Abstract
Background:
To address the need for improved virologic suppression among youth living with HIV (YLH) on antiretroviral treatment (ART), we evaluated peer navigation plus TXTXT daily text message ART reminders.
Setting:
YLH aged 15–24 years on ART for at least 3 months at 6 research sites in 4 Nigerian cities.
Methods:
Using a stepped-wedge design, cluster 1 was nonrandomized, whereas clusters 2 and 3 were randomized to sequences of routine care (control period) and 48 weeks of the combination intervention (intervention period). The primary end point was viral suppression (HIV-1 RNA <200 copies/mL) at week 48 of the intervention. Secondary end points included adherence measured by self-report ( 90% considered adherent). Post hoc analysis assessed virologic control at <50 copies per milliliter and <1000 copies per milliliter. Generalized estimating equations determined the difference between intervention and control periods in the intention-to-treat population.
Results:
We enrolled 558 YLH and followed 541 over time, mean age 18 years, 53.8% female, 71.7% perinatally infected, and 38.6% virologically nonsuppressed at enrollment. For the primary end point, the intervention periods displayed a small, nonsignificant increase in viral suppression < 200 copies per milliliter [odds ratio (OR) = 1.16 (0.88–1.54), P = 0.297]. There was a significant effect of the combination intervention on virologic control <1000 copies per milliliter (OR = 1.42 [1.03–1.94], P = 0.030). Self-reported adherence also improved (OR = 2.07 [1.46–2.95], P < 0.001).
Conclusions:
Peer navigation plus daily text message ART reminders demonstrated limited benefit among ART-experienced, predominantly perinatally infected YLH, with no significant effect on viral suppression below 200 copies per milliliter despite improvement in self-reported adherence.
Key Words: text messaging, youth, HIV, viral suppression, peer navigation, adherence
INTRODUCTION
Virologic suppression among youth living with HIV (YLH) aged 15–24 years lags behind the progress made among adults globally.1 In Nigeria, YLH have lower virologic suppression rates than all other age groups except 0–14 year olds.2 New interventions addressing youth-specific cognitive and decisional capacity, emotional development, communication preferences, and contextual influences on health behavior are needed to optimize antiretroviral treatment (ART) outcomes in YLH.1–4
Because multiple interventions delivered in tandem may be more effective than single interventions,5 we developed a youth-specific combination intervention comprising peer navigation plus daily text message ART reminders in the Intensive Combination Approach to Rollback the HIV Epidemic in Nigeria (iCARE Nigeria) study. Peer navigation is recommended by the World Health Organization (WHO) as an evidence-based strategy to improve HIV outcomes in youth, as peers can help YLH cope with fear, hopelessness, stigma, and discrimination and facilitate problem solving.6–8 The daily text message reminder component of the combination intervention was adapted from Text Messaging Intervention to Improve Antiretroviral Adherence among HIV-Positive Youth (TXTXT), an individual-level intervention that is based on social cognitive theory,9,10 and leverages the preference of young persons to communicate through text messaging.11 TXTXT was first shown to promote ART adherence among YLH in the United States12 and is endorsed by the US Centers for Disease Control as evidence based.13 Both components of the combination intervention were adapted to the Nigerian context through the engagement of local advisors from academia, community, and governmental sectors, followed by focus groups with YLH and HIV service providers from AIDS service organizations in Ibadan, Nigeria.14
In a pilot, pre–post study conducted among 40 YLH on ART at the Infectious Diseases Institute, College of Medicine, University of Ibadan, Nigeria (IDI),15 we demonstrated that the combination intervention was feasible, acceptable, and efficacious. There was a 14-fold and 6-fold higher odds of virologic suppression at 24 and 48 weeks of the combination intervention, respectively. Self-reported adherence ( 90%) also improved by 63% and 68% at weeks 24 and 48, respectively. An effect was seen across subgroups by birth sex, mode of transmission (perinatal or behavioral), and ART regimen (first line or later). All the 37 participants at week 48 were fully or mostly satisfied with the intervention. We herein present our multisite full-scale trial conducted to definitively evaluate the effects of the combination intervention on adherence and viral suppression among YLH on ART in Nigeria.
METHODS
Study Design and Participants
A stepped-wedge, cluster-randomized study was conducted at 6 government-owned tertiary health care sites across 4 states in Nigeria, consisting of the pilot site (IDI) and the HIV outpatient clinics at the Lagos University Teaching Hospital, Lagos State University Teaching Hospital, Jos University Teaching Hospital, Olabisi Onabanjo University Teaching Hospital, and the Nigerian Institute of Medical Research. These main study sites recruited additional participants at 11 satellite secondary health care facilities. All the study sites operated similar HIV care protocols based on national guidelines. The study was approved by the Institutional Review Board at Northwestern University, the ethics committees at the main study sites, and the local health authority for the satellite sites. A written informed consent was obtained from each participant (or their parent if aged 15 years and not emancipated) before study participation.
