Abstract
Background
Ventral hernias are a common but heterogeneous disease. Communication among key stakeholders (eg, patients, clinicians, administrators, payers, and researchers) can be augmented by a widely utilized classification system. The European Hernia Society (EHS) developed an expert-opinion-based hernia classification system organized by hernia type (primary versus incisional) and size. We sought to assess what components of the EHS ventral hernia classification system were correlated to real-world clinical outcomes.
Methods
This was a multicenter cohort study. All hospitals contributing to the database were affiliated with 1 of 6 academic institutions. All adult patients who underwent ventral hernia repair over a 4-year period were included. The primary endpoint was adverse events defined as any major (deep or organ space) surgical site infection (SSI), abdominal reoperation, or hernia recurrence. Utilizing a multivariable Cox regression, factors associated with adverse events were identified. Accuracy was assessed using Harrell's C concordance statistic.
Results
Of the 2385 patients who underwent repair of ventral hernias (primary n = 810, 34.0% and incisional n = 1575, 66%), with a median follow-up of 11.1 months, 27.5% suffered adverse events including major SSIs (5.7%), hernia recurrences (12.1%), and abdominal reoperations (9.7%). In the overall cohort and the primary ventral hernia subgroup, all hernia-specific variables were associated with adverse events. American Society of Anesthesiologist score, low albumin, and prior SSI were associated with adverse events in the overall cohort and primary ventral hernia subgroup while surgical approach was associated with adverse events in the overall cohort and incisional ventral hernia subgroup. On multivariable Cox regression analyses, incisional ventral hernia and larger hernia width were independently associated with adverse events.
Conclusion
Hernia size and type (primary versus incisional) from the EHS ventral hernia classification system were associated with clinical outcomes. Additional factors, including patient and operative factors, also impact outcomes. Our model allows key stakeholders to communicate more clearly regarding the challenges and outcomes of various patients with diverse ventral hernias.
Keywords: ventral hernias, EHS classification system, classification, primary ventral hernia, incisional ventral hernia, surgical wound infection, retrospective studies
Introduction
Abdominal wall or ventral hernias are among the most common surgical diseases and one-third of all patients are born with or will develop a ventral hernia. If left untreated, these hernias can affect a patient’s quality of life, diminish their function, increase in size, and risk incarceration and strangulation of bowel or other intra-abdominal organs. Ventral hernias are a diverse disease process with substantial heterogeneity in hernias types and the patients who develop them. Without validated and widely accepted nomenclature, key stakeholders (eg, patients, clinicians, administrators, payers, and researchers) often struggle to clearly communicate about the patient, their hernia, and clinical outcomes. Ventral hernias can range from a first-time umbilical hernia in young, healthy individuals that can be repaired in less than an hour in an outpatient operative setting to a large, contaminated, and recurrent ventral incisional hernia in comorbid individuals that requires hours in the operating suite and an extended inpatient stay.1,2 Not only is common nomenclature necessary for communication during clinical practice and health care resource utilization, but it is also important in hernia research.3 For example, the synthesis of studies comparing techniques for ventral hernia repair is difficult if primary (first-time umbilical or epigastric hernias) and ventral incisional hernias are not differentiated, given their different rates for complications and outcomes.4
Multiple classification systems have been developed for ventral hernias. All have limitations and few have been externally validated (Table 1).4–9 Recently, the European Hernia Society (EHS) developed a staging system using hernia-specific variables (hernia type, size, and location).4 While there is substantial interest in the use of this staging system, it was developed on expert opinion (Table 1), is theoretical and has limited patient data assessing the system. The aim of our study was to assess which components of the EHS classification system are associated with clinical outcomes along with identifying any relevant factors not included in the EHS classification system.
Table 1.
Previously Published Classification Systems*
| Author/Group year | Hernia type | Primary outcome | Primary purpose | Hernia specific parameters | Patient and treatment parameters | Developed on patient data | External validation |
|---|---|---|---|---|---|---|---|
| Muysoms et al. 20094 | Primary and incisional | Recurrence | Staging*** | Size of hernia, location of hernia, recurrent hernia | None | No | No |
| Ammaturo and Bassi. 20056 | Incisional | Recurrence | Staging | Size of hernia, ratio of anterior wall surface/wall defect surface, number of previous repairs, location | None | Yes | No |
| Dietz et al. 20077 | Primary and incisional | Recurrence | Classification | Size of hernia, number of previous repairs, location of hernia | Sternocostal angle, BMI > 25, male, nicotine abuse, age > 45, underlying disease, anemia, wound contamination, postoperative contamination, second intervention <1 month, >2 interventions within past year | No | Yes |
| Korenkov et al. 20018 | Incisional | Recurrence | Staging | Size of hernia, number of previous repairs, symptoms of hernia, reducibility, location of hernia | None | No | No |
| Berger and Liang. 201312,13 | Primary and incisional | Surgical site infection | Risk-stratification** | None | ASA, skin flaps, concomitant procedure, BMI, wound class | Yes | Yes |
| Ventral Hernia Working Group 201021 | Incisional | Surgical site occurrence | Risk-stratification | None | Smoker, BMI, diabetes, immunosuppressed, COPD, previous wound infection, stoma present, wound class (violation of gastrointestinal tract, infected mesh, septic dehiscence) | No | Yes |
| Swedish Registry. 200822 | Incisional | Recurrence | Classification | Size of hernia, number of defects, number of previous repairs, previous mesh implantation, reducibility of hernia | BMI, indication for operation, type and location of incision | Yes | No |
| Chevrel and Rath. 200026 | Incisional | Recurrence | Staging | Size of hernia, number of previous repairs, location of hernia | None | Yes | Yes |
| Schumpelick. 200027 | Incisional | Recurrence | Staging | Size of hernia, number of defects, number of previous repairs, reducibility, location of hernia | None | No | No |
Classification system is defined as: the systematic arrangement of similar entities on the basis of certain differing characteristics.
