Abstract
Background and aim:
Sarcoidosis can affect every organ with varying frequency based on ethnicity, gender and age. We aimed to evaluate the health-related quality of life (HRQoL) and fatigue levels of our sarcoidosis patients. However, our second aim is to determine whether patient or disease markers correlate with quality-of-life assessment questionnaires.
Materials and Methods:
Pulmonary sarcoidosis patients who were followed up in the chest diseases outpatient clinic of our hospital were included in this prospective study. In the follow-up of 2023, routine blood tests, angiotensin-converting enzyme (ACE), posteroanterior lung (thorax computed tomography in patients with parenchymal involvement) were requested from the patients. Participants were asked to fill out the short-form 36-item questionnaire (SF-36), fatigue assessment scale (FAS) and fatigue severity scale (FSS) under the control of the outpatient clinic.
Results:
A total of 189 patients, 139 (73.5%) female and 50 (26.5%) male, diagnosed with Sarcoidosis were included. The mean age of our patients was 53.1±13.6. ACE (IU/L) 68.5±44.5. Of the patients we followed up for pulmonary sarcoidosis, 111 (58.7%) had single organ involvement and 78 (41.3%) had additional organ involvement. FAS was high in 103 (64.3%) patients and 29 (15.3%) were very tired. FSS value was 4.45±0.7 (3-5.88). All SF-36 parameters were lower than expected; MH and SF was minimally lower, PF, RP, BP, GH, RE and VT were significantly lower. SF-36 scores were found to be lower in the women and additional organ involvement participating.
Conclusion:
We found a significant decrease in HRQoL and an increase in fatigue scores of our patients. These changes were more pronounced in women participating in the study than in men. In addition, the involvement of additional organs with the lung negatively affected the quality of life.
Keywords: sarcoidosis, health-related quality of life, age, lung, additional organ involvement
Introduction
Sarcoidosis is a multi-system disease characterized by unidentified inflammation of the affected areas and most commonly involving the lungs. It affects people of all ethnicities and occurs at any time in life, but is more common in African Americans and Scandinavians, as well as adults in the middle-age group. Sarcoidosis can affect every organ with varying frequency based on ethnicity, gender and age. In the majority of patients with unilateral or bilateral hilar lymphadenopathy of the lung and lung parenchymal micronodules, the lymphatic structures are mainly the most affected system, and involvement along the intrathoracic lymphatic chain occurs frequently. Its annual incidence is reported at rates ranging from 0.1-109 per 100,000 and is 4 per 100,000 in our country (1-3). Sarcoidosis requires special care as it can be seen in every organ. According to research, the lung is the most affected organ in sarcoidosis. Skin involvement is the most common after lung and is found alone or in combination with other system involvements in approximately one-third of patients (1,4). It is reported that eye involvement varies between 10% and 50% (5). In earlier studies, lung lymphadenopathies could be detected on chest X-rays (CXRs) taken for other reasons in more than half of the patients. Incidental discovery in CXR is now very rare and is reported in 8.4% of patients (6,7). Löfgren’s syndrome, described by Swedish Professor Sven Löfgren in 1952, is a different phenotype of sarcoidosis (1). Respiratory symptoms are present at the time of admission in 30-53% of patients; 27-53% have cough, 18-51% have shortness of breath and 9-23% have chest pain (6). Respiratory distress is frequently seen in patients with interstitial fibrosis due to sarcoidosis and in patients with delayed diagnosis (8). General symptoms are common in sarcoidosis; In particular, fatigue has been found to be a positive relationship between dyspnea and fatigue, with values such as two-thirds of patients in some studies (9). The detection of fatigue in sarcoidosis is extremely important because, on the one hand, research has shown that fatigue has a negative relationship with quality of life (10). Other nonspecific structural symptoms include fever, sweating, and weight loss (11). Health-related quality of life (HRQoL) is defined as the impact of physical and mental health on an individual’s quality of life. Having sarcoidosis has an impact on the quality of life of family members and partners apart from the patient (12,13). Improving the quality of life of almost all patients with sarcoidosis should be one of the goals of treatment. Quality of life is a multifaceted concept in sarcoidosis due to single or multiple involvement of different organs of the disease. A large European study in 2018 assessed patient priorities in the treatment of sarcoidosis. It was observed that the most important outcome for the patients was the quality of life, followed by functionality. Objective measurements such as pulmonary function tests, laboratory results, imaging, and side effects have been reported to be less important (14). HRQoL and symptoms are considered an important part of new treatment plans (15). In 2011, a group working on sarcoidosis developed new proposals. One of his main recommendations was the inclusion of HRQoL as an endpoint in all clinical trials (16). This recommendation was highlighted in a recent Delphi survey conducted by the Sarcoidosis Research Foundation (FSR) (15). It is thought that sarcoidosis may reduce the HRQoL of patients (13). Patients may suffer from relationship problems, role changes (e.g., in family life), fear of stigma, and social isolation (12). Sarcoidosis can affect many aspects of daily life, including participation in work, as it often affects people during their working lives. Due to general symptoms that are not specific to the organ involved, such as fatigue and depression, the ability to work is often reduced, and they may be forced to work fewer hours and pay less. It has been stated that low income can be significantly associated with poor outcomes and worse HRQoL (17). In general, in chronic diseases, the severity of the disease is one of the main factors thought to be associated with HRQoL. However, this relationship has been observed less clearly in sarcoidosis (12). Fatigue is a global problem experienced by most sarcoidosis patients. Fatigue has been linked to chronic diseases, including those with sarcoidosis. And it has a significant impact on patients’ quality of life (QoL) (18). In one study, half of sarcoidosis patients complained of fatigue causing deterioration in quality of life. And it was added that the reason for this distressing situation was unclear (19). Sarcoidosis is a chronic multisystem disease that most commonly affects the lung. Although quality of life assessments come to the fore in other chronic diseases in our country, it is rare in sarcoidosis patients. We aimed to evaluate the quality of life and fatigue levels of our patients by applying a quality-of-life questionnaire, fatigue assesment scale and fatigue severity scale. Thus, we thought that we could increase the treatment satisfaction and HRQoL of our patients. However, our second aim is to find out whether patient or disease markers correlate with quality-of-life assessment questionnaires.
