Abstract
The highly conserved human and mouse SLC39A14 and SLC39A8 genes encode the ZIP14 and ZIP8 transporters, respectively—functioning as divalent cation/bicarbonate symporters and expressed in dozens of tissues. Due to alternative splicing of exons 4, human and mouse SLC39A14 genes each encode two distinct gene products, whereas SLC39A8 produces a single product. This lab previously noted that ZIP14A and ZIP14B show highly variable expression in different cell types, suggesting differences in metal uptake function. We ligated mouse ZIP14A, ZIP14B and ZIP8 cDNA coding regions into the Xenopus-specific vector pXFRM, transcribed these in vitro, and microinjected the capped RNAs into Xenopus oocytes. Km and Vmax values for Cd, Zn and Fe uptake were determined. Electrogenicity studies using a potassium gradient confirmed that (just as we found previously for ZIP8) ZIP14A- and ZIP14B-mediated divalent Cd– or Zn–bicarbonate complexes are electroneutral. Competitive inhibition of Cd and Zn uptake with ten additional divalent cations showed a unique gradient of patterns for each of ZIP14A, ZIP14B and ZIP8. ZIP14 proteins are prominent in the gastrointestinal tract and ZIP8 protein is located on the surface of renal proximal tubular epithelial cells. It is known that renal Fanconi syndrome can be caused by five nonessential heavy metals: Cd2+, Hg2+, Pb2+, Pt2+ and U2+. In the present study we show that these five divalent cations are usually competitors of ZIP14- and/or ZIP8-mediated Zn uptake; our data thus support the possible involvement of intestinal ZIP14 for uptake of these five metals into the body and ZIP8 for efficient uptake into the kidney.
Introduction
Cadmium (Cd, Cd2+) is a nonessential heavy metal which is both toxic and carcinogenic. Acute doses of Cd can damage the central nervous system, lung, bone, gastrointestinal (GI) tract, liver, ovary, testis, placenta, and developing embryo [reviewed in ref. 1–3]. Chronic low doses of Cd can cause proximal renal tubular metabolic acidosis (RTA) and osteomalacia (renal Fanconi syndrome); Cd accumulates with age, predominantly in the kidney.1 Cd is classified by IARC as a “Category I” human lung carcinogen. Persons at highest risk for Cd-induced lung cancer and renal disease include cigarette smokers, those ingesting excessive amounts of Cd-contaminated shellfish, and those having anemia, malnutrition, and/or infection.2,3
To be toxic or carcinogenic, Cd must enter the cell. Until this past decade, little molecular biology was known about Cd influx into cells. Starting with mouse inbred strain differences in Cd-induced testicular necrosis as the phenotype,4 this laboratory proceeded via positional cloning to identify and characterize the Cdm locus;5–7 ultimately, the Cdm locus was proven to be the Slc39a8 gene8 which encodes ZIP8, an apical-surface-located Zn2+/(HCO3−)2 symporter that moves one cation and two anions into the cell as an electroneutral complex.9 Fe2+ and Mn2+ are also likely endogenous substrates for ZIP8.1
Mouse Slc39a8 is one of fourteen genes in the ZIP (SLC39) family; the human genome also has fourteen SLC39 genes, confirming a high degree of conservation in sequence and function between mouse and human.1 Evolutionarily, ZIP14 is most closely related to ZIP8—relative to the other twelve SLC39 genes that are more diverged from this pair. This lab determined that ZIP14 is also an apical-surface-located Zn2+/(HCO3−)2 symporter;10 again, Fe2+ and Mn2+ are likely endogenous substrates for ZIP14.10,11
The mouse Slc39a14 gene (Fig. 1) encodes two proteins, due to alternative splicing of exons 4: ZIP14A and ZIP14B both contain 289 amino acids, yet have molecular weights of 53 754 and 53 962 Daltons, respectively.1 Whereas both are ubiquitously expressed in innumerable mammalian tissues, ZIP8 expression predominates in kidney, lung and testis, whereas ZIP14 is most highly expressed in the GI tract and hepatocyte.1,8,10 Intriguingly, distinct differences in cell-type expression exist among ZIP14A and ZIP14B.10 ZIP14A expression is highest in liver, duodenum, kidney, and testis; in contrast, ZIP14B activity is highest in liver, duodenum, brain, and testis.10 ZIP8 expression is highest in lung, testis, and kidney.8 These findings suggest that both ZIP14 transporters derived from the same gene, as well as ZIP8 from its own gene, might all uniquely participate in specific functions, as well as in specific cell types.
Fig. 1.
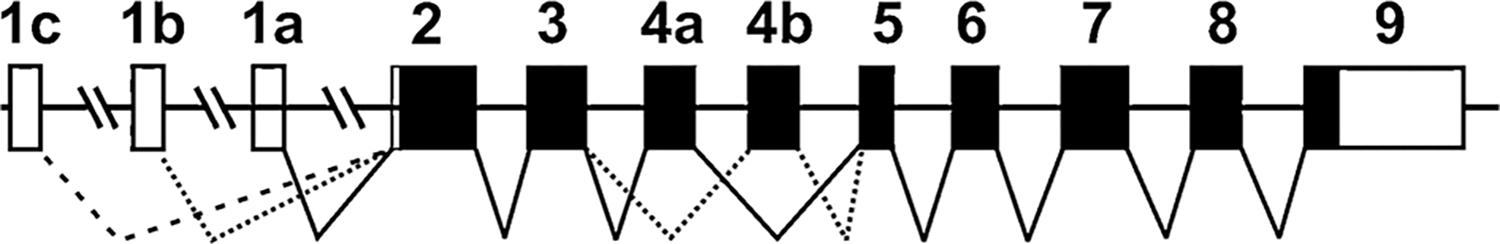
Structure of mouse Slc39a14 genomic gene (introns not drawn to scale)—illustrating the alternatively spliced exons 4, which encode the ZIP14A and ZIP14B proteins, respectively. Similar to Slc39a8,6 the Slc39a14 gene also has three alternatively spliced nontranslated exons 1 (shown as exons 1a, 1b and 1c, moving upstream from exon 2, as standardized nomenclature dictates). Within the exons (rectangles), the coding region is closed, and the 5′ and 3′ UTRs are open. The human SLC39A14 gene has the same highly conserved structure as the mouse gene, except that exons 1b and 1c have not been identified.10
Cell-type-specific expression levels of each transporter—coupled with route-of-uptake and distribution, local concentration of each nonessential heavy metal, and binding affinity—are all clinically important parameters for understanding the etiology of heavy metal-induced disease. For each essential divalent cation, cell-type-specific expression levels of each transporter, coupled with the amount of ingested metal and distribution of each cation that might compete with environmental toxic metals, are clinically relevant to the balance between nutrition and disease. Uptake Km values can be determined in mammalian cell culture;7,10 however, generally these values are artificially high and inaccurate due to competition with dozens if not hundreds of other transporters on mammalian cell surface membranes. Hence, Xenopus oocytes are commonly used, due to small numbers of interfering transporters on their cell surface, i.e. less background “noise”.