Staging system is defined as the classification of phases, quantities, or periods of a disease.
Risk-stratification is defined as: the constellation of activities to determine an individual’s risk for suffering a particular condition and need—or lack thereof—for intervention.
Abbreviations: ASA = American Society of Anesthesiologists Physical Status Classification System; BMI = body-mass index; COPD = chronic obstructive pulmonary disease
Methods
Following Institutional Review Board approval, a retrospective database of all consecutive ventral hernia repairs performed at 8 HCA Healthcare hospitals in the United States between January 2008 and December 2011 was created and evaluated. Hospitals contributing to the database were affiliated with 1 of the 6 academic institutions that participate as part of the Ventral Hernia Outcomes Collaborative. The primary outcome was chosen through a Delphi survey of 10 surgeons who were considered hernia surgery experts at their institutions and in their regions, had performed at least 50 ventral hernia repairs in the previous year, and who had presented or published hernia-related research at a national or international forum. Following interactive discussion and iterative voting, consensus for the primary outcome was adverse events defined as any major surgical site infection (SSI), hernia recurrence, or abdominal reoperation. Major SSI was defined as the Center for Disease Control’s definition of deep or organ space SSI including any prosthetic infection.10 Hernia recurrence was determined by clinical evaluation at the time of reoperation or on radiographic follow-up. 11 Abdominal reoperation was defined as any procedure following the ventral hernia repair that involved the mesh, fascia, or peritoneal cavity.12 Examples of abdominal reoperation include mesh explantation, debridement of the fascia, lysis of adhesions for small bowel obstruction, or repair of a hernia recurrence.
All data were classified as hernia-specific data (eg, hernia type, size, location, recurrent), patient-specific variables (eg, gender, ethnicity, body mass index [BMI], American Society of Anesthesiologist [ASA] score, prostate disease, chronic obstructive pulmonary disease, immunosuppression, diabetes mellitus, hemoglobin A1c [HbA1c] within 6 months of surgery, active smoking, history of prior SSI, number of prior abdominal surgeries, and albumin within 3 months of surgery), or treatment-specific variables (eg, institution, acute repair, laparoscopic versus open procedure, concomitant procedure, wound class, elevation of skin flaps, fascial release, use of mesh, mesh location, and operative duration). These variable definitions have been previously reported and are in concordance with the EHS and National Surgical Quality Improvement Project (NSQIP).4,13,14 Data collection was performed following guidelines established by NSQIP using trained abstractors and whenever possible, pre-established, local NSQIP databases.
Statistical Analysis
Categorical variables were compared using chi-square tests. Continuous variables that were normally distributed were analyzed using 2-tailed t tests, while nonparametric continuous and ordinal data variables were analyzed using Wilcoxon rank-sum or Kruskal-Wallis tests. Whenever feasible, continuous data (BMI, HbA1c, albumin, etc) were summarized as both continuous and categorical variables using standard categories.15,16
Because adverse events were a time-to-event outcome, multivariable Cox regression analyses that accounted for the duration of follow-up were utilized to identify associated variables. For all variables, the proportional hazard assumption was checked graphically. Variables violating the proportionality assumption were treated as time-dependent covariates. Using a backward-stepwise elimination based on the Aikake information criterion and Bayesian information criterion, clinically and statistically significant variables were included in the final model. Initially, models based upon EHS classification variables were utilized both as categorical data as well as continuous data. Different categories were also assessed and models with the lowest Aikake and Bayesian information criteria were selected. Model fit was assessed using Harrell's C concordance statistic. The C-statistic provides a global assessment of a fitted outcome model for the continuous event time. We utilized generally established guidelines that Harrell’s C concordance of 0.7 or greater would be considered a good fit.17,18 Subgroup analyses were determined a priori and included primary ventral hernias, incisional hernias, large incisional hernias (greater than 10 cm in width), and patients with a follow-up of greater than 1 year. All statistics were performed using StataCorp. 2013. (Stata: Release 13. Statistical Software. College Station, TX: StataCorp LP).
Results
Overall, a total of 2385 patients who underwent repair of their ventral hernias (primary n = 810, 34.0% and incisional n = 1575, 66.0%) were followed for a median of 11.1 (range 1–61) months. In addition, there were 469 (19.7%) patients who suffered 657 (27.5%) adverse events including 137 (5.7%) major SSIs, 288 (12.1%) hernia recurrences, and 232 (9.7%) abdominal reoperations.