Materıals and Methods
Participants and study design
This prospective study included patients diagnosed with sarcoidosis. Patients with pulmonary sarcoidosis who were followed up in the Chest Diseases Outpatient Clinic of the University of Health Sciences Şişli Hamidiye Etfal Training and Research Hospital were admitted to our study. Patient records were extracted through the hospital computer data system. The files of patients who were investigated with a preliminary diagnosis of sarcoidosis in the clinics and outpatient clinics of our hospital and diagnosed with sarcoidosis were examined. Patients over the age of 18, diagnosed with sarcoidosis in accordance with the 2020 American Thoracic Society clinical practice guideline, followed up by the chest diseases clinic, and with complete file information were admitted to our study (11). Patients under the age of 18, patients who did not come to follow-up regularly, who had missing files, who had immune system disorders and malignancies were not included in the study (Figure 1).
Figure 1.
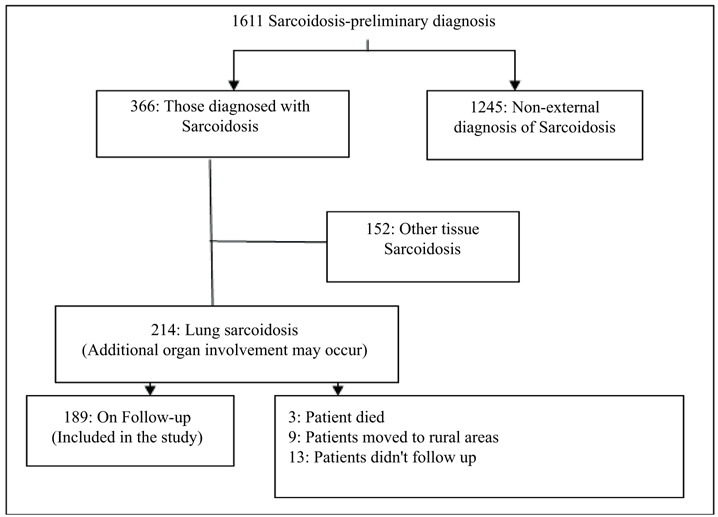
Patient participation scheme.
In the follow-up of 2023, routine laboratory tests and angiotensin-converting enzyme (ACE) tests were requested. Posteroanterior chest X-ray was required for patients and thoracic computed tomography was required for patients with parenchymal involvement. Participants were asked to fill out the short-form 36-item questionnaire (SF-36) and a fatigue assessment scale (FAS) under the control of the outpatient clinic. Patients with high FAS scale were required to fill in the fatigue severity scale (FSS). Demographic data, laboratory data, ACE result, SF-36 scale form FAS and FSS data of patients diagnosed with sarcoidosis were uploaded to excel program and statistics were made.
Short form 36-point questionnaire (SF-36)
With social life arrangements and renewals in medical care-service systems, interest and skills for health-related quality of life are developing. HRQoL is defined as the subjective feeling of the multifaceted effect of a disease by patients (20). SF-36 is a frequently used questionnaire to assess HRQoL. In this study, we used the Turkish version of SF-36 to evaluate HRQoL in our patients (21). This questionnaire included eight parameters: physical function (PF), physical role difficulty (RP), pain (BP), general perception of health (GH), emotional role difficulty (RE), energy-vitality (VT), mental health (MH) and social functioning (SF). The first 4 parameters are physical health and the last 4 are mental health values. Scores for each parameter range from 0 to 100, with higher scores indicating quality of life associated with better health.
Fatigue assessment scale (FAS) ve Fatigue Severity Scale (FSS)
The FAS assesses fatigue by examining physical and psychological fatigue. The scale consists of 10 questions. While a FAS score of less than 22 is considered as no fatigue, a score between 22-34 is considered as fatigue, and a score of 35 and above is considered as extreme fatigue (22). In the evaluation of the fatigue levels of the individuals, FSS, which has been shown to be valid and reliable in Turkish, was applied. This scale consists of nine items. Each item is scored on a scale of 1 to 7, and as the total score decreases, so does fatigue (23).
Analysis of data
IBM SPSS 22.0 package program was used in the study. Descriptive statistics of the participants’ demographic data and all parameters (frequency, percentage, median, interquartile range, min-max values, mean and standard deviation) were calculated. In the comparison of SF-36 parameters according to gender and additional organ involvement, independent samples t test was used if there was conformity with normal distribution, and mann whitney U test was used if there was no conformity. In the relationship analysis, Pearson Correlation Analysis was used if there was conformity to the normal distribution, and Spearman Correlation Analysis was used if there was no conformity. Whether the parameters were suitable for normal distribution was determined by Shapiro wilk test. All statistical analyses were evaluated at 95% confidence interval and significance was evaluated at p<0.05.