YLH aged 15–24 years were eligible for this study; those aged 15 years had to have parental consent or be emancipated based on a prior court order, independent living without parental guidance for at least a year, marriage, or living on the street. The other eligibility criteria were being on ART for least 3 months, ability to understand and read basic English or the main local languages (Pidgin English, Hausa, or Yoruba), and intention to remain a patient at the study site throughout the study duration. To identify potential participants, we created a sampling frame from the medical records at each site, stratified by virologic status as virologically suppressed (most recent plasma HIV-1 RNA less than 200 copies/mL), or nonsuppressed. Eligible YLH were contacted and screened until the accrual target was reached. We enrolled both virologically suppressed and nonsuppressed YLH to evaluate the combination intervention in the clinical scenarios encountered in routine care. Enrollment was monitored to ensure the enrolled population reflected the proportion of virologically suppressed and nonsuppressed YLH on ART at the study sites.
We grouped the study sites into 3 clusters. Cluster 1 (IDI), being the pilot site, was not randomized, whereas cluster 2 (Lagos University Teaching Hospital, Lagos State University Teaching Hospital, Nigerian Institute of Medical Research) and cluster 3 (Olabisi Onabanjo University Teaching Hospital and Jos University Teaching Hospital) were randomized to sequences of routine care (control period) and 48 weeks of the combination intervention while continuing routine care (intervention period). Participants recruited at the secondary sites were enrolled under their respective main sites and were assigned to clusters and randomized accordingly. The control and intervention periods of observation across the 3 clusters lasted a total of 96 weeks. This design ensured exposure of participants in each of the 3 clusters to the combination intervention for the same duration of 48 weeks, which we considered important for research equity because the combination intervention had shown promising results in the pilot study. Study visits occurred every 24 weeks for viral load monitoring and completion of study questionnaires. A data safety and monitoring board provided oversight.
Control Period
When a cluster was in the control period, participants in that cluster received routine care based on national guidelines.4 All the sites were in the process of programmatic transition to dolutegravir-based first-line ART when the study started. Antiretroviral drugs were dispensed at on-site pharmacies every 3 to 6 months, and ART adherence was supported by trained adherence counselors and youth clubs that provided social interaction and peer support to promote ART engagement and adherence. The national guidelines included viral load measurement 6 months after ART initiation, then every 12 months if suppressed and every 6 months if unsuppressed. If HIV-1 RNA was above 1000 copies per milliliter, the threshold in the WHO-recommended public health approach to ART,16 intensive adherence counseling was provided for 3 months followed by switch to a protease inhibitor–based second-line regimen if the repeat HIV-1 RNA remained above 1000 copies per milliliter. Resistance testing was rarely performed, as it was restricted to those experiencing virologic failure on second-line ART.
Intervention Period
During intervention periods, all participants in the cluster received the iCARE combination intervention of peer navigation plus the TXTXT daily text message ART reminders while remaining exposed to routine care. The peer navigation component of the combination intervention was conducted by YLH volunteers at the site, selected using prespecified criteria, including being virologically suppressed within the preceding 12 months, clinically stable and subjectively assessed as doing well by their physician, willing to volunteer as a navigator, and completion of comprehensive training. Based on feedback from YLH during local adaption of the combination intervention, the peer navigators were aged 18–30 years, and each navigator was assigned 4 or 5 study participants with whom they shared characteristics, such as gender, level of education, mode of infection, sexual orientation, age, and religion. Peer support was designed to be dynamic and flexible, and it started with a needs assessment and mental health assessment conducted by trained clinic staff assisted by the peer navigator. A customized action plan was then developed, which identified specific tasks for the peer navigator to promote participant ART adherence, such as providing peer-to-peer encouragement, and helping with antiretroviral drug pick-up, clinic attendance, disclosure of HIV status, and linkage to mental health services as may be needed. Navigators initiated a minimum of 1 encounter every 2 weeks with each of their assigned participants in person or by texting, telephone call, or social media to follow-up on the action plan. To ensure intervention fidelity, the peer navigators were trained using lectures and role-plays on basic principles of ART, privacy, confidentiality, and best practices for disclosure.16 A procedures manual was used to define the scope of potential peer navigation. The peer navigation activities were integrated into clinic operations through in-person or telephone meeting between the navigator and a site nurse or adherence counselor at least every other week. Based on the recommendations from YLH, peer navigators received a monthly stipend of up to 20,000 Naira (about $50) to cover their communication and transportation costs. Participants also received a stipend of 4000 Naira per month (about $10) to cover the costs of telecommunication, including text messaging and phone calls.
The TXTXT daily ART reminder messages were sent automatically through the Dimagi Commcare platform.17 Each participant created their own reminder message, avoiding language that might lead to unintended HIV disclosure. Each participant also chose a preferred delivery time for the text message reminder based on their daily ART schedule. Participants were asked to text a response after receiving each reminder message to indicate whether they had taken their ART according to schedule. If the participant responded “yes,” an automated affirmative message such as “well done!” was sent in reply; a “no” response triggered an automated acknowledging and encouraging message such as “You can do it!” Participants were expected to use their own phones for text messaging. Cell phones were provided to participants who did not have access to a personal phone.