Univariate Analyses
In the overall cohort, all of the hernia-specific variables were associated with adverse events, as well as the primary ventral hernia subgroup (Table 2). However, none of the hernia-specific variables were associated with adverse events for the ventral incisional hernia subgroup. For patient- and treatment-specific variables, only concomitant procedures, emergent repairs, wound class, skin flaps, and operative duration were associated with adverse events in all 3 cohorts (overall, primary, and incisional). ASA score, low albumin, and prior SSI were associated with adverse events in the overall cohort and primary ventral hernia cohort. The surgical approach was associated with adverse events in the overall cohort and the incisional ventral hernia cohort (Table 3).
Table 2.
Disease (Hernia)-Specific Factors
| Variable | Overall cohort N = 2385 |
Primary ventral hernia N = 810 |
Incisional ventral hernia N = 1575 |
||||||
|---|---|---|---|---|---|---|---|---|---|
| AE n = 469 (19.7%) | No AE n = 1916 (80.3%) | P value | AE n = 107 (13.2%) | No AE n = 703 (86.8%) | P value | AE n = 362 (22.9%) | No AE n = 1213 (77.0%) | P value | |
| Hernia type | - | - | <.001 | - | - | - | - | - | - |
| Primary | 107 (22.8) | 703 (36.7) | - | - | - | - | - | - | - |
| Incisional | 362 (77.2) | 1213 (63.3) | - | - | - | - | - | - | - |
| Hernia width (cm) * | 6.4 ± 5.5 | 5.4 ± 5.0 | <.001 | 2.9 ± 2.3 | 2.2 ± 1.9 | <.001 | 7.4 ± 5.7 | 7.2 ± 5.3 | .542 |
| Primary <2, Incisional <4 | - | - | - | 53 (49.5) | 465 (66.1) | <.001 | 107 (29.6) | 359 (29.6) | .956 |
| Primary 2–4, Incisional 4–10 | - | - | - | 17 (15.9) | 110 (15.7) | - | 155 (42.8) | 528 (43.5) | - |
| Primary >4, Incisional >10 | - | - | - | 37 (34.6) | 128 (18.2) | - | 100 (27.6) | 326 (26.9) | - |
| Hernia length (cm) * | 7.3 ± 6.5 | 6.0 ± 5.9 | <.001 | 3.2 ± 2.7 | 2.4 ± 2.1 | <.001 | 8.6 ± 6.8 | 8.2 ± 6.4 | .299 |
| Hernia area (cm 2 ) * | 77.9 ± 125.7 | 59.0 ± 108.2 | <.001 | 14.2 ± 26.2 | 9.0 ± 23.8 | .047 | 96.8 ± 136.9 | 87.2 ± 125.7 | .232 |
| Hernia Location | - | - | .040 | - | - | .043 | - | - | .445 |
| Medial | 418 (89.1) | 1,746 (91.1) | - | 104 (97.2) | 693 (98.6) | - | 314 (86.7) | 1053 (86.8) | - |
| Lateral | 43 (9.2) | 93 (4.9) | - | 3 (2.8) | 5 (0.7) | - | 31 (8.6) | 88 (7.3) | - |
Mean ± SD
Abbreviation: AE = Adverse events
Table 3.
Patient and Treatment-Specific Factors
| Variable | Overall cohort N = 2385 |
Primary ventral hernia N = 810 |
Incisional ventral hernia N = 1575 |
||||||
|---|---|---|---|---|---|---|---|---|---|
| AE n = 469 (19.7%) | No AE n = 1916 (80.3%) | P value | AE n = 107 (13.2%) | No AE n = 703 (86.8%) | P value | AE n = 362 (22.9%) | No AE n = 1213 (77.0%) | P value | |
| Gender | |||||||||
| Male | 245 (52.2) | 1085 (56.6) | .086 | 85 (79.4) | 542 (77.1) | .590 | 160 (44.2) | 543 (44.8) | .849 |
| Female | 224 (47.8) | 831 (43.4) | 22 (20.6) | 161 (22.9) | 202 (55.8) | 670 (55.2) | |||
| Age * | 53.1 ± 12.2* | 52.1 ± 12.8* | .136 | 52.9 ± 12.1* | 49.6 ± 12.9* | .011 | 53.2 ± 12.2* | 53.6 ± 12.6* | .539 |
| Race | |||||||||
| Caucasian | 290 (61.8) | 1,245 (65.0) | .202 | 58 (54.2) | 390 (55.5) | .805 | 232 (64.1) | 855 (70.5) | .021 |
| Others⋄ | 179 (38.2) | 671 (35.0) | 49 (45.8) | 313 (44.5) | 130 (35.9) | 358 (29.5) | |||
| ASA score | |||||||||
| 1–2 | 257 (54.8) | 933 (48.7) | .018 | 60 (56.1) | 252 (35.8) | <.001 | 197 (54.4) | 681 (56.1) | .563 |