Using Xenopus oocytes, we found that ZIP8 has Km values of 0.26 μM and 0.48 μM for Zn and Cd, respectively.9 Depending on local concentrations of Cd in a specific cell type, therefore, Cd due to its high affinity appears likely to displace the endogenous Zn (or Mn or Fe) cation and enter the cell via ZIP8. In the present Xenopus oocyte study, we compared kinetics and inhibition by other divalent cations—as well as electrogenicity of ZIP14A- and ZIP14B-mediated Cd2+/bicarbonate and Zn2+/bicarbonate—with that of ZIP8-mediated parameters.
Materials and methods
Chemicals
Most divalent cations were purchased as chloride or acetate salts from Fisher Scientific (Pittsburgh, PA): Cd, Zn, Fe, Mn, cobalt (Co), copper (Cu), nickel (Ni), calcium (Ca), mercury (Hg), and lead (Pb). For platinum (Pt), the chemotherapeutic agent cisplatin [cis-diamminedichloroplatinum(II)] (absorbed as valence Pt2+) was purchased from Sigma-Aldrich (St Louis, MO). For depleted uranium (U), uranyl acetate (valence U2+) was a generous gift from Dr Marian L. Miller (University Cincinnati, Ohio). Tetramethylammonium (TMA+) chloride, collagenase, and Chelex-100 were bought from Sigma (St Louis, MO). 65ZnCl2 [4.28 mCi mg−1 in 0.1 M HCl] and 109CdCl2 [159.42 mCi mg−1 in 0.1 M HCl] were purchased from Perkin Elmer (Waltham, MA), and 55FeCl2 [120 mCi mg−1 in 0.1 M HCl] from National Laboratory of Oak Ridge (Oak Ridge, TN); [1 mCi = 37 mBq]. ND-96 uptake medium (96 mM NaCl, 2.0 mM KCl, 1.0 mM MgCl2, 1.8 mM CaCl2, 5.0 mM HEPES, 100 U mL−1 penicillin, and 100 μg mL−1 streptomycin, pH 7.5) was made fresh and filtered in our laboratory. For collecting oocytes, OR2 medium (82.5 mM NaCl, 2 mM KCl, 1 mM MgCl2, 5 mM HEPES buffer, pH 7.5) was made fresh and filtered in our laboratory.
Generation of ZIP8, 14A and 14B capped RNA (cRNA) from cDNA-containing vectors
ZIP8, ZIP14A and ZIP14B cDNAs7,10 were excised from pBluescript vector, using BamHI and SalI sites and ligated into the multiple-cloning site of pXFRM (Fig. 2). For in vitro transcription, plasmids were linearized with BspQI. The cRNAs were transcribed in vitro from the linearized cDNA template using the mMESSAGE mMACHINE SP6 kit (Ambion; Austin, TX), according to manufacturer’s instructions. Size and quality of purified transcription products were evaluated by gel electrophoresis and quantified by spectrophotometry (NanoDrop ND-1000; Thermo Fisher). The cRNAs were dissolved in RNase-free water and stored at −80 °C until use, but for no longer than 15 days.
Fig. 2.
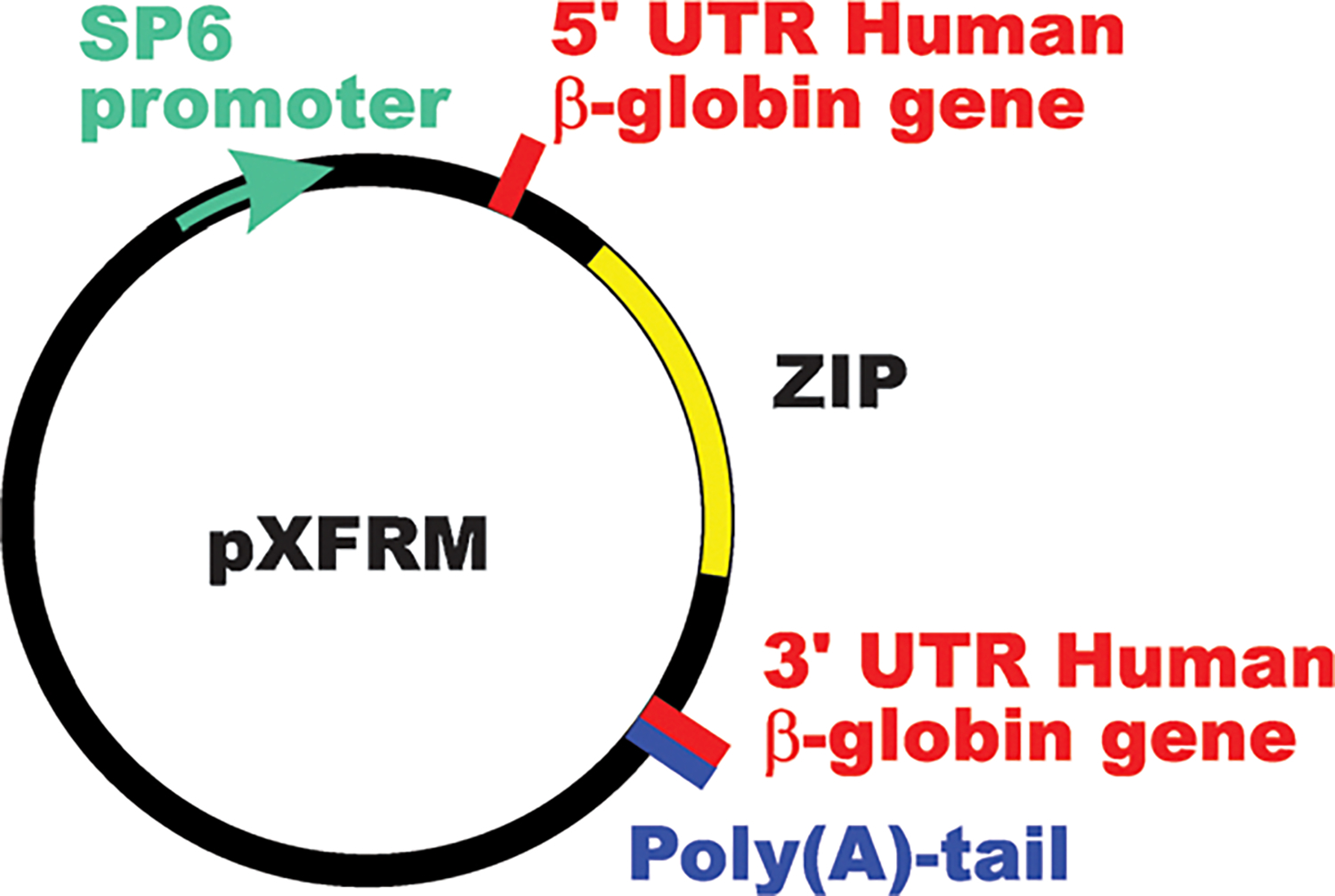
Diagram of pXFRM, a specific Xenopus vector28,29 which was a generous gift of William F. Marzluff (University of North Carolina, Chapel Hill). The coding regions of mouse ZIP14A, ZIP14B or ZIP8 cDNA were inserted into the fragment labeled “ZIP”. UTR, untranslated region. Official name of human β-globin gene is ACTB.