Results
The study included 189 patients who were followed up with the diagnosis of sarcoidosis, regardless of whether they were male or female. The mean age of our patients was 53.1±13.6 years. Of the 189 patients, 139 (73.5%) were female and 50 (26.5%) were male. The BMI of the patients was 28.2±5.4. Laboratory results of the patients: Glucose 140.4±61.1, alanine aminotransferase 31.4±21.3, aspartate aminotransferase 39.3±28.5, urea 34.6±19.4, creatinine 0.9±0.6, lactic dehydrogenase 315.7±116.3, ACE(IU/L) 68.5±44.5, C-reactive protein 100.3± 69.3, white blood cell count 9.6± 4.1 (Table 1). Of the 189 patients who participated in our study, 129 (68.3) had respiratory system complaints. Cough was present in 110 (58.2%) patients, shortness of breath in 75 (39.7%) patients, wheezing in 25 (13.2%) patients, chest pain in 17 (9.0%) patients and hemoptysis in 3 (1.6%) patients. The mean follow-up year was 7.6±4.8 years. Of the patients we followed up for pulmonary sarcoidosis, 111 (58.7%) had single organ involvement and 78 (41.3%) had additional organ involvement. Of the patients, 73 (38.6%) were current smokers, 82 (43.4%) had quit smoking and 34 (18.0%) had never smoked. Forced expiratory volume in 1 s (FEV1) % value 80.6±14.6, forced vital capacity (FVC)% value 81.7±12.4 and forced expiratory volume in 1 s /forced vital capacity (FEV1/FVC) values of patients 85.2±8.6 were found (Table 1).
Table 1.
Baseline demographic of the study population.
| Characteristics | Patients (n=189) | |
|---|---|---|
| Gender, n (%) | ||
| Female | 139 (%73.5) | |
| Male | 50 (%26.5) | |
| Age, year, mean±SD | 53.1±13.6 | |
| (min-max) | (18-71) | |
| BMI, year, mean±SD | 28.8±5.9 | |
| ACE mean±SD | 68.5±44.5 | |
| (minimum-maximum) | (2-259) | |
| Complaint, n (%) | ||
| No | 60 (31.7) | |
| Yes | Cough | 110 (58.2) |
| Dispnea | 75 (39.7) | |
| Wheezing | 25 (13.2) | |
| Chest pain | 17 (9.0) | |
| Haemoptysis | 3 (1.6) | |
| Smoking, n (%) | ||
| Smoking | 73 (%38.6) | |
| Qut | 82 (%43.4) | |
| Never Smoked | 34 (%18.0) | |
| Duration of illness, year, mean±SD | 7.6±4.8 | |
| (min-max) | (1-25) | |
| Organ involvement | ||
| Lung | 111 (58.7) | |
| Additional Organ Involvement | 78 (41.3) | |
| Spirometry, mean±SD | ||
| FEV1(%) | 80.6±14.6 | |
| FVC (%) | 81.7±12.4 | |
| FEV1/FVC | 85.2±8.6 | |
| FAS | 24.52±7.2 (14-42) | |
Abbreviations: SD: Standard deviation, BMI: Body mass index, min-max: Minimum-maximum ACE: Angiotensin-converting enzyme, FEV1: Forced expiratory volume in 1 s, FVC: Forced vital capacity, FEV1/FVC: Forced expiratory volume in 1 s/Forced vital capacity, FAS: Fatigue assessment scale.
Short form 36-point questionnaire (SF-36)
SF-36 was applied to all 189 patients. The PF parameter was calculated as 68.9±12.3, the RP parameter was calculated as 57.8±12.2, the BP parameter was calculated as 64.3±11.2, the GH parameter was calculated as 55.8±9.6, the RE parameter was calculated as 63.8±9.8, the VT parameter was calculated as 53.5±10.7, the MH parameter was calculated as 64.3±8.9, the SF parameter was calculated as 68.4±9.1. All parameters were lower than expected; MH and SF were minimally lower than expected and PF, RP, BP, GH, RE and VT were significantly lower than expected (p < 0.05) (Figure 2).
Figure 2.
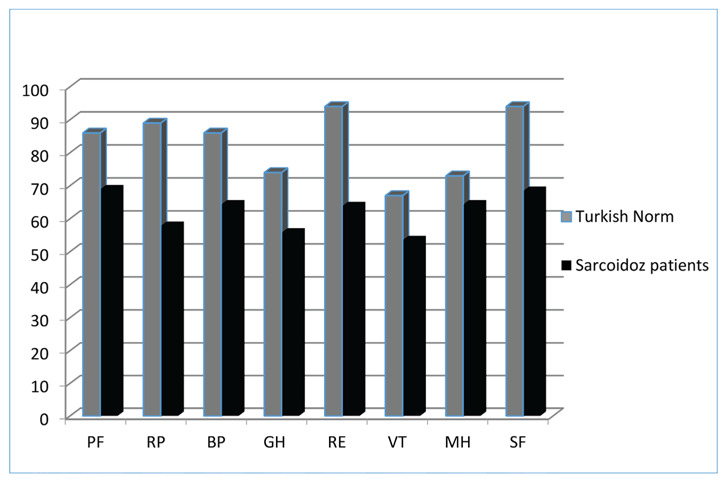
Comparison of SF-36 scores of Turkish population and Sarcoidosis patients.
The vertical axis represents mean (SD) SF-36 domain scores from 0 to 100. The horizontal axis shows SF-36 subparameters. Abbreviations: PF: physical function, RP: physical role difficulties, BP: pain, GH: general health perception, RE: emotional role diff RE: emotional role difficulties, VT: energy vitality, MH: mental health, SF: social functionality.