Outcomes
The primary outcome measure was virologic suppression, defined as plasma HIV-1 RNA less than 200 copies per milliliter. A cutoff of 200 copies per milliliter was selected because persistent viremia above this level has been associated with virologic failure and resistance evolution.18,19 Secondary outcome measures included self-reported adherence based on 30-day recall reported on a visual analogue scale of 0%–100%,20 dichotomized as ≥90% (adherent) or <90% (nonadherent). In post hoc analysis, we assessed virologic control below 1000 copies per milliliter, the threshold currently used in Nigeria's ART programs, and below 50 copies per milliliter, which was recently proposed by Nigerian researchers as a better benchmark to strengthen clinical outcomes and track progress in Nigeria's HIV control efforts.21
Statistical Analysis
With a small-to-moderate within-cluster correlation (ICC = 0.10) and moderate individual autocorrelation (0.50), this analysis will have adequate power (power = 0.945) to detect an OR of 1.5 in the proportion of participants achieving viral suppression with at least 90 participants per site. Our effect size estimate is conservative given that the iCARE UG3 pilot trial findings (OR = 14.0) was not randomized and not controlled. The primary analysis population was the intention-to-treat population. We also evaluated the per protocol population, which excluded missing or withdrawn cases. We used a generalized estimating equation approach to determine the difference between intervention and control periods, accounting for the clustering of individuals over time. Each model used a binomial distribution and an exchangeable correlation structure. An indicator for cluster membership was used to control for mean differences in the outcome across sites, whereas categorical indicators for each step were included in the model to account for secular trends in each outcome over time. The main variable of interest was an indicator identifying if the intervention had been introduced for each cluster. A sensitivity analysis was conducted to examine if time had a confounding influence on the intervention by examining the statistical significance of the interaction between the intervention and time, as suggested by recent reviews of stepped wedge randomized trials.22
Role of the Funding Source
Research reported in this publication was supported by the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health under Award Number UH3HD096920, as part of the Prevention and Treatment through a Comprehensive Care Continuum for HIV-affected Adolescents in Resource Constrained Settings (PATC3H) consortium. The research was also supported by the Fogarty International Center of the National Institutes of Health under Award Number D43TW009608. The content of this article is solely the responsibility of the authors and does not represent the official views of the National Institutes of Health.
RESULTS
Between April and October 2021, we screened 790 and assigned 550 YLH to 3 clusters (Fig. 1). Of these, a total of 541 participants were followed over time after withdrawing 9 individuals who were determined to be ineligible based on age or interest in serving as a peer navigator: cluster 1 (nonrandomized, n = 60), cluster 2 (randomized, n = 309), and cluster 3 (randomized, n = 172). Baseline characteristics of the participants in the 3 clusters (Table 1) indicate a mean age of 18 years, 53.8% female, 71.7% perinatally infected, and 38.6% virologically nonsuppressed (HIV-1 RNA of 200 copies/mL or greater). Most (71%) of the participants were on a dolutegravir-based, first-line regimen. One hundred and twenty-seven (23.5%) of the participants were provided cell phones. As shown in Figure 2, cluster 1 initiated the 48-week combination intervention first, followed by cluster 2 then cluster 3. An average of 96.5% of the participants across the 3 clusters completed study follow-up. Fourteen serious adverse events (12 deaths and 2 hospitalizations) were observed, none of which were related to the combination intervention. On average, participants had 71.3 (SD = 44.8) encounters with a peer navigator over the 48-week intervention period or about 3 per two-week period (goal was 1 per 2-week period).
FIGURE 1.
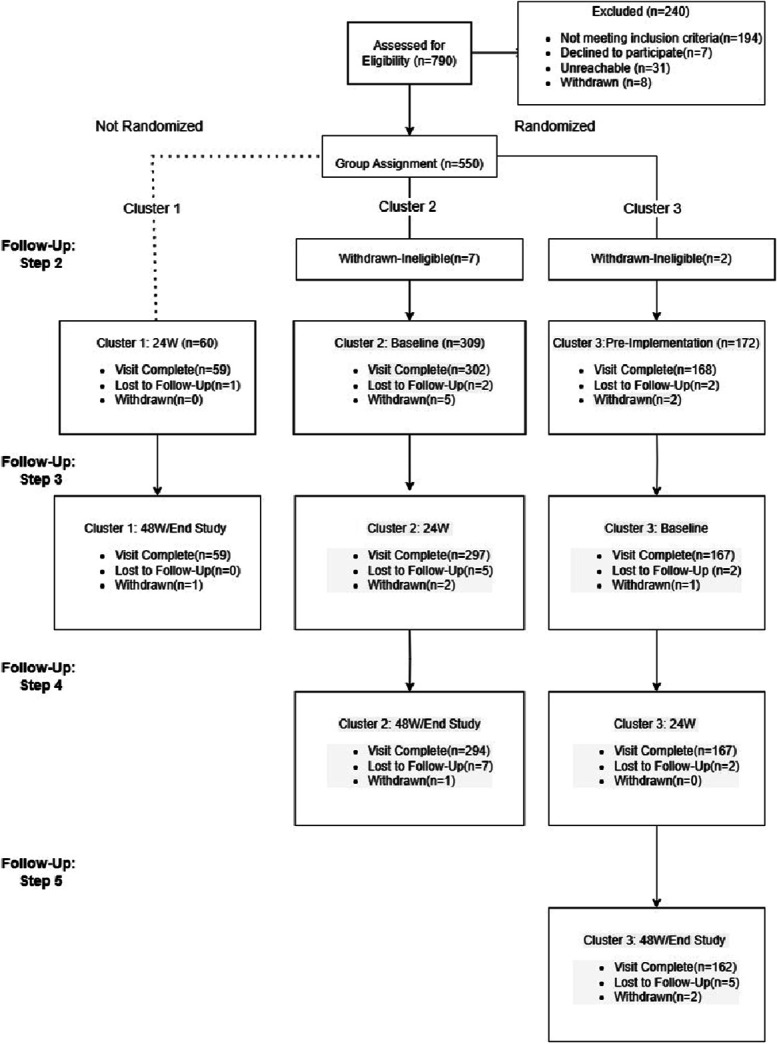
Flowchart of participants in the 3 clusters and 5 study steps.