Microinjection of cRNA into Xenopus laevis oocytes
All frog experiments were approved by, and conducted in accordance with, the National Institutes of Health standards for care and use of experimental animals and the University Cincinnati Medical Center Institutional Animal Care and Use Committee. Frogs were anesthetized with 0.2% tricaine methane sulfonate. Clumps of oocytes were removed, and washed in OR2 buffer. Single healthy oocytes were dissociated by type II collagenase (3 mg mL−1) in Ca2+-free ND-96 solution at 20 °C. After digestion, the follicular layer was removed mechanically with a fire-polished Pasteur pipette. The cRNA (50 nL of 0.5 μg−1 μL−1 solution) was injected with a Drummond 510 micro-dispenser having a sterile glass pipette (Drummond NANOJET). After injection, oocytes were maintained in ND96 solution, as described.12 All assays were carried out at least 2 days after cRNA microinjection. Incubation medium was maintained at 22 °C (pH 7.5), with eight to 12 oocytes per time-point, or per concentration-point. Because protein content of oocytes varied widely (range = 266 to 1250 μg per oocyte, all kinetics data are expressed as “per oocyte”.
Radiolabeled divalent cation uptake and culture conditions
Because ZIP14A, ZIP14B and ZIP8 symporters are bicarbonate-dependent,7,10 the ND-96 medium always included 25 mM NaHCO3− (pH 7.5). Oocytes grown in ND-96 were otherwise treated with radiolabeled 109Cd2+, 65Zn2+, or 55Fe2+ as previously described.7,10
Competition of Cd or Zn uptake by other divalent cations
The competing divalent cation was added to ND-96 medium simultaneously with addition of (radiolabeled) 0.5 μM Cd, Zn or Fe—at concentrations 3, 10 or 30 times greater than that of Cd or Zn—and incubated 20 min. The chosen concentration (0.5 μM) and length of incubation (20 min) were based on dose and time-curves by which uptake linearity had been determined for each metal and each transporter. Due to precipitation problems, Hg2+ and Pb2+ studies were carried out in chloride-free ND-96 in which Cl anion had been replaced by gluconate anion. Likewise, Ca-free ND-96 was used for Ca competition studies: Ca2+ was replaced by Na1+. In order to maintain reduced Fe2+, 1 mM ascorbate acid was added to the ND-96 medium for all studies involving Fe. After a 20 min incubation, oocytes were washed three times with cold ND-96, lysed with 0.1 M NaOH at 60 °C for 30 min, neutralized with HCl, and radioactivity was determined using a Perkin Elmer Scintillator Counter 2200. Experiments with each competitor were performed a minimum of three times.
Electrogenicity studies
Modified ND-96 medium (containing 25 mM NaHCO3−) was adjusted with tetramethylammonium (TMA+) to block water permeability, and the medium was maintained at a constant 98 mM isosmolar replacement of monovalent cations: i.e. if K+ = 60 mM, then Na+ = 38 mM; if K+ = 20 mM, then Na+ = 38 mM and TMA+ = 40 mM; if K+ = 2 mM, then Na+ = 38 mM and TMA+ = 58 mM; if K+ = 1 mM, then Na+ = 38 mM and TMA+ = 59 mM. Cd or Zn uptake by ZIP-containing oocytes was compared with that by water-injected oocytes at each K+ concentration.
Statistical analysis
Graphs and all calculations were generated using Microsoft Windows Excel (Microsoft Corporation). Student’s t-test and analysis of variance (ANOVA) were used. P-values of <0.05 were regarded as statistically significant.
Results
Comparison of Cd, Zn and Fe uptake kinetics
ZIP14A-, ZIP14B- and ZIP8-mediated Cd uptake was first determined as a function of time (Fig. 3A). Cd (or Zn or Fe; data not shown) uptake carried out by each of the three transporters was linear over ~60 min of incubation time. A 20-min incubation time was thus chosen for all remaining studies.
Fig. 3.