Fatigue assessment scale (FAS) ve Fatigue Severity Scale (FSS)
FAS was applied to all 189 patients. FAS was normal in 86 (45.5%) patients and high in 103 (54.5%) patients. Of the patients with high FAS, 74 (39.2%) were fatigue and 29 (15.3%) were extreme fatigue. The mean FSS value of 103 patients with fatigue was 4.45±0.7 (3-5.88).
Comparison of parameters and SF-36
Age: There is a statistically significant, negative, high-level relationship between the age of the study participants and the MH score. There is a moderate negative correlation with VT and SF scores. There is a low level of negative correlation with PF, BP and GH scores. There is no statistically significant relationship with RP and RE scores (Table 2). Duration of illness: There is a statistically significant, negative, low-level relationship between the duration of illness and PF, BP, GH, VT, MH, SF scores of the study participants. There is no statistically significant relationship with RP and RE scores (Table 2). Spirometry: There is a statistically significant, negative, moderate correlation between FEV1 values and GH and VT scores of the study participants. There was a statistically significant, negative, low-level correlation between FVC and FEV1/FVC values of the study participants and all F-36 parameters (Table 2). ACE: There is a statistically significant, negative, low-level correlation between the ACE values of the study participants and all F-36 parameters (Table 2).
Table 2.
Comparison of SF-36 parameters and age, duration of illness, FEV1(%), FVC (%), FEV1/FVC, ACE.
| PF | RP | BP | GH | RE | VT | MH | SF | ||
|---|---|---|---|---|---|---|---|---|---|
| Age | r | -.342** | 0.126 | -.408** | -.355** | 0.105 | -.514** | -.834** | -.640** |
| p | 0.0001 | 0.083 | 0.0001 | 0.0001 | 0.151 | 0.0001 | 0.0001 | 0.0001 | |
| N | 189 | 189 | 189 | 189 | 189 | 189 | 189 | 189 | |
| Duration of illness | r | -.270** | -0.062 | -.305** | -.231** | -0.096 | -.333** | -.412** | -.369** |
| p | 0.0002 | 0.396 | 0.0001 | 0.001 | 0.190 | 0.0001 | 0.0001 | 0.0001 | |
| N | 189 | 189 | 189 | 189 | 189 | 189 | 189 | 189 | |
| FEV1 (%) | r | .460** | .425** | .390** | .549** | .279** | .551** | .274** | .474** |
| p | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | |
| N | 189 | 189 | 189 | 189 | 189 | 189 | 189 | 189 | |
| FVC (%) | r | .376** | .383** | .363** | .493** | .200** | .469** | .217** | .410** |
| p | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.006 | 0.0001 | 0.003 | 0.0001 | |
| N | 189 | 189 | 189 | 189 | 189 | 189 | 189 | 189 | |
| FEV1/FVC | r | .392** | .377** | .328** | .418** | .300** | .457** | .257** | .382** |
| p | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0003 | 0.0001 | |
| N | 189 | 189 | 189 | 189 | 189 | 189 | 189 | 189 | |
| ACE | r | -.194** | -.325** | -0.119 | -.210** | -.272** | -0.116 | 0.061 | -.144* |
| p | 0.008 | 0.0001 | 0.102 | 0.004 | 0.0002 | 0.112 | 0.407 | 0.048 | |
| N | 189 | 189 | 189 | 189 | 189 | 189 | 189 | 189 | |
Abbreviations: The vertical axis, ACE: Angiotensin-converting enzyme, FEV1: Forced expiratory volume in 1 s, FVC: Forced vital capacity, FEV1/FVC: Forced expiratory volume in 1 s/Forced vital capacity. The horizontal axis shows SF-36 subparameters. PF: physical function, RP: physical role difficulties, BP: pain, GH: general health perception, RE: emotional role difficulties, VT: energy vitality, MH: mental health, SF: social functionality, *: p<0,05; **: p<0,01.
Gender: There is a statistically significant difference between the participants in terms of RP, BP, GH, VT and MH according to their gender. Men’s RP scores were significantly lower than women’s scores. Women’s BP, GH, VT and MH scores were significantly lower than men’s scores (Table 3).
Table 3.
Comparison of gender and SF-36 parameters.
| Female (n=139) | Male (n=50) | p | |||||
|---|---|---|---|---|---|---|---|
| Median (IQR) | Min-Max | mean±SD | Median (IQR) | Min-Max | mean±SD | ||
| PF | 69 (12) | 41-94 | 67.73±11.1 | 71 (19) | 39-99 | 72.2±15 | 0.0581 |
| RP | 55 (17) | 39-92 | 58.87±12.2 | 52 (17) | 38-91 | 54.8±12,2 | 0.015*2 |
| BP | 62 (12) | 44-91 | 62.63±10 | 68 (20) | 44-92 | 69.08±13 | 0.002**2 |
| GH | 53 (9) | 41-80 | 54.46±7.4 | 59 (22) | 37-83 | 59.5±13.4 | 0.033*2 |
| RE | 64 (10) | 44-83 | 63.88±8.1 | 61 (23) | 41-88 | 63.66±13.5 | 0.9151 |
| VT | 51 (11) | 36-76 | 51.48±8.4 | 59.5 (23) | 31-48 | 59.12±14.1 | 0.001**2 |
| MH | 63 (12) | 46-83 | 63.13±7.8 | 71.5 (16) | 45-86 | 67.64±11 | 0.002**2 |
| SF | 67 (11) | 52-88 | 67.56±7.8 | 72 (20) | 49-89 | 70.8±11.8 | 0.0682 |
Abbreviations: PF: physical function, RP: physical role difficulties, BP: pain, GH: general health perception, RE: emotional role difficulties, VT: energy vitality, MH: mental health, SF: social functionality, SD: Standard deviation, BMI: Body mass index, min-max: Minimum-maximum, *: p<0,05; **: p<0,01; 1 : independent samples t test; 2 : mann Whitney U test.
