Abstract
Background:
Sarcoidosis is a granulomatous disease of unknown origin. Conventional laboratory and imaging modalities may lead to equivocal conclusions for sarcoidosis diagnosis. 68Ga-citrate PET/CT has been utilized in the diagnosis of inflammatory and infectious diseases due to its better diagnostic yield.
Objectives:
This study was designed to determine the clinical trenchancy of a novel tracer 68Ga-citrate in sarcoidosis patients as an alternative radiopharmaceutical that binds to the somatostatin receptors of the inflammatory cells in sarcoid granulomas because current laboratory and imaging modalities may occasionally lack accuracy for sarcoidosis diagnosis. Conventional laboratory data including the clinic, serum biochemistry, and thorax CT findings were compared with the 18F-FDG PET/CT and 68Ga-citrate PET/CT imaging manifestations to evaluate the diagnostic yield of each modality in sarcoidosis.
Methods:
Forty-four sarcoidosis patients were included in the study. Conventional laboratory investigation and thorax CT were performed in 44 while 68Ga-citrate PET/CT and 18F-FDG PET/CT were done in 22 patients. Findings of each modality were analyzed in regard to the diagnostic yield for granulomatous activity assessment, extrapulmonary organ involvement identification, and detection of coexistent malignant disease in sarcoidosis patients.
Results:
68Ga-citrate PET/CT revealed a significantly higher diagnostic yield for sarcoidosis compared to conventional laboratory and 18F-FDG-PET/CT imaging modalities. 68Ga-citrate PET/CT revealed 72.7% sensitivity for the detection of granulomatous inflammation that was consistent with the final diagnosis of sarcoidosis. Diagnostic yield for biopsy site determination, discrimination between active inflammation and fibrosis was significantly higher by 68Ga-citrate PET/CT. 18F-FDG only displayed a slightly higher clinical expediency over 68Ga-citrate for diagnosis of malignancy.
Conclusions:
68Ga-citrate PET/CT was conclusive for the diagnosis, activity assessment, and identification of extrapulmonary organ involvement in sarcoidosis patients. As an innovative novel tracer, 68Ga-citrate identified disease activity, extrapulmonary organ involvement, and biopsy sites with a higher statistical diagnostic yield compared to the conventional laboratory findings in sarcoidosis due to its more advanced chemical properties. 18F-FDG only provided a slightly higher clinical yield with a negligible significant statistical difference compared to 68Ga-citrate for the detection of coexistent malignant disease among sarcoidosis patients.
Keywords: Sarcoidosis, 68Ga-citrate PET/CT, 18F-FDG PET/CT, Malignancy, Non-caseating granulomatous inflammation
Introduction
Sarcoidosis is a chronic, granulomatous disease of unknown origin that can involve any organ in the body with approximately more than 90% lung involvement. The airways and interstitium are the most common areas of inflammation (1-4). Accurate diagnosis, assessment of granulomatous inflammation activity, and identification of extrapulmonary organ involvement in sarcoidosis patients is a diagnostic challenge due to the revealed equivocal manifestations of the currently utilized laboratory or imaging modalities. Determination of granulomatous inflammatory activity and extent of sarcoidosis emerge as a crucial defiance in clinical practice while 18F-FDG-PET/CT exhibited a significant contribution in this regard. Existence of activated leukocytes, macrophages, and CD4 T-lymphocytes with epithelioid and multinucleated giant cells lead to significantly increased 18F-FDG uptake intensity in sarcoidosis that may reverberate disease activity. 18F-FDG PET/CT may also detect occult disease sites that are undetectable clinically or by conventional imaging modalities in 15% of the patients. Despite its beneficial clinical use, 18F-FDG PET/CT may yield substantial equivocal findings for the diagnosis and assessment of sarcoidosis (5-12) that may entail the use of other tracers with a better uptake by the noncaseating granulomatous lesions.
68Ga-citrate labeled somatostatin-based receptor hybrid imaging emerges to be a sensitive nuclear modality for the diagnosis of inflammatory or infectious diseases that appears to be a significant alternative to other previous nuclear tracers such as 67Ga-citrate and 18F-FDG both for diagnosis and disease activity assessment in sarcoidosis patients along with the detection of extrapulmonary organ involvement. With similar chemical properties, the high diagnostic yield of 68Ga-citrate relevant to its high-energy protons, better spatial resolution, lower radiation dose, short half-life, low dosimetry, and short application time may be used to yield higher image resolution of PET/CT scanners compared to the conventional tracers formerly used in sarcoidosis patients. In addition, the homologous ferric cation of 68Ga-citrate binding to plasma proteins with its stability in the blood pool for up to two hours is another feature that may provide a much better visualization of lymph nodes and other involved organs in sarcoidosis (13-17). This new nuclear imaging modality that utilizes an innovative novel radiopharmaceutical 68Ga-citrate may lead to a significant contribution in terms of diagnosis and activity assessment of granulomatous inflammation in clinical practice with a significantly higher yield as well as the detection of coexistent occult malignancy in sarcoidosis patients.
