Abstract
Introduction
While male breast carcinoma is a relatively uncommon occurrence, its incidence is on the rise, potentially attributed to sporadic pathophysiological mechanisms, primarily involving hormonal imbalances. Invasive apocrine carcinoma represents a small fraction of global breast malignancies, with limited instances reported among male patients in the literature. The clinical presentation of an apocrine breast carcinoma closely resembles that of other breast cancer subtypes, as it is most often described as a solitary ulcerative nodular lesion occupying a retro-areolar region of the breast. Herein, we describe a novel case of an apocrine male breast carcinoma metastasizing to the skin, given that past cases had presented ab initio in the subcutaneous breast tissue. Furthermore, we discuss the unusual histopathology encountered in this entity such as the presence of psammoma bodies.
Case Presentation
In this case report, we outline the clinical presentation of an 80-year-old male with a history of prior breast cancer and mastectomy, performed 9 years before this dermatologic consultation. Upon a physical examination, a singular nodular lesion on his right breast was discovered, leading to a biopsy. Subsequent histological analysis identified an apocrine cell carcinoma characterized by numerous psammoma bodies, an unusual occurrence in breast tissue. Consequently, the patient received a diagnosis of relapsing breast apocrine carcinoma.
Conclusion
Cutaneous metastases arising from apocrine breast carcinoma are infrequent in male patients. The precise diagnosis of invasive apocrine breast carcinoma hinges on the accurate identification of immunohistochemical markers and a clear morphological profile. A notable correlation has been observed, particularly in positive expressions of gross cystic disease fluid protein-15 (GCDFP-15) and androgen receptor. However, due to the rarity of this presentation, there is limited data on treatment modalities. Ongoing studies are investigating the potential role of anti-androgens in the treatment of apocrine breast carcinomas.
Keywords: breast neoplasms, male, apocrine glands, carcinoma, case reports, male breast carcinoma, invasive apocrine carcinoma, cutaneous metastasis, psammoma body
Introduction
In the United States, breast cancer is the second-leading cause of malignancy in females. However, it occurs in less than 1% of males.1,2 Nonetheless, male and female breast cancers have both increased in incidence worldwide over the past 2 decades.3 However, male breast cancer has been found predominantly in African American and Israeli populations.4 Moreover, the majority of male breast malignancies tend to manifest later in life in contrast to their female counterparts.5
Pure apocrine carcinoma of the breast is an uncommon finding in female patients, and it is an exceptionally rare presentation in male patients. Invasive apocrine carcinoma constitutes between 0.3% and 4.0% of all invasive breast malignancies worldwide.6 The reported difference is so wide since there have been no strict criteria for diagnosing an apocrine breast carcinoma due to the rarity of its presentation. Fewer cases of invasive apocrine carcinomas have been reported in the literature among male patients,6 with the average age of onset around 60–70 years in male patients.7 The clinical presentation of an apocrine breast carcinoma closely resembles that of other breast cancer subtypes, as it is most often described as a solitary ulcerative nodular lesion occupying a retro-areolar region of the breast.7 Upon diagnosing a patient with such a lesion, mammography and ultrasound are frequently utilized to establish an initial diagnosis.7 However, imaging results do not differentiate between apocrine breast carcinoma and other carcinoma subtypes. Therefore, histological analysis plays a pivotal role in diagnosing a male patient with apocrine breast cancer due to the broad range of differential diagnoses.7
To the best of our knowledge, fewer than 20 cases of male apocrine breast carcinoma are reported in the medical literature.7 Indeed, we describe the very first case of an apocrine male breast carcinoma presenting in the skin, given that the past cases had presented ab initio in the subcutaneous breast tissue. Herein, we present a recurrent invasive ductal breast carcinoma with apocrine differentiation following antecedent chemotherapy, radiotherapy, and a mastectomy 9 years prior to this presentation. Moreover, our case highlights the presence of psammoma bodies, which, despite being frequently found in these cases with female patients, are unprecedented in male breast apocrine carcinoma.