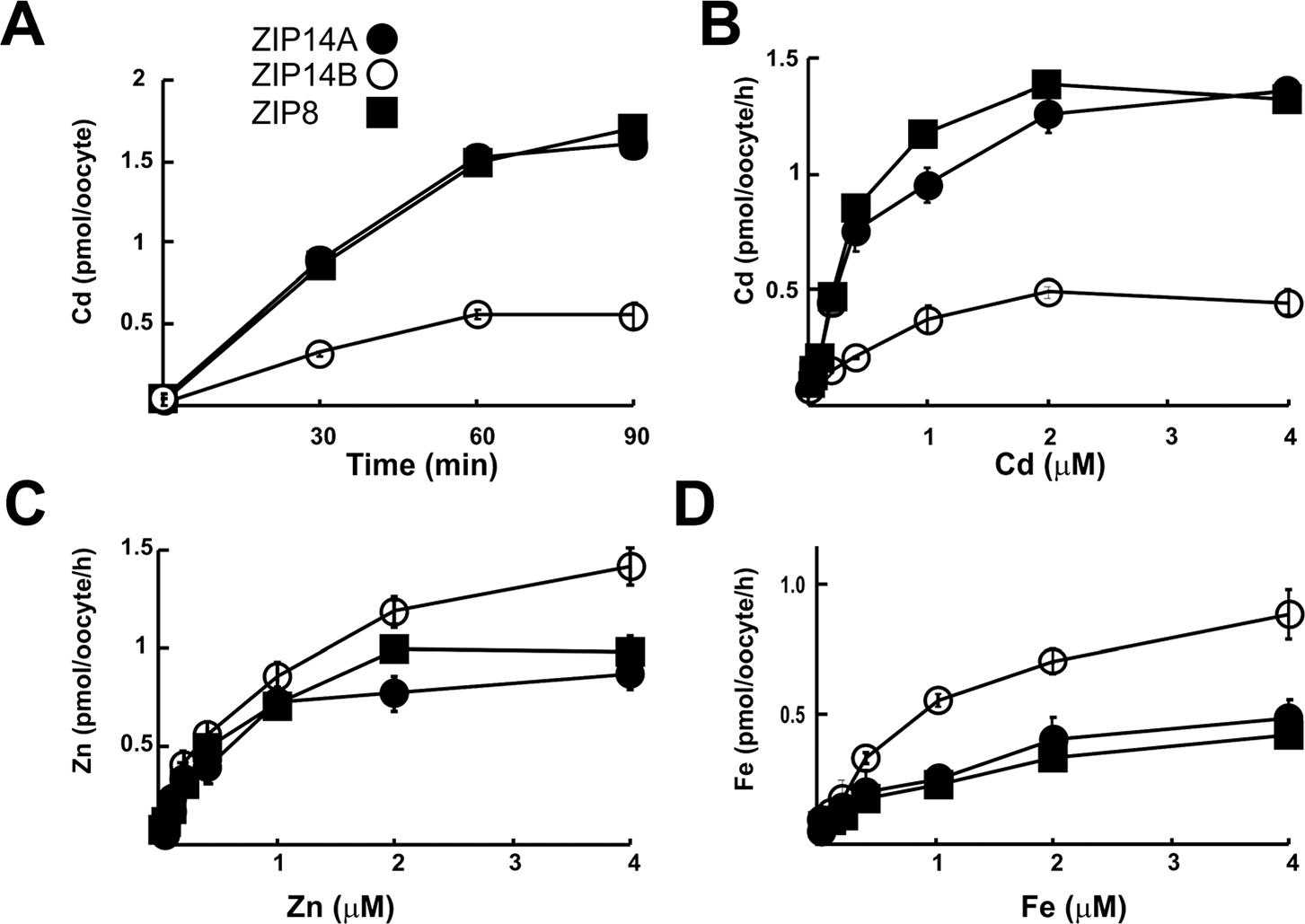
Divalent cation uptake kinetics by ZIP14A, ZIP14B and ZIP8. (A) Cd uptake as a function of time. In this and in subsequent panels, values of control oocytes (water-injected only) are subtracted from those of cRNA-injected oocytes. Metal uptake values in empty-vector-injected oocytes were similar to those by water-injected oocytes [data not shown]. (B) Cd uptake as function of concentration. (C) Zn uptake as a function of concentration. (D) Uptake as a function of concentration. In this and remaining figures, data are expressed as means (symbols) ± S.E.M. (brackets). Note different values on the Y-axes.
With regard to divalent cation uptake as a function of metal concentration, the ZIP14A and ZIP8 curves were similar for Cd (Fig. 3B), curves for each of the three transporters were distinctly different for Zn (Fig. 3C), and similar ZIP14A and ZIP8 curves were much lower than the ZIP14B curve for Fe (Fig. 3D). Km and Vmax uptake values for all three divalent cations mediated by the three transporters are listed in Table 1. Rank order of affinity for Cd uptake was ZIP14B (highest) > ZIP8 > ZIP14A, but the range of all three Km values was < 2-fold. Rank order of affinity for Zn uptake was ZIP8 (highest) > ZIP14A > ZIP14B, over a 3-fold range. Rank order of affinity for Fe uptake was ZIP8 (highest) ~/= ZIP14A ≫ ZIP14B, with a 4-fold range in Km values.
Table 1.
Km and Vmax values for Cd, Zn and Fe uptake by ZIP14A, ZIP14B and ZIP8
| Divalent cation | ZIP14A |
ZIP14B |
ZIP8 |
||||
|---|---|---|---|---|---|---|---|
| Km (μM) | Vmax a | Km (μM) | Vmax a | Km (μM) | Vmax a | ||
|
| |||||||
| Cd | 0.54 μM ± 0.78 | 1.60 ± 0.07 | 0.31 μM ± 0.01 | 0.51 ± 0.01 | 0.48 μM ± 0.08 | 1.81 ± 0.07 | |
| Zn | 0.36 μM ± 0.03 | 1.05 ± 0.01 | 0.78 μM ± 0.027 | 1.67 ± 0.10 | 0.26 μM ± 0.09 | 1.00 ± 0.07 | |
| Fe | 0.19 μM ± 0.12 | 0.38 ± 0.07 | 0.64 μM ± 0.16 | 0.92 ± 0.08 | 0.16 μM ± 0.10 | 0.24 ± 0.05 | |
Vmax Values are expressed as “pmol per oocyte per h”.
Rank order of Vmax values of the three cations for ZIP14A was Cd > Zn > Fe; for ZIP14B was Zn > Fe > Cd; and for ZIP8 was Cd > Zn > Fe. Curiously, the ZIP14A and ZIP8 characteristics of divalent cation uptake seem to be more similar to one another, whereas ZIP14B is distinctly different from the other two symporters.