In the current study, 68Ga-citrate was utilized as a novel tracer for diagnosis, assessment of disease activity and detection of extrapulmonary organ involvement in sarcoidosis patients. The results were compared with the conventional laboratory manifestations including the traditional imaging modalities, and the 18F-FDG PET/CT findings. The second aim of the study was to identify the presence of extrapulmonary organ involvement to determine the biopsy sites in sarcoidosis patients. The third goal was the diagnosis of occult malignant disease that may emerge with a considerable incidence among sarcoidosis patients. Although preliminary and introductory, our study reveals that PET/CT utilizing 68Ga-citrate as a tracer appears to be a useful alternative imaging modality for diagnosis, activity assessment of granulomatous inflammation, and biopsy site detection in sarcoidosis with an explicit diagnostic yield for recondite malignancy.
Materials and methods
The study was done at the Cerrahpasa Medical Faculty of Istanbul Cerrahpasa University between 2020 May and June 2022. The Ethical Committee authorized the experiment for human patients and approved (Ethical Committee registration number of is 604.01.02-73300 and the inscription date is 12/04/2021) the virtuous aspects of the study. A total of 44 patients, 26 female (59.1%), with a mean age of 52, 6±14,8 years were included in the study. Thirty patients were smokers (32±16.4 pack-year) while none had a comorbid disease. Patients with a previous diagnosis of sarcoidosis dubious for a relapse or suspicious for sarcoidosis diagnosis were included in the study to determine granulomatous inflammatory activity, organ involvement, and biopsy sites. The patients were not under any kind of anti-inflammatory or immunosuppressive treatment during the study period and were randomly assigned for 18F-FDG and 68Ga-citrate PET/CT scanning.
Twenty-four patients (54.5%) presented with dyspnea, 20 (45.4%) dry cough, 18 (40.9%) lassitude, 14 (31.8%) cutaneous lesions, 12 (27.3%) had ocular manifestations, 8 patients (18.1%) with myalgia, and six (13.6%) had night sweats. All subjects had routine serum biochemistry, ECG, chest x-ray, pulmonary function tests, DLCO, and thorax CT. ACE, serum, and 24/h urine Ca levels were measured in each subject. For the assessment of sarcoidosis activity, conventional clinical criteria including worsening of patient symptoms, laboratory findings consisting of serum ACE, gamma-globulins, serum calcium levels, 24/h urinary Ca along with chest x-ray and thorax CT manifestations were evaluated. A definitive diagnosis of sarcoidosis was confirmed in all patients: by assessment pathologic findings compatible with sarcoidosis in the biopsy samples taken from at least two organs; exclusion of other similar granulomatous, infectious, autoimmune lung, or systemic disorders in the differential diagnosis; and a minimum of three years follow-up period in every case. Malignancy was confirmed by pathologic assessment of the tissue samples from the involved organs.
Different chelators such as DOTA, NOTA, and DTPA aim at different molecular targets for the diagnosis of bone infections or the imaging neuroendocrine tumors. Accordingly, 68Ga-ctirate was synthesized by Modular-Lab Standart (Eckert Zieckler) in the Nuclear Medicine department of the Cerrahpasa Medical faculty and was utilized for the evaluation of sarcoidosis patients for the first time in literature to identify disease activity, extrapulmonary organ involvement, and to determine the biopsy sites that could not be identified with the conventional imaging modalities. A five mm scan thickness was performed with PET/CT (Siemens Biograph Horizon) and emission scanning was obtained in the caudo-cranial direction with a 10 for thorax and 1.5 minutes duration for extrapulmonary organs following intravenous tracer injection, respectively.
Forty-four patients, 16 with a previously and 28 with recently diagnosed sarcoidosis patients were included in the study. All patients had conventional laboratory, pulmonary function tests and radiologic modalities including chest x-ray and thorax CT. Of these patients 22 underwent 68Ga-citrate PET/CT imaging while 22 had 18F-FDG PET/CT. The patients were categorized into three groups as the conventional, 18F-FDG PET/CT, and the 68Ga-citrate PET/CT category. The three groups were compared for the yield of the conventional laboratory, 18F-FDG PET/CT, and the 68Ga-citrate PET/CT imaging manifestations for sarcoidosis diagnosis, assessment of disease activity, and organ involvement along with the identification of coexistent malignancy. SSPS version 21 was used for statistical analysis. The variables were denoted as mean and standard deviation. Kolmogrov-Smirnov, Shapiro Wilk tests, Q-Q plot and histogram graphics were performed to evaluate normal distribution. Pearson Chi-square test was used for adequate, and Fisher’s exact test was done for inadequate survey situations. For multiple comparison, Bonferroni correction was applied for statistically significant analytic data. Spearman correlation was utilized for the evaluation of data with an abnormal distribution. A p value of less than 0.05 was accepted statistically significant.