Case Presentation
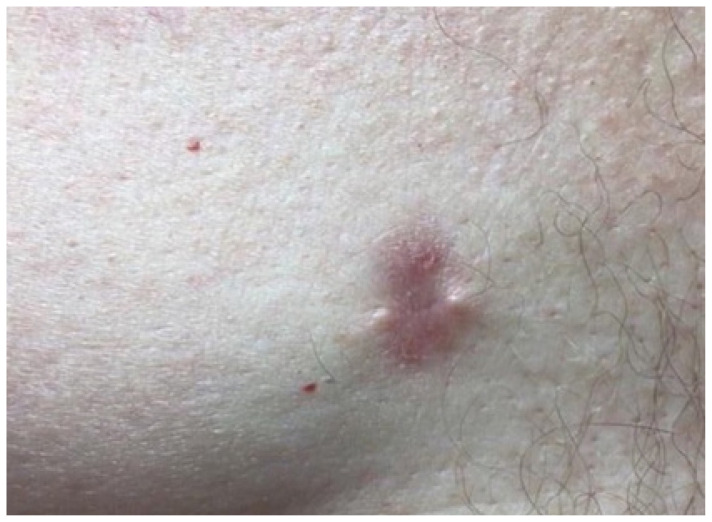
An 80-year-old White male with a past medical history of prostate and right breast cancers presented to the clinic for an annual skin evaluation without any complaints. The patient was initially diagnosed with an invasive ductal high-grade estrogen receptor (ER)/androgen receptor (AR) positive breast cancer 9 years earlier and had been on adjuvant hormonal therapy since then. At the time of this diagnosis, he underwent a mastectomy and radiation therapy to the right breast. The patient was offered genetic counseling and testing but did not undergo further evaluation for any predisposing genes, and his family history was negative for breast cancer, prostate cancer, or colon cancer. At the time of his presentation to the clinic for a dermatologic consultation, he had completed a total of 9 years of hormone therapy. The patient did not report any skin changes or complaints. However, upon a physical examination, a solitary 2.0 cm papulonodular lesion was found on his right medial breast (Figure 1). A 4.0 mm punch biopsy was performed to rule out breast cancer relapse or potential cutaneous metastases from other organs.
Figure 1.
Cutaneous lesions from the malignant male breast carcinoma presented as a 2.0 cm erythematous, papulonodular lesion on the right medial breast.
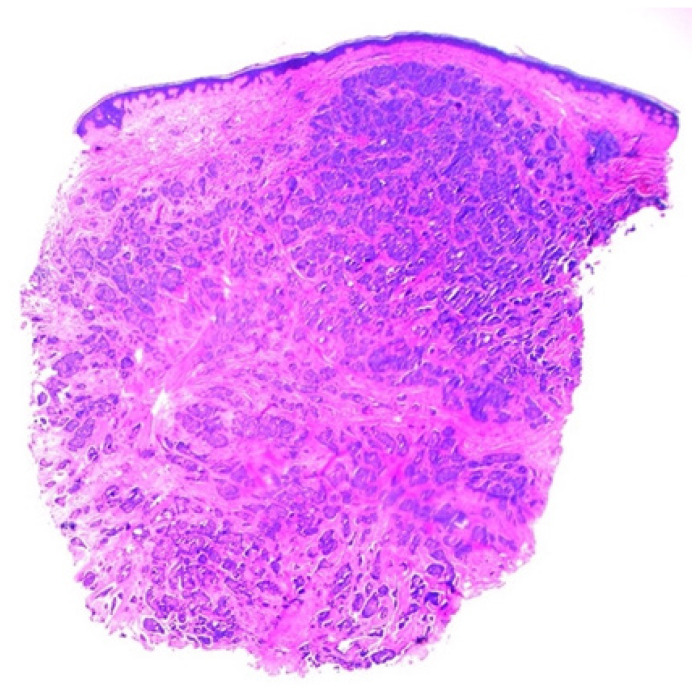
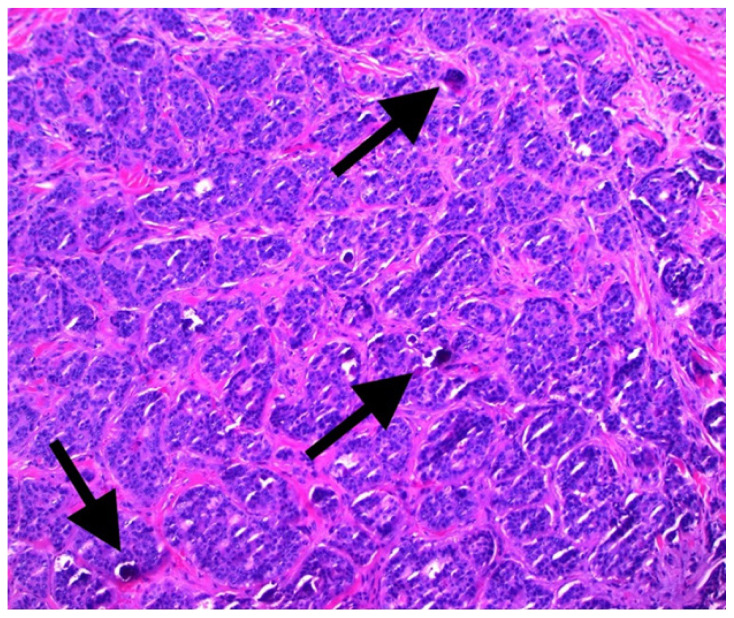
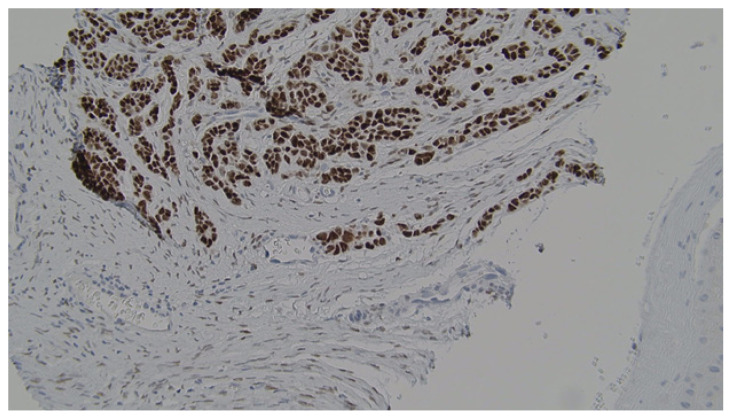