Competition studies of Cd or Zn uptake with ten additional metals
Competition of Cd uptake (Fig. 4) and Zn uptake (Fig. 5) by ten additional divalent cations was assessed. We chose to list the order of metals in three groups of four: Cd, Zn, Fe and Mn—previously shown to be excellent substrates for these transporters;1,6,7,10 Co, Cu, Ni and Ca—heretofore unknown divalent cation substrates; and lastly, Hg, Pb, Pt and U—the four metals (along with Cd) known to cause renal Fanconi syndrome.13
Fig. 4.

Inhibition of Cd uptake by competitor cations. Competitor cation was added (concomitantly with Cd) at 3-fold, 10-fold and 30-fold greater concentrations than Cd. Uptake mediated by ZIP14A (top), ZIP14B (middle) and ZIP8 (bottom). To avoid clutter in this figure and the next, P-values and brackets denoting S.E.M. are removed, but are available on request.
Fig. 5.
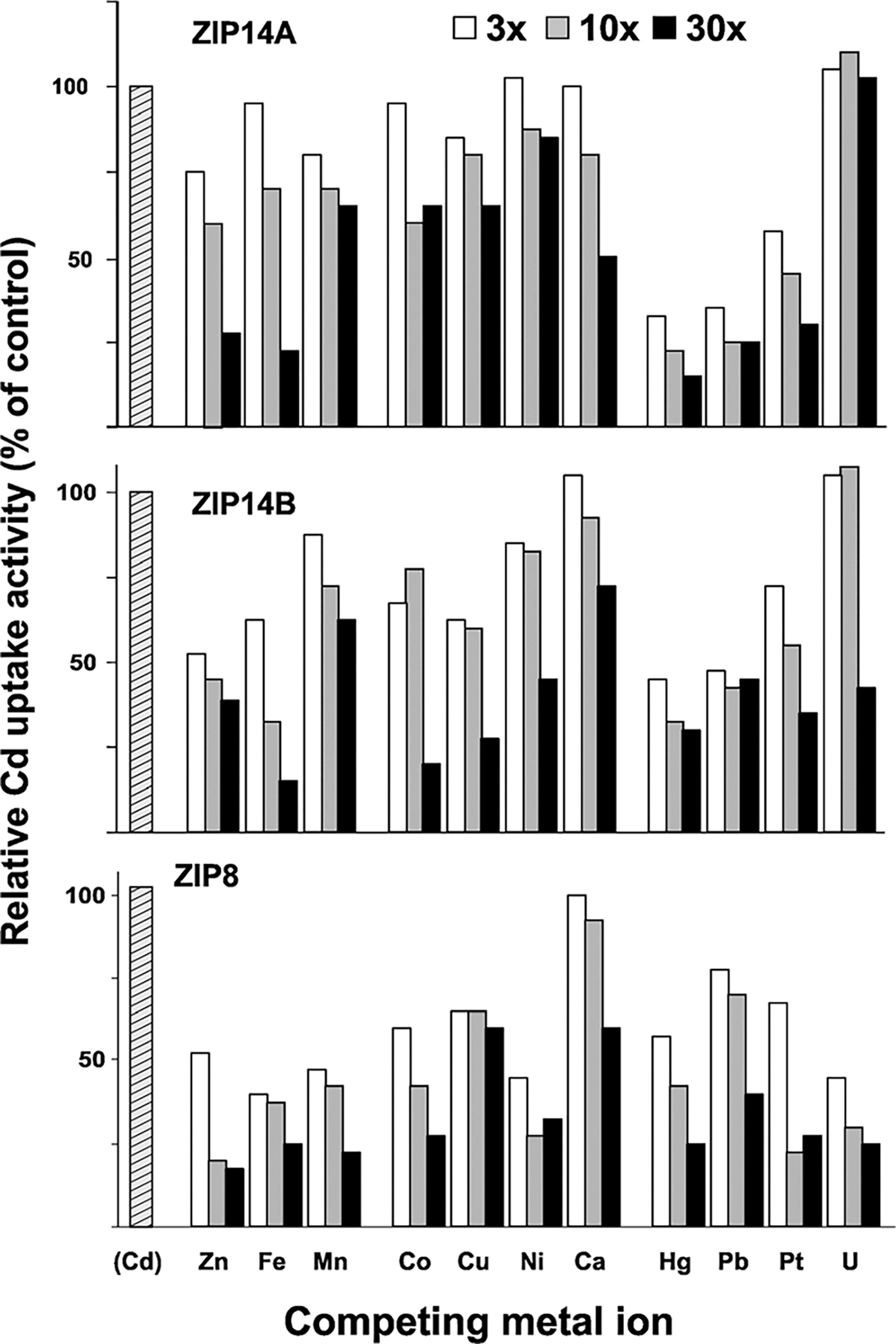
Inhibition of Zn uptake by competitor cations. Experimental methods are identical to those in Fig. 4.
Each candidate divalent cation inhibitor was added at 3-, 10- and 30-fold excess, relative to the Cd or Zn concentration (0.5 μM). To attempt quantification of the degree of competition, we combined the amounts of inhibition—for all three levels of competitor studied—in order to achieve a somewhat “quantitative number” for each metal. In rank order, Cd uptake (Fig. 4) by ZIP14A was most highly inhibited by Hg > Pb > Pt = Zn = Fe (P-values ranging from <0.0001 to 0.033), whereas Ca, Co, Mn, Cu, Ni, and U inhibition was not significant (P > 0.05). Cd uptake by ZIP14B was inhibited by Fe > Hg > Co = Cu > Zn = Pt = Pb > Ni (P-values ranging from 0.001 to 0.045), whereas U, Mn and Ca inhibition was not significant (P > 0.05). Cd uptake by ZIP8 was inhibited by Zn > U = Mn = Fe = Pt > Hg = Ni = Co > Pb (P-values ranging from o0.0001 to 0.037), whereas Cu and Ca inhibition was not significant (P > 0.05).