Results
Twenty-eight were diagnosed with active sarcoidosis according to the conventional clinical criteria. Mean ACE, serum calcium and the mean 24 h urinary calcium was 28±16.4 IU/L, 9.31±0.45 mg/dL and 151±46 mg/dL, respectively. Chest x-ray revealed stage I in 14 (32.1 %), stage II in 18 (%40.1), stage III in eight (18.2%), and stage IV sarcoidosis in two (4.5%) patients. Mean PFT percentages were as follows: FVC: 92.4±18.6% FEV1: 82.8±17.9%, FEV1/FVC: 71.5±18.4, and DLCO/VA: 87.6±16.4. Pulmonary function tests revealed obstructive in eight (19.0%), restrictive defect in six (28.6%), and diffusion abnormality in 20 (48%) patients. Thorax CT revealed mediastinal lymphadenopathy in 32 (82.1%) with upper lobe predominant parahilar, peribronchovascular, or subpleural nodules in 26 (66.7%) subjects.
Bronchoscopic examination was normal in 12 (28.6%) while BAL lymphocyte mean ratio and CD4/CD8 value was 50±18% and 3.78±1.82, respectively. Noncaseating granulomatous inflammation was detected by bronchial biopsy in 24 (57.1%) and by transbronchial biopsy in 20 (47.6%) of the specimens. Histopathologic examination revealed granulomatous inflammation compatible with sarcoidosis in 12 lymph nodes, one open lung, one liver, one bone marrow, and one bone biopsy of the tissue samples. The percentage distribution of findings consistent with the definitive diagnosis of sarcoidosis, activity of granulomatous inflammation, organ involvement, and detection of malignant disease data identified by the conventional criteria, 18F-FDG, and 68Ga-citrate PET/CT imaging are shown in Table 1. Active sarcoidosis was identified in 28 (63.6%) patients by clinical criteria including symptoms, PFTs, conventional laboratory, chest x-ray, thorax CT, BAL, and bronchoscopy findings.
Table 1.
Distribution of conventional laboratory, 18F-FDG, and 68Ga-citrate PET/CT imaging manifestations among sarcoidosis patients.
| Conventional laboratory* | 18F-FDG PET/CT | 68Ga-citrate PET/CT | ||||
|---|---|---|---|---|---|---|
| Patient # | % Dx | Patient # | %Dx | Patient # | %Dx | |
| Granulomatous inflammation | 12 | 27.2 | 14 | 63.6 | 18 | 81.6 |
| Extrapulmonary organ disease | 7 | 15.9 | 10 | 45.4 | 16 | 72.7 |
| Biopsy site identification | 8 | 18.1 | 6 | 27.2 | 12 | 54.5 |
| Discrimination of fibrotic and active parenchymal disease | 4 | 9.1 | 5 | 22.7 | 14 | 63.6 |
| Detection of occult malignancy | 6 | 13.6 | 8 | 36.4 | 6 | 27.3 |
*Conventional laboratory includes blood count, serum biochemistry, chest x-ray, and thorax CT.
68Ga-citrate PET/CT revealed compatible findings with active granulomatous inflammation in 18 (81.8%) and identified extrapulmonary organ involvement in 16 (72.7%) patients (p<0.001). Mean SUVmax was 6.8±2.4 ranging from 3.2 to 9.6 among sarcoidosis patients. Of these patients one had cerebral, two had liver, two had lacrimal gland, three had parotid, and eight had lymph node involvement compible with granulomatous inflammation (Figure 1, Figure 2). 18F-FDG PET/CT revealed a mean SUVmax of 4.6±2.8 and identified active granulomatous inflammation in 14 (63.6%), and extrapulmonary organ involvement in 10 (45.4%) patients (p<0.05). A statistically significant (p<0.001) difference between 68Ga-citrate and 18F-FDG PET/CT was observed for the detection of active granulomatous inflammation and organ involvement due to sarcoidosis exhibiting a clinically noteworthy discrepancy. 18F-FDG PET/CT identified occult malignancy in six (27.2%) while 68Ga-citrate PET/CT displayed high tracer uptake in four (18.2%) patients (Figure 3, Figure 4). The overall sensitivity of 68Ga and 18F-FDG PET/CT for the detection active granulomatous inflammation was found to be 72.7% and 63.6%, respectively (p<0.05). Identification of extrapulmonary organ sarcoidosis substantially higher by 18Ga-citrate compared to 18FDG- PET/CT imaging. The statistical denotation revealed an acceptable or a mildly distinctive or an explicit difference (p<0.062) was observed between the two nuclear imaging modalities for the diagnosis of malignant disease (Table 2). A clinically slight distinctive difference was noted on behalf of 18F-FDG PET/CT compared to 18Ga-citrate PET/CT for occult malignancy diagnosis among sarcoidosis patients with a trivial statistical difference.
Figure 1.
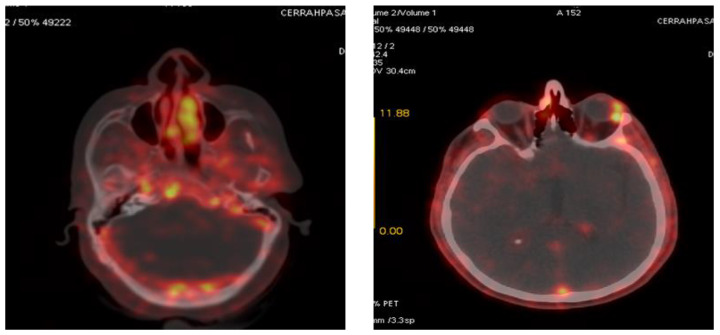
68Ga-citrate PET/CT revealing significantly increased avidity within a granulomatous lesion at the right posterior cavernous sinus and a focal granuloma with a distinctive uptake at the left anterior orbita.