Histopathological analysis (Figure 2) revealed voluminous cells with eosinophilic cytoplasm showing apical blebs and containing enlarged hyperchromatic nuclei with prominent nucleoli. Increased numbers of typical and atypical mitosis figures with ductular necrosis were also identified. The stroma surrounding the tumor foci was sclerotic and rare foci of dystrophic calcification were observed (Figure 3). Additionally, many psammoma bodies were recognized within the gland lumina of the tumor. Immunohistochemical staining revealed strong positivity for ER (Figure 4), AR (Figure 5), and gross cystic disease fluid protein-15 (GCDFP-15), which strongly suggested a diagnosis of an apocrine cell carcinoma. Additionally, immunostaining for progesterone receptor (PR) was done which revealed a lack of expression. Shortly thereafter, the patient had his annual PET/CT scanning that revealed a new atypical uptake originating inferiorly to the right breast with a standardized uptake value of 5.1. The diagnosis of a relapsing breast carcinoma with apocrine differentiation was established. Per our patient’s protocol, he was being treated with fulvestrant, palbociclib, and leuprolide as chemotherapy agents.
Figure 2.
Low-power view of an apocrine breast carcinoma showed dense connective tissue stroma (hematoxylin and eosin stain, 40X).
Figure 3.
High-power view demonstrated psammoma bodies (black arrows) appearing as purple flecks of calcification within the lumina of the breast gland (hematoxylin and eosin stain, 100X).
Figure 4.