TABLE 1.
Demographic Characteristics for Cluster-Randomized Participants at Enrollment (N = 541)
| Characteristic | IDI | JUTH | LASUTH | LUTH | NIMR | OOUTH | Total |
| Age (mean, SD) | 18.85 (2.4) | 18.15 (2.1) | 18.25 (2.5) | 18.95 (2.7) | 18.37 (2.4) | 18.47 (2.6) | 18.41 (2.5) |
| CD4 (mean, SD), (cells/mm3) | 458.25 (248.21) | 450.73 (220.29) | 511.52 (270.40) | 528.67 (288.04) | 468.20 (282.46) | 401.89 (341.03) | 476.08 (274.20) |
| Gender (n, %) | |||||||
| Male | 19 (31.7) | 54 (47.0) | 44 (43.1) | 52 (51.0) | 57 (54.3) | 23 (40.4) | 249 (46.0) |
| Female | 41 (68.3) | 61 (53.0) | 58 (56.9) | 50 (49.0) | 48 (45.7) | 34 (59.6) | 292 (54.0) |
| Mode of infection (n, %) | |||||||
| Perinatal | 39 (65.0) | 97 (84.3) | 75 (73.5) | 68 (66.7) | 83 (79.0) | 26 (45.6) | 388 (71.7) |
| Sexual risk | 11 (18.3) | 6 (5.2) | 6 (5.9) | 9 (8.8) | 7 (6.7) | 1 (1.8) | 40 (7.4) |
| Other* | 6 (10.0) | 8 (7.0) | 18 (17.6) | 21 (20.6) | 5 (4.8) | 15 (26.3) | 73 (13.5) |
| Do not know | 4 (6.7) | 4 (4.5) | 3 (2.9) | 3 (2.9) | 10 (9.5) | 15 (26.3) | 39 (7.2) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (1.0) | 0 (0.0) | 0 (0.0) | 1 (0.2) |
| HIV-1 RNA (n, %) | |||||||
| Suppressed (<200 copies/mL) | 40 (67.8) | 75 (65.2) | 68 (66.7) | 55 (53.9) | 60 (57.1) | 33 (57.9) | 331 (61.2) |
| Nonsuppressed | 19 (32.2) | 40 (34.8) | 34 (33.3) | 47 (46.1) | 45 (42.9) | 24 (42.1) | 209 (38.6) |
| Missing | 1 (1.7)† | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.2) |
Other includes reported modes such as exposure to contaminated sharp objects.
Missing viral load data due to specimen insufficiency or error.
FIGURE 2.
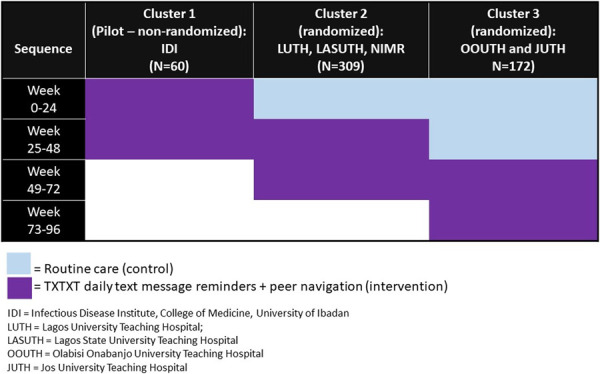
Sequences of exposure of participants in the 3 study clusters to routine care (control) and the combination intervention of peer navigation plus TXTXT daily ART reminder text messages (intervention).