Figure 2.
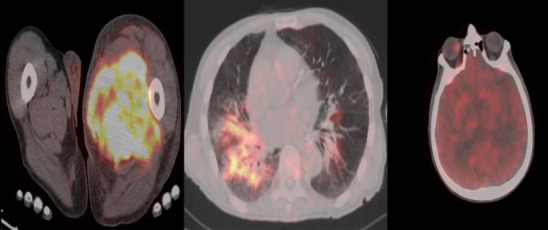
68Ga-citrate PET/CT images showing increased tracer uptake in the left leg muscles, right lung parenchyma, and right orbita.
Figure 3.
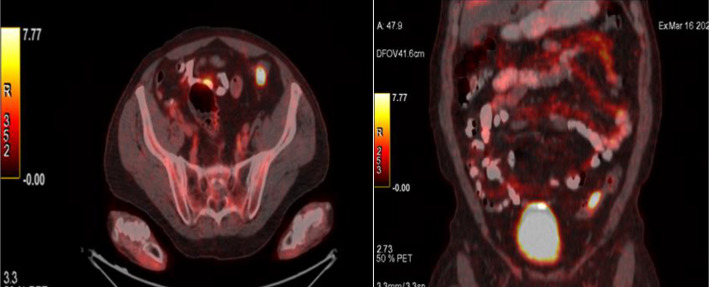
Axial and coronal 68Ga-citrate PET/CT revealing a high tracer uptake at the left lower quadrant lesion identifying colon carcinoma.
Figure 4.
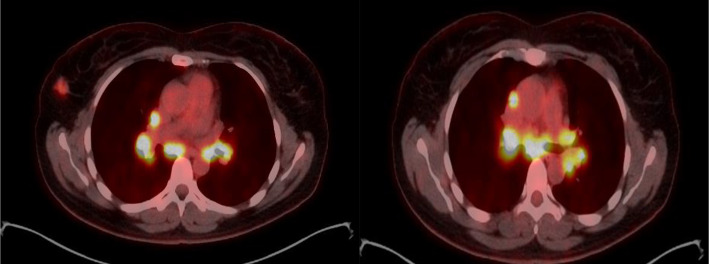
Axial 68Ga-citrate PET/CT showing a high tracer uptake at the hilar lymph nodes associated with sarcoidosis and at the right breast due to carcinoma.
Table 2.
Statistical analysis and correlation between the conventional laboratory findings, 18F-FDG, and 68Ga PET/CT findings.
| Conventional laboratory | 18F-FDG PET/CT | 68Ga-citrate PET/CT | ||||
|---|---|---|---|---|---|---|
| r | p | r | p | r | p | |
| Granulomatous inflammation | 0.32 | <0.18 | 0.68 | <0.05 | 0.86 | <0.01 |
| Extrapulmonary organ disease | 0.36 | <0.24 | 0.72 | <0.05 | 0.84 | <0.05 |
| Biopsy site identification | 0.28 | <0.32 | 0.74 | <0.01 | 0.82 | <0.01 |
| Discrimination of fibrotic and active parenchymal lung disease | 0.34 | <0.28 | 0.68 | <0.05 | 0.88 | <0.01 |
| Detection of occult malignancy | 0.62 | <0.08 | 0.86 | <0.01 | 0.72 | <0.05 |
r: correlation coefficient, p: probability value.
Discussion
Sarcoidosis is a chronic disease of unknown origin characterized by the presence of noncaseating granulomas most commonly in the lungs. Any other extrapulmonary organ including the lymph nodes, eyes, skin, liver, spleen, brain, muscle, or bone may also be involved in sarcoidosis. None of the organs have immunity against the granulomatous inflammation of sarcoidosis (1,3). Current laboratory and imaging modalities have not achieved sufficient sensitivity relevant to the outstanding drawbacks of diagnosis, activity assessment of sarcoidosis along with the identification of biopsy sites for feasible granulomatous inflammation. Consequently, diagnosis of sarcoidosis and detection of granulomatous inflammation arise as a diagnostic challenge for clinicians. The results of our study indicate that 68Ga-citrate PET/CT may lead to new horizons in terms of diagnosis, identification of disease activity, and biopsy sites in sarcoidosis patients. It is also extremely evident that 68Ga-citrate PET/CT may provide great benefit for the identification of extrapulmonary organ involvement for occult granulomatous inflammation that cannot be diagnosed by the conventional laboratory or imaging modalities. 68Ga-citrate PET/CT imaging may also determine accompanying delitescent malignant disease coexistent with sarcoidosis. Because there are no definitive or specific laboratory and imaging features that can identify sarcoidosis, reveal asymptomatic extrapulmonary organ involvement, and detect occult coexisting malignant disease, this stalemate arises as the most explicit indication for the application of 68Ga-citrate PET/CT in sarcoidosis patients.