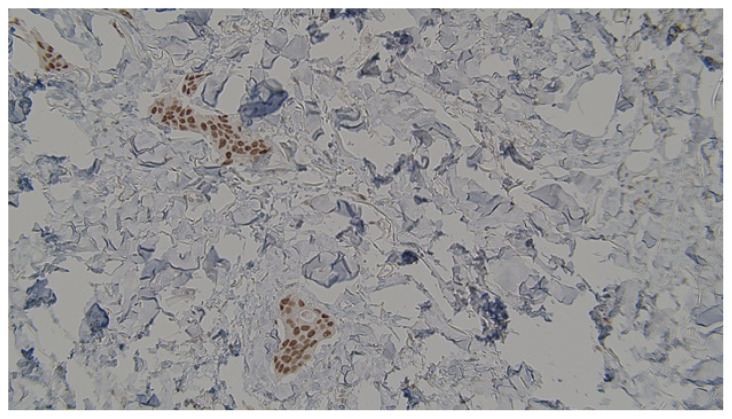
Immunostaining revealed strong positivity towards estrogen receptors.
Figure 5.
Immunostaining revealed strong positivity towards androgen receptors.
Discussion
Mechanisms underlying a state of estrogen dominance in the male body seem to play an integral role in breast tissue carcinogenesis. Obesity, exogenous hormone intake, hereditary syndromes, genetic mutations, and certain drugs are among the well-recognized risk factors involved in the progression of male breast cancer.1 Genetic mutations comprise the most common cause of male breast malignancies. Up to one-quarter of male breast carcinomas are associated with the positive expression of the breast cancer 2 (BRCA2) gene.8 Patients with a mutated BRCA2 gene are at a higher risk of developing a higher stage and grade of breast cancer, along with faster metastases.3 Additionally, the partner and localizer of the BRCA2 gene (PALB2) are closely associated with both the BRCA1 and BRCA2 genes. Thus, the positive expression of PALB2 might suggest a higher probability of developing breast cancer in male patients.3 Additionally, an active 1100delC mutation within the checkpoint kinase 2 (CHEK2) gene has been found to be associated with the growth of male breast cancer.9 It has been suggested that observing a CHEK2 mutation should raise strong clinical suspicion of breast cancer in male patients.9 Furthermore, hereditary syndromes are equally implicated in the pathogenesis of male breast cancer. Diagnosing a patient with either the Klinefelter, Lynch, Cowden, or Li-Fraumeni syndromes puts the patient at a higher risk for developing breast cancer.9 However, genetic predisposition is not solely responsible for breast carcinogenesis in a male patient. Many documented risk factors are often modifiable and associated with the patient’s lifestyle choices. Severe obesity, a sedentary lifestyle, and diabetes mellitus type 2 are strongly associated with a wide array of diseases, including breast cancer among males.10 Particularly, hormonal disequilibrium in adipose and breast tissues leads to the phenomenon of estrogen prevalence.10 It has been proposed that a high estrogen-to-androgen ratio in the male body may contribute to the progression of breast carcinomas.10 Additionally, infections that affect the testicles might exacerbate the hormonal imbalance by disrupting testosterone conversion and synthesis.3 Fortunately, certain lifestyles have been found to be protective against the development of breast cancer. Increased physical workload, an active lifestyle, and a low-carbohydrate plant-based diet are thought to act as protective mechanisms against the state of estrogen dominance in males.10
Apocrine breast carcinoma was previously classified as a subtype of NST breast carcinoma.11 Nonetheless, recent advances in the molecular classification of breast tumors have led to the discovery of “luminal androgen receptor” cancers that include a pure apocrine carcinoma type.12 Accurate expression of immunohistochemical markers in these tumors, along with an appropriate morphological picture, have been utilized to establish an accurate diagnosis of an invasive apocrine breast carcinoma.13 To meet the requirements, a pure apocrine tumor must contain 90% or more malignant apocrine cells and stain positive for AR but stain negative for ER and PR.11 If both criteria are not satisfied simultaneously, the tumor is classified as an “apocrine-like” instead of a pure breast apocrine carcinoma.13 Additionally, male apocrine breast neoplasms were found to be strongly associated with the positive expression of mammaglobin and GCDFP-15.14 Furthermore, an overexpression of both GCDFP-15 and p21 genes serve as a positive indicator for apocrine differentiation in male breast carcinomas. 14 Finally, the degree of cancer metastases and lymph node spread has been primarily predicted by the presence of these biomarkers. 