhodopsin (bRho), which endogenously couples to transducin, a Gαi/o-type protein, although previous studies have shown that bRho is also able to activate other G proteins of the same family (Kanaho et al. 1984). Cao et al. (2012) demonstrated that pan-neuronal expression and illumination of bRho in C. elegans efficiently inhibits locomotion, the effect of which is relatively long-lasting compared to the duration of the stimulus, as 1 s high-intensity light stimulation was sufficient to fully inhibit movement of the animals for ca. 20 min. Normal crawling behavior resumed after ca. 80 min. Analysis of various mutant strains provided evidence that the signaling occurs selectively through GOA-1, the C. elegans Gαi/o ortholog, and is dependent on cAMP-specific phosphodiesterases (Cao et al. 2012).
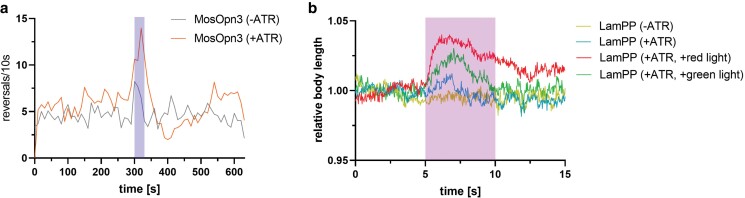
Expression and activation of a different GPCR, mosquito Opn3 (MosOpn3), in ASH neurons elicited light-dependent avoidance behavior (see Fig. 3a for quantification of light-evoked reversals of animals expressing MosOpn3 in ASH), which is in agreement with activation of ODR-3-dependent signaling, a C. elegans G protein which shows similarity to members of the Gi/o family. MosOpn3 is bleach-resistant, which could enable repeated stimulation with less reduction in the intensity of the response (Koyanagi et al. 2022). Similar effects were shown by these authors when bRho was expressed in ASH, arguing that it is a Gi/o-coupled GPCR. ASH neurons can also be photoactivated by channelrhodopsin (Schmitt et al. 2012), a depolarizing light-gated channel, indicating that there must be a “sign inversion” of GPCR-induced signals downstream of ODR-3, leading to depolarization of the neuron. Hilliard et al. (2005) also showed that repellent-induced Ca2+ signals in ASH were diminished in odr-3(n2150) mutants. Earlier work has shown that the TRP channels OSM-9 and OCR-2 are required for Ca2+ transients and depolarization of ASH (Colbert et al. 1997; Tobin et al. 2002). How exactly ODR-3 signaling, supposedly via lipid mobilization of polyunsaturated fatty acids (PUFAs), activates OSM-9/OCR-2 (Kahn-Kirby et al. 2004) is not well understood, and whether ODR-3 is a genuine Gi/o protein, i.e. inactivating adenylate cyclase, has not been shown in vitro. bRho requires supplementation of 11-cis-retinal, or as a substitute, 9-cis-retinal, for its activity in C. elegans neurons. In contrast, MosOpn3 is functional both in its 11-cis-retinal- and its 13-cis-retinal-bound form. Thus, it is also active when all-trans-retinal (ATR) is fed to the worms as it is in equilibrium with the 13-cis isomer (Cao et al. 2012; Koyanagi et al. 2022).
Fig. 3.
Examples of light-sensitive GPCRs (rhodopsins), used for inhibition: a) light-evoked reversals of animals expressing MosOpn3 in ASH neurons. The multiworm tracker (Swierczek et al. 2011) was used to observe the crawling behavior of worms expressing MosOpn3 in ASH neurons. Upon blue light illumination (30 s, 100 µW/mm2, 470 nm, shaded area, 300–330 s), worms treated with ATR (100 µM) showed an increase in reversals, indicating an avoidance response upon MosOpn3 activation. b) LamPP activation in cholinergic neurons induces a wavelength-dependent increase of body length, due to muscle relaxation. Body length of animals either without ATR (−), or treated with ATR (+), were compared. Additionally, animals treated with ATR were either kept in the dark until the measurement, or preilluminated with red light (620–660 nm; overnight), or illuminated with green light (520–535 nm) for 30 s prior to the start of the experiment. Violet light illumination (5 s, 100 µW/mm2, 373–387 nm, shaded area, 5–10 s) of animals without ATR evoked no change in body length, while ATR-treated worms exhibited a slight increase. To convert the active ATR-bound state back to its inactive state, worms were illuminated with green light before the start of the measurements, resulting in a body length increase upon subsequent violet light illumination. To test whether red light illumination of the LamPP active state causes photoregeneration, worms were fed ATR under red light illumination overnight. These animals showed an increase in body length upon violet light illumination.
The same study also investigated the UV-sensitive bistable lamprey parapinopsin (LamPP) in C. elegans. The peculiarity of LamPP is that it exhibits large spectral differences between the inactive and active state, allowing sustained Gi/o-pathway activation and controlled deactivation (Koyanagi et al. 2004; Eickelbeck et al. 2020). Thus, expression of LamPP in C. elegans cholinergic motor neurons allowed color-dependent control of behavior (Koyanagi et al. 2022). We could show that activation of LamPP also induces color-dependent changes of body length (Fig. 3b). Red light illumination of the LamPP active state led to photoregeneration and a state with an absorption maximum almost identical to the dark state (Koyanagi et al. 2004). Hence, worms were fed under red light, which resulted in relaxation, and body length increase upon subsequent violet light exposure (Fig. 3b). Green light, right before the measurement, which should convert LamPP back to its active state, resulted in reduction of crawling upon illumination with violet light and a significant increase in body length, which returned to baseline levels. However, most animals showed reduced locomotion during the entire duration of observation, indicating continued activity and Gi/o signaling in cholinergic motor neurons.
Another rhodopsin mediating inhibition in C. elegans was recently described, the Platynereis dumerilii opsin (PdCO), which is a bistable opsin that can be turned on and off with 405 and 525 nm light, respectively (Wietek et al. 2024). PdCO was expressed pan-neuronally, and as it couples to Gαo, and through Gβ/γ may activate GIRK (G-protein activated, inward rectifier potassium) channels, caused rapid and reversible reduction of locomotion speed by ca. 75%, in an ATR-dependent manner.
An approach related to activation of inhibitory GPCRs has been recently introduced by Lockyer et al. (2023). The authors could inhibit Gαq signaling in C. elegans neurons as well as in other organisms by using an engineered version of a mammalian Gαq-specific regulator of G protein signaling (RGS2), modified with a cryptochrome CRY2 domain, that could be recruited to plasma membrane-bound CIBN protein in a light-induced manner. Using this so-called CRY2-CIB1 system (Kennedy et al. 2010), the authors were able to reduce motility of the worms in a light-dependent way (Lockyer et al. 2023).
Activation of Gi/o protein signaling for cellular inhibition is useful especially when intending to target specific intracellular pathways, inhibiting activity without directly affecting membrane potential, or to mimic endogenous GPCR-induced inhibitory processes. GOA-1 was found to be expressed in all or nearly all neurons in C. elegans (Mendel et al. 1995); thus, the potential application of GOA-1 addressing tools is not limited to only some cells. However, due to the potentially different intracellular pathways in different cell types, being mediated and amplified by various intracellular effectors, the effects as well as the mechanism of inhibition might differ slightly when a light-reactive GPCR is utilized in different cells. Hence, results obtained with such tools, as well as their G protein coupling preference, need to be evaluated carefully for the cell type under study.
Silencing by optogenetic activation of inhibitory neurons
Another intriguing possibility to inhibit neuronal or muscle function is through activation of upstream inhibitory neurons, for example, by expression of cation ChRs (CCRs) in GABAergic motor neurons (Liewald et al. 2008; Bergs et al. 2018). GABA release upon CCR gating will lead to an inhibition of BWMs and thus relaxation in animals treated with ATR (Schultheis et al. 2011; Lu et al. 2022). Next to directly targeting synaptic transmission by optogenetics, release of inhibitory neuromodulators from interneurons may be triggered to achieve a widespread and long-term inhibition of excitable cells. Examples include the photo-evoked release of FLP-11 neuropeptides from the RIS neuron to inhibit motor neurons or the release of serotonin from sensory neurons to inhibit interneurons (Flavell et al. 2013; Steuer Costa et al. 2019). The major advantage of this approach is the use of endogenous transmission to study the effect of inhibition in a more physiological paradigm. However, in order to draw causal conclusions, one must keep in mind the complexity, interconnectivity, and feedback mechanisms of most neuronal signaling pathways, which requires combining optogenetic experiments with a range of other assays and (genetic) manipulations, as exemplified in this work by Sordillo and Bargmann (2021) and further summarized in a review by Fang-Yen et al. (2015).
Conclusions
The range of methods and approaches to inhibit or eliminate (excitable) cells in C. elegans, or individual proteins within these cells, is very broad, and it may thus be challenging to choose the right method. However, this should be decided based upon the respective process or cell type that is targeted and whether the manipulation needs to be fast, i.e. to observe acute effects, or to avoid compensatory changes to occur. Also, it may be important to consider whether the manipulation needs to take place during or following the end of development, e.g. depending on whether the cell or protein targeted is essential for the same. While some of these methods can be applied to single or few cells at a time, other methods, which include a genetically encoded tool, allow to eliminate entire cell types and can be applied in populations of animals. For some methods, temporal control of the moment of inhibition or ablation is poor, i.e. defined by the time of expression, while others can be precisely timed by administration of a chemical compound, or with light. Some methods are permanent, others are immediately reversible, and even others require long recovery times, e.g. when de novo protein synthesis is needed. It may be advisable to test more than one approach to achieve the desired outcome. We hope this review provides readers with a good basis for their decision.
Supplementary Material
Acknowledgments
This work summarizes a community wide effort and would not have been possible without this community and their willingness to share information. Also, this effort is tremendously supported by community resources like WormBase (Sternberg et al. 2024), the Alliance of Genome Resources (Consortium 2024), or the Caenorhabditis Genetics Center (CGC), which is funded by the NIH Office of Research Infrastructure Programs (P40 OD010440).
Contributor Information
Dennis Rentsch, Buchmann Institute for Molecular Life Sciences, Goethe University, Max-von-Laue Strasse 15, D-60438 Frankfurt, Germany; Institute for Biophysical Chemistry, Goethe University, Max-von-Laue Strasse 9, D-60438 Frankfurt, Germany.