18F-FDG PET/CT has been used to identify granulomatous inflammation activity and to detect organ involvement lesions in sarcoidosis as well as the evaluation of cardiac sarcoidosis with variable sensitivity (18-22). Currently, novel radiotracers may improve the utility of PET/CT imaging for sarcoidosis diagnosis by procuring better image resolution due to more advanced chemical properties. In a small study (24 sarcoid, 17 lung cancer), the combination of 18F-FDG and fluorine-18-methyltyrosine (18F-FMT) PET/CT differentiated sarcoidosis from malignancy; sarcoid lesions were positive on 18F-FDG PET/CT, but negative on 18F-FMT PET while both were positive in cancer patients (23). None of these nuclear imaging modalities have reached an adequate sensitivity and specificity for sarcoidosis diagnosis or differentiation from other diseases such as malignancy or infection since they may yield undistinctive positive findings in a significant number of cases. Consequently, we introduced the 68Ga-citrate labelled tracer for the assessment of sarcoidosis patients that has been used previously for the diagnosis of various inflammatory and infectious diseases. As a novel tracer with similar properties, 68Ga-citrate may set forth a higher diagnostic yield in sarcoidosis due to its high energy protons, short half-life, low radiation, short application duration along with a low dosimetry. The main theme was that the unique properties of this tracer would lead to much better imaging yield for the diagnosis of granulomatous inflammation in sarcoidosis due to its higher image resolution properties. The other crucial aspect was that the stability of this tracer for two hours in the blood pool would better discriminate lymph nodes or other involved organs in these patients (13-17). As the results of our study indicate, 68Ga-citrate as a novel tracer may set forth a great excellence over other previously used agents in the diagnosis of sarcoidosis.
The innovative tracer 68Ga-citrate will most likely increase the diagnostic yield for granulomatous inflammation, extrapulmonary organ involvement, and the identification of biopsy sites with a better discrimination from infectious diseases or malignancy that emerges as a dilemma among sarcoidosis patients. Another hallmark was the simultaneous detection of occult asymptomatic malignant disease accompanying sarcoidosis with 68Ga-citrate imaging. 68Ga-citrate PET/CT revealed a higher potential diagnostic yield for all aspects of sarcoidosis than the 18F-FDG PET/CT including diagnosis, determination of granulomatous inflammatory activity, and detection of extrapulmonary organ involvement. On the other hand, the two nuclear imaging modalities did not exhibit a remarkable difference for malignancy diagnosis while 18F-FDG PET/CT revealed a a slightly higher but an imperceptible statistical significance than the 68Ga-citrate PET/CT that may indicate a more noteworthy or prevailing diagnostic accuracy in clinical practice. The hallmark of our study was the detection of granulomatous inflammation in the extrapulmonary organs that were asymptomatic, clinically silent, and undetected by any of the current laboratory or imaging modalities. Such a result would completely change the patient treatment profile when the extrapulmonary organ involvement is considered, particularly when the treatment of sarcoidosis is considered.
The first limitation of our study is the small sample size consisting of only forty-four patients as a preliminary study. Second, it is well-known that sarcoidosis shows a variable course or disease prognosis among patients due to individual patient profile, hereditary or racial factors. Genetic features are the hallmark for the clinical perspective and prognosis of sarcoidosis patients. The Caucasian origin of all the participants may have influenced the corollary of our study. Thirdly, activity of granulomatous inflammation at the time of PET/CT imaging may have affirmatively or adversely effected the sensitivity because the presence of active granulomatous inflammation provides a much more pronounced image resolution than the fibrotic disease or for cases of suppressed inflammation by the treatment due to the decreased current intensity of the granulomatous inflammation. 18F-FDG PET/CT revealed a slightly higher statistical and clinical significance compared to 68Ga-citrate for the detection of occult malignancy in sarcoidosis patients. It seems highly probable that such a difference may have arisen due to the small number of subjects. Consequently, studies with larger sample sizes involving patients with different genetic, racial, and disease profiles along with patients under treatment or with fibrotic disease are required for further assessment of 18Ga-citrate utility as a novel tracer in sarcoidosis. Stages of sarcoidosis at the time of imaging may also have affected the sensitivity as negative pulmonary 18F-FDG-PET/CT findings were common in patients with radiographic stage 0, I, and IV sarcoidosis while best results have been achieved among patients with stage II or III disease. A similar theoretical explanation may also be pertinent for the 68Ga-citrate PET/CT findings (9-12). Other studies have achieved higher diagnostic yield with 18F-FDG-PET/CT in stage 0, I and IV sarcoidosis patients compared to our study (8-10) that can be explained by the intensity of the active granulomatous inflammation, fibrotic granulomatous tissue load, treatment status during the study period, as well as racial or genetic characteristics of the patients. Our findings for 18F-FDG-PET/CT may be associated with the low intensity of granulomatous inflammation or the high fibrotic tissue load at these stages. The diagnostic superiority of 68Ga-citrate can be explained by the more effective and superior uptake of this novel radioactive tracer in granulomatous foci that ensures a better image resolution during stage 0, and I where granulomatous inflammation is at its initial phase with a low intensity and stage IV where active granulomatous inflammation is replaced by fibrosis.