The proportions of participants with virologic suppression (HIV-1 RNA <200 copies/mL) in clusters 1, 2, and 3 at baseline were 63.1%, 60.3%, and 64.7%, respectively; at week 24 of intervention, they were 70.7%, 65.0%, and 66.1%, respectively; at week 48 of intervention, the proportions were 61.4%, 62.0%, and 68.4%, respectively. Results of the generalized linear models are displayed in Table 2. The predicted probability of virologic success is shown in Figure 3A. In the primary intention-to-treat analysis (participantsn = 541, observationsn = 2276), intervention periods witnessed a small but nonsignificant increase in viral suppression {odds ratio, OR = 1.16 [95% confidence interval (CI): 0.88 to 1.52], P = 0.298} compared with control periods. When using the post hoc virologic control cutoff of HIV-1 RNA <1000 copies per milliliter, there was a significant increase in virologic control comparing intervention and control periods (OR = 1.37 [95% CI: 1.02 to 1.85], P = 0.037). There was no significant difference in virologic control at <50 copies per milliliter. With respect to adherence, intervention periods displayed a significant increase in self-reported adherence (OR = 2.03 [95% CI: 1.44 to 2.84], P < 0.001) compared with control periods and controlling for cluster membership and secular trends (Fig. 3B). Sensitivity analysis revealed no significant interaction between the intervention and time for the measures of viral suppression. With respect to adherence, there was a significant interaction between the intervention and time (OR = 0.69 [95% CI: 0.56 to 0.86], P < 0.001), indicating a smaller increase in the later intervention periods.
TABLE 2.
Results of Generalized Estimating Equations for Viral Suppression and Adherence
| Intention to Treat | HIV-1 RNA (<50) | HIV-1 RNA (<200) | HIV-1 RNA (<1000) | Adherence (90%) |
| Intercept | 1.00 [0.66–1.52] | 1.68 [1.07–2.63] | 2.34 [1.45–3.78] | 0.85 [0.56–1.28] |
| iCare | 1.25 [0.96–1.64] | 1.16 [0.88–1.52] | 1.37 [1.02–1.85] | 2.03 [1.44–2.84] |
| Step | ||||
| 1 (reference) | — | — | — | — |
| 2 | 0.99 [0.84–1.17] | 0.93 [0.78–1.11] | 0.85 [0.71–1.02] | 1.25 [1.01–1.55] |
| 3 | 0.89 [0.69–1.14] | 0.95 [0.73–1.24] | 0.86 [0.65–1.16] | 1.03 [0.73–1.46] |
| 4 | 0.79 [0.57–1.09] | 0.85 [0.60–1.19] | 0.66 [0.46–0.96] | 0.77 [0.50–1.19] |
| 5 | 0.81 [0.53–1.25] | 0.87 [0.57–1.33] | 0.65 [0.40–1.06] | 0.62 [0.34–1.16] |
| Cluster | ||||
| 1 (reference) | — | — | — | — |
| 2 | 0.95 [0.61–1.47] | 0.89 [0.55–1.42] | 0.92 [0.56–1.52] | 0.91 [0.59–1.39] |
| 3 | 1.18 [0.73–1.92] | 1.00 [0.60–1.67] | 1.11 [0.64–1.92] | 3.62 [2.19–6.00] |
| Per-protocol | HIV-1 RNA (<50) | HIV-1 RNA (<200) | HIV-1 RNA (<1000) | Adherence (90%) |
| Intercept | 1.03 [0.67–1.56] | 1.75 [1.11–2.76] | 2.47 [1.51–4.04] | 0.83 [0.55–1.26] |
| iCare | 1.26 [0.96–1.67] | 1.16 [0.88–1.54] | 1.42 [1.03–1.94] | 2.07 [1.46–2.95] |
| Step | ||||
| 1 (reference) | — | — | — | — |
| 2 | 1.03 [0.87–1.22] | 0.97 [0.81–1.17] | 0.90 [0.75–1.08] | 1.32 [1.06–1.64] |
| 3 | 0.93 [0.73–1.20] | 1.02 [0.78–1.33] | 0.95 [0.70–1.28] | 1.11 [0.78–1.59] |
| 4 | 0.84 [0.60–1.18] | 0.94 [0.66–1.34] | 0.74 [0.51–1.09] | 0.84 [0.54–1.32] |
| 5 | 0.95 [0.61–1.48] | 1.07 [0.69–1.67] | 0.81 [0.48–1.38] | 0.81 [0.42–1.55] |
| Cluster | ||||
| 1 (reference) | — | — | — | — |
| 2 | 0.93 [0.60–1.44] | 0.85 [0.53–1.37] | 0.87 [0.52–1.46] | 0.92 [0.60–1.42] |
| 3 | 1.16 [0.71–1.89] | 0.95 [0.57–1.60] | 1.04 [0.59–1.83] | 3.84 [2.30–6.42] |
Bold indicates statistically significant at P < 0.05.
FIGURE 3.