| 3–4 | 212 (45.2) | 983 (51.3) | 47 (43.9) | 451 (64.2) | 165 (45.6) | 532 (43.9) | |||
| COPD | 40 (8.5) | 194 (10.1) | .298 | 10 (9.3) | 45 (6.4) | .259 | 30 (8.3) | 149 (12.3) | .036 |
| Prostate disease | 29 (6.2) | 161 (8.4) | .112 | 9 (8.4) | 51 (7.3) | .670 | 20 (5.5) | 110 (9.1) | .032 |
| Active smoking | 125 (26.7) | 490 (25.6) | .632 | 31 (29.0) | 172 (24.5) | .316 | 94 (26.0) | 318 (26.2) | .925 |
| Immunosuppression | 21 (4.5) | 79 (4.1) | .731 | 2 (1.9) | 16 (2.3) | .790 | 19 (5.3) | 63 (5.2) | .967 |
| Pre-operative albumin (mg/dl) * | 3.57 ± 0.7* | 3.65 ± 0.6* | <.001 | 3.7 ± 0.7* | 3.9 ± 0.4* | .030 | 3.5 ± 0.7* | 3.5 ± 0.6* | .641 |
| <3.5 mg/dl | 132 (28.2) | 444 (23.2) | .002 | 18 (16.8) | 69 (9.8) | <.001 | 114 (31.5) | 375 (30.9) | .769 |
| ≥3.5 mg/dl | 277 (59.1) | 1295 (67.6) | 68 (63.6) | 574 (81.7) | 209 (57.7) | 721 (59.4) | |||
| Unknown | 60 (12.8) | 177 (9.2) | 21 (19.6) | 60 (8.5) | 39 (10.8) | 117 (9.7) | |||
| Diabetes mellitus | 122 (26.0) | 453 (23.6) | .282 | 33 (30.8) | 133 (18.9) | <.001 | 89 (31.5) | 320 (26.4) | .494 |
| HgA1c (mg/dL) * | 6.7 ± 1.6* | 6.6 ± 1.6* | .632 | 6.8 ± 1.2* | 6.5 ± 1.3* | .203 | 6.6 ± 1.8* | 6.7 ± 1.8* | .819 |
| ≤6.5 (mg/dL) | 74 (15.8) | 287 (14.9) | .438 | 20 (18.7) | 120 (17.1) | .03 | 54 (14.9) | 167 (13.8) | .852 |
| >6.5 (mg/dL) | 48 (10.2) | 164 (8.6) | 19 (17.8) | 68 (9.7) | 29 (8.0) | 96 (7.9) | |||
| Unknown | 347 (74.0) | 1465 (76.5) | 68 (63.6) | 515 (73.3) | 279 (77.1) | 950 (78.3) | |||
| BMI (kg/m 2 ) * | 33.2 ± 7.7* | 33.0 ± 8.0* | .652 | 32.2 ± 6.0* | 32.2 ± 7.5* | .988 | 33.6 ± 8.1* | 33.6 ± 8.3* | .991 |
| ≤40 (kg/m2) | 386 (82.3) | 1621 (84.6) | .221 | 92 (86.0) | 632 (89.9) | .220 | 294 (81.2) | 989 (81.5) | .891 |
| >40 (kg/m2) | 83 (17.7) | 295 (15.4) | 15 (14.0) | 71 (10.1) | 68 (18.8) | 224 (18.5) | |||
| Prior SSI | 52 (11.1) | 144 (7.5) | .012 | 3 (2.8) | 1 (0.1) | <.001 | 49 (13.5) | 143 (11.8) | .373 |
| Prior abdominal surgery | 379 (80.8) | 1276 (66.6) | <.001 | 18 (16.8) | 71 (10.1) | .038 | 361 (99.7) | 1205 (99.3) | .396 |
| Concomitant procedures | 117 (24.9) | 293 (15.3) | <.001 | 29 (27.1) | 97 (13.8) | <.01 | 88 (24.3) | 196 (16.2) | <.001 |
| Emergent repair | 84 (17.9) | 225 (11.7) | <.001 | 22 (20.6) | 89 (12.7) | .027 | 62 (17.1) | 136 (11.2) | <.001 |
| Surgical approach | |||||||||
| Laparoscopic | 94 (20.0) | 516 (26.9) | <.001 | 23 (21.5) | 143 (20.3) | .449 | 71 (19.6) | 373 (30.8) | <.001 |
| Open | 369 (78.7) | 1372 (71.6) | 81 (75.7) | 551 (78.4) | 288 (79.6) | 821 (67.7) | |||
| Converted to open | 6 (1.3) | 28 (1.5) | 3 (2.8) | 9 (1.3) | 3 (0.8) | 19 (1.6) | |||
| Wound class ≥ 3 | 66 (14.1) | 142 (7.4) | <.001 | 10 (9.3) | 26 (3.4) | <.001 | 56 (15.5) | 116 (9.6) | <.001 |
| Elevation of skin flaps | 189 (40.3) | 548 (28.6) | <.001 | 26 (24.3) | 101 (14.4) | <.001 | 163 (45.0) | 447 (36.9) | <.001 |
| Fascial release | 88 (18.8) | 268 (14.0) | <.001 | 4 (3.7) | 19 (2.7) | .548 | 84 (23.2) | 249 (20.5) | .274 |
| Mesh | 326 (69.5) | 1381 (72.1) | .269 | 47 (43.9) | 367 (52.2) | .110 | 279 (77.1) | 1014 (83.6) | <.001 |
| Mesh location | |||||||||
| Underlay1 | 250 (53.3) | 1,014 (52.9) | .350 | 36 (33.6) | 263 (37.4) | .356 | 215 (59.4) | 750 (61.8) | .082 |
| Sublay2 | 28 (6.0) | 164 (8.6) | 7 (6.5) | 70 (9.9) | 21 (5.8) | 95 (7.8) | |||
| Inlay3 | 6 (1.3) | 26 (1.4) | 1 (0.9) | 9 (1.3) | 5 (1.4) | 17 (1.4) | |||
| Onlay4 | 13 (2.8) | 43 (2.2) | 3 (2.8) | 11(1.6) | 10 (2.8) | 35 (2.9) | |||