Dimension or the structural nature of the granulomatous or inflammatory lesions may have a negative impact on the image nature as 18F- FDG PET/CT reveals lack and loss of tracer uptake in nodules smaller than 8 to 10 mm in diameter or for infiltrative lesions such as adenocarcinoma as well as the prevalence and the consistence of the granulomatous nodules. As this is the first study using 68Ga-citrate as a radiotracer, application technique may also involve discordant features although a standard implementation technique was utilized. In future studies, with a more advanced 68Ga-citrate application procedure, the diagnostic yield may yet be further promoted in sarcoidosis patients. Despite the inadequate of our potential data, 68Ga-citrate may come out as a novel tracer for the diagnosis of sarcoidosis along with possible identification of occult malignant disease thereby eliminating the redundant interventions that may lead to a diagnostic delay in sarcoidosis patients. Likewise, it should not be forgotten that the simultaneous application of 68Ga-citrate and 18F-FDG PET/CT in the same patients would reveal much more accurate results in terms of statistical assessment, but such an application was unfortunately not be possible due to the high radiation dose patients would receive and the increased financial burden that will arise from simultaneous application of two similar nuclear imaging modalities in the same patient.
As a noninvasive and an easily applicable intervention, revealing high image quality, able to scan all body parts simultaneously with enduement of expeditious consequences appear to be the most auspicious or encouraging factors of 68Ga-citrate PET/CT for the diagnosis and activity assessment of sarcoidosis patients in clinical practice. Although if not diagnostic by itself, 68Ga-citrate PET/CT displays a great diagnostic potential in cases of equivocal sarcoidosis cases by revealing unexpected and rarely involved organ biopsy sites that cannot be detected by any other current laboratory and imaging modalities (24,25). 68Ga-citrate PET/CT may reveal a congruent significance and an appreciable diagnostic effect as the Kveim test (26) but may be more efficient for detecting concomitant organ involvement that may be revealed by future prospective studies. In cases where 68Ga-citrate PET/CT reveals inconclusive results for sarcoidosis, it is probable to achieve a plausible result with collaboration of other conventional laboratory or imaging manifestations. Another potential beneficial effect of 68Ga-citrate administration is that it may eliminate the requirement for 18F-FDG PET/CT for sarcoidosis patients with a coexistent occult or suspicious malignant disease. This novel non-invasive nuclear imaging procedure displays a better image quality for granulomatous inflammation and can scan all body parts simultaneously as a time-saving modality that reveals ultimately expeditious results along with a significant diagnostic potential for malignant diseases.
Detection of occult extrapulmonary organ involvement in sarcoidosis patients emerges as the most relevant and expedient trenchancy of this nuclear modality. The relatively high incidence of occult malignancy may be associated with the conspicuous prevalence of smoking and advanced age among our patients or may have emerged due to the relatively small sample size of our study. With this aspect, the new tracer will provide outstanding utility for both in detecting biopsy sites for cases with suspected diagnosis and in determining treatment requirement. We firmly believe that the novel 68Ga-citrate PET/CT will take its place as the most successful, convenient, and the practical implementation for the diagnosis and activity assessment of sarcoidosis patients as it will also obviate the use of 18F-FDG PET/CT for a probable occult malignant disease coexistent with sarcoidosis. Discrimination of active granulomatous inflammation and fibrotic lung disease due to the high tracer uptake in active disease and the negligible or lack of 68Ga-citrate tracer activity detected in the fibrotic lung parenchyma of stage IV patients will also discriminate between active and fibrotic lung disease thereby determining an optimal treatment option. Although this aspect was not evaluated in our study, we believe that this imaging modality will also provide useful data for the assessment of treatment response in sarcoidosis by detection of inflammatory and fibrotic foci in the lung as well as any organ of the body.
Conclusions
This is the first study to evaluate the diagnostic yield of 68Ga-citrate PET/CT for the diagnostic assessment of sarcoidosis patients. Fundamental features of 68Ga-citrate as an innovative tracer are its low cost, the shorter acquisition time that may lead to a much better granulomatous inflammation image achievement, its contribution to advanced image resolution and quality due its chemical properties, as well as the determination of biopsy sites along with the identification of an occult coexistent malignant disease. Our study has revealed that 68Ga-citrate PET/CT can lead to new horizons in clinical practice that emerges as a comprehensive and integral imaging modality for a full assessment of sarcoidosis patients. The yield of the 68Ga-citrate PET/CT may further be increased in terms of sarcoidosis diagnosis and activity assessment when this modality is collaborated with the conventional clinical procedures. Another crucial aspect of 68Ga-citrate as a tracer is the detection of tissue biopsy sites for sarcoidosis that cannot be identified by the conventional laboratory or imaging modalities, particularly for relevant organ involvement in asymptomatic patients. Diagnosis of occult malignancy that may emerge occasionally in sarcoidosis patients appears to be another potential utility of this implementation. In the diagnosis of occult malignancy accompanying sarcoidosis, approximately equivalent results were obtained by 68Ga-citrate but with a slightly less sensitivity compared to 18FDG PET CT imaging with a negligible statistical significance. 68Ga-citrate seems to be a very useful screening diagnostic modality, as a significant proportion of sarcoidosis patients pose a challenge relevant to disease diagnosis, extrapulmonary organ involvement, and activity assessment in clinical practice. Other outstanding features of this new approach can be listed as its non-invasiveness and easy applicability along with the expeditious introduction of analytic conclusions. Even in cases where diagnostic accuracy is equivocal with 68Ga-citrate PET/CT by itself alone, its collaboration with other conventional clinical criteria may further increase the diagnostic yield for the appraisement of sarcoidosis patients. As a novel tracer, yielding high image quality, being able to scan all body parts at the same time, and enduement of results in a short time are the hallmark of 68Ga-citrate for diagnosis and activity assessment of sarcoidosis patients along with its high potential for detecting occult malignant disease. Furthermore, utility of 68Ga-citrate PET/CT may determine the treatment indication in sarcoidosis patients by unveiling the active granulomatous inflammation especially for occultly involved extrapulmonary organs where detection of active disease is extremely unlikely with the conventional laboratory and imaging methods. Furthermore, the distinction between fibrotic disease and active inflammation emerges as another essential and decisive utility for a treatment indication along with assessment of a post-treatment response.