14 Microscopically, apocrine tumor cells are defined by the accumulation of lipids in their cytoplasm, which gives the cells a foamy appearance. 7 Neoplastic cells can be distinguished by the presence of intranuclear helioid inclusion bodies.6 Moreover, 2 distinct apocrine cell types are typically found within the tumor, known as type A and type B cells.7 Type A cells are characterized by their eosinophilic appearance due to an accumulation of PAS-positive granular cells and an abundance of mitochondria.7 Conversely, type B cells resemble sebaceous cells or foamy histiocytes due to their microvacuolar cytoplasm.7 Some apocrine neoplasms are purely composed of type A cells and present similarly to granular cell breast tumors.7 In contrast, apocrine neoplasms containing a majority of type B cells are commonly misdiagnosed with inflammatory reactions of the breast tissue.7 The ultimate distinction is made by immunophenotyping, which reveals positive staining for potential apocrine markers. In our patient’s case, the tumor had a strong positivity towards AR, ER, and GCDFP-15. Furthermore, a single case report was published in 1981 about an apocrine carcinoma of the male breast demonstrating psammoma bodies.15 In this case study reported by Bryant, the patient had no significant past medical history or previous malignancies before his diagnosis of an apocrine breast carcinoma.15 Our patient would be the second reported case of a male who developed an apocrine breast carcinoma with numerous psammoma bodies on histology. Psammoma bodies are commonly encountered in thyroid papillary cancer, meningioma, and ovarian papillary cystadenocarcinoma, but they are rarely observed in other tumors.15 It has been suggested that the formation of psammoma bodies in apocrine breast carcinoma stems from the widespread cellular degeneration followed by dystrophic calcification.15
There is a wide range of differential diagnoses exhibiting analogous skin findings, including various types of cutaneous metastases from breast cancer, which makes our case more perplexing. There are 4 classical types of cutaneous metastases from breast cancer reported in the literature, such as erysipelas carcinomatosa, carcinoma telangiectaticum, carcinoma en cuirasse, and nodular carcinoma.16–18 The timeframe of cutaneous metastases varies greatly based on the original tumor site, but it is not uncommon to present late in the disease course due to a retrograde lymphatic spread.16 Our patient was first diagnosed with ER- and PR-positive invasive ductal breast carcinoma 9 years prior to the new finding. The newly discovered cutaneous lesion was considered to be a metastasis from a relapsed right breast cancer that developed an apocrine morphology based on an independent review by a pathologist. Given the gross presentation of the lesion, it was classified as a nodular type of cutaneous breast cancer metastases. It would be the first reported occurrence of psammoma bodies found in a cutaneous metastatic lesion originating from an apocrine male breast carcinoma.
Conclusion
Cutaneous metastases originating from apocrine breast carcinoma are rarely encountered in male patients. There are fewer than 20 case studies of apocrine male breast carcinomas reported in the literature and no reported cases demonstrating metastatic lesions to the skin. Due to the rarity of this presentation, there is also limited data on potential treatment modalities. There are ongoing studies investigating the role of anti-androgens in the care of patients with apocrine breast carcinomas that require substantial research on possible resistance to chemotherapy in these patients. The mainstay of current treatment includes conservative protocols that have been routinely utilized by clinicians, such as surgical excision and adjuvant chemotherapy.
Footnotes
Conflicts of Interest: The authors declare they have no conflicts of interest.