| Operative duration * | 154.7 ± 103.8* | 124.0 ± 110.7* | <.001 | 109.6 ± 88.7* | 82.0 (60.3)* | <.001 | 166.7 ± 104.4* | 147.4 ± 124.6* | .010 |
Mean ± SD
Others include mainly Hispanic, Black, Asian (single digit), and Indian patients, along with other races.
Abbreviations: AE = Adverse events; ASA = American Society of Anesthesiologists Physical Status Classification System; BMI = body-mass index; COPD = chronic obstructive pulmonary disease; HbA1c = hemoglobin A1c; SD=standard deviation
Table 4.
Multivariable Cox Regression for Adverse Events*
| Entire cohort n = 2385 | Primary VH n = 810 | Incisional VH n = 1575 | Large incisional VH (>10 cm width) n = 426 | Follow-up >1 year n = 1100 | |
|---|---|---|---|---|---|
| Variables | HR (95% CI) | HR(95% CI) | HR(95% CI) | HR(95% CI) | HR(95% CI) |
| Hernia-specific | |||||
| Secondary (ref: primary) | 1.59 (1.23–2.05) | - | - | 1.58 (1.14–2.21) | |
| Hernia size (width) | |||||
| Primary (ref: < 1) | |||||
| 2–4 | 1.02 (0.62–1.97) | 1.13 (0.63–2.02) | - | 1.04 (0.50–2.15) | |
| >4 | 2.37 (1.53–4.25) | 2.36 (1.41–3.96) | - | 2.25 (1.03–4.92) | |
| Secondary (ref: < 4) | |||||
| 4–10 | 1.35 (0.64–2.85) | - | 0.98 (0.75–1.29) | 0.97 (0.69–1.37) | |
| > 10 | 1.91 (1.02–3.56) | - | 1.38 (1.00–1.90) | 1.39 (0.90–2.16) | |
| Patient- and treatment-specific | |||||
| ASA class ≥3 (ref: 1 & 2) | 1.19 (0.96–1.46) | 1.69 (1.02–2.27) | 1.35 (1.08–1.69) | 1.53 (1.15–2.03) | 1.36 (1.0–1.85) |
| Albumin < 3.5 mg/dL | 1.39 (1.04–1.85) | 1.56 (0.99–2.17) | 1.11 (0.69–1.41) | 1.35 (0.95–1.91) | 1.59 (1.07–2.35) |
| Emergent repair | 1.49 (1.12–1.92) | 1.97 (1.17–3.31) | 1.33 (1.01–1.78) | 1.29 (0.89–1.87) | 1.37 (0.95–1.96) |
| Laparoscopic | Ref | Ref | Ref | Ref | Ref |
| Open repair | 0.60 (0.46–0.79) | 0.79 (0.42–1.47) | 0.76 (0.56–1.05) | 0.75 (0.52–1.1) | 0.72 (0.48–1.07) |
| Converted to open | 1.23 (0.53–2.85) | 6.52 (1.72–24.59) | 0.70 (0.21–2.03) | 1.77 (0.41–7.58) | 0.41 (0.05–3.04) |
| Skin flaps | 1.50 (1.17–1.94) | 1.11 (0.51–2.41) | 1.58 (1.20–2.07) | 1.26 (0.88–1.81) | 1.18 (0.83–1.68) |
| Mesh reinforcement | 0.77 (0.60–0.99) | 0.77 (0.47–1.25) | 0.81 (0.60–1.09) | 1.24 (0.87–1.78) | 1.00 (0.68–1.49) |
| Concomitant procedure | 1.37 (1.09–1.73) | 1.78 (1.09–2.90) | 1.25 (0.967–1.619) | 1.46 (1.03–2.06) | 1.52 (1.09–1.96) |
| Harrell's C concordance ** | 0.71 | 0.65 | 0.69 | 0.67 | 0.68 |
Adverse events = deep or organ space surgical site infection, hernia recurrence, or reoperation
Harrell’s C concordance quantifies the capacity of the estimated risk score in discriminating among subjects with different event times
Abbreviations: CI = confidence intervals; HR = hazard ratios; VH = ventral hernia
Multivariable Model
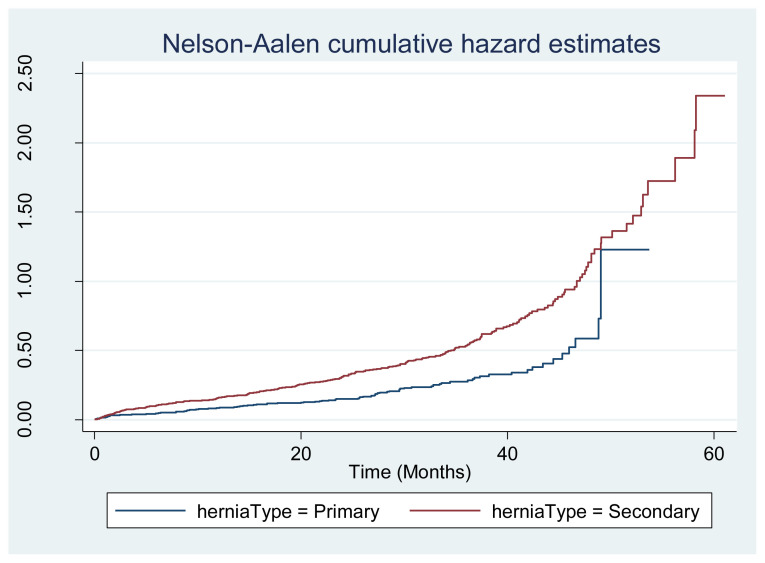
Using multivariable Cox regression analyses, several hernia factors included in the EHS classification system—hernia type (incisional hazard ratio [HR] 1.59) and larger hernia width (HR 2.37 for primary and 1.35 for incisional hernias)—were independently associated with adverse events. However, other disease factors (recurrent and medial/lateral) were not shown to be associated with adverse events in this data set. For the overall cohort, pertinent patient and treatment factors included ASA class (HR 1.19), lower albumin (HR 1.39), emergency repair (HR 1.49), repair technique (open, laparoscopic, converted to open), skin flaps (HR 1.50), mesh reinforcement (HR 0.77 decreases adverse events), and concomitant procedure (HR 1.37). The model fit was good for the overall cohort (Harrell’s C = 0.71) and maintained a Harrell’s C of 0.65–0.69 for the analyzed subgroups. Figure 1 demonstrates the HR for adverse events for primary and secondary hernias. Results were similar for primary and incisional hernias with slight variations in HRs and confidence intervals.