Author Contributions:
Cuneyt Tetikkurt designed the study and wrote the manuscript. Bahar Kubat wrote the clinical findings of the patients. Haluk Burcak Sayman prepared the nuclear medicine data. Halil Yanardag performed the statistical analysis. Muammer Bilir produced the figures and the references of the manuscript.
Ethical Statement:
The study was done at the Cerrahpasa Medical Faculty of Istanbul Cerrahpasa University between 2020 May and June 2022. The Ethical Committee authorized the experiment for human patients and approved (Ethical Committee registration number of is 604.01.02-73300 and the inscription date is 12/04/2021) the virtuous aspects of the study.
Conflicts of Interest:
The authors declare that they do not have any conflicts of interest.
References
- Baughman RP, Teirstein AS, Judson MA, et al. Clinical characteristics of patients in a case control study of sarcoidosis. Am J Respir Crit Care Med. 2001;164(10):1885–9. doi: 10.1164/ajrccm.164.10.2104046. [DOI] [PubMed] [Google Scholar]
- Statement on sarcoidosis. Joint Statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS) and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee. February. Am J Respir Crit Care Med. 1999;160(2):736–55. doi: 10.1164/ajrccm.160.2.ats4-99. [DOI] [PubMed] [Google Scholar]
- Costabel U, Hunninghake GW. ATS/ERS/WASOG statement on sarcoidosis. Sarcoidosis Statement Committee. American Thoracic Society. European Respiratory Society. World Association for Sarcoidosis and Other Granulomatous Disorders. Eur Respir J. 1999;14(4):735–7. doi: 10.1034/j.1399-3003.1999.14d02.x. [DOI] [PubMed] [Google Scholar]
- Hunninghake GW, Costabel U, Ando M, et al. ATS/ERS/WASOG statement on sarcoidosis. American Thoracic Society/European Respiratory Society/World Association of Sarcoidosis and other Granulomatous Disorders. Sarcoidosis Vasc Diffuse Lung Dis. 1999;16(2):149–73. [PubMed] [Google Scholar]
- Youssef G, Leung E, Mylonas I, et al. The use of 18F-FDG PET in the diagnosis of cardiac sarcoidosis: a systematic review and metaanalysis including the Ontario experience. J Nucl Med. 2012;53(2):241–8. doi: 10.2967/jnumed.111.090662. [DOI] [PubMed] [Google Scholar]
- Akaike G, Itani M, Shah H, et al. PET/CT in the Diagnosis and Workup of Sarcoidosis: Focus on Atypical Manifestations. RadioGraphics. 2018;38:1536–49. doi: 10.1148/rg.2018180053. [DOI] [PubMed] [Google Scholar]
- Rubini G, Ferrari C, Altini C, Cimino A, Fanelli M, Asabella AN. Diagnostic Performance of 18F-FDG PET/CT Semiquantitative Analysis in the Management of Sarcoidosis. Curr Med Imaging Rev. 2019;15(1):32–8. doi: 10.2174/1573405614666180522075828. [DOI] [PubMed] [Google Scholar]
- Mostard RLM, Verschakelen JA, Van Kroonenburgh MJPG, et al. Severity of pulmonary involvement and (18)F-FDG PET activity in sarcoidosis. Respiratory Medicine. 2013;107:439–47. doi: 10.1016/j.rmed.2012.11.011. [DOI] [PubMed] [Google Scholar]
- Sobic-Saranovic D, Grozdic I, Videnovic-Ivanov J, et al. The utility of 18F-FDG PET/CT for diagnosis and adjustment of therapy in patients with active chronic sarcoidosis. J Nucl Med. 2012;53(10):3–1549. doi: 10.2967/jnumed.112.104380. [DOI] [PubMed] [Google Scholar]
- Ambrosini V, Zompatori M, Fasano L, et al. 18F-FDG PET/CT for the assessment of disease extension and activity in patients with sarcoidosis: results of a preliminary prospective study. Clin Nucl Med. 2013;38(4):171–7. doi: 10.1097/RLU.0b013e31827a27df. [DOI] [PubMed] [Google Scholar]
- Sobic-Saranovic D, Artiko V, Obradovic V. FDG PET imaging in sarcoidosis. Semin Nucl Med. 2013;43(6):404–11. doi: 10.1053/j.semnuclmed.2013.06.007. [DOI] [PubMed] [Google Scholar]