Presence of additional organ involvement: There is a statistically significant difference between the participants in terms of PF, RP, BP, GH, RE, VT and SF according to their additional organ involvement status (Table 4).
Table 4.
Comparison of Organ Involvement and SF-36 parameters.
| Lung (n=111) | Additional Organ Involvement (n=78) | p | |||||
|---|---|---|---|---|---|---|---|
| Median (IQR) | Min-Max | mean±SD | Median (IQR) | Min-Max. | mean±SD | ||
| PF | 70 (14) | 52-99 | 73.76±10.6 | 62 (17) | 39-94 | 62.01±11.5 | 0.0001**2 |
| RP | 61 (22) | 41-92 | 62.58±13.1 | 51 (9) | 38-70 | 50.99±6.4 | 0.0001**2 |
| BP | 66 (16) | 47-92 | 67.83±10.5 | 58 (16) | 44-91 | 59.37±10.4 | 0.0001**2 |
| GH | 56 (14) | 39-83 | 57.84±9.5 | 51.5 (10) | 37-80 | 52.88±9.1 | 0.0001**2 |
| RE | 66 (12) | 42-88 | 67.62±8.5 | 59 (15) | 41-87 | 58.41±8.9 | 0.0001**1 |
| VT | 54 (15) | 39-84 | 56.19±10.8 | 48 (12) | 31-81 | 49.68±9.3 | 0.0001**2 |
| MH | 65 (14) | 49-85 | 64.95±8.8 | 63 (12) | 45-86 | 63.42±9.1 | 0.362 |
| SF | 71 (13) | 54-89 | 70.86±8.5 | 64 (12) | 49-89 | 64.95±8.8 | 0.0001**1 |
Abbreviations: PF: physical function, RP: physical role difficulties, BP: pain, GH: general health perception, RE: emotional role difficulties, VT: energy vitality, MH: mental health, SF: social functionality, SD: Standard deviation, BMI: Body mass index, min-max: Minimum-maximum, **: p<0,01; 1 : independent samples t test; 2 : mann Whitney U test.
Comparison of parameters with FAS and FSS
Gender: There was no statistically significant difference between the participants in terms of FAS according to their gender. There is a statistically significant difference in terms of FSS. Women’s FSS scores were significantly higher than men’s FSS scores (Independent samples t test; t=2.209; p=0.029<0.05, this comparison was made with 75 females and 28 males.) (Table 5). Presence of additional organ involvement: The FAS scores of those with more than one organ involvement who participated in the study were significantly higher than the FAS scores of those with single organ involvement. There was no statistically significant difference between the participants in terms of FSS according to organ involvement (Independent samples t test; p>0.05, this comparison was made with 42 females and 61 males) (Table 5).
Table 5.
Comparison of FAS and FSS with organ involvement and gender.
| Female | Male | p | |||||
|---|---|---|---|---|---|---|---|
| Median (IQR) | Min-Max | Mean±SD | Medyan (IQR) | Min-Max | Mean±SD | ||
| FAS | 23 (9) | 14-41 | 24.56±7.4 | 22.5 (11) | 15-42 | 24.4±6.9 | 0.9342 |
| FSS | 4.66 (0.89) | 3-5.88 | 4.54±0.7 | 4,33 (0.84) | 3.11-5.77 | 4.21±0.7 | 0.029*1 |
| Lung | Additional Organ Involvement | p | |||||
| Median (IQR) | Min-Max | Mean±SD | Median (IQR) | Min-Max. | Mean±SD | ||
| FAS | 20 (6) | 14-38 | 21.53±5.8 | 28 (10) | 17-42 | 28.77±7 | 0.0001**2 |
| FSS | 4.33 (1) | 3.11-5.66 | 4.31±0.7 | 4,66 (0.89) | 3-5.88 | 4.45±0.7 | 0.0741 |
Abbreviations: FAS: Fatigue assessment scale, FSS: Fatigue severity scale, SD: Standard deviation, min-max: Minimum-maximum, 1: Independent samples t test; 2 : Mann whitney U test; *: p<0,05; **: p<0,01.
Duration of illness: There is a statistically significant, positive, low-level relationship between FAS and disease duration. There is a statistically significant, positive, low-level correlation between FSS and disease duration (Table 6). ACE: There is a statistically significant, positive, low-level correlation between FAS and ACE (Table 6). Spirometry: There is a statistically significant, negative, low-level correlation between FAS and FEV1 (%) (Table 6).
Table 6.
Comparison of FAS, FSS and age, duration of illness, FEV1(%), ACE.
| Age | Duration of illness | FEV1(%) | ACE | ||
|---|---|---|---|---|---|
| FAS | r | -0.057 | .362** | -.409** | .233** |
| p | 0.437 | 0.0001 | 0.0001 | 0.001 | |
| N | 189 | 189 | 189 | 189 | |
| FSS | r | -0.024 | .247* | -0.145 | 0.095 |
| p | 0.808 | 0.012 | 0.144 | 0.342 | |
| N | 103 | 103 | 103 | 103 | |
Abbreviations: The vertical axis, FAS: Fatigue assessment scale, FSS: Fatigue Severity Scale, the horizontal axis shows FEV1: Forced expiratory volume in 1 s, FVC: Forced vital, ACE: Angiotensin-converting enzyme.