Figure 1.
Cumulative hazard estimates of adverse events include deep or organ-space surgical site infections, hernia recurrence, and reoperation.
Discussion
We assessed the EHS ventral hernia classification system in this study, yielding a good Harrell’s C score (0.71), which suggests that the model is moderately accurate in its predictions, specifically regarding hernia type (primary versus incisional) as well as with hernia size. However, hernia location and recurrence were not associated with adverse events. In addition to hernia-specific details, patient and operative characteristics had a substantial impact on outcomes and can be utilized to augment the EHS classification system. Disease severity utilizing the ASA score accounts for common variables associated with ventral hernia outcomes including BMI, smoking, and diabetes. Operative details such as emergent as opposed to elective procedures, use of mesh, and contamination captured by concomitant procedures were all associated with adverse events.
In choosing an endpoint, we sought to identify a variable that assessed the harms and the benefits of the disease process and treatment. With the Delphi process, it initially appeared that hernia recurrence would be a straightforward selection; however, hernia recurrence failed to address many of the potentially catastrophic outcomes of ventral hernia repair, such as mesh infection with mesh explantation (without “hernia recurrence”), enterocutaneous fistula, major wound complications, and abdominal reoperation not due to recurrence (ie, bowel obstruction). An alternative was to use abdominal reoperation as an endpoint, however, recent data suggest only 1 of 4 recurrences are reoperated on and surgeons/patients can easily choose not to reoperate despite serious complications.19 Based upon these challenges, the Delphi panel of 10 hernia surgeons chose a composite endpoint of adverse events (major SSI, hernia recurrence, or hernia reoperation) because it was believed that adverse events encompass all of the major and relevant clinical harms and benefits by assessing for an intact hernia repair without major infection or abdominal reoperation.
An important aspect of this dataset was the inclusion of hernia-specific data. Many large datasets such as the NSQIP or nationwide inpatient samples lack hernia-specific details such as hernia type or hernia size.20,21 Models derived from these datasets are difficult to assess given the lack of important disease-specific information.19,22 Alternatively, other large national databases including the American Core Health Quality Collaborative (formerly Americas Hernia Society Quality Collaborative) and a variety of national hernia datasets from across the world also provide hernia-specific information in addition to patient and procedural details. Given these limitations, the American College of Surgeons (ACS) NSQIP seeks to gather hernia-specific data to augment their information for one of the most commonly performed surgeries in the world.