Amelie Bergs, Buchmann Institute for Molecular Life Sciences, Goethe University, Max-von-Laue Strasse 15, D-60438 Frankfurt, Germany; Institute for Biophysical Chemistry, Goethe University, Max-von-Laue Strasse 9, D-60438 Frankfurt, Germany.
Jiajie Shao, Buchmann Institute for Molecular Life Sciences, Goethe University, Max-von-Laue Strasse 15, D-60438 Frankfurt, Germany; Institute for Biophysical Chemistry, Goethe University, Max-von-Laue Strasse 9, D-60438 Frankfurt, Germany.
Nora Elvers, Buchmann Institute for Molecular Life Sciences, Goethe University, Max-von-Laue Strasse 15, D-60438 Frankfurt, Germany; Institute for Biophysical Chemistry, Goethe University, Max-von-Laue Strasse 9, D-60438 Frankfurt, Germany.
Christiane Ruse, Buchmann Institute for Molecular Life Sciences, Goethe University, Max-von-Laue Strasse 15, D-60438 Frankfurt, Germany; Institute for Biophysical Chemistry, Goethe University, Max-von-Laue Strasse 9, D-60438 Frankfurt, Germany.
Marius Seidenthal, Buchmann Institute for Molecular Life Sciences, Goethe University, Max-von-Laue Strasse 15, D-60438 Frankfurt, Germany; Institute for Biophysical Chemistry, Goethe University, Max-von-Laue Strasse 9, D-60438 Frankfurt, Germany.
Ichiro Aoki, Buchmann Institute for Molecular Life Sciences, Goethe University, Max-von-Laue Strasse 15, D-60438 Frankfurt, Germany; Institute for Biophysical Chemistry, Goethe University, Max-von-Laue Strasse 9, D-60438 Frankfurt, Germany.
Alexander Gottschalk, Buchmann Institute for Molecular Life Sciences, Goethe University, Max-von-Laue Strasse 15, D-60438 Frankfurt, Germany; Institute for Biophysical Chemistry, Goethe University, Max-von-Laue Strasse 9, D-60438 Frankfurt, Germany.
Data availability
Strains and plasmids are available upon request, as far as they have been generated by the authors or former Gottschalk lab members. The authors affirm that all data necessary for confirming the conclusions of the article are present within the article, figures, and tables.
Funding
This work was funded by the Deutsche Forschungsgemeinschaft (grants GO1011/12-2, GO1011/13-2, CRC1080-B02, and CRC1507-P06) and by funds from Goethe University to A.G.
Literature cited
- Abiusi E, D’Alessandro M, Dieterich K, Quevarec L, Turczynski S, Valfort A-C, Mezin P, Jouk PS, Gut M, Gut I, et al. 2017. Biallelic mutation of UNC50, encoding a protein involved in AChR trafficking, is responsible for arthrogryposis. Hum Mol Genet. 26(20):3989–3994. doi: 10.1093/hmg/ddx288. [DOI] [PubMed] [Google Scholar]
- Abraham C, Bai L, Leube RE. 2011. Synaptogyrin-dependent modulation of synaptic neurotransmission in Caenorhabditis elegans. Neuroscience. 190:75–88. doi: 10.1016/j.neuroscience.2011.05.069. [DOI] [PubMed] [Google Scholar]
- Abraham C, Hutter H, Palfreyman MT, Spatkowski G, Weimer RM, Windoffer R, Jorgensen EM, Leube RE. 2006. Synaptic tetraspan vesicle membrane proteins are conserved but not needed for synaptogenesis and neuronal function in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 103(21):8227–8232. doi: 10.1073/pnas.0509400103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ache BW, Young JM. 2005. Olfaction: diverse species, conserved principles. Neuron. 48(3):417–430. doi: 10.1016/j.neuron.2005.10.022. [DOI] [PubMed] [Google Scholar]
- Airan RD, Thompson KR, Fenno LE, Bernstein H, Deisseroth K. 2009. Temporally precise in vivo control of intracellular signalling. Nature. 458(7241):1025–1029. doi: 10.1038/nature07926. [DOI] [PubMed] [Google Scholar]
- Alberio L, Locarno A, Saponaro A, Romano E, Bercier V, Albadri S, Simeoni F, Moleri S, Pelucchi S, Porro A, et al. 2018. A light-gated potassium channel for sustained neuronal inhibition. Nat Methods. 15(11):969–976. doi: 10.1038/s41592-018-0186-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki I, Golinelli L, Dunkel E, Bhat S, Bassam E, Beets I, Gottschalk A. 2023. Hierarchical regulation of functionally antagonistic neuropeptides expressed in a single neuron pair. bioRxiv 568473. 10.1101/2023.11.23.568473, preprint: not peer reviewed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arata Y, Hirabayashi J, Kasai K. 1997. The two lectin domains of the tandem-repeat 32-kDa galectin of the nematode Caenorhabditis elegans have different binding properties. Studies with recombinant protein. J Biochem (Tokyo). 121(6):1002–1009. doi: 10.1093/oxfordjournals.jbchem.a021686. [DOI] [PubMed] [Google Scholar]
- Arena JP, Liu KK, Paress PS, Frazier EG, Cully DF, Mrozik H, Schaeffer JM, 1995. The mechanism of action of avermectins in Caenorhabditis elegans: correlation between activation of glutamate-sensitive chloride current, membrane binding, and biological activity. J Parasitol. 81(2):286–294. doi: 10.2307/3283936. [DOI] [PubMed] [Google Scholar]
- Atanas AA, Kim J, Wang Z, Bueno E, Becker M, Kang D, Park J, Kramer TS, Wan FK, Baskoylu S, et al. 2023. Brain-wide representations of behavior spanning multiple timescales and states in C. elegans. Cell. 186(19):4134–4151.e31. doi: 10.1016/j.cell.2023.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audhya A, Hyndman F, McLeod IX, Maddox AS, YatesJR, III, Desai A, Oegema K. 2005. A complex containing the Sm protein CAR-1 and the RNA helicase CGH-1 is required for embryonic cytokinesis in Caenorhabditis elegans. J Cell Biol. 171:267–279. doi: 10.1083/jcb.200506124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avery L, Horvitz HR. 1990. Effects of starvation and neuroactive drugs on feeding in Caenorhabditis elegans. J Exp Zool. 253:263–270. doi: 10.1002/jez.1402530305. [DOI] [PubMed] [Google Scholar]
- Bargmann CI, Avery L. 1995. Laser killing of cells in Caenorhabditis elegans. Methods Cell Biol. 48:225–250. doi: 10.1016/S0091-679X(08)61390-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bargmann CI, Hartwieg E, Horvitz HR. 1993. Odorant-selective genes and neurons mediate olfaction in C. elegans. Cell. 74(3):515–527. doi: 10.1016/0092-8674(93)80053-H. [DOI] [PubMed] [Google Scholar]
- Bargmann CI, Horvitz HR. 1991a. Chemosensory neurons with overlapping functions direct chemotaxis to multiple chemicals in C. elegans. Neuron. 7(5):729–742. doi: 10.1016/0896-6273(91)90276-6. [DOI] [PubMed] [Google Scholar]
- Bargmann CI, Horvitz HR. 1991b. Control of larval development by chemosensory neurons in Caenorhabditis elegans. Science. 251(4998):1243–1246. doi: 10.1126/science.2006412. [DOI] [PubMed] [Google Scholar]
- Beck S, Yu-Strzelczyk J, Pauls D, Constantin OM, Gee CE, Ehmann N, Kittel RJ, Nagel G, Gao S. 2018. Synthetic light-activated ion channels for optogenetic activation and inhibition. Front Neurosci. 12:643. doi: 10.3389/fnins.2018.00643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Béjà O, Aravind L, Koonin EV, Suzuki MT, Hadd A, Nguyen LP, Jovanovich SB, Gates CM, Feldman RA, Spudich JL. et al. 2000. Bacterial rhodopsin: evidence for a new type of phototrophy in the sea. Science. 289(5486):1902–1906. doi: 10.1126/science.289.5486.1902. [DOI] [PubMed] [Google Scholar]
- Bendesky A, Tsunozaki M, Rockman MV, Kruglyak L, Bargmann CI. 2011. Catecholamine receptor polymorphisms affect decision-making in C. elegans. Nature. 472(7343):313–318. doi: 10.1038/nature09821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berendzen KM, Durieux J, Shao L-W, Tian Y, Kim H-E, Wolff S, Liu Y, Dillin A. 2016. Neuroendocrine coordination of mitochondrial stress signaling and proteostasis. Cell. 166(6):1553–1563.e1510. doi: 10.1016/j.cell.2016.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergs A, Schultheis C, Fischer E, Tsunoda SP, Erbguth K, Husson SJ, Govorunova E, Spudich JL, Nagel G, Gottschalk A, et al. 2018. Rhodopsin optogenetic toolbox v2.0 for light-sensitive excitation and inhibition in Caenorhabditis elegans. PLoS One. 13(2):e0191802. doi: 10.1371/journal.pone.0191802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergs ACF, Liewald JF, Rodriguez-Rozada S, Liu Q, Wirt C, Bessel A, Zeitzschel N, Durmaz H, Nozownik A, Dill H, et al. 2023. All-optical closed-loop voltage clamp for precise control of muscles and neurons in live animals. Nat Commun. 14(1):1939. doi: 10.1038/s41467-023-37622-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernal Sierra YA, Rost BR, Pofahl M, Fernandes AM, Kopton RA, Moser S, Holtkamp D, Masala N, Beed P, Tukker JJ, et al. 2018. Potassium channel-based optogenetic silencing. Nat Commun. 9(1):4611. doi: 10.1038/s41467-018-07038-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berndt A, Lee SY, Wietek J, Ramakrishnan C, Steinberg EE, Rashid AJ, Kim H, Park S, Santoro A, Frankland PW, et al. 2016. Structural foundations of optogenetics: determinants of channelrhodopsin ion selectivity. Proc Natl Acad Sci U S A. 113(4):822–829. doi: 10.1073/pnas.1523341113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhoi A, Palladino F, Fabrizio P. 2021. Auxin confers protection against ER stress in Caenorhabditis elegans. Biol Open. 10(2):bio057992. doi: 10.1242/bio.057992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi L, Gerstbrein B, Frokjaer-Jensen C, Royal DC, Mukherjee G, Royal MA, Xue J, Schafer WR, Driscoll M. 2004. The neurotoxic MEC-4(d) DEG/ENaC sodium channel conducts calcium: implications for necrosis initiation. Nat Neurosci. 7(12):1337–1344. doi: 10.1038/nn1347. [DOI] [PubMed] [Google Scholar]