Discussion
Sarcoidosis is a multi-system disease of unknown etiology, characterized by non-caseating inflammation in the affected areas and most commonly involving the lungs. Sarcoidosis can affect every organ with varying frequency according to ethnicity, gender and age (1). It is thought that sarcoidosis may reduce the HRQoL of patients (13). We evaluated the HRQoL of our patients by applying a quality-of-life questionnaire. The SF-36 parameters of our patients were compared with Turkish norms, and it was determined that they were low. There is a high level of correlation between the age of the study participants and the MH score. SF-36 scores of women with sarcoidosis were lower than men. In addition, the presence of additional organ involvement was associated with lower SF-36 scores. Having sarcoidosis has a negative impact on a person’s quality of life (13). The main element of care and treatment in sarcoidosis is to reduce or treat organ damage that develops in the disease and to keep HRQoL high of the patient who continues his life. Cox et al. They observed overall declines in HRQoL and mental health indices in outpatients for sarcoidosis, and they found lower HRQoL in patients receiving oral corticosteroid therapy (13). In a study, it was stated that the quality of life in sarcoidosis decreased significantly, and it was stated that this observed change was associated with a decrease in physical function, pain, severe loss of income, absenteeism from work and tension in personal relationships (24). Vis et al. found a decrease in various areas of HRQoL in sarcoidosis patients and reported that fatigue and decreased HRQoL may be serious and long-lasting problems even with clinical remission in these patients (25). In their review, Vries and Drent stated that sarcoidosis patients, especially those with clinical symptoms, have a deterioration in HRQoL and health status, and this is more pronounced in terms of mobility, working capacity and activities of daily living (26). Jastrzębski et al. reported that HRQoL was low in patients with sarcoidosis and that dyspnea levels and fatigue scores were blunted with sf-36 parameters (27). In our study, we used the SF-36 scale to measure HRQoL. All SF-36 parameters were low in our sarcoidosis patients. Mental health scores were more conservative than physical health scores. The most significant lows were physical role difficulty and general health perception parameters. Although sarcoidosis can be seen in all ages and both sexes, it is most common at the age of 30-50 years. In a study conducted in the US, being female and being of lower age was associated with lower HRQoL (28). In another study, it was stated that female patients had a lower quality of life in the areas of physical and psychological health, especially in terms of pain, sleep, positive emotions, self-esteem, body image, mobility and activities of daily living (29). Bourbonnais et al. showed that women with sarcoidosis have a lower HRQoL score and more functional impairment than men (30). In response, Cox et al. stated that gender, age, ethnicity, and smoking status were not associated with HRQoL (13). A statistically significant, negative, low-level correlation was found between the disease duration and ACE values of the study participants and SF-36 parameters. In our study, there was a negative correlation between age and quality of life. It is generally thought that there is a substantial link between HRQoL and disease severity in chronic diseases. In a study conducted in Germany, FEV1, not FVC or diffusion capacity (DLCO), was found to be associated with deterioration of general health status and reduced quality of life (31). In Wirnsberger’s study, no relationship was found between pulmonary function tests and HRQoL (32). A correlation has been shown between Bourbonnais, DLCO and a decrease in 6-minute walking test distance and an increase in shortness of breath and quality of life, but no link has been established with spirometry (30). In our study, there was a low correlation between spirometry and HRQoL parameters. Organ manifestations may adversely affect HRQoL in sarcoidosis. The Serbia study showed that patients with multiple organ involvement had the lowest average quality of life scores in terms of daily functioning, physical functioning, and emotional functioning (33). Hinz et al. reported that people with multisystem involvement may be associated with a poorer HRQoL and a higher rate of psychiatric comorbidities (34). In our study, HRQoL was found to be lower in sarcoidosis patients who participated in the study. The MH parameter was conserved, all other parameters were lower than normal. Fatigue has been linked to chronic diseases, including cancer, autoimmune disorders, and sarcoidosis patients. And it has a significant impact on patients’ quality of life (QoL) (18). Very little data are currently available on the specific treatment of fatigue associated with sarcoidosis. Since the causes of this symptom are usually multifactorial, treatment requires the investigation of many reversible and irreversible causes. Proper identification and treatment of anemia, diabetes mellitus, and thyroid disorders can improve the altered quality of life secondary to fatigue. Depression, anxiety, and stress are closely intertwined with fatigue (35). Fatigue has been identified as a prominent problem in sarcoidosis, and the presence of fatigue has often been associated with impaired quality of life compared to patients without fatigue (36). Bloem et al. noted that fatigue was quite common in 117 patients with IPF or sarcoidosis. They added that patients with severe fatigue experienced more severe dyspnea, sleepiness, anxiety, depression, fatigue-related catastrophicization, functional activity disorders, and lower quality of life (37). We found fatigue in more than half of our patients. The fatigue severity scale of the patients with fatigue complaints was also high, and this was more pronounced in women. In addition, FAS was higher in patients with additional organ involvement.
Conclusion
Many patients with sarcoidosis have a deteriorating quality of life. And the complaint of fatigue of these individuals is also pronounced. Both clinicians and patients are looking for ways to improve HRQoL and get rid of fatigue. We found a significant decrease in the quality-of-life parameters of our patients. The scores of the women participating in the study were significantly lower than the scores of the men. In addition, the involvement of additional organs with the lung negatively affected the quality of life. Reducing fatigue and increasing the quality of life is prioritized by many patients as the most important treatment goal. There is a need for the structural use and development of patient-reported outcome measures (PROMs) in order to better identify individual needs and to have a positive impact on their treatment.