In the Centers for Medicare and Medicaid Services (CMS) current procedural terminology (CPT), ventral hernia repair is classified by hernia type (primary versus incisional), recurrent, incarceration/strangulation, approach (open versus laparoscopic), and use of mesh (for open incisional only). While these types account for many of the key variables, they do not account for hernia defect size. For example, a 1 cm ventral hernia port site, a hernia that may require 1 hour to repair, has the same CPT code as a 10 cm ventral hernia from a prior open colectomy. Only the utilization of another CPT code such as for myofascial release, bowel resection, or advancement flap can account for the differences in surgeon effort and case complexity. CPT codes for ventral hernia repair changed in 2023 and the new CPT stratifies hernia repairs based on size (< 3 cm, 3–10 cm, and > 10 cm), presence of incarceration/strangulation, and prior repairs while eliminating stratification based upon surgical approach (laparoscopic vs. open).23 The new CPT does not account for hernia type (adhesions, complexity). As the CPT code changes to reflect practice patterns, it will be interesting to evaluate the impact of these newly trackable hernia characteristics upon outcome measures utilizing large databases such as NSQIP. Integrating hernia defect size along with the other variables reported in our Cox regression model into the CPT code can more accurately account for case complexity for the patient and surgeon.
Our classification system was based on a multi-institutional study with hernia-specific data; therefore, the findings should be validated and potentially refined with other datasets. We acknowledge that our findings may be outdated due to our data search date range. However, we utilized an older database to achieve longer follow-up. In addition, due to the absence of a radiographic evaluation of all patients, small recurrences may not be detected on clinical exam. However, small hernias undetectable on clinical exam have unknown clinical relevance.22 Although we used a Delphi panel of hernia experts to determine the outcome measure of interest, there are other outcomes that may be of interest to others, in particular patient-centered outcomes. Furthermore, all 8 institutions were academic teaching institutions. While a wide variety of surgeons, hernia types, and techniques were represented, the applicability of the results of this study to other institution types and patients remains to be evaluated.
Conclusion
Ventral hernias represent a diverse and complex disease. By utilizing the EHS classification system along with the clinical and operative details included, the risk of adverse events can be more clearly assessed. In addition, stakeholders have a standardized method to clearly communicate and discuss the complexity of the care involved. While further external validation is needed, our study has the potential to trigger and stimulate changes in patient selection and to allow more accurate patient counseling regarding peri-operative risk. Furthermore, it remains to be seen if potentially modifiable risk factors such as the creation of skin flaps or staging surgeries and avoiding concomitant procedures can improve surgical outcomes.
Funding Statement
This research was supported (in whole or in part) by HCA Healthcare and/or an HCA Healthcare-affiliated entity
Footnotes
Conflicts of Interest: The authors declare they have no conflicts of interest.
Drs Ali, Barrientes, Hogan, Liang, and Ms Anwoju are employees of HCA Houston Healthcare Kingwood, a hospital affiliated with the journal’s publisher.
This research was supported (in whole or in part) by HCA Healthcare and/or an HCA Healthcare-affiliated entity. The views expressed in this publication represent those of the author(s) and do not necessarily represent the official views of HCA Healthcare or any of its affiliated entities.
References
- 1. Jenkins ED, Yom VH, Melman L, et al. Clinical predictors of operative complexity in laparoscopic ventral hernia repair: a prospective study. Surg Endosc. 2010;24(8):1872–1877. doi: 10.1007/s00464-009-0863-y.. [DOI] [PubMed] [Google Scholar]
- 2. Holihan JL, Alawadi Z, Martindale RG, et al. Adverse events after ventral hernia repair: the vicious cycle of complications. J Am Coll Surg. 2015;221(2):478–485. doi: 10.1016/j.jamcollsurg.2015.04.026.. [DOI] [PubMed] [Google Scholar]
- 3. Köckerling F, Schug-Paß C, Adolf D, Reinpold W, Stechemesser B. Is pooled data analysis of ventral and incisional hernia repair acceptable? Front Surg. 2015;2:15. doi: 10.3389/fsurg.2015.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Muysoms FE, Miserez M, Berrevoet F, et al. Classification of primary and incisional abdominal wall hernias. Hernia. 2009;13(4):407–414. doi: 10.1007/s10029-009-0518-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Liang MK, Goodenough CJ, Martindale RG, Roth JS, Kao LS. External validation of the ventral hernia risk score for prediction of surgical site infections. Surg Infect (Larchmt) 2015;16(1):36–40. doi: 10.1089/sur.2014.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ammaturo C, Bassi G. The ratio between anterior abdominal wall surface/wall defect surface: a new parameter to classify abdominal incisional hernias. Hernia. 2005;9(4):316–321. doi: 10.1007/s10029-005-0016-8. [DOI] [PubMed] [Google Scholar]
- 7. Dietz UA, Hamelmann W, Winkler MS, et al. An alternative classification of incisional hernias enlisting morphology, body type and risk factors in the assessment of prognosis and tailoring of surgical technique. J Plast Reconstr Aesthet Surg. 2007;60(4):383–388. doi: 10.1016/j.bjps.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 8. Korenkov M, Paul A, Sauerland S, et al. Classification and surgical treatment of incisional hernia. Results of an experts' meeting. Langenbecks Arch Surg. 2001;386(1):65–73. doi: 10.1007/s004230000182. [DOI] [PubMed] [Google Scholar]
- 9.Bendavid R, Abrahamson J, Arregui ME, editors. Abdominal Wall Hernias: Principles and Management. 1 ed. Springer; 2001. [Google Scholar]
- 10.Surgical Site Infection (SSI) Healthcare-associated Infections (HAIs) 2010. [Accessed November 25, 2014]. https://www.cdc.gov/surgical-site-infections .