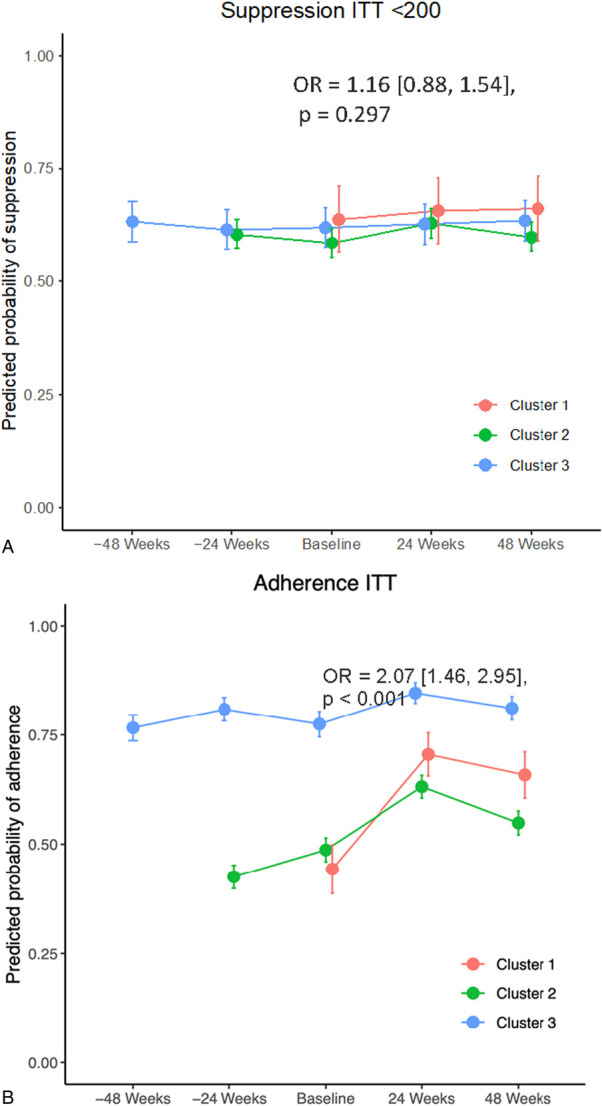
Probability of a successful outcome—virologic suppression below 200 copies per milliliter (A) and at least 90% adherence based on a 30-day visual analog scale (B).
Similar results were obtained in per protocol analysis (participantsn = 540, observationsn = 2203). Specifically, intervention periods demonstrated a small but nonsignificant increase in viral suppression below 200 copies per milliliter (OR = 1.16 [0.88, 1.54], P = 0.297) compared with control periods. When using an HIV-1 RNA cutoff of < 1000 copies per milliliter, there was a significant increase in virologic control comparing intervention and control periods (OR = 1.42 [1.03–1.94], P = 0.030). No significant difference was seen for HIV-1 RNA below 50 copies per milliliter. For adherence in the per-protocol analysis, intervention periods demonstrated a significant increase in adherence (OR = 2.07 [1.46–2.95], P < 0.001) compared with control periods and controlling for cluster membership and secular trends.
DISCUSSION
New interventions are needed to close the viral suppression gap between youth and adults living with HIV. We addressed this in the iCARE Nigeria study by evaluating the combination of peer navigation and daily text message ART reminders in a stepped-wedge, cluster-randomized, multisite trial. The study was successfully implemented in ART clinics located in tertiary and secondary health care centers that are representative of government-owned ART delivery sites in Nigeria. Although part of the study occurred during the Coronavirus 2019 lockdown, more than 95% of the randomized participants completed all the study visits. The proportion of participants who achieved the primary outcome (viral suppression defined as HIV-1 RNA below 200 copies/mL) ranged between 60.3% and 70.7% across the 3 clusters during the study. There was no significant difference between the control and intervention periods.
The challenging clinical context likely contributed to the absence of a significant effect of the combination intervention on the primary outcome in this study. Notably, we recruited predominantly perinatally infected youth, consistent with the population of YLH receiving ART at the study sites. Perinatally infected YLH have greater barriers to ART adherence and viral suppression than other YLH, including increased risks for antiretroviral drug resistance, neurocognitive impairment, and treatment fatigue.23 The mental health barriers to ART adherence commonly experienced by YLH are exacerbated by chronic illness, parental loss, and the difficult transition from pediatric to adult clinics in the perinatally infected subgroup.24 Our results suggest that more effective interventions addressing the unique challenges of youth are needed, particularly for perinatally infected youth in Nigeria and similar settings where perinatal infections continue to occur.25,26
The routine adherence programs at the study sites may have also muted the potential impact of the iCARE combination intervention because of overlapping mechanisms of change, such as provision of peer support and improvement of self-efficacy. The routine adherence activities were coordinated by trained counselors, who organized regular youth clubs with funding from governmental and donor agencies. By contrast, the TXTXT text message reminder component of the iCARE combination intervention employs different mechanisms of change, including the provision of direct and repetitive reminders with immediate positive reinforcement for adherence; hence, it was expected to have a measurable independent impact. However, it is possible that some participants' response to the daily text message reminders dwindled over time as demonstrated in our previous trial.12 Reinforcing participant responses to the daily text messages, which was not done consistently as part of our research protocol, may be one way to boost the impact of this intervention approach on study outcomes.