Acknowledgments:
We thank the participants of this research.
Ethics Committee Approval:
The study was carried out with the permission of Health Sciences University Şişli Hamidiye Etfal Training and Research Hospital Ethics Committee (Date: 10.10.2023, Decision No:2464).
Informed Consent:
Because the study was designed retrospectively, no written informed consent form was obtained from patients.
Conflict of Interest:
Each author declares that he or she has no commercial associations (e.g. consultancies, stock ownership, equity interest, patent/licensing arrangement etc.) that might pose a conflict of interest in connection with the submitted article.
Funding:
The authors declared that this study has received no financial support.
Author Contributions:
All the authors declare that they have all participated in the design, execution, and analysis of the paper and that they have approved the final version.
References
- Sève P, Pacheco Y, Durupt F, et al. Sarcoidosis: A clinical overview from symptoms to diagnosis. Cells. 2021;10(4):766. doi: 10.3390/cells10040766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arkema EV, Grunewald J, Kullberg S, Eklund A, Askling J. Sarcoidosis incidence and prevalence: a nationwide register-based assessment in Sweden. Eur Respir J. 2016 Dec;48(6):1690–99. doi: 10.1183/13993003.00477-2016. [DOI] [PubMed] [Google Scholar]
- Musellim B, Kumbasar OO, Ongen G, et al. Epidemiological features of Turkish patients with sarcoidosis. Respir Med. 2009;103(6):907–12. doi: 10.1016/j.rmed.2008.12.011. [DOI] [PubMed] [Google Scholar]
- Caplan A, Rosenbach M, Imadojemu S. Cutaneous Sarcoidosis. Semin. Respir. Crit. Care Med. 2020;41(5):689–99. doi: 10.1055/s-0040-1713130. [DOI] [PubMed] [Google Scholar]
- Bodaghi B, Touitou V, Fardeau C, Chapelon C, LeHoang P. Ocular sarcoidosis. Presse Med. 2012 Jun;41(6 Pt 2):e349–54. doi: 10.1016/j.lpm.2012.04.004. [DOI] [PubMed] [Google Scholar]
- Mañá J, Rubio-Rivas M, Villalba N, et al. Multidisciplinary approach and long-term follow-up in a series of 640 consecutive patients with sarcoidosis: Cohort study of a 40-year clinical experience at a tertiary referral center in Barcelona, Spain. Medicine (Baltimore) 2017 Jul;96(29):e7595. doi: 10.1097/MD.0000000000007595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valeyre D, Bernaudin JF, Jeny F, et al. Pulmonary Sarcoidosis. Clin. Chest Med. 2015;36(4):631–41. doi: 10.1016/j.ccm.2015.08.006. [DOI] [PubMed] [Google Scholar]
- Nardi A, Brillet PY, Letoumelin P, et al. Stage IV Sarcoidosis: Comparison of Survival with the General Population and Causes of Death. Eur. Respir. J. 2011;38(6):1368–73. doi: 10.1183/09031936.00187410. [DOI] [PubMed] [Google Scholar]
- Drent M, Strookappe B, Hoitsma E, De Vries J. Consequences of Sarcoidosis. Clin Chest Med. 2015 Dec;36(4):727–37. doi: 10.1016/j.ccm.2015.08.013. [DOI] [PubMed] [Google Scholar]
- Michielsen HJ, Drent M, Peros-Golubicic T, De Vries J. Fatigue is associated with quality of life in sarcoidosis patients. Chest. 2006 Oct;130(4):989–94. doi: 10.1378/chest.130.4.989. [DOI] [PubMed] [Google Scholar]
- Crouser ED, Maier LA, Wilson KC, et al. Diagnosis and Detection of Sarcoidosis. An Official American Thoracic Society Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2020;201(8):e26–e51. doi: 10.1164/rccm.202002-0251ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moor CC, Obi ON, Kahlmann V, Buschulte K, Wijsenbeek MS. Quality of life in sarcoidosis. J Autoimmun. 2023 Oct 7:103123. doi: 10.1016/j.jaut.2023.103123. [DOI] [PubMed] [Google Scholar]
- Cox CE, Donohue JF, Brown CD, Kataria YP, Judson MA. Health-related quality of life of persons with sarcoidosis. Chest. 2004 Mar;125(3):997–1004. doi: 10.1378/chest.125.3.997. [DOI] [PubMed] [Google Scholar]
- Baughman RP, Barriuso R, Beyer K, et al. Sarcoidosis: patient treatment priorities. ERJ Open Res. 2018 Dec 21;4(4):00141–2018. doi: 10.1183/23120541.00141-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judson MA, Spagnolo P, Stanfel R, et al. Living with sarcoidosis: Virtual roundtable dialogue with patients and healthcare professionals. Respir Med. 2023 Apr-May;210:107174. doi: 10.1016/j.rmed.2023.107174. [DOI] [PubMed] [Google Scholar]
- Baughman RP, Drent M, Culver DA, et al. Endpoints for clinical trials of sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2012 Oct;29(2):90–8. [PubMed] [Google Scholar]