In rank order, Zn uptake (Fig. 5) by ZIP14A was inhibited by U > Co = Cd = Ni > Mn = Pb > Hg > Fe = Cu (P-values ranging from < 0.001 to 0.037), whereas Pt and Ca inhibition was not significant (P > 0.05). Zn uptake by ZIP14B was inhibited by Mn > Fe > Pb > Cd = Hg > Cu > Ni (P-values ranging from <0.001 to 0.042), whereas Co, U, Pt and Ca inhibition was not significant (P > 0.05). Zn uptake by ZIP8 was inhibited by Cd > Hg > U = Cu = Pt > Ni > Fe = Co > Pb (P-values ranging from <0.001 to 0.048), whereas Mn and Ca inhibition was not significant (P > 0.05).
Electrogenicity studies
When 1, 2, 20 and 60 mM K+ in the incubation medium were compared, no significant differences in Cd uptake were seen in water-injected Xenopus oocytes (Fig. 6, top panel). Similar data were found for Zn uptake in water-injected oocytes (data not shown). Whether it was ZIP14A- or ZIP14B-mediated Cd influx (Fig. 6, 2nd and 3rd panels) or ZIP14A- or ZIP14Bmediated Zn influx (Fig. 6, bottom two panels), no statistically significant differences in divalent cation uptake were seen—across the range from 1 mM to 60 mM extracellular K+. Similar results for ZIP8-mediated Cd and Zn uptake had been reported previously.9
Fig. 6.
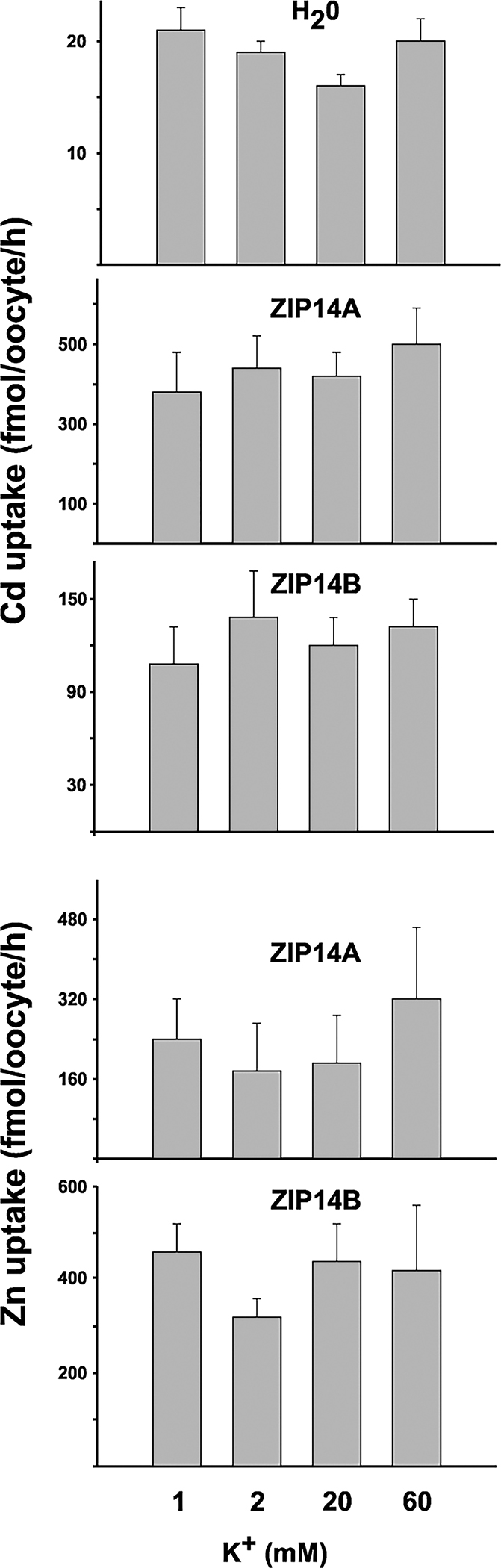
Electrogenicity studies. Cd or Zn was 0.5 μM in uptake buffer, and K+ concentrations were varied between 1 and 60 mM. Cd uptake in water-injected oocytes (top panel) is compared with that in ZIP14A-expressing oocytes (2nd panel) and ZIP14B-expressing oocytes (3rd panel). Zn uptake is shown in ZIP14A-expressing oocytes (4th panel) and ZIP14B-expressing oocytes (bottom panel). Zn uptake in water-injected oocytes was similar to that for Cd uptake in water-injected oocytes (not shown). Values represent means + S.E.M. (brackets). Note different values on the Y-axes.
Discussion
Our goals of the present study in Xenopus oocytes included: to determine Km and Vmax values for divalent cation uptake, to measure competitive inhibition of Cd or Zn uptake by ten additional metals, and to assess electrogenicity during uptake of the Cd–bicarbonate and Zn–bicarbonate complex. Three transporters were compared—ZIP14A, ZIP14B and ZIP8. Some of the ZIP8 data were previously reported.9
Zn, Fe, Mn, Co, Cu and Ca are known essential metals, i.e. required nutrients and/or metals used as enzyme cofactors and transcription factors. Abnormally high or low levels of these essential metals can lead to disease. Cd, Ni, Hg, Pb, Pt and U are regarded as nonessential metals; any amount of these exogenous cations that enter the body is undesirable and thus potentially dangerous to health. A general rule for all toxicological research is that foreign chemicals and metals (xenobiotics) are able to enter the body because they can displace an endogenous substrate from its receptor, enzyme or transporter molecule. Following internalization, the xenobiotic then carries out actions differently from normal endogenous compounds, and these actions often can lead to disease.
Uptake Km values
Comparison of uptake Km values for the essential metals Zn and Fe with that for the nonessential metal Cd (Fig. 3 and Table 1) suggests that ZIP14A- and ZIP8-mediated uptake would favor Zn and Fe over Cd. On the other hand, ZIP14B shows greater uptake affinity for Cd than for Fe or Zn. Thus, at identical concentrations of these two endogenous and one foreign metal, high expression levels of ZIP14B in a particular cell type would result in the highest rate of Cd uptake—possibly leading to toxicity or cancer. The total-body pharmacokinetics of a metal (absorption, distribution, excretion), combined with the precise concentration of each metal, competing in the milieu at the surface of a specific cell type—will be pivotal in heavy-metal-induced disease. For example, ZIP14B-mediated Cd uptake from the GI tract will increase blood Cd levels, which in turn can lead to ZIP8-mediated Cd uptake into bronchoalveolar cells (to cause lung cancer) and to ZIP8-mediated Cd uptake into kidney proximal tubular epithelial cells (to cause proximal RTA and/or renal failure).