- Blasi J, Chapman ER, Link E, Binz T, Yamasaki S, Camilli PD, Südhof TC, Niemann H, Jahn R. 1993. Botulinum neurotoxin A selectively cleaves the synaptic protein SNAP-25. Nature. 365(6442):160–163. doi: 10.1038/365160a0. [DOI] [PubMed] [Google Scholar]
- Bounoutas A, O’Hagan R, Chalfie M. 2009. The multipurpose 15-protofilament microtubules in C. elegans have specific roles in mechanosensation. Curr Biol. 19(16):1362–1367. doi: 10.1016/j.cub.2009.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks VB, Curtis DR, Eccles JC. 1957. The action of tetanus toxin on the inhibition of motoneurones. J Physiol. 135(3):655–672. doi: 10.1113/jphysiol.1957.sp005737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulina ME, Chudakov DM, Britanova OV, Yanushevich YG, Staroverov DB, Chepurnykh TV, Merzlyak EM, Shkrob MA, Lukyanov S, Lukyanov KA. 2006a. A genetically encoded photosensitizer. Nat Biotechnol. 24:95–99. doi: 10.1038/nbt1175. [DOI] [PubMed] [Google Scholar]
- Bulina ME, Lukyanov KA, Britanova OV, Onichtchouk D, Lukyanov S, Chudakov DM. 2006b. Chromophore-assisted light inactivation (CALI) using the phototoxic fluorescent protein KillerRed. Nat Protoc. 1:947–953. doi: 10.1038/nprot.2006.89. [DOI] [PubMed] [Google Scholar]
- Busack I, Bringmann H. 2023. A sleep-active neuron can promote survival while sleep behavior is disturbed. PLoS Genet. 19(3):e1010665. doi: 10.1371/journal.pgen.1010665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busack I, Jordan F, Sapir P, Bringmann H. 2020. The OptoGenBox—a device for long-term optogenetics in C. elegans. J Neurogenet. 34:466–474. doi: 10.1080/01677063.2020.1776709 [DOI] [PubMed] [Google Scholar]
- Cao P, Sun W, Kramp K, Zheng M, Salom D, Jastrzebska B, Jin H, Palczewski K, Feng Z. 2012. Light-sensitive coupling of rhodopsin and melanopsin to G(i/o) and G(q) signal transduction in Caenorhabditis elegans. FASEB J. 26(2):480–491. doi: 10.1096/fj.11-197798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalfie M, Wolinsky E. 1990. The identification and suppression of inherited neurodegeneration in Caenorhabditis elegans. Nature. 345(6274):410–416. doi: 10.1038/345410a0. [DOI] [PubMed] [Google Scholar]
- Chandra R, Farah F, Muñoz-Lobato F, Bokka A, Benedetti KL, Brueggemann C, Saifuddin MFA, Miller JM, Li J, Chang E, et al. 2023. Sleep is required to consolidate odor memory and remodel olfactory synapses. Cell. 186(13):2911–2928.e2920. doi: 10.1016/j.cell.2023.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang AJ, Chronis N, Karow DS, Marletta MA, Bargmann CI. 2006. A distributed chemosensory circuit for oxygen preference in C. elegans. PLoS Biol. 4(9):e274. doi: 10.1371/journal.pbio.0040274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase DL, Koelle MR. 2007. Biogenic amine neurotransmitters in C. elegans. In: WormBook, editor. The C. elegans Research Community. WormBook. doi: 10.1895/wormbook.1.132.1, http://www.wormbook.org. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chelur DS, Chalfie M. 2007. Targeted cell killing by reconstituted caspases. Proc Natl Acad Sci U S A. 104(7):2283–2288. doi: 10.1073/pnas.0610877104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Bharill S, Altun Z, O’Hagan R, Coblitz B, Isacoff EY, Chalfie M.. 2016a. Caenorhabditis elegans paraoxonase-like proteins control the functional expression of DEG/ENaC mechanosensory proteins. Mol Biol Cell. 27(8):1272–1285. doi: 10.1091/mbc.E15-08-0561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y-C, Chen H-J, Tseng W-C, Hsu J-M, Huang T-T, Chen C-H, Pan C-L. 2016b. A C. elegans thermosensory circuit regulates longevity through crh-1/CREB-dependent flp-6 neuropeptide signaling. Dev Cell. 39(2):209–223. doi: 10.1016/j.devcel.2016.08.021. [DOI] [PubMed] [Google Scholar]
- Choi U, Wang H, Hu M, Kim S, Sieburth D. 2021. Presynaptic coupling by electrical synapses coordinates a rhythmic behavior by synchronizing the activities of a neuron pair. Proc Natl Acad Sci U S A. 118(20):e2022599118. doi: 10.1073/pnas.2022599118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow BY, Han X, Dobry AS, Qian X, Chuong AS, Li M, Henninger MA, Belfort GM, Lin Y, Monahan PE, et al. 2010. High-performance genetically targetable optical neural silencing by light-driven proton pumps. Nature. 463(7277):98–102. doi: 10.1038/nature08652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung SH, Mazur E. 2009. Femtosecond laser ablation of neurons in C. elegans for behavioral studies. Appl Phys A. 96(2):335–341. doi: 10.1007/s00339-009-5201-7. [DOI] [Google Scholar]
- Colbert HA, Smith TL, Bargmann CI. 1997. OSM-9, a novel protein with structural similarity to channels, is required for olfaction, mechanosensation, and olfactory adaptation in Caenorhabditis elegans. J Neurosci. 17(21):8259–8269. doi: 10.1523/JNEUROSCI.17-21-08259.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conradt B, Horvitz HR. 1998. The C. elegans protein EGL-1 is required for programmed cell death and interacts with the bcl-2-like protein CED-9. Cell. 93(4):519–529. doi: 10.1016/S0092-8674(00)81182-4. [DOI] [PubMed] [Google Scholar]
- Consortium TAoGR. 2024. Updates to the alliance of genome resources central infrastructure. Genetics. 227(1):iyae049. doi: 10.1093/genetics/iyae049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornell R, Cao W, Liu J, Pocock R. 2022. Conditional degradation of UNC-31/CAPS enables spatiotemporal analysis of neuropeptide function. J Neurosci. 42(46):8599–8607. doi: 10.1523/JNEUROSCI.1368-22.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosentino C, Alberio L, Gazzarrini S, Aquila M, Romano E, Cermenati S, Zuccolini P, Petersen J, Beltrame M, Van Etten JL, et al. 2015. Optogenetics. Engineering of a light-gated potassium channel. Science. 348(6235):707–710. doi: 10.1126/science.aaa2787. [DOI] [PubMed] [Google Scholar]
- Cully DF, Vassilatis DK, Liu KK, Paress PS, Van der Ploeg LH, Schaeffer JM, Arena JP. 1994. Cloning of an avermectin-sensitive glutamate-gated chloride channel from Caenorhabditis elegans. Nature. 371(6499):707–711. doi: 10.1038/371707a0. [DOI] [PubMed] [Google Scholar]
- Damijonaitis A, Broichhagen J, Urushima T, Hüll K, Nagpal J, Laprell L, Schönberger M, Woodmansee DH, Rafiq A, Sumser MP, et al. 2015. AzoCholine enables optical control of alpha 7 nicotinic acetylcholine receptors in neural networks. ACS Chem Neurosci. 6(5):701–707. doi: 10.1021/acschemneuro.5b00030. [DOI] [PubMed] [Google Scholar]
- Daniel K, Icha J, Horenburg C, Müller D, Norden C, Mansfeld J.. 2018. Conditional control of fluorescent protein degradation by an auxin-dependent nanobody. Nat Commun. 9(1):3297. doi: 10.1038/s41467-018-05855-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davletov B, Bajohrs M, Binz T. 2005. Beyond BOTOX: advantages and limitations of individual botulinum neurotoxins. Trends Neurosci. 28(8):446–452. doi: 10.1016/j.tins.2005.06.001. [DOI] [PubMed] [Google Scholar]
- Denning DP, Hatch V, Horvitz HR. 2013. Both the caspase CSP-1 and a caspase-independent pathway promote programmed cell death in parallel to the canonical pathway for apoptosis in Caenorhabditis elegans. PLoS Genet. 9(3):e1003341. doi: 10.1371/journal.pgen.1003341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dent JA, Davis MW, Avery L. 1997. avr-15 encodes a chloride channel subunit that mediates inhibitory glutamatergic neurotransmission and ivermectin sensitivity in Caenorhabditis elegans. Embo J. 16(19):5867–5879. doi: 10.1093/emboj/16.19.5867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driscoll M, Chalfie M. 1991. The mec-4 gene is a member of a family of Caenorhabditis elegans genes that can mutate to induce neuronal degeneration [see comments]. Nature. 349(6310):588–593. doi: 10.1038/349588a0. [DOI] [PubMed] [Google Scholar]
- Edwards SL, Charlie NK, Milfort MC, Brown BS, Gravlin CN, Knecht JE, Miller KG.. 2008. A novel molecular solution for ultraviolet light detection in Caenorhabditis elegans. PLoS Biol. 6(8):0060198. doi: 10.1371/journal.pbio.0060198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickelbeck D, Rudack T, Tennigkeit SA, Surdin T, Karapinar R, Schwitalla JC, Mücher B, Shulman M, Scherlo M, Althoff P, et al. 2020. Lamprey parapinopsin (“UVLamP”): a bistable UV-sensitive optogenetic switch for ultrafast control of GPCR pathways. ChemBioChem. 21(5):612–617. doi: 10.1002/cbic.201900485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Mouridi S, AlHarbi S, Frokjaer-Jensen C. 2021. A histamine-gated channel is an efficient negative selection marker for C. elegans transgenesis. MicroPubl Biol. 2021. doi: 10.17912/micropub.biology.000349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emtage L, Gu G, Hartwieg E, Chalfie M. 2004. Extracellular proteins organize the mechanosensory channel complex in C. elegans touch receptor neurons. Neuron. 44(5):795–807. doi: 10.1016/j.neuron.2004.11.010. [DOI] [PubMed] [Google Scholar]