- 11. Holihan JL, Karanjawala B, Ko A, et al. Use of computed tomography in diagnosing ventral hernia recurrence: a blinded, prospective, multi-specialty evaluation. JAMA Surg. 2016;151(1):7–13. doi: 10.1001/jamasurg.2015.2580. [DOI] [PubMed] [Google Scholar]
- 12. Liang MK, Li LT, Nguyen MT, Berger RL, Hicks SC, Kao LS. Abdominal reoperation and mesh explantation following open ventral hernia repair with mesh. Am J Surg. 2014;208(4):670–676. doi: 10.1016/j.amjsurg.2013.10.024. [DOI] [PubMed] [Google Scholar]
- 13. Berger RL, Li LT, Hicks SC, Davila JA, Kao LS, Liang MK. Development and validation of a risk-stratification score for surgical site occurrence and surgical site infection after open ventral hernia repair. J Am Coll Surg. 2013;217(6):974–982. doi: 10.1016/j.jamcollsurg.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 14. Goodenough CJ, Ko TC, Kao LS, et al. Development and validation of a risk stratification score for ventral incisional hernia after abdominal surgery: hernia expectation rates in intra-abdominal surgery (the HERNIA Project) J Am Coll Surg. 2015;220(4):405–413. doi: 10.1016/j.jamcollsurg.2014.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tsereteli Z, Pryor BA, Heniford BT, Park A, Voeller G, Ramshaw BJ. Laparoscopic ventral hernia repair (LVHR) in morbidly obese patients. Hernia. 2008;12(3):233–238. doi: 10.1007/s10029-007-0310-8. [DOI] [PubMed] [Google Scholar]
- 16. Goodenough CJ, Liang MK, Nguyen MT, et al. Preoperative glycosylated hemoglobin and postoperative glucose together predict major complications after abdominal surgery. J Am Coll Surg. 2015;221(4):854–61e1. doi: 10.1016/j.jamcollsurg.2015.07.013. [DOI] [PubMed] [Google Scholar]
- 17. Longato E, Vettoretti M, Di Camillo B. A practical perspective on the concordance index for the evaluation and selection of prognostic time-to-event models. J Biomed Inform. 2020;108:103496. doi: 10.1016/j.jbi.2020.103496. [DOI] [PubMed] [Google Scholar]
- 18.Glen S.C-Statistic: Definition, Examples, Weighting and Significance. Statistics How To. https://www.statisticshowto.com/c-statistic/
- 19. Helgstrand F, Jørgensen LN, Rosenberg J, Kehlet H, Bisgaard T. Nationwide prospective study on readmission after umbilical or epigastric hernia repair. Hernia. 2013;17(4):487–492. doi: 10.1007/s10029-013-1120-9. [DOI] [PubMed] [Google Scholar]
- 20.ACS National Surgical Quality Improvement Program. American College of Surgeons; [Accessed November 14, 2024]. https://www.facs.org/quality-programs/data-and-registries/acs-nsqip/ [Google Scholar]
- 21.Abdominal Core Health Quality Collaborative (ACHQC) [Accessed November 14, 2024]. https://achqc.org/
- 22.The Swedish Abdominal Wall Hernia Registry Data. International Congress of the European Association for Endoscopic Surgery; June 2008; [Accessed November 21, 2015]. https://svenskt-brackregister.se/en . [Google Scholar]
- 23. American College of Surgeons. New 2023 CPT coding changes impact general surgery, related specialties. Bulletin of the American College of Surgeons. 2023;108(1) [Google Scholar]
- 24. Ah-Kee EY, Kallachil T, O'Dwyer PJ. Patient awareness and symptoms from an incisional hernia. Int Surg. 2014;99(3):241–246. doi: 10.9738/INTSURG-D-14-00039.1.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ventral Hernia Working G. Breuing K, Butler CE, et al. Incisional ventral hernias: review of the literature and recommendations regarding the grading and technique of repair. Surgery. 2010;148(3):544–558. doi: 10.1016/j.surg.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 26. Chevrel JP, Rath AM. Classification of incisional hernias of the abdominal wall. Hernia. 2000;4(2):94. doi: 10.1007/BF02353754. [DOI] [Google Scholar]
- 27.Schumpelick V. Narbenhernie. In: Schumpelick V, editor. Hernien. Stuttgart: Thieme; 2000. pp. 266–269. [Google Scholar]