Vmax Values
For Cd uptake, ZIP14B shows the lowest Vmax value, and ZIP8 the highest (Table 1). These data suggest that ZIP14B binding-sites would be the quickest, and ZIP8 the slowest, to become saturated with Cd. For Zn uptake, ZIP14A and ZIP8 show the lowest Vmax values, and ZIP14B the highest—suggesting that ZIP14A and ZIP8 binding-sites would be the quickest, and ZIP8 the slowest, to become saturated with Zn. For Fe uptake, ZIP8 shows the lowest Vmax value, and ZIP14B the highest (Table 1). These results indicate that ZIP8 binding-sites would be the quickest, and ZIP14B the slowest, to become saturated with Fe.
SLC11A2 less important than ZIP14 or ZIP8 as the Cd influxor
Xenopus oocytes expressing human SLC11A2 (also called NRAMP2 and DMT1) were reported to have a Km value of 1.04 mM for Cd uptake.14 The Km value of 0.31 mM for ZIP14B-mediated Cd uptake (Table 1) indicates a 3-fold greater affinity than SLC11A2. Normally, one would expect environmental levels of Cd to be extremely low (e.g. nM, pM, or even fM concentrations); moreover, environmental Cd is taken up largely by the GI tract, and ZIP14B is highly expressed in the GI tract.10 It would therefore be expected that ZIP14B is considerably more important than SLC11A1 for bringing Cd into the body. As mentioned above, Cd would be taken up by ZIP14B, displacing essential metals such as Zn, Fe or Mn. Km values for ZIP14A- and ZIP8-mediated Cd uptake (Table 1) also confirm a 2-fold greater affinity than SLC11A2 for Cd uptake.
SLC11A2 is best known for its role as an iron transporter.15–17 Contrary to ZIP14 or ZIP8, moreover, SLC11A2 is a proton-coupled transporter,18 which operates most effectively at pH 5.5 and would operate less effectively under pH 7.4 physiological conditions. SLC11A2 is also known to participate in Cd transport in renal distal tubular cells and GI tract enterocytes.15,16
Competition with Cd or Zn uptake
The general patterns of each competitor can be visually appreciated (Fig. 3 and 4), but appeared highly unpredictable. Clearly, inhibition is not an all-or-none phenomenon but rather a gradient of degrees of inhibition for ZIP14A-, ZIP14B- and ZIP8-mediated uptake of Cd or Zn.
The most consistent finding was that Ca was not a significant inhibitor of ZIP14A-, ZIP14B- or ZIP8-mediated Cd or Zn uptake; in fact, Ca appeared to slightly enhance Cd or Zn uptake. Cd uptake by mammalian cells had been suggested to involve Ca channels;19–21 hence, this was our reason to include Ca as a competitor in the present study. Data herein confirm that ZIP14 and ZIP8 are far more important than Ca channels in Cd uptake.
Might size (ionic radius) play a role in the successful transport of a cation by ZIP proteins? Cd and Zn have ionic radii of 109 and 88 pm, respectively. Given that Ca has a larger ionic radius (114 pm), we looked into the sizes of ionic radii for the other nine metal cations: these varied from small (Ni, Cu, & Zn = 83, 87, 88 pm, respectively) to large (Hg, U, Pb = 116, 116.5 and 133 pm, respectively). Assessing the patterns of each competitor, we conclude there is no relationship between size of the metal cation (ionic radius) and its effectiveness at ZIP-mediated transport or inhibition of Cd or Zn uptake.
Mn (ionic radius of 97 pm) was demonstrated to be a strong competitor of ZIP8-mediated Cd uptake in mouse fetal fibroblast cultures in Hank’s balanced salt solution.7 Curiously, Mn was a good inhibitor of ZIP8-mediated, but not ZIP14A- or ZIP14B-mediated, Cd uptake (Fig. 4); also, Mn was a good inhibitor of ZIP14A- and ZIP14B-mediated, but not ZIP8-mediated, Zn uptake (Fig. 5). ZIP14- and ZIP8-mediated Mn and Cd uptake was recently demonstrated by siRNA “knockdown” studies in renal proximal tubule cell cultures.22
Fe was the most significant competitor of ZIP14B-mediated Cd uptake (Fig. 4), but was a statistically significant competitor in all studies: ZIP14A-, ZIP14B- and ZIP8-mediated Cd uptake (Fig. 4) and ZIP14A-, ZIP14B- and ZIP8-mediated Zn uptake (Fig. 5). ZIP14 has been shown to mediate non-transferrin-bound Fe2+ uptake in human HEK 293H cells, Spodoptera frugiperda (insect) Sf9 cells, and AML12 mouse hepatocyte cultures11 and in Xenopus oocytes.23 In a recent study with a hypomorphic Slc39a8 mouse line,24 the clinical importance of in utero Fe transport by ZIP8 was shown most likely to contribute to the severe anemia observed in the embryo, fetus and newborn.25
A very recent study was reported in Xenopus oocytes,26 showing that ZIP8-mediated Fe uptake was inhibited by zinc and that ZIP8 significantly transported radiolabeled Cd, Co, Zn and Mn, but not Cu. All these findings are consistent with those in the present study, except perhaps for Cu. Whereas we found Cu was not a significant inhibitor of ZIP8-mediated Cd uptake (Fig. 3), it was a significant inhibitor of ZIP8-mediated Zn uptake (Fig. 4); the reason for this discrepancy will require further experiments.
Electrogenicity studies
Using the Nernst equation, as done previously for ZIP8-mediated Cd and Zn uptake,9 we calculated the intracellular charges to be −132, −114, −53, and −24 mV at extracellular K+ concentrations of 1, 2, 20 and 60 mM, respectively. Because Cd or Zn uptake is no more favorable with an electrochemical gradient of −24 mV than at −132 mV (Fig. 5), we conclude that (similar to ZIP8) the complexes moving through the ZIP14A- and ZIP14B-injected Xenopus oocyte membranes are electroneutral, i.e. Cd2+/[HCO3−]2 and Zn2+/[HCO3−]2 complexes.