- Fang-Yen C, Alkema MJ, Samuel AD. 2015. Illuminating neural circuits and behaviour in Caenorhabditis elegans with optogenetics. Philos Trans R Soc Lond B Biol Sci. 370(1677):20140212. doi: 10.1098/rstb.2014.0212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang-Yen C, Gabel CV, Samuel AD, Bargmann CI, Avery L. 2012. Laser microsurgery in Caenorhabditis elegans. Methods Cell Biol. 107:177–206. doi: 10.1016/B978-0-12-394620-1.00006-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flavell SW, Pokala N, Macosko EZ, Albrecht DR, Larsch J, Bargmann CI.. 2013. Serotonin and the neuropeptide PDF initiate and extend opposing behavioral states in C. elegans. Cell. 154(5):1023–1035. doi: 10.1016/j.cell.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frokjaer-Jensen C, Davis MW, Hopkins CE, Newman BJ, Thummel JM, Olesen S-P, Grunnet M, Jorgensen EM.. 2008. Single-copy insertion of transgenes in Caenorhabditis elegans. Nat Genet. 40(11):1375–1383. doi: 10.1038/ng.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frøkjær-Jensen C, Davis MW, Ailion M, Jorgensen EM. 2012. Improved Mos1-mediated transgenesis in C. elegans. Nat Methods. 9(2):117–118. doi: 10.1038/nmeth.1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry AL, Laboy JT, Norman KR. 2014. VAV-1 acts in a single interneuron to inhibit motor circuit activity in Caenorhabditis elegans. Nat Commun. 5(1):5579. doi: 10.1038/ncomms6579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao S, Nagpal J, Schneider MW, Kozjak-Pavlovic V, Nagel G, Gottschalk A. 2015. Optogenetic manipulation of cGMP in cells and animals by the tightly light-regulated guanylyl-cyclase opsin CyclOp. Nat Commun. 6(1):8046. doi: 10.1038/ncomms9046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh DD, Sanders T, Hong S, McCurdy LY, Chase DL, Cohen N, Koelle MR, Nitabach MN.. 2016. Neural architecture of hunger-dependent multisensory decision making in C. elegans. Neuron. 92(5):1049–1062. doi: 10.1016/j.neuron.2016.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gisselmann G, Pusch H, Hovemann BT, Hatt H. 2002. Two cDNAs coding for histamine-gated ion channels in D. melanogaster. Nat Neurosci. 5(1):11–12. doi: 10.1038/nn787. [DOI] [PubMed] [Google Scholar]
- Glauser DA, Chen WC, Agin R, Macinnis BL, Hellman AB, Garrity PA, Tan MW, Goodman MB.. 2011. Heat avoidance is regulated by transient receptor potential (TRP) channels and a neuropeptide signaling pathway in Caenorhabditis elegans. Genetics. 188(1):91–103. doi: 10.1534/genetics.111.127100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbachev DA, Staroverov DB, Lukyanov KA, Sarkisyan KS. 2020. Genetically encoded red photosensitizers with enhanced phototoxicity. Int J Mol Sci. 21(22):8800. doi: 10.3390/ijms21228800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govorunova EG, Gou Y, Sineshchekov OA, Li H, Lu X, Wang Y, Brown LS, St-Pierre F, Xue M, Spudich JL.. 2022. Kalium channelrhodopsins are natural light-gated potassium channels that mediate optogenetic inhibition. Nat Neurosci. 25:967–974. doi: 10.1038/s41593-022-01094-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govorunova EG, Sineshchekov OA, Hemmati R, Janz R, Morelle O, Melkonian M, Wong GK, Spudich JL.. 2018. Extending the time domain of neuronal silencing with cryptophyte anion channelrhodopsins. eNeuro. 5:ENEURO.0174-0118.2018. doi: 10.1523/ENEURO.0174-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govorunova EG, Sineshchekov OA, Janz R, Liu X, Spudich JL. 2015. Natural light-gated anion channels: a family of microbial rhodopsins for advanced optogenetics. Science. 349(6248):647–650. doi: 10.1126/science.aaa7484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govorunova EG, Sineshchekov OA, Li H, Wang Y, Brown LS, Spudich JL.. 2020. RubyACRs, nonalgal anion channelrhodopsins with highly red-shifted absorption. Proc Natl Acad Sci U S A. 117(37):22833–22840. doi: 10.1073/pnas.2005981117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govorunova EG, Sineshchekov OA, Rodarte EM, Janz R, Morelle O, Melkonian M, Wong GK-S, Spudich JL.. 2017. The expanding family of natural anion channelrhodopsins reveals large variations in kinetics, conductance, and spectral sensitivity. Sci Rep. 7(1):43358. doi: 10.1038/srep43358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gradinaru V, Thompson KR, Deisseroth K. 2008. eNpHR: a Natronomonas halorhodopsin enhanced for optogenetic applications. Brain Cell Biol. 36(1–4):129–139. doi: 10.1007/s11068-008-9027-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gradinaru V, Zhang F, Ramakrishnan C, Mattis J, Prakash R, Diester I, Goshen I, Thompson KR, Deisseroth K.. 2010. Molecular and cellular approaches for diversifying and extending optogenetics. Cell. 141(1):154–165. doi: 10.1016/j.cell.2010.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JM, Hill JJ, Bargmann CI. 2005. A circuit for navigation in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 102(9):3184–3191. doi: 10.1073/pnas.0409009101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guntas G, Hallett RA, Zimmerman SP, Williams T, Yumerefendi H, Bear JE, Kuhlman B.. 2015. Engineering an improved light-induced dimer (iLID) for controlling the localization and activity of signaling proteins. Proc Natl Acad Sci U S A. 112(1):112–117. doi: 10.1073/pnas.1417910112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X, Boyden ES. 2007. Multiple-color optical activation, silencing, and desynchronization of neural activity, with single-spike temporal resolution. PLoS One. 2(3):e299. doi: 10.1371/journal.pone.0000299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbinder S, Tavernarakis N, Herndon LA, Kinnell M, Xu SQ, Fire A, Driscoll M. 1997. Genetically targeted cell disruption in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 94(24):13128–13133. doi: 10.1073/pnas.94.24.13128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henss T, Nagpal J, Gao S, Scheib U, Pieragnolo A, Hirschhäuser A, Schneider-Warme F, Hegemann P, Nagel G, Gottschalk A. 2022. Optogenetic tools for manipulation of cyclic nucleotides functionally coupled to cyclic nucleotide-gated channels. Br J Pharmacol. 179:2519–2537. doi: 10.1111/bph.15445. [DOI] [PubMed] [Google Scholar]
- Hermann A, Liewald JF, Gottschalk A. 2015. A photosensitive degron enables acute light-induced protein degradation in the nervous system. Curr Biol. 25(17):R749–R750. doi: 10.1016/j.cub.2015.07.040. [DOI] [PubMed] [Google Scholar]
- Hibbs RE, Gouaux E. 2011. Principles of activation and permeation in an anion-selective cys-loop receptor. Nature. 474(7349):54–60. doi: 10.1038/nature10139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilliard MA, Apicella AJ, Kerr R, Suzuki H, Bazzicalupo P, Schafer WR.. 2005. In vivo imaging of C. elegans ASH neurons: cellular response and adaptation to chemical repellents. Embo J. 24(1):63–72. doi: 10.1038/sj.emboj.7600493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hills-Muckey K, Martinez MAQ, Stec N, Hebbar S, Saldanha J, Medwig-Kinney TN, Moore FEQ, Ivanova M, Morao A, Ward JD, et al. 2021. An engineered, orthogonal auxin analog/AtTIR1(F79G) pairing improves both specificity and efficacy of the auxin degradation system in Caenorhabditis elegans. Genetics. 220(2):iyab174. doi: 10.1093/genetics/iyab174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobert O. 2013. The neuronal genome of Caenorhabditis elegans. In: WormBook, editor. The C. elegans Research Community. WormBook. doi: 10.1895/wormbook.1.161.1, http://www.wormbook.org. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin AL, Huxley AF. 1952. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoerndli FJ, Wang R, Mellem JE, Kallarackal A, Brockie PJ, Thacker C, Madsen DM, Maricq AV.. 2015. Neuronal activity and CaMKII regulate kinesin-mediated transport of synaptic AMPARs. Neuron. 86(2):457–474. doi: 10.1016/j.neuron.2015.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosono R, Kuno S, Midsukami M. 1985. Temperature-sensitive mutations causing reversible paralysis in Caenorhabditis elegans. J Exp Zool. 235(3):409–421. doi: 10.1002/jez.1402350313. [DOI] [PubMed] [Google Scholar]
- Huang Y-C, Luo J, Huang W, Baker CM, Gomes MA, Meng B, Byrne AB, Flavell SW.. 2023. A single neuron in C. elegans orchestrates multiple motor outputs through parallel modes of transmission. Curr Biol. 33(20):4430–4445.e4436. doi: 10.1016/j.cub.2023.08.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husson SJ, Liewald JF, Schultheis C, Stirman JN, Lu H, Gottschalk A.. 2012. Microbial light-activatable proton pumps as neuronal inhibitors to functionally dissect neuronal networks in C. elegans. PLoS One. 7(7):e40937. doi: 10.1371/journal.pone.0040937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda M, Nakano S, Giles AC, Xu L, Costa WS, Gottschalk A, Mori I.. 2020. Context-dependent operation of neural circuits underlies a navigation behavior in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 117(11):6178–6188. doi: 10.1073/pnas.1918528117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson K, Rajfur Z, Vitriol E, Hahn K. 2008. Chromophore-assisted laser inactivation in cell biology. Trends Cell Biol. 18(9):443–450. doi: 10.1016/j.tcb.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan LY, Jan YN. 2012. Voltage-gated potassium channels and the diversity of electrical signalling. J Physiol. 590(11):2591–2599. doi: 10.1113/jphysiol.2011.224212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jánosi B, Liewald JF, Seidenthal M, Yu S-C, Umbach S, Redzovic J, Rentsch D, Alcantara IC, Bergs ACF, Schneider MW, et al. 2024. RIM and RIM-binding protein localize synaptic CaV2 channels to differentially regulate transmission in neuronal circuits. J Neurosci. e0535222024. doi: 10.1523/JNEUROSCI.0535-22.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone DB, Wei A, Butler A, Salkoff L, Thomas JH. 1997. Behavioral defects in C. elegans egl-36 mutants result from potassium channels shifted in voltage-dependence of activation. Neuron. 19(1):151–164. doi: 10.1016/S0896-6273(00)80355-4. [DOI] [PubMed] [Google Scholar]