The combination intervention did significantly improve virologic control below 1000 copies per milliliter but not below 50 copies per milliliter. Although 1000 copies per milliliter is the least stringent threshold evaluated, it is important because it is the virologic benchmark used in the WHO public health approach to ART and is tracked by many countries and the Joint United Nations Program on HIV/AIDS as a key indicator for ART programmatic outcomes. The 1000 copies per milliliter cutoff is also used programmatically in Nigeria and similar settings to make clinical decisions, such as when to switch to a new ART regimen. Furthermore, this threshold may be relevant to transmission prevention efforts, as a recent study reported that viral suppression below 1000 copies per milliliter can reduce the risk of sexual transmission to almost zero,27 consistent with another study where the risk of transmission per sexual act without a condom when viral load was <1000 copies per milliliter was estimated to be as low as 0.00028.28
The discordant effect of the intervention on viral suppression below 1000 copies per milliliter compared with 200 copies per milliliter (the primary outcome measure) and 50 copies per milliliter is notable because we saw a significant increase (approximately doubling) in self-reported adherence during the intervention period. One potential explanation for the discordance is that the level or pattern of adherence improvement lowered viremia modestly to below 1000 copies per milliliter but was inadequate to lower viremia to levels below 200 copies per milliliter. It is also plausible that the level and pattern of adherence improvement were robust, but antiretroviral drug resistance limited the efficacy of the treatment regimens and blunted the potential effects of improved adherence on viral suppression. This scenario is particularly plausible in perinatally infected participants with extensive histories of ART and failure of prior regimens. The lack of resistance data limits our ability to fully investigate the potential impact of drug resistance on our results. On the other hand, it is possible that self-reported adherence was inflated due in part to social desirability bias during the intervention period29 and that actual changes in adherence were modest and hence the modest effect of the intervention on viral suppression. An objective biomarker of adherence, such as the level of tenofovir in clinical samples, might have been helpful in improving the precision of our adherence assessment. Another limitation of this study is that the combination intervention had 2 independent components (peer navigation and TXTXT), and we are unable to ascertain which component worked or did not work. However, we designed the iCARE intervention as a combination of 2 interventions because single interventions have been reported to have limited success.5 Finally, peer navigation may have introduced some dependency in our data that was not accounted for in the analysis. Taken together, the lack of a significant effect of the iCARE intervention on viral suppression below 200 copies per milliliter suggests that the intervention has limited benefits in the population studied, especially because there are concerns about the potential for increased risk of virological nonsuppression and failure among persons with low-level viremia, including from Nigerian researchers.21
In summary, we evaluated a combination intervention comprising peer navigation plus TXTXT daily text message ART reminders in the iCARE Nigeria study. In clinics with concurrent routine adherence activities, the addition of the iCARE combination intervention did not significantly improve virologic suppression below 200 copies per milliliter among predominantly perinatally infected YLH on ART, despite significant improvement in self-reported adherence. An improvement was seen in virologic control to levels below 1000 copies per milliliter. These results highlight the complexities of improving viral suppression among ART-experienced YLH, the population evaluated in this study. The iCARE Nigeria combination intervention may produce different results among treatment-naive youth who acquired HIV behaviorally. As viral suppression must be high across age groups to achieve the United Nations Program on HIV/AIDS 95% target, efforts should be redoubled to close the gap between youth and adults living with HIV. It is noteworthy that a recent study showed superior virologic suppression rates with long-acting cabotegravir plus rilpivirine compared with standard oral ART among adults experiencing virologic failure.30 Studies of long-acting ART are also needed among YLH to expand the treatment options for those struggling to achieve and maintain viral suppression.
Footnotes
Funding: National Institutes of Health.
B. O. Taiwo has received consultant fees in the past from ViiV/GSK, Gilead, and Johnson and Johnson. The remaining authors have no funding or conflicts of interest to disclose.
Accepted for presentation at the 25th International AIDS Conference, Munich, Germany on July 24, 2024.
REFERENCES
- 1.UNAIDS . Young Peole and HIV; 2021. Available at https://www.unaids.org/sites/default/files/media_asset/young-people-and-hiv_en.pdf. Accessed February 20, 2024. [Google Scholar]
- 2.Tomescu S, Crompton T, Adebayo J, et al. Factors associated with viral load non-suppression in people living with HIV on ART in Nigeria: cross-sectional analysis from 2017 to 2021. BMJ Open. 2023;13:e065950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Federal Ministry of Health Nigeria. Process report on Integrated biological & behavioural surveillance survey (IBBSS), 2020. Available at: https://wacphd.org/projects/ibbss-2020/. Accessed February 20, 2024.
- 4.Federal Ministry of Health Nigeria. National guidelines for HIV prevention, treatment and care. National AIDS and STIs Control Programme. 2020. Available at: ///Users/tba936/Downloads/17%20(3).pdf. Accessed February 10, 2024. [Google Scholar]
- 5.Kanters S, Park JJ, Chan K, et al. Interventions to improve adherence to antiretroviral therapy: a systematic review and network meta-analysis. Lancet HIV. 2017;4:e31–e40. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization. HIV and Adolescents: Guidance for HIV Testing and Counselling and Care for Adolescents Living with HIV. Geneva, Switzerland: World Health Organization, 2013. Available at: https://www.who.int/publications/i/item/9789241506168. Accessed January 20, 2024. [Google Scholar]
- 7.Simoni JM, Nelson KM, Franks JC, et al. Are peer interventions for HIV efficacious? A systematic review. AIDS Behav. 2011;15:1589–1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simoni JM, Franks JC, Lehavot K, et al. Peer interventions to promote health:conceptual considerations. Am J Orthopsychiatry. 2011;81:351–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bandura A. The anatomy of stages of change. Am J Health Promot. 1997;12:8–10. [DOI] [PubMed] [Google Scholar]
- 10.Bandura A. Health promotion by social cognitive means. Health Educ Behav. 2004;31:143–164. [DOI] [PubMed] [Google Scholar]
- 11.Militello LK, Kelly SA, Melnyk BM. Systematic review of text-messaging interventions to promote healthy behaviors in pediatric and adolescent populations: implications for clinical practice and research. Worldviews Evid Based Nurs. 2012;9:66–77. [DOI] [PubMed] [Google Scholar]
- 12.Garofalo R, Kuhns LM, Hotton A, et al. A randomized controlled trial of personalized text message reminders to promote medication adherence among HIV-positive adolescents and young adults. AIDS Behav. 2016;20:1049–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention. Effective behavioral interventions. 2019. Available at: https://www.cdc.gov/hiv/research/interventionresearch/ebis/index.html. Accessed February 12, 2024.