References
- 1. Weiss JR, Moysich KB, Swede H. Epidemiology of male breast cancer. Cancer Epidemiol Biomarkers Prev. 2005;14(1):20–26. [PubMed] [Google Scholar]
- 2. Miao H, Verkooijen HM, Chia KS, et al. Incidence and outcome of male breast cancer: an international population-based study. J Clin Oncol. 2011;29(33):4381–4386. doi: 10.1200/JCO.2011.36.8902. [DOI] [PubMed] [Google Scholar]
- 3. Fox S, Speirs V, Shaaban AM. Male breast cancer: an update. Virchows Arch. 2022;480(1):85–93. doi: 10.1007/s00428-021-03190-7. [DOI] [PubMed] [Google Scholar]
- 4. Rizzolo P, Silvestri V, Tommasi S, et al. Male breast cancer: genetics, epigenetics, and ethical aspects. Ann Oncol . 2013;24(Suppl 8):viii75–viii82. doi: 10.1093/annonc/mdt316. [DOI] [PubMed] [Google Scholar]
- 5. Anderson WF, Althuis MD, Brinton LA, Devesa SS. Is male breast cancer similar or different than female breast cancer? Breast Cancer Res Treat. 2004;83(1):77–86. doi: 10.1023/B:BREA.0000010701.08825.2d. [DOI] [PubMed] [Google Scholar]
- 6. Vranic S, Schmitt F, Sapino A, et al. Apocrine carcinoma of the breast: a comprehensive review. Histol Histopathol. 2013;28(11):1393–1409. doi: 10.14670/HH-28.1393. [DOI] [PubMed] [Google Scholar]
- 7. Sekal M, Znati K, Harmouch T, Riffi AA. Apocrine carcinoma of the male breast: a case report of an exceptional tumor. Pan Afr Med J. 2014;19:294. doi: 10.11604/pamj.2014.19.294.2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cree IA, Lokuhetty D, Tan PH. The World Health Organization classification of tumors and external quality assurance for immunohistochemistry and molecular pathology. Arch Pathol Lab Med. 2022;146(11):1303–1307. doi: 10.5858/arpa.2021-0491-RA. [DOI] [PubMed] [Google Scholar]
- 9. Hallamies S, Pelttari LM, Poikonen-Saksela P, et al. CHEK2 c.1100delC mutation is associated with an increased risk for male breast cancer in Finnish patient population. BMC Cancer. 2017;17(1):620. doi: 10.1186/s12885-017-3631-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brinton LA, Key TJ, Kolonel LN, et al. Prediagnostic sex steroid hormones in relation to male breast cancer risk. J Clin Oncol. 2015;33(18):2041–2050. doi: 10.1200/JCO.2014.59.1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. D’Arcy C, Quinn CM. Apocrine lesions of the breast: part 2 of a two-part review. Invasive apocrine carcinoma, the molecular apocrine signature and utility of immunohistochemistry in the diagnosis of apocrine lesions of the breast. J. Clin Pathol. 2019;72(1):7–11. doi: 10.1136/jclinpath-2018-205485. [DOI] [PubMed] [Google Scholar]
- 12. Lehmann BD, Bauer JA, Chen X, et al. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest. 2011;121(7):2750–2767. doi: 10.1172/JCI45014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vranic S, Tawfik O, Palazzo J, et al. EGFR and HER-2/neu expression in invasive apocrine carcinoma of the breast. Mod Pathol. 2010;23(5):644–653. doi: 10.1038/modpathol.2010.50. [DOI] [PubMed] [Google Scholar]
- 14. Kornegoor R, Verschuur-Maes AH, Buerger H, et al. Immunophenotyping of male breast cancer. Histopathology. 2012;61(6):1145–1155. doi: 10.1111/j.1365-2559.2012.04330.x. [DOI] [PubMed] [Google Scholar]
- 15. Bryant J. Male breast cancer: a case of apocrine carcinoma with psammoma bodies. Hum Pathol. 1981;12(8):751–753. doi: 10.1016/s0046-8177(81)80178-5. [DOI] [PubMed] [Google Scholar]
- 16. Baum EM, Omura EF, Payne RR, Little WP. Alopecia neoplastica--a rare form of cutaneous metastasis. J Am Acad Dermatol. 1981;4(6):688–694. doi: 10.1016/s0190-9622(81)80201-0. [DOI] [PubMed] [Google Scholar]
- 17. Mehregan AH. Metastatic carcinoma to the skin. Dermatologica. 1961;123:311–325. doi: 10.1159/000255148. [DOI] [PubMed] [Google Scholar]
- 18. Leavell UW, Jr, Tillotson FW. Metastatic cutaneous carcinoma from the breast; a clinical and pathologic study of a case showing three types of lesions. AMA Arch Derm Syphilol. 1951;64(6):774–782. doi: 10.1001/archderm.1951.01570120109012. [DOI] [PubMed] [Google Scholar]