- Kahn-Kirby AH, Dantzker JL, Apicella AJ, Schafer WR, Browse J, Bargmann CI, Watts JL.. 2004. Specific polyunsaturated fatty acids drive TRPV-dependent sensory signaling in vivo. Cell. 119(6):889–900. doi: 10.1016/j.cell.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Kanaho Y, Tsai SC, Adamik R, Hewlett EL, Moss J, Vaughan M.. 1984. Rhodopsin-enhanced GTPase activity of the inhibitory GTP-binding protein of adenylate cyclase. J Biol Chem. 259(12):7378–7381. doi: 10.1016/S0021-9258(17)42799-2. [DOI] [PubMed] [Google Scholar]
- Kanke M, Nishimura K, Kanemaki M, Kakimoto T, Takahashi TS, Nakagawa T, Masukata H. 2011. Auxin-inducible protein depletion system in fission yeast. BMC Cell Biol. 12(18):1471–2121. doi: 10.1186/1471-2121-12-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano T, Po MD, Gao S, Leung G, Ryu WS, Zhen M.. 2011. An imbalancing act: gap junctions reduce the backward motor circuit activity to bias C. elegans for forward locomotion. Neuron. 72(4):572–586. doi: 10.1016/j.neuron.2011.09.005. [DOI] [PubMed] [Google Scholar]
- Kennedy MJ, Hughes RM, Peteya LA, Schwartz JW, Ehlers MD, Tucker CL.. 2010. Rapid blue-light-mediated induction of protein interactions in living cells. Nat Methods. 7(12):973–975. doi: 10.1038/nmeth.1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennis JT, van Stokkum IH, Crosson S, Gauden M, Moffat K, van Grondelle R.. 2004. The LOV2 domain of phototropin: a reversible photochromic switch. J Am Chem Soc. 126(14):4512–4513. doi: 10.1021/ja031840r. [DOI] [PubMed] [Google Scholar]
- Kerk SY, Kratsios P, Hart M, Mourao R, Hobert O. 2017. Diversification of C. elegans motor neuron identity via selective effector gene repression. Neuron. 93(1):80–98. doi: 10.1016/j.neuron.2016.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K, Sato K, Shibuya M, Zeiger DM, Butcher RA, Ragains JR, Clardy J, Touhara K, Sengupta P.. 2009. Two chemoreceptors mediate developmental effects of dauer pheromone in C. elegans. Science. 326(5955):994–998. doi: 10.1126/science.1176331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi J, Shidara H, Morisawa Y, Kawakami M, Tanahashi Y, Hotta K, Oka K.. 2013. A method for selective ablation of neurons in C. elegans using the phototoxic fluorescent protein, KillerRed. Neurosci Lett. 548:261–264. doi: 10.1016/j.neulet.2013.05.053. [DOI] [PubMed] [Google Scholar]
- Koutsoumparis A, Welp LM, Wulf A, Urlaub H, Meierhofer D, Börno S, Timmermann B, Busack I, Bringmann H.. 2022. Sleep neuron depolarization promotes protective gene expression changes and FOXO activation. Curr Biol. 32(10):2248–2262.e9. doi: 10.1016/j.cub.2022.04.012. [DOI] [PubMed] [Google Scholar]
- Koyanagi M, Kawano E, Kinugawa Y, Oishi T, Shichida Y, Tamotsu S, Terakita A.. 2004. Bistable UV pigment in the lamprey pineal. Proc Natl Acad Sci U S A. 101(17):6687–6691. doi: 10.1073/pnas.0400819101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyanagi M, Shen B, Nagata T, Sun L, Wada S, Kamimura S, Kage-Nakadai E, Terakita A.. 2022. High-performance optical control of GPCR signaling by bistable animal opsins MosOpn3 and LamPP in a molecular property-dependent manner. Proc Natl Acad Sci U S A. 119(48):e2204341119. doi: 10.1073/pnas.2204341119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhara A, Ohnishi N, Shimowada T, Mori I. 2011. Neural coding in a single sensory neuron controlling opposite seeking behaviours in Caenorhabditis elegans. Nat Commun. 2(1):355. doi: 10.1038/ncomms1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel MT, Johnstone DB, Thomas JH, Salkoff L. 2000. Mutants of a temperature-sensitive two-P domain potassium channel. J Neurosci. 20(20):7517–7524. doi: 10.1523/JNEUROSCI.20-20-07517.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Kim SA, Coakley S, Mugno P, Hammarlund M, Hilliard MA, Lu H.. 2014. A multi-channel device for high-density target-selective stimulation and long-term monitoring of cells and subcellular features in C. elegans. Lab Chip. 14(23):4513–4522. doi: 10.1039/C4LC00789A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leinwand SG, Chalasani SH. 2013. Neuropeptide signaling remodels chemosensory circuit composition in Caenorhabditis elegans. Nat Neurosci. 16(10):1461–1467. doi: 10.1038/nn.3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeMasurier M, Heginbotham L, Miller C. 2001. KCSA: it's a potassium channel. J General Physiol. 118(3):303–314. doi: 10.1085/jgp.118.3.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis JA, Wu CH, Berg H, Levine JH. 1980a. The genetics of levamisole resistance in the nematode Caenorhabditis elegans. Genetics. 95(4):905–928. doi: 10.1093/genetics/95.4.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis JA, Wu CH, Levine JH, Berg H. 1980b. Levamisole-resistant mutants of the nematode Caenorhabditis elegans appear to lack pharmacological acetylcholine receptors. Neuroscience. 5(6):967–989. doi: 10.1016/0306-4522(80)90180-3. [DOI] [PubMed] [Google Scholar]
- Li S, Prasanna X, Salo VT, Vattulainen I, Ikonen E. 2019. An efficient auxin-inducible degron system with low basal degradation in human cells. Nat Methods. 16(9):866–869. doi: 10.1038/s41592-019-0512-x. [DOI] [PubMed] [Google Scholar]
- Liewald JF, Brauner M, Stephens GJ, Bouhours M, Schultheis C, Zhen M, Gottschalk A. 2008. Optogenetic analysis of synaptic function. Nat Methods. 5(10):895–902. doi: 10.1038/nmeth.1252. [DOI] [PubMed] [Google Scholar]
- Lin JY, Knutsen PM, Muller A, Kleinfeld D, Tsien RY. 2013a. Reachr: a red-shifted variant of channelrhodopsin enables deep transcranial optogenetic excitation. Nat Neurosci. 16(10):1499–1508. doi: 10.1038/nn.3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JY, Sann SB, Zhou K, Nabavi S, Proulx CD, Malinow R, Jin Y, Tsien RY.. 2013b. Optogenetic inhibition of synaptic release with chromophore-assisted light inactivation (CALI). Neuron. 79(2):241–253. doi: 10.1016/j.neuron.2013.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P, Chen B, Mailler R, Wang ZW. 2017. Antidromic-rectifying gap junctions amplify chemical transmission functionally mixed at electrical-chemical synapses. Nat Commun. 8(1):14818. doi: 10.1038/ncomms14818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Hollopeter G, Jorgensen EM. 2009. Graded synaptic transmission at the Caenorhabditis elegans neuromuscular junction. Proc Natl Acad Sci U S A. 106(26):10823–10828. doi: 10.1073/pnas.0903570106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Sinnen BL, Boxer EE, Schneider MW, Grybko MJ, Buchta WC, Gibson ES, Wysoczynski CL, Ford CP, Gottschalk A, et al. 2019. A photoactivatable botulinum neurotoxin for inducible control of neurotransmission. Neuron. 101(5):863–875.e6. doi: 10.1016/j.neuron.2019.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu WW, Wilson RI. 2013. Transient and specific inactivation of Drosophila neurons in vivo using a native ligand-gated ion channel. Curr Biol. 23(13):1202–1208. doi: 10.1016/j.cub.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockyer J, Reading A, Vicenzi S, Delandre C, Marshall O, Gasperini R, Foa L, Lin JY. 2023. Optogenetic inhibition of Gα signalling alters and regulates circuit functionality and early circuit formation. bioRxiv 539674. 10.1101/2023.05.06.539674, preprint: not peer reviewed. [DOI] [Google Scholar]
- Loose JA, Ghazi A. 2021. Auxin treatment increases lifespan in Caenorhabditis elegans. Biol Open. 10(5):bio058703. doi: 10.1242/bio.058703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Cruz A, Sordillo A, Pokala N, Liu Q, McGrath PT, Bargmann CI.. 2019. Parallel multimodal circuits control an innate foraging behavior. Neuron. 102(2):407–419.e8. doi: 10.1016/j.neuron.2019.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Ahamed T, Mulcahy B, Meng J, Witvliet D, Guan SA, Holmyard D, Hung W, Wen Q, Chisholm AD, et al. 2022. Extrasynaptic signaling enables an asymmetric juvenile motor circuit to produce symmetric undulation. Curr Biol. 32(21):4631–4644.e4635. doi: 10.1016/j.cub.2022.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo L, Cook N, Venkatachalam V, Martinez-Velazquez LA, Zhang X, Calvo AC, Hawk J, MacInnis BL, Frank M, Ng JHR, et al. 2014. Bidirectional thermotaxis in Caenorhabditis elegans is mediated by distinct sensorimotor strategies driven by the AFD thermosensory neurons. Proc Natl Acad Sci U S A. 111(7):2776–2781. doi: 10.1073/pnas.1315205111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macosko EZ, Pokala N, Feinberg EH, Chalasani SH, Butcher RA, Clardy J, Bargmann CI.. 2009. A hub-and-spoke circuit drives pheromone attraction and social behaviour in C. elegans. Nature. 458(7242):1171–1175. doi: 10.1038/nature07886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahn M, Gibor L, Patil P, Cohen-Kashi Malina K, Oring S, Printz Y, Levy R, Lampl I, Yizhar O.. 2018. High-efficiency optogenetic silencing with soma-targeted anion-conducting channelrhodopsins. Nat Commun. 9(1):4125. doi: 10.1038/s41467-018-06511-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahn M, Prigge M, Ron S, Levy R, Yizhar O. 2016. Biophysical constraints of optogenetic inhibition at presynaptic terminals. Nat Neurosci. 19(4):554–556. doi: 10.1038/nn.4266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maluck E, Busack I, Besseling J, Masurat F, Turek M, Busch KE, Bringmann H.. 2020. A wake-active locomotion circuit depolarizes a sleep-active neuron to switch on sleep. PLoS Biol. 18(2):e3000361. doi: 10.1371/journal.pbio.3000361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malyshev AY, Roshchin MV, Smirnova GR, Dolgikh DA, Balaban PM, Ostrovsky MA.. 2017. Chloride conducting light activated channel GtACR2 can produce both cessation of firing and generation of action potentials in cortical neurons in response to light. Neurosci Lett. 640:76–80. doi: 10.1016/j.neulet.2017.01.026. [DOI] [PubMed] [Google Scholar]