- Harper LJ, Gerke AK, Wang XF, et al. Income and Other Contributors to Poor Outcomes in U.S. Patients with Sarcoidosis. Am J Respir Crit Care Med. 2020 Apr 15;201(8):955–64. doi: 10.1164/rccm.201906-1250OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendriks C, Drent M, Elfferich M, De Vries J. The Fatigue Assessment Scale: quality and availability in sarcoidosis and other diseases. Curr Opin Pulm Med. 2018 Sep;24(5):495–503. doi: 10.1097/MCP.0000000000000496. [DOI] [PubMed] [Google Scholar]
- Drent M, Lower EE, De Vries J. Sarcoidosis-associated fatigue. Eur Respir J. 2012 Jul;40(1):255–63. doi: 10.1183/09031936.00002512. [DOI] [PubMed] [Google Scholar]
- Caballero T, Prior N. Burden of Illness and Quality-of-Life Measures in Angioedema Conditions. Immunol Allergy Clin North Am. 2017 Aug;37(3):597–616. doi: 10.1016/j.iac.2017.04.005. [DOI] [PubMed] [Google Scholar]
- Kocyigit H, Aydemir O, Fisek G, Olmez N, Memis A. Validity and reliability of the Turkish version of Short Form 36: a study of patients with rheumatoid disorder. Turkish J Drugs Therap. 1999;12(2):102–6. [Google Scholar]
- Marcellis RG, Lenssen A, Elfferich M, et al. Exercise capacity, muscle strength and fatigue in sarcoidosis. Eur Respir J. 2011;38(3):628–34. doi: 10.1183/09031936.00117710. [DOI] [PubMed] [Google Scholar]
- Armutlu K, Korkmaz NC, Keser I, et al. The validity and reliability of the Fatigue Severity Scale in Turkish multiple sclerosis patients. Int J Rehabil Res. 2007;30(1):81–5. doi: 10.1097/MRR.0b013e3280146ec4. [DOI] [PubMed] [Google Scholar]
- Saketkoo LA, Russell AM, Jensen K, et al. Health-related quality of life (HRQoL) in sarcoidosis: diagnosis, management, and health outcomes. Diagnostics. 2021;11(6):1089. doi: 10.3390/diagnostics11061089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vis R, van de Garde EM, Meek B, Korenromp IH, Grutters JC. Randomised, placebo-controlled trial of dexamethasone for quality of life in pulmonary sarcoidosis. Respir Med. 2020;165:105936. doi: 10.1016/j.rmed.2020.105936. [DOI] [PubMed] [Google Scholar]
- De Vries J, Drent M. Quality of life and health status in sarcoidosis: a review of the literature. Clin Chest Med. 2008;29(3):525–32. doi: 10.1016/j.ccm.2008.03.003. [DOI] [PubMed] [Google Scholar]
- Jastrzębski D, Ziora D, Lubecki M, et al. Fatigue in sarcoidosis and exercise tolerance, dyspnea, and quality of life. Lung Cancer. 2015:31–6. doi: 10.1007/5584_2014_18. [DOI] [PubMed] [Google Scholar]
- Harper LJ, Gerke AK, Wang XF, et al. Income and other contributors to poor outcomes in US patients with sarcoidosis. Am J Respir Crit Care Med. 2020;201(8):955–64. doi: 10.1164/rccm.201906-1250OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vries J, Van Heck GL, Drent M. Gender differences in sarcoidosis: symptoms, quality of life, and medical consumption. Women Health. 2000;30(2):99–114. doi: 10.1300/j013v30n02_07. [DOI] [PubMed] [Google Scholar]
- Bourbonnais JM, Samavati L. Effect of gender on health-related quality of life in sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2010;27(2):96–102. [PubMed] [Google Scholar]
- Frye BC, Potasso L, Farin-Glattacker E, Birring S, Müller-Quernheim J, Schupp JC. FEV1 and BMI influence King’s Sarcoidosis Questionnaire score in sarcoidosis patients. BMC Pulm Med. 2021;21:395. doi: 10.1186/s12890-021-01761-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirnsberger RM, de Vries J, Breteler MH, Van Heck GL, Wouters EFM, Drent M. Evaluation of quality of life in sarcoidosis patients. Respir Med. 1998;92(5):750–6. doi: 10.1016/s0954-6111(98)90007-5. [DOI] [PubMed] [Google Scholar]
- Mihailović-Vučinić V, Gvozdenović B, Stjepanović M, et al. Administering the Sarcoidosis Health Questionnaire to sarcoidosis patients in Serbia. J Bras Pneumol. 2016;42(2):99–105. doi: 10.1590/S1806-37562015000000063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinz A, Brähler E, Möde R, Wirtz H, Bosse-Henck A. Anxiety and depression in sarcoidosis: the influence of age, gender, affected organs, concomitant diseases, and dyspnea. Sarcoidosis Vasc Diffuse Lung Dis. 2012;9(2):139–46. [PubMed] [Google Scholar]
- De Vries J, Drent M. Relationship between perceived stress and sarcoidosis in a Dutch patient population. Sarcoidosis Vasc Diffuse Lung Dis. 2004;21:57–63. [PubMed] [Google Scholar]
- De Kleijn WP, De Vries J, Lower EE, et al. Fatigue in sarcoidosis: a systematic review. Curr Opin Pulm Med. 2009;15(5):499–506. doi: 10.1097/MCP.0b013e32832d0403. [DOI] [PubMed] [Google Scholar]
- Bloem AE, Mostard RL, Stoot N, et al. Severe fatigue is highly prevalent in patients with IPF or sarcoidosis. J Clin Med. 2020;9(4):1178. doi: 10.3390/jcm9041178. [DOI] [PMC free article] [PubMed] [Google Scholar]