- 14.Kuhns LM, Johnson AK, Adetunji A, et al. Adaptation of evidence-based approaches to promote HIV testing and treatment engagement among high-risk Nigerian youth. PLoS One. 2021;16:e0258190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taiwo BO, Kuti KM, Kuhns LM, et al. Effect of text messaging plus peer navigation on viral suppression among youth with HIV in the iCARE Nigeria pilot study. J Acquir Immune Defic Syndr. 2021;87:1086–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.World Health Organisation (WHO). Consolidated guidelines on HIV prevention, testing, treatment, service delivery and monitoring: recommendations for a public health approach 2021. Available at: https://www.who.int/publications/i/item/9789240031593. Accessed February 21, 2024. [PubMed]
- 17.CommCare. Dimagi app builder. Available at: https://www.dimagi.com/commcare/. Accessed February 29, 2024.
- 18.Antiretroviral Therapy Cohort Collaboration ART-CC; Vandenhende MA, Ingle S, et al. Impact of low-level viremia on clinical and virological outcomes in treated HIV-1-infected patients. AIDS. 2015;29:373–383. [DOI] [PubMed] [Google Scholar]
- 19.Boillat-Blanco N, Darling KE, Schoni-Affolter F, et al. Virological outcome and management of persistent low-level viraemia in HIV-1-infected patients: 11 years of the Swiss HIV Cohort Study. Antivir Ther. 2015;20:165–175. [DOI] [PubMed] [Google Scholar]
- 20.Finitsis DJ, Pellowski JA, Huedo-Medina TB, et al. Visual analogue scale (VAS) measurement of antiretroviral adherence in people living with HIV (PLWH): a meta-analysis. J Behav Med. 2016;39:1043–1055. [DOI] [PubMed] [Google Scholar]
- 21.Chun HM, Abutu A, Milligan K, et al. Low-level viraemia among people living with HIV in Nigeria: a retrospective longitudinal cohort study. Lancet Glob Health. 2022;10:e1815–e1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hemming K, Haines TP, Chilton PJ, et al. The stepped wedge cluster randomised trial: rationale, design, analysis, and reporting. BMJ. 2015;350:h391. [DOI] [PubMed] [Google Scholar]
- 23.Fields EL, Bogart LM, Thurston IB, et al. Qualitative comparison of barriers to antiretroviral medication adherence among perinatally and behaviorally HIV-infected youth. Qual Health Res. 2017;27:1177–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patel K, Seage GR, Burchett SK, et al. Disparities in HIV viral suppression among adolescents and young adults by perinatal infection. Am J Public Health. 2019;109:e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.UNAIDS. Miles to go closing gaps breaking barriers righting injustice. 2018. Available at: https://www.unaids.org/en/resources/documents/2018/global-aids-update#:∼:text=The%20global%20AIDS%20response%20is,not%20matching%20the%20global%20ambition. Accessed February 12, 2024.
- 26.UNICEF (Press release). A child was infected with HIV every two minutes in 2020. Available at: https://www.unicef.org/nigeria/press-releases/child-was-infected-hiv-every-two-minutes-2020-unicef#:∼:text=Nigeria%20HIV%2FAIDS%20data%3A,compared%20with%201%2C575%20adolescent%20boys. Accessed February 29, 2024.
- 27.Broyles LN, Luo R, Boeras D, et al. The risk of sexual transmission of HIV in individuals with low-level HIV viraemia: a systematic review. Lancet. 2023;402:464–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hughes JP, Baeten JM, Lingappa JR, et al. Determinants of per-coital-act HIV-1 infectivity among African HIV-1-serodiscordant couples. J Infect Dis. 2012;205:358–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kagee A, Nel A. Assessing the association between self-report items for HIV pill adherence and biological measures. AIDS Care. 2012;24:1448–1452. [DOI] [PubMed] [Google Scholar]
- 30.Rana AI, Bao Y, Zhen L, et al. Long-acting injectable CAB/RPV is superior to oral ART in PWH with adherence challenges: ACTG A5359. Abstract 212. Conference on Retroviruses and Opportunistic Infections in Denver, Colorado. March 6, 2024.