- Mauss AS, Busch C, Borst A. 2017. Optogenetic neuronal silencing in Drosophila during visual processing. Sci Rep. 7(1):13823. doi: 10.1038/s41598-017-14076-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon HT, Ushkaryov YA, Edelmann L, Link E, Binz T, Niemann H, Jahn R, Südhof TC.. 1993. Cellubrevin is a ubiquitous tetanus-toxin substrate homologous to a putative synaptic vesicle fusion protein. Nature. 364(6435):346–349. doi: 10.1038/364346a0. [DOI] [PubMed] [Google Scholar]
- Mendel JE, Korswagen HC, Liu KS, Hajdu-Cronin YM, Simon MI, Plasterk RHA, Sternberg PW.. 1995. Participation of the protein Go in multiple aspects of behavior in C. elegans [see comments]. Science. 267(5204):1652–1655. doi: 10.1126/science.7886455. [DOI] [PubMed] [Google Scholar]
- Meng J, Ahamed T, Yu B, Hung W, Mouridi SE, Wang Z, Zhang Y, Wen Q, Boulin T, Gao S, et al. 2024. A tonically active master neuron modulates mutually exclusive motor states at two timescales. Sci Adv. 10(15):eadk0002. doi: 10.1126/sciadv.adk0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelassi F, Liu H, Hu Z, Dittman JS. 2017. A C1-C2 module in Munc13 inhibits calcium-dependent neurotransmitter release. Neuron. 95(3):577–590.e5. doi: 10.1016/j.neuron.2017.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller KG, Alfonso A, Nguyen M, Crowell JA, Johnson CD, Rand JB.. 1996. A genetic selection for Caenorhabditis elegans synaptic transmission mutants. Proc Natl Acad Sci U S A. 93(22):12593–12598. doi: 10.1073/pnas.93.22.12593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mironenko A, Zachariae U, de Groot BL, Kopec W. 2021. The persistent question of potassium channel permeation mechanisms. J Mol Biol. 433(17):167002. doi: 10.1016/j.jmb.2021.167002. [DOI] [PubMed] [Google Scholar]
- Mohamed GA, Cheng R-K, Ho J, Krishnan S, Mohammad F, Claridge-Chang A, Jesuthasan S.. 2017. Optical inhibition of larval zebrafish behaviour with anion channelrhodopsins. BMC Biol. 15(1):103. doi: 10.1186/s12915-017-0430-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammad F, Stewart JC, Ott S, Chlebikova K, Chua JY, Koh T-W, Ho J, Claridge-Chang A.. 2017. Optogenetic inhibition of behavior with anion channelrhodopsins. Nat Methods. 14(3):271–274. doi: 10.1038/nmeth.4148. [DOI] [PubMed] [Google Scholar]
- Molgo J, Comella JX, Angaut-Petit D, Pecot-Dechavassine M, Tabti N, Faille L, Mallart A, Thesleff S.. 1990. Presynaptic actions of botulinal neurotoxins at vertebrate neuromuscular junctions. J Physiol (Paris). 84:152–166. [PubMed] [Google Scholar]
- Molina-García L, Lloret-Fernández C, Cook SJ, Kim B, Bonnington RC, Sammut M, O'Shea JM, Gilbert SP, Elliott DJ, et al. 2020. Direct glia-to-neuron transdifferentiation gives rise to a pair of male-specific neurons that ensure nimble male mating. eLife. 9:e48361. doi: 10.7554/eLife.48361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montecucco C, Schiavo G. 1994. Mechanism of action of tetanus and botulinum neurotoxins. Mol Microbiol. 13(1):1–8. doi: 10.1111/j.1365-2958.1994.tb00396.x. [DOI] [PubMed] [Google Scholar]
- Mori I, Ohshima Y. 1995. Neural regulation of thermotaxis in Caenorhabditis elegans. Nature. 376(6538):344–348. doi: 10.1038/376344a0. [DOI] [PubMed] [Google Scholar]
- Mueller BD, Merrill SA, Watanabe S, Liu P, Niu L, Singh A, Maldonado-Catala P, Cherry A, Rich MS, Silva M, et al. 2023. Cav1 and CaV2 calcium channels mediate the release of distinct pools of synaptic vesicles. Elife. 12:e81407. doi: 10.7554/eLife.81407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel G, Ollig D, Fuhrmann M, Kateriya S, Musti AM, Bamberg E, Hegemann P.. 2002. Channelrhodopsin-1: a light-gated proton channel in green algae. Science. 296(5577):2395–2398. doi: 10.1126/science.1072068. [DOI] [PubMed] [Google Scholar]
- Nakashiba T, Young JZ, McHugh TJ, Buhl DL, Tonegawa S. 2008. Transgenic inhibition of synaptic transmission reveals role of CA3 output in hippocampal learning. Science. 319(5867):1260–1264. doi: 10.1126/science.1151120. [DOI] [PubMed] [Google Scholar]
- Nance J, Frøkjær-Jensen C. 2019. The Caenorhabditis elegans transgenic toolbox. Genetics. 212(4):959–990. doi: 10.1534/genetics.119.301506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natsume T, Kiyomitsu T, Saga Y, Kanemaki MT. 2016. Rapid protein depletion in human cells by auxin-inducible degron tagging with short homology donors. Cell Rep. 15(1):210–218. doi: 10.1016/j.celrep.2016.03.001. [DOI] [PubMed] [Google Scholar]
- Negishi T, Kitagawa S, Horii N, Tanaka Y, Haruta N, Sugimoto A, Sawa H, Hayashi K-I, Harata M, Kanemaki MT.. 2022. The auxin-inducible degron 2 (AID2) system enables controlled protein knockdown during embryogenesis and development in Caenorhabditis elegans. Genetics. 220(2):iyab218. doi: 10.1093/genetics/iyab218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson MD, Lee KH, Churgin MA, Hill AJ, Van Buskirk C, Fang-Yen C, Raizen DM.. 2014. FMRFamide-like FLP-13 neuropeptides promote quiescence following heat stress in Caenorhabditis elegans. Curr Biol. 24(20):2406–2410. doi: 10.1016/j.cub.2014.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura K, Fukagawa T, Takisawa H, Kakimoto T, Kanemaki M. 2009. An auxin-based degron system for the rapid depletion of proteins in nonplant cells. Nat Methods. 6(12):917–922. doi: 10.1038/nmeth.1401. [DOI] [PubMed] [Google Scholar]
- Oesterhelt D, Tittor J. 1989. Two pumps, one principle: light-driven ion transport in halobacteria. Trends Biochem Sci. 14(2):57–61. doi: 10.1016/0968-0004(89)90044-3. [DOI] [PubMed] [Google Scholar]
- Okazaki A, Takagi S. 2013. An optogenetic application of proton pump ArchT to C. elegans cells. Neurosci Res. 75(1):29–34. doi: 10.1016/j.neures.2012.09.002. [DOI] [PubMed] [Google Scholar]
- Oranth A, Schultheis C, Tolstenkov O, Erbguth K, Nagpal J, Hain D, Brauner M, Wabnig S, Steuer Costa W, McWhirter RD, et al. 2018. Food sensation modulates locomotion by dopamine and neuropeptide signaling in a distributed neuronal network. Neuron. 100(6):1414–1428 e1410. doi: 10.1016/j.neuron.2018.10.024. [DOI] [PubMed] [Google Scholar]
- Ott S, Xu S, Lee N, Hong IHK, Anns J, Suresh DD, Zhang Z, Zhang X, Harion R, Ye W, et al. 2024. Kalium channelrhodopsins effectively inhibit neurons. Nat Commun. 15:6088. doi: 10.1038/s41467-024-50250-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei Y, Rogan SC, Yan F, Roth BL. 2008. Engineered GPCRs as tools to modulate signal transduction. Physiology (Bethesda). 23:313–321. doi: 10.1152/physiol.00025.2008. [DOI] [PubMed] [Google Scholar]
- Pohl F, Germann AL, Mao J, Hou S, Bakare B, Kong TLP, Yates K, Nonet ML, Akk G, Kornfeld K, et al. 2023. UNC-49 is a redox-sensitive GABAA receptor that regulates the mitochondrial unfolded protein response cell nonautonomously. Sci Adv. 9(44):eadh2584. doi: 10.1126/sciadv.adh2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pokala N, Liu Q, Gordus A, Bargmann CI. 2014. Inducible and titratable silencing of Caenorhabditis elegans neurons in vivo with histamine-gated chloride channels. Proc Natl Acad Sci U S A. 111(7):2770–2775. doi: 10.1073/pnas.1400615111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi YB, Garren EJ, Shu X, Tsien RY, Jin Y. 2012. Photo-inducible cell ablation in Caenorhabditis elegans using the genetically encoded singlet oxygen generating protein miniSOG. Proc Natl Acad Sci U S A. 8(19):7499–7504. doi: 10.1073/pnas.1204096109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauthan M, Morck C, Pilon M. 2007. The C. elegans M3 neuron guides the growth cone of its sister cell M2 via the Kruppel-like zinc finger protein MNM-2. Dev Biol. 311(1):185–199. doi: 10.1016/j.ydbio.2007.08.037. [DOI] [PubMed] [Google Scholar]