If the rate of Cd or Zn uptake had been several-fold significantly greater at −24 mV than at −132 mV, this would have been consistent with electronegative complexes, i.e. (Cd2+/[HCO3−]3)− and (Zn2+/[HCO3−]3)−. Conversely, if the rate of Cd or Zn uptake had been several-fold significantly lower at −24 mV than at −132 mV, this would have been consistent with electropositive complexes, i.e. [Zn2+/HCO3−]+ and [Cd2+/HCO3−]+.
Renal Fanconi syndrome
Proximal RTA (renal Fanconi syndrome) can be caused by sporadic or familial events, carbonic anhydrase inhibition from e.g. acetazolamide or sulfamylon, or enzyme deficiency (e.g. osteopetrosis with mutations in CA2 gene). Other causes of proximal RTA include: various inborn errors of metabolism (e.g. cystinosis, Lowe syndrome, tyrosinemia, galactosemia, Wilson disease); dysproteinemic states (e.g. multiple myeloma, monoclonal gammopathy, amyloidosis); interstitial renal disease (e.g. Sjögren syndrome, dysplastic kidney, Balkan nephropathy); and miscellaneous disorders (e.g. congenital heart disease, malignancies, nephrotic syndrome). The final category of proximal RTA causes includes those that are toxicant-induced: gentamicin, maleic acid, coumarin, streptozotocin, outdated tetracyclines, and inadvertent exposure to any of five nonessential heavy metals—Cd2+, Pb2+, Hg2+, Pt2+ and U2+.13 Cisplatin, a common treatment of solid malignancies (and the form of Pt that was used in the present study), is a major cause of Pt-induced nephrotoxicity and renal tubular dysfunction.28
ZIP8 mRNA and protein was demonstrated to be located on the apical surface of renal proximal tubular epithelial cells, and to participate in Cd uptake and Cd-mediated renal failure.8 ZIP14 likewise was shown to be located on the apical surface of polarized epithelial cells in culture.10 ZIP8 levels have been shown to be highest in kidney, lung and testis,1,6,8 and ZIP14 levels are highest in GI tract enterocytes and hepatocytes.1,10 Metal-induced proximal RTA usually occurs via ingestion. Given extremely low environmental levels of nonessential metals2,3 and the extremely high-affinity of Cd for both ZIP8 and ZIP14 (Fig. 2 and Table 1), combined with the competitive inhibition of ZIP14- and ZIP8-mediated Cd uptake by most, if not all, of the five metals listed above (Fig. 3), we propose that heavy-metal-induced renal Fanconi syndrome might be mediated by ZIP transporters—ZIP14 in the GI tract for uptake into the body and ZIP8 for efficient uptake into the kidney.
It should be noted, however, that this hypothesis remains to be proven further—by specifically studying ZIP14A-, ZIP14B- and ZIP8-mediated uptake of radiolabeled Pb2+, Hg2+, Pt2+ and U2+ in mammalian cell culture or Xenopus oocytes. In other words, competition for Cd or Zn uptake cannot prove absolutely that the transporter does indeed transport these other four metals; competitive inhibitors might simply block Cd or Zn uptake via interaction with thiol groups or by plugging up the transport channel. For example, Ni was shown to bind to human ZIP4 and prevent Zn uptake, but Ni is not transported by human ZIP4.27
Acknowledgements
This work was funded, in part, by NIH R01 ES010416 (D.W.N.), T32 ES016646 (M.G.-P.), R01 DK62809 (M.S), and P30 ES06096 (D.W.N.) and an Undergraduate Research Fellowship, University of Cincinnati Research Council (C.Y.). We thank our colleagues for valuable discussions and careful reading of this manuscript. We are grateful to Professors Maripali B. Rao and Mario Medvedovic for helpful statistical suggestions. We appreciate the help of Professor Emerita Marian L. Miller with graphics.
References
- 1.He L, Wang B, Hay EB and Nebert DW, Discovery of ZIP transporters that participate in cadmium damage to testis and kidney, Toxicol. Appl. Pharmacol, 2009, 238, 250–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Waalkes MP, Cadmium carcinogenesis, Mutat. Res, 2003, 533, 107–120. [DOI] [PubMed] [Google Scholar]
- 3.Järup L, Hazards of heavy metal contamination, Br. Med. Bull, 2003, 68, 167–182. [DOI] [PubMed] [Google Scholar]
- 4.Taylor BA, Heiniger HJ and Meier H, Genetic analysis of resistance to cadmium-induced testicular damage in mice, Proc. Soc. Exp. Biol. Med, 1973, 143, 629–633. [DOI] [PubMed] [Google Scholar]
- 5.Dalton TP, Miller ML, Wu X, Menon A, Cianciolo E, McKinnon RA, Smith PW, Robinson LJ and Nebert DW, Refining the mouse chromosomal location of Cdm, the major gene associated with susceptibility to cadmium-induced testicular necrosis, Pharmacogenetics, 2000, 10, 141–151. [DOI] [PubMed] [Google Scholar]
- 6.Dalton TP, He L, Wang B, Miller ML, Jin L, Stringer KF, Chang X, Baxter CS and Nebert DW, Identification of mouse SLC39A8 as the transporter responsible for cadmium-induced toxicity in the testis, Proc. Natl. Acad. Sci. U. S. A, 2005, 102, 3401–3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.He L, Girijashanker K, Dalton TP, Reed J, Li H, Soleimani M and Nebert DW, ZIP8, member of the solute-carrier-39 (SLC39) metal-transporter family: characterization of transporter properties, Mol. Pharmacol, 2006, 70, 171–180. [DOI] [PubMed] [Google Scholar]
- 8.Wang B, Schneider SN, Dragin N, Girijashanker K, Dalton TP, He L, Miller ML, Stringer KF, Soleimani M, Richardson DD and Nebert DW, Enhanced cadmium-induced testicular necrosis and renal proximal tubule damage caused by gene-dose increase in a Slc39a8-transgenic mouse line, Am. J. Physiol, 2007, 292, C1523–C1535. [DOI] [PubMed] [Google Scholar]
- 9.Liu Z, Li H, Soleimani M, Girijashanker K, Reed JM, He L, Dalton TP and Nebert DW, Cd2+ versus Zn2+ uptake by the ZIP8 (HCO3−)-dependent symporter: kinetics, electrogenicity and trafficking, