Table S1.
Blank format of questions and possible answers provided to all participants in the online survey system.
| Do you cooperate with a Pathology Department for diagnosis of sarcoidosis? |
|
| Do you have access to a multidisciplinary team (MDD) to confirm diagnosis of sarcoidosis? |
|
| Do you have a sarcoidosis- specific institutional registry in place with clinical, functional and imaging data? |
|
| If you answered no to the previous question, do you have a database with clinical, functional and imaging data which is approved by the EC of your Institution? |
|
| Do you have in place blood sample collection? |
|
| Do you have in place BAL collection? |
|
| Do you have access to a flow cytometry and availability to store cells block? |
|
| Do you have access to or perform Bronchoscopy for BAL, EBUS and TBB? |
|
| Do you have access to cryobiopsy? |
|
| What kind of tissue specimens do you collect? |
|
| Do you have the methods in place for DNA/RNA extraction from blood samples? |
|
| Do you have the methods in place for DNA/RNA extraction from tissue sections? |
|
| What kind of sample storage conditions do you have in place? |
|
Table S2.
Geographical distributions of the participating ERN HCPs.
| Country | HCPs (N) | ERN Core Network |
|---|---|---|
| Germany | 1 | Sarcoidosis |
| Spain | 1 | Sarcoidosis |
| Italy | 3 | Sarcoidosis |
| France | 1 | Sarcoidosis |
| Netherlands | 2 | Sarcoidosis |
| Belgium | 1 | Sarcoidosis |
| Poland | 1 | Sarcoidosis |
| United Kingdom | 1 | Sarcoidosis |
| Germany | 2 | ILD |
| Spain | 1 | ILD |
| Italy | 2 | ILD |
| France | 1 | ILD |
| Netherlands | 1 | ILD |
| Ireland | 1 | ILD |
| Hungary | 2 | ILD |
| Lithuania | 1 | ILD |
| Latvia | 1 | ILD |
| Denmark | 1 | ILD |
| Austria | 1 | ILD |
| Finland | 1 | ILD |
To the editor,
Sarcoidosis is a systemic granulomatous disease included in the wide group of interstitial lung diseases (ILD). Due to its systemic nature, sarcoidosis needs a multidisciplinary approach for diagnosis and clinical management. Although significant progress has been made in understanding the pathogenesis of sarcoidosis, progression factors and treatment response still cannot be reliably predicted. Bio specimens play a key role both in clinical and translational research since they elucidate pathogenesis and pathophysiology of the various diseases. Biobanks are specialized pathology laboratories in which various types of biological samples are collected and stored. Research protocols that involve biobanking must be approved by Medical Ethics Committee and/or a relevant Institutional Review Board (1). Existing registries for ILD worldwide generally, but not systematically, include patients with sarcoidosis. In the US, a web-based sarcoidosis registry for patient-entered data has been set up and is actively collecting data (2). In Europe, ProSar, the Danish Sarcoidosis registry, started as a single center, non-randomized and observational study but can be potentially extended to other national centers (3). The present survey, promoted by the European Reference Network on rare respiratory diseases (ERN-Lung, Core Networks Sarcoidosis and ILD), aims to assess the existing sarcoidosis registries and biobanks across Europe and to compare the various types of biospecimen collected, the different procedures performed, and the sample storage conditions applied. This survey was initiated by the European Reference Network on rare respiratory diseases (ERN-Lung) Core Network “Sarcoidosis” in April 2023. The survey was launched by ERN-Lung Core Network “Sarcoidosis” in August 2023 and remained active until end of February 2024. It was disseminated to all ERN-Lung Core Network “Sarcoidosis” members (first round) and to “ILD” CN members (second round) via mail. Consent to completing the questionnaire was expressly requested before starting the online procedure. The submitted answers were registered through an online format published via online survey system (LimeSurvey, Open-Source Software provided by the University of Duisburg-Essen) and were checked for duplication. The questionnaire could be completed using any electronic or portable device such as personal computers, iPhones, or any other device equipped with internet connectivity. Collected data was subsequently included in an electronical database guaranteeing the anonymity of every participant; descriptive statistical analysis was conducted through GraphPad Prism software (version 8.0). The single questions and possible answers are shown in the supplementary Table S1. Twenty-six physicians from different healthcare providers (HCPs) responded the survey. The Figure 1 shows a flowchart of responders’ type and origin, and of all the provided answers. The geographical distribution of the centers is shown in the Table S2. Since an informed consent to publish the name of the affiliations was not collected, the results of the survey should remain anonymized. The majority of HCPs (n=14, 54%) declared a sarcoidosis-specific institutional registry in place.
Figure 1.