- Ravi B, Garcia J, Collins KM. 2018. Homeostatic feedback modulates the development of two-state patterned activity in a model serotonin motor circuit in Caenorhabditis elegans. J Neurosci. 38(28):6283–6298. doi: 10.1523/JNEUROSCI.3658-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner DJ, Weinshenker D, Thomas JH. 1995. Analysis of dominant mutations affecting muscle excitation in Caenorhabditis elegans. Genetics. 141(3):961–976. doi: 10.1093/genetics/141.3.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riani YD, Matsuda T, Takemoto K, Nagai T. 2018. Green monomeric photosensitizing fluorescent protein for photo-inducible protein inactivation and cell ablation. BMC Biol. 16(1):50. doi: 10.1186/s12915-018-0514-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Rozada S, Wietek J, Tenedini F, Sauter K, Dhiman N, Hegemann P, Soba P, Wiegert JS.. 2022. Aion is a bistable anion-conducting channelrhodopsin that provides temporally extended and reversible neuronal silencing. Commun Biol. 5(1):687. doi: 10.1038/s42003-022-03636-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu M-H, Kang I-H, Nelson MD, Jensen TM, Lyuksyutova AI, Siltberg-Liberles J, Raizen DM, Gomelsky M.. 2014. Engineering adenylate cyclases regulated by near-infrared window light. Proc Natl Acad Sci U S A. 111(28) 10167–10172. doi: 10.1073/pnas.1324301111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkisyan KS, Zlobovskaya OA, Gorbachev DA, Bozhanova NG, Sharonov GV, Staroverov DB, Egorov ES, Ryabova AV, Solntsev KM, Mishin AS, et al. 2015. KillerOrange, a genetically encoded photosensitizer activated by blue and green light. PLoS ONE. 10(12):e0145287. doi: 10.1371/journal.pone.0145287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathyan KM, McKenna BD, Anderson WD, Duarte FM, Core L, Guertin MJ.. 2019. An improved auxin-inducible degron system preserves native protein levels and enables rapid and specific protein depletion. Genes and Development. 33(19–20):1441–1455. doi: 10.1101/gad.328237.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer JM, Bergstrom AR. 1988. Identification of gamma-aminobutyric acid and its binding sites in Caenorhabditis elegans. Life Sci. 43(21):1701–1706. doi: 10.1016/0024-3205(88)90481-X. [DOI] [PubMed] [Google Scholar]
- Schiavo CG, Benfenati F, Poulain B, Rossetto O, de Laureto PP, DasGupta BR, Montecucco C.. 1992. Tetanus and botulinum-B neurotoxins block neurotransmitter release by proteolytic cleavage of synaptobrevin. Nature. 359(6398):832–835. doi: 10.1038/359832a0. [DOI] [PubMed] [Google Scholar]
- Schiksnis EC, Nicholson AL, Modena MS, Pule MN, Arribere JA, Pasquinelli A.. 2020. Auxin-independent depletion of degron-tagged proteins by TIR1. MicroPubl Biol. 2020. doi: 10.17912/micropub.biology.000213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt C, Schultheis C, Pokala N, Husson SJ, Liewald JF, Bargmann CI, Gottschalk A.. 2012. Specific expression of channelrhodopsin-2 in single neurons of Caenorhabditis elegans. PLoS One. 7(8):e43164. doi: 10.1371/journal.pone.0043164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultheis C, Brauner M, Liewald JF, Gottschalk A. 2011. Optogenetic analysis of GABAB receptor signaling in Caenorhabditis elegans motor neurons. J Neurophysiol. 106(2):817–827. doi: 10.1152/jn.00578.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidel HS, Ailion M, Li J, van Oudenaarden A, Rockman MV, Kruglyak L.. 2011. A novel sperm-delivered toxin causes late-stage embryo lethality and transmission ratio distortion in C. elegans. PLoS Biol. 9(7):e1001115. doi: 10.1371/journal.pbio.1001115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidel HS, Rockman MV, Kruglyak L. 2008. Widespread genetic incompatibility in C. elegans maintained by balancing selection. Science. 319(5863):589–594. doi: 10.1126/science.1151107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepers JJ, Verstappen NHM, Vo AA, Ragle JM, Ruijtenberg S, Ward JD, Boxem M.. 2022. The mIAA7 degron improves auxin-mediated degradation in Caenorhabditis elegans. G3 (Bethesda). 12(10):jkac222. doi: 10.1093/g3journal/jkac222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setty H, Salzberg Y, Karimi S, Berent-Barzel E, Krieg M, Oren-Suissa M.. 2022. Sexually dimorphic architecture and function of a mechanosensory circuit in C. elegans. Nat Commun. 13(1):6825. doi: 10.1038/s41467-022-34661-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao L-W, Niu R, Liu Y. 2016. Neuropeptide signals cell non-autonomous mitochondrial unfolded protein response. Cell Res. 26(11):1182–1196. doi: 10.1038/cr.2016.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen E-Z, Chen H, Ozturk AR, Tu S, Shirayama M, Tang W, Ding Y-H, Dai S-Y, Weng Z, Mello CC.. 2018. Identification of piRNA binding sites reveals the argonaute regulatory landscape of the C. elegans germline. Cell. 172(5):937–951.e918. doi: 10.1016/j.cell.2018.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinkai Y, Yamamoto Y, Fujiwara M, Tabata T, Murayama T, Hirotsu T, Ikeda DD, Tsunozaki M, Iino Y, Bargmann CI, et al. 2011. Behavioral choice between conflicting alternatives is regulated by a receptor guanylyl cyclase, GCY-28, and a receptor tyrosine kinase, SCD-2, in AIA interneurons of Caenorhabditis elegans. J Neurosci. 31(8):3007–3015. doi: 10.1523/JNEUROSCI.4691-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu X, Lev-Ram V, Deerinck TJ, Qi Y, Ramko EB, Davidson MW, Jin Y, Ellisman MH, Tsien RY.. 2011. A genetically encoded tag for correlated light and electron microscopy of intact cells, tissues, and organisms. PLoS Biol. 9(4):e1001041. doi: 10.1371/journal.pbio.1001041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sordillo A, Bargmann CI. 2021. Behavioral control by depolarized and hyperpolarized states of an integrating neuron. eLife. 10:e67723. doi: 10.7554/eLife.67723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanakis N, Jiang J, Liang Y, Shaham S. 2024. LET-381/FoxF and its target UNC-30/Pitx2 specify and maintain the molecular identity of C. elegans mesodermal glia that regulate motor behavior. EMBO J. 43(6):956–992. doi: 10.1038/s44318-024-00049-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhardt RA, Bi G, Alderton JM. 1994. Cell membrane resealing by a vesicular mechanism similar to neurotransmitter release. Science. 263(5145):390–393. doi: 10.1126/science.7904084. [DOI] [PubMed] [Google Scholar]
- Sternberg PW, Van Auken K, Wang Q, Wright A, Yook K, Zarowiecki M, Arnaboldi V, Becerra A, Brown S, Cain S, et al. 2024. WormBase 2024: status and transitioning to alliance infrastructure. Genetics. 227(1):iyae050. doi: 10.1093/genetics/iyae050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steuer Costa W, Van der Auwera P, Glock C, Liewald JF, Bach M, Schüler C, Wabnig S, Oranth A, Masurat F, Bringmann H, et al. 2019. A GABAergic and peptidergic sleep neuron as a locomotion stop neuron with compartmentalized Ca2+ dynamics. Nat Commun. 10(1):4095. doi: 10.1038/s41467-019-12098-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steuer Costa W, Yu SC, Liewald JF, Gottschalk A. 2017. Fast cAMP modulation of neurotransmission via neuropeptide signals and vesicle loading. Curr Biol. 27(4):495–507. doi: 10.1016/j.cub.2016.12.055. [DOI] [PubMed] [Google Scholar]
- Stojanovski K, Gheorghe I, Lenart P, Lanjuin A, Mair WB, Towbin BD.. 2023. Maintenance of appropriate size scaling of the C. elegans pharynx by YAP-1. Nat Commun. 14(1):7564. doi: 10.1038/s41467-023-43230-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulston JE, White JG. 1980. Regulation and cell autonomy during postembryonic development of Caenorhabditis elegans. Dev Biol. 78(2):577–597. doi: 10.1016/0012-1606(80)90353-X. [DOI] [PubMed] [Google Scholar]
- Sweeney ST, Broadie K, Keane J, Niemann H, O’Kane CJ. 1995. Targeted expression of tetanus toxin light chain in Drosophila specifically eliminates synaptic transmission and causes behavioral defects. Neuron. 14(2):341–351. doi: 10.1016/0896-6273(95)90290-2. [DOI] [PubMed] [Google Scholar]
- Swierczek NA, Giles AC, Rankin CH, Kerr RA. 2011. High-throughput behavioral analysis in C. elegans. Nat Methods. 8(7):592–598. doi: 10.1038/nmeth.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajima S, Kim YS, Fukuda M, Jo Y, Wang PY, Paggi JM, Inoue M, Byrne EFX, Kishi KE, Nakamura S, et al. 2023. Structural basis for ion selectivity in potassium-selective channelrhodopsins. Cell. 186(20):4325–4344.e26. doi: 10.1016/j.cell.2023.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemoto K, Matsuda T, Sakai N, Fu D, Noda M, Uchiyama S, Kotera I, Arai Y, Horiuchi M, Fukui K, et al. 2013. SuperNova, a monomeric photosensitizing fluorescent protein for chromophore-assisted light inactivation. Sci Rep. 3(1):2629. doi: 10.1038/srep02629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi J, Chen H, Hoyt MA, Coffino P. 2008. Structural elements of the ubiquitin-independent proteasome degron of ornithine decarboxylase. Biochem J. 410(2):401–407. doi: 10.1042/BJ20071239. [DOI] [PubMed] [Google Scholar]
- Taslimi A, Vrana JD, Chen D, Borinskaya S, Mayer BJ, Kennedy MJ, Tucker CL.. 2014. An optimized optogenetic clustering tool for probing protein interaction and function. Nat Commun. 5(1):4925. doi: 10.1038/ncomms5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taslimi A, Zoltowski B, Miranda JG, Pathak GP, Hughes RM, Tucker CL.. 2016. Optimized second-generation CRY2-CIB dimerizers and photoactivatable Cre recombinase. Nat Chem Biol. 12(6):425–430. doi: 10.1038/nchembio.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teale WD, Paponov IA, Palme K. 2006. Auxin in action: signalling, transport and the control of plant growth and development. Nat Rev Mol Cell Biol. 7(11):847–859. doi: 10.1038/nrm2020. [DOI] [PubMed] [Google Scholar]