- Treglia G, Annunziata S, Sobic-Saranovic D, Bertagna F, Caldarella C, Giovanella L. The role of 18F-FDG-PET and PET/CT in patients with sarcoidosis: an updated evidence-based review. Acad Radiol. 2014;21(5):675–84. doi: 10.1016/j.acra.2014.01.008. [DOI] [PubMed] [Google Scholar]
- Slart RHJA, Koopmans KP, van Geel PP, et al. Somatostatin receptor based hybrid imaging in sarcoidosis. Eur J Hybrid Imaging. 2017;1(1):1–5. doi: 10.1186/s41824-017-0014-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S, Singh AD, Sharma SK, Tripathi M, Das CJ, Kumar R. Gallium-68 DOTA-NOC PET/CT as an alternate predictor of disease activity in sarcoidosis. Nucl Med Commun. 2018;39(8):768–78. doi: 10.1097/MNM.0000000000000869. [DOI] [PubMed] [Google Scholar]
- Kamphuis LS, Kwekkeboom DJ, Missotten TO, et al. Somatostatin receptor scintigraphy patterns in patients with sarcoidosis. Clin Nucl Med. 2015;40(12):925–9. doi: 10.1097/RLU.0000000000000977. [DOI] [PubMed] [Google Scholar]
- Lapa C, Reiter T, Kircher M, et al. Somatostatin-receptor based PET/CT in patients with the suspicion of cardiac sarcoidosis: an initial comparison to cardiac MRI. Oncotarget. 2016;7(47):77807–14. doi: 10.18632/oncotarget.12799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobashi T, Nakamoto Y, Kubo T, et al. The utility of PET/CT with (68)Ga-DOTATOC in sarcoidosis: comparison with (67) Ga-scintigraphy. Ann Nucl Med. 2016;30(8):544–52. doi: 10.1007/s12149-016-1095-6. [DOI] [PubMed] [Google Scholar]
- Teirstein AS, Machac J, Almeida O, Lu P, Padilla ML, Iannuzzi MC. Results of 188 whole-body fluorodeoxyglucose positron emission tomography scans in 137 patients with sarcoidosis. Chest. 2007;132(6):49–1953. doi: 10.1378/chest.07-1178. [DOI] [PubMed] [Google Scholar]
- Mostard RLM, Vöö S, van Kroonenburgh MJ, et al. Inflammatory activity assessment by F18FDG-PET/CT in persistent symptomatic sarcoidosis. Respir Med. 2011;105:1917–24. doi: 10.1016/j.rmed.2011.08.012. [DOI] [PubMed] [Google Scholar]
- Keijsers RG, Verzijlbergen FJ, van den Bosch JM, et al. 18F-FDG PET as a predictor of pulmonary function in sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2011;28:123–9. [PubMed] [Google Scholar]
- Brudin LH, Valind SO, Rhodes CG, et al. Fluorine-18 deoxyglucose uptake in sarcoidosis measured with positron emission tomography. Eur J Nucl Med. 1994;21:297–305. doi: 10.1007/BF00947964. [DOI] [PubMed] [Google Scholar]
- Nishiyama Y, Yamamoto Y, Fukunaga K, et al. Comparative evaluation of 18F-FDG PET and 67Ga scintigraphy in patients with sarcoidosis. J Nucl Med. 2006;47:1571–6. [PubMed] [Google Scholar]
- Kaira K, Oriuchi N, Otani Y, et al. Diagnostic usefulness of fluorine-18-alpha-methyltyrosine positron emission tomography in combination with 18F-fluorodeoxyglucose in sarcoidosis patients. Chest. 2007;131:1019–27. doi: 10.1378/chest.06-2160. [DOI] [PubMed] [Google Scholar]
- Tetikkurt C, Yanardag H, Sayman BH, et al. Diagnostic utility of 68Ga-citrate and 18FDG PET/CT in sarcoidosis patients. Monaldi Arch Chest Dis. 2020;90(4):729–37. doi: 10.4081/monaldi.2020.1509. [DOI] [PubMed] [Google Scholar]
- Tetikkurt C, Sayman H, Dedeoglu SE, Kubat B, Tetikkurt S. Simultaneous use of FDG-18 and 68Ga-citrate PET/CT for the differential diagnosis of sarcoidosis and malignant disease. Monaldi Arch Chest Dis. 2020;90(3):469–72. doi: 10.4081/monaldi.2020.1320. [DOI] [PubMed] [Google Scholar]
- Tetikkurt C, Yanardag E, Bilir M, Yanardag H, Kimyon U. Diagnostic yield of the Kveim test in sarcoidosis patients. Sarcoidosis Vasc Diffuse Lung Dis. 2024 Mar 26;41(1):e2024003. doi: 10.36141/svdld.v41i1.15253. [DOI] [PMC free article] [PubMed] [Google Scholar]