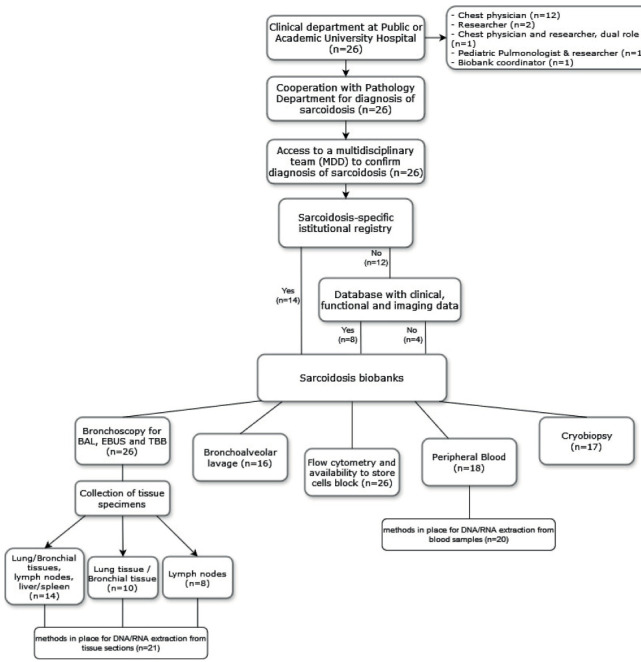
Flowchart showing the origin and type of responders, as well as a summary of the provided answers.
Our study collected the information about existing sarcoidosis registries and biobanks in centers affiliated to ERN-Lung Core Network “Sarcoidosis” and “ILD”. Despite all centers collect samples from diagnostic procedures, official biobanks are rare. None of the centers declared to actively participate into national or international registries or biobanks. At present, few examples of multi-centric national or international registries for ILD exist. All the 26 HCPs who responded our survey work at public or academic university hospital and has a multidisciplinary approach for diagnosis and management of sarcoidosis, as recommended by the most recent guidelines (4,5). This is an expected finding, since ERN affiliated HCPs undergo assessment of expertise based on caseload, number of diagnostic procedures and multidisciplinary approach, on regular basis (6). More than half of HCPs (54%) collect clinical, functional and imaging data in a sarcoidosis-specific institutional registry, the other HCPs report a local sarcoidosis or ILD database where patients’ demographics with or without functional and imaging data are collected. This finding should trigger cooperation to provide a standardisation of data collection and an agreement of which variables at baseline and over time are meaningful fur such a sarcoidosis registry. For this purpose, the experience gained by the ProSar Danish Sarcoidosis registry (3) could serve as a platform for a European Sarcoidosis Registry coordinated by ERN-LUNG. Biobanks are a crucial component of biomedical research. Biobanks facilitate sample sharing by systematically inventorying the samples and ensuring the quality of collection and storage. More than half of our participants reported to routinely collect BAL fluid, peripheral blood and lung/bronchial tissue, lymph nodes and liver/spleen tissue from sarcoidosis patients having access to freezers with temperatures ranging from low temperature to ultra-low temperature (as described in Table S1). About 80% of participants had methods in place for DNA/RNA extraction from tissue sections and blood samples. However, only few HCPs reported to collect the samples into an official certified biobank, no one into a national or international biobank. This finding points out the urgent need to connect all the local institutional biobanks to create an accredited network for sarcoidosis research. An interesting finding of the survey is related to the access to flow cytometry and cells sort facilities, which was reported by all the participating HCPs. The use of flow cytometry in the clinical laboratory has grown substantially in the past decade, but we didn’t expected such a high-rate percentage of centers applying that in the routine. This may be related to the fact that flow cytometry has been introduced as diagnostic tool in various lung diseases, including the diagnosis of sarcoidosis (7,8). This survey raises important questions about future developments. The creation of registries and biobanks specific for sarcoidosis is mandatory to improve the knowledge of sarcoidosis pathogenesis and its management. The next step should be to create a map of existing registries/databases and biobanks and connect them each other under the umbrella of ERN-LUNG. In addition, a strategy to merge all the registries or at least to provide a common template for data collection should be planned. Although there are some agreements within the European Union about data and biological material sharing policies, the centres must comply with national or even local laws and regulations, which represent a huge and time-consuming hurdle for international cooperations. The time for a call to action aimed at speeding up common regulation for data and samples sharing within Europe is now. Concluding, this survey, performed across ERN-LUNG affiliated HCPs, shows that the map of registries and biobanks specific for sarcoidosis in Europe is fragmented and needs merging, funding and coordination to allow high quality and structured research in sarcoidosis.
Acknowledgements:
This work has been conducted in accordance with the aim shared within ERN-Lung network and ERN-Lung Core Network “Sarcoidosis”.
Funding:
This research received no external funding.
Conflicts of Interest:
Each author declares that he or she has no commercial associations (e.g. consultancies, stock ownership, equity interest, patent/licensing arrangement etc.) that might pose a conflict of interest in connection with the submitted article.