- Teo JH-M, Kurokawa I, Onishi Y, Sato N, Kitazono T, Tokunaga T, Fujiwara M, Ishihara T.. 2022. Behavioral forgetting of olfactory learning is mediated by interneuron-regulated network plasticity in Caenorhabditis elegans. eNeuro. 9(4):ENEURO.0084-0022.2022. doi: 10.1523/ENEURO.0084-22.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobin D, Madsen D, Kahn-Kirby A, Peckol E, Moulder G, Barstead R, Maricq AV, Bargmann CI.. 2002. Combinatorial expression of TRPV channel proteins defines their sensory functions and subcellular localization in C. elegans neurons. Neuron. 35(2):307–318. doi: 10.1016/S0896-6273(02)00757-2. [DOI] [PubMed] [Google Scholar]
- Tolstenkov O, Van der Auwera P, Steuer Costa W, Bazhanova O, Gemeinhardt TM, Bergs ACf, Gottschalk A.. 2018. Functionally asymmetric motor neurons contribute to coordinating locomotion of Caenorhabditis elegans. Elife. 7:e34997. doi: 10.7554/eLife.34997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner CD, Stuhr NL, Ramos CM, Van Camp BT, Curran SP. 2023. A dicer-related helicase opposes the age-related pathology from SKN-1 activation in ASI neurons. Proc Natl Acad Sci U S A. 120(52):e2308565120. doi: 10.1073/pnas.2308565120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida N, Takahashi K, Iwasaki R, Yamada R, Yoshimura M, Endo TA, Kimura S, Zhang H, Nomoto M, Tada Y, et al. 2018. Chemical hijacking of auxin signaling with an engineered auxin–TIR1 pair. Nat Chem Biol. 14(3):299–305. doi: 10.1038/nchembio.2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vettkotter D, Schneider M, Goulden BD, Dill H, Liewald J, Zeiler S, Guldan J, Ateş YA, Watanabe S, Gottschalk A.. 2022. Rapid and reversible optogenetic silencing of synaptic transmission by clustering of synaptic vesicles. Nat Commun. 13(1):7827. doi: 10.1038/s41467-022-35324-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vierock J, Peter E, Grimm C, Rozenberg A, Chen IW, Tillert L, Castro Scalise AG, Casini M, Augustin S, Tanese D, et al. 2022. Wichr, a highly potassium-selective channelrhodopsin for low-light one- and two-photon inhibition of excitable cells. Sci Adv. 8(49):eadd7729. doi: 10.1126/sciadv.add7729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissenberger S, Schultheis C, Liewald JF, Erbguth K, Nagel G, Gottschalk A.. 2011. PACalpha–an optogenetic tool for in vivo manipulation of cellular cAMP levels, neurotransmitter release, and behavior in Caenorhabditis elegans. J Neurochem. 116(4):616–625. doi: 10.1111/j.1471-4159.2010.07148.x. [DOI] [PubMed] [Google Scholar]
- Westberg M, Holmegaard L, Pimenta FM, Etzerodt M, Ogilby PR. 2015. Rational design of an efficient, genetically encodable, protein-encased singlet oxygen photosensitizer. J Am Chem Soc. 137(4):1632–1642. doi: 10.1021/ja511940j. [DOI] [PubMed] [Google Scholar]
- White JG, Horvitz HR. 1979. Laser microbeam techniques in biological research. Electro-Opt Syst Des. 11(8):23–24. [Google Scholar]
- Wiegert JS, Mahn M, Prigge M, Printz Y, Yizhar O. 2017. Silencing neurons: tools, applications, and experimental constraints. Neuron. 95:504–529. doi: 10.1016/j.neuron.2017.06.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wietek J, Beltramo R, Scanziani M, Hegemann P, Oertner TG, Wiegert JS.. 2015. An improved chloride-conducting channelrhodopsin for light-induced inhibition of neuronal activity in vivo. Sci Rep. 5(1):14807. doi: 10.1038/srep14807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wietek J, Nozownik A, Pulin M, Saraf-Sinik I, Matosevich N, Gowrishankar R, Gat A, Malan D, Brown BJ, Dine J, et al. 2024. A bistable inhibitory optoGPCR for multiplexed optogenetic control of neural circuits. Nat Methods. 21(7):1275–1287. doi: 10.1038/s41592-024-02285-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wietek J, Rodriguez-Rozada S, Tutas J, Tenedini F, Grimm C, Oertner TG, Soba P, Hegemann P, Wiegert JS. 2017. Anion-conducting channelrhodopsins with tuned spectra and modified kinetics engineered for optogenetic manipulation of behavior. Sci Rep. 7(1):14957. doi: 10.1038/s41598-017-14330-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wietek J, Wiegert JS, Adeishvili N, Schneider F, Watanabe H, Tsunoda SP, Vogt A, Elstner M, Oertner TG, Hegemann P.. 2014. Conversion of channelrhodopsin into a light-gated chloride channel. Science. 344(6182):409–412. doi: 10.1126/science.1249375. [DOI] [PubMed] [Google Scholar]
- Williams DC, Bejjani RE, Ramirez PM, Coakley S, Kim SA, Lee H, Wen Q, Samuel A, Lu H, Hilliard MA, et al. 2013. Rapid and permanent neuronal inactivation in vivo via subcellular generation of reactive oxygen with the use of KillerRed. Cell Rep. 5(2):553–563. doi: 10.1016/j.celrep.2013.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Y, Yee C, Zhao CZ, Martinez MAQ, Zhang W, Shen K, Matus DQ, Hammell C.. 2023. An expandable FLP-ON::TIR1 system for precise spatiotemporal protein degradation in Caenorhabditis elegans. GENETICS. 223(4):iyad013. doi: 10.1093/genetics/iyad013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu S, Chisholm AD. 2016. Highly efficient optogenetic cell ablation in C. elegans using membrane-targeted miniSOG. Sci Rep. 6(1):21271. doi: 10.1038/srep21271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu T, Huo J, Shao S, Po M, Kawano T, Lu Y, Wu M, Zhen M, Wen Q.. 2018. Descending pathway facilitates undulatory wave propagation in Caenorhabditis elegans through gap junctions. Proc Natl Acad Sci U S A. 115(19):E4493–E4502. doi: 10.1073/pnas.1717022115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M, Wada N, Kitabatake Y, Watanabe D, Anzai M, Yokoyama M, Teranishi Y, Nakanishi S.. 2003. Reversible suppression of glutamatergic neurotransmission of cerebellar granule cells in vivo by genetically manipulated expression of tetanus neurotoxin light chain. J Neurosci. 23(17):6759–6767. doi: 10.1523/JNEUROSCI.23-17-06759.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanashi T, Maki M, Kojima K, Shibukawa A, Tsukamoto T, Chowdhury S, Yamanaka A, Takagi S, Sudo Y.. 2019. Quantitation of the neural silencing activity of anion channelrhodopsins in Caenorhabditis elegans and their applicability for long-term illumination. Sci Rep. 9(1):7863. doi: 10.1038/s41598-019-44308-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yesbolatova A, Saito Y, Kitamoto N, Makino-Itou H, Ajima R, Nakano R, Nakaoka H, Fukui K, Gamo K, Tominari Y, et al. 2020. The auxin-inducible degron 2 technology provides sharp degradation control in yeast, mammalian cells, and mice. Nat Commun. 11(1):5701. doi: 10.1038/s41467-020-19532-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young LEA, Shoben C, Ricci K, Williams DC. 2019. Genetic analysis of KillerRed in C. elegans identifies a shared role of calcium genes in ROS-mediated neurodegeneration. J Neurogenet. 33(1):1–9. doi: 10.1080/01677063.2018.1531857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu B, Wang X, Wei S, Fu T, Dzakah EE, Waqas A, Walthall WW, Shan G.. 2017. Convergent transcriptional programs regulate cAMP levels in C. elegans GABAergic motor neurons. Dev Cell. 43(2):212–226.e217. doi: 10.1016/j.devcel.2017.09.013. [DOI] [PubMed] [Google Scholar]
- Zhan X, Chen C, Niu L, Du X, Lei Y, Dan R, Wang Z-W, Liu P.. 2023. Locomotion modulates olfactory learning through proprioception in C. elegans. Nat Commun. 14(1):4534. doi: 10.1038/s41467-023-40286-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Wang LP, Brauner M, Liewald JF, Kay K, Watzke N, Wood PG, Bamberg E, Nagel G, Gottschalk A, et al. 2007. Multimodal fast optical interrogation of neural circuitry. Nature. 446(7136):633–639. doi: 10.1038/nature05744. [DOI] [PubMed] [Google Scholar]
- Zhang T, Liu C, Li W, Kuang J, Qiu X-Y, Min L, Zhu L.. 2022. Targeted protein degradation in mammalian cells: a promising avenue toward future. Comput Struct Biotechnol J. 20:5477–5489. doi: 10.1016/j.csbj.2022.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Köhler S, Rillo-Bohn R, Dernburg AF. 2018. A compartmentalized signaling network mediates crossover control in meiosis. eLife. 7:e30789. doi: 10.7554/eLife.30789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Ward JD, Cheng Z, Dernburg AF. 2015. The auxin-inducible degradation (AID) system enables versatile conditional protein depletion in C. elegans. Development (Cambridge). 142:4374–4384. doi: 10.1242/dev.129635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Brockie PJ, Mellem JE, Madsen DM, Maricq AV. 1999. Neuronal control of locomotion in C. elegans is modified by a dominant mutation in the GLR-1 ionotropic glutamate receptor. Neuron. 24(2):347–361. doi: 10.1016/S0896-6273(00)80849-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Strains and plasmids are available upon request, as far as they have been generated by the authors or former Gottschalk lab members. The authors affirm that all data necessary for confirming the conclusions of the article are present within the article, figures, and tables.