Abbreviations:
BAL, bronchoalveolar lavage; ILD, interstitial lung disease; ERN-Lung, European Reference Network on rare respiratory diseases; MDD, multidisciplinary discussion team; EBUS-TBNA, endobronchial ultrasound-guided transbronchial needle aspiration; TBB, transbronchial biopsy
Author Contributions:
M.d., E. Bal, F.B., conceptualization and designing research studies; M.d., E. Bal, F.B., acquiring data; M.d., analyzing data; M.d., T.P., L.B., P.C., E. Bar., E. Bal., E. Bor, F.B., visualization and supervision; all authors, writing the manuscript. All authors have read and agreed to the published version of the manuscript.
References
- Kang B, Park J, Cho S, et al. Current status, challenges, policies, and bioethics of biobanks. Genomics Inform. 2013 Dec;11(4):211–7. doi: 10.5808/GI.2013.11.4.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerke AK, Tang F, Cozier YC, et al. A web-based registry for patients with sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2017;34(1):26–34. doi: 10.36141/svdld.v34i1.5129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- M⊘ller J, Hilberg O, Bendstrup E. Design and rationale of ProSar, the first Danish sarcoidosis registry. Sarcoidosis Vasc Diffuse Lung Dis. 2022;38(4):e2021044. doi: 10.36141/svdld.v38i4.11605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crouser ED, Maier LA, Wilson KC, et al. Diagnosis and Detection of Sarcoidosis. An Official American Thoracic Society Clinical Practice Guideline. Am J Respir Crit Care Med. 2020 Apr 15;201(8):e26–51. doi: 10.1164/rccm.202002-0251ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baughman RP, Valeyre D, Korsten P, et al. ERS clinical practice guidelines on treatment of sarcoidosis. Eur Respir J. 2021 Dec 16;58(6):2004079. doi: 10.1183/13993003.04079-2020. [DOI] [PubMed] [Google Scholar]
- ERN-LUNG | Rare Respiratory Diseases [Internet]. [cited 2024 May 28]. How to join ERN-LUNG. Available from: https://ern-lung.eu/for-clinicians/join-ern-lung/ [Google Scholar]
- Mortaz E, Gudarzi H, Tabarsi P, et al. Flow cytometry applications in the study of immunological lung disorders. Iran J Allergy Asthma Immunol. 2015 Feb;14(1):12–8. [PubMed] [Google Scholar]
- Magallon RE, Harmacek LD, Arger NK, et al. Standardization of flow cytometry and cell sorting to enable a transcriptomic analysis in a multi-site sarcoidosis study. PLoS One. 2023;18(3):e0281210. doi: 10.1371/journal.pone.0281210. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.
Blank format of questions and possible answers provided to all participants in the online survey system.
| Do you cooperate with a Pathology Department for diagnosis of sarcoidosis? |
|
| Do you have access to a multidisciplinary team (MDD) to confirm diagnosis of sarcoidosis? |
|
| Do you have a sarcoidosis- specific institutional registry in place with clinical, functional and imaging data? |
|
| If you answered no to the previous question, do you have a database with clinical, functional and imaging data which is approved by the EC of your Institution? |
|
| Do you have in place blood sample collection? |
|
| Do you have in place BAL collection? |
|
| Do you have access to a flow cytometry and availability to store cells block? |
|
| Do you have access to or perform Bronchoscopy for BAL, EBUS and TBB? |
|
| Do you have access to cryobiopsy? |
|
| What kind of tissue specimens do you collect? |
|
| Do you have the methods in place for DNA/RNA extraction from blood samples? |
|
| Do you have the methods in place for DNA/RNA extraction from tissue sections? |
|
| What kind of sample storage conditions do you have in place? |
|
Table S2.
Geographical distributions of the participating ERN HCPs.
| Country | HCPs (N) | ERN Core Network |
|---|---|---|
| Germany | 1 | Sarcoidosis |
| Spain | 1 | Sarcoidosis |
| Italy | 3 | Sarcoidosis |
| France | 1 | Sarcoidosis |
| Netherlands | 2 | Sarcoidosis |
| Belgium | 1 | Sarcoidosis |
| Poland | 1 | Sarcoidosis |
| United Kingdom | 1 | Sarcoidosis |
| Germany | 2 | ILD |
| Spain | 1 | ILD |
| Italy | 2 | ILD |
| France | 1 | ILD |
| Netherlands | 1 | ILD |
| Ireland | 1 | ILD |
| Hungary | 2 | ILD |
| Lithuania | 1 | ILD |
| Latvia | 1 | ILD |
| Denmark | 1 | ILD |
| Austria | 1 | ILD |
| Finland | 1 | ILD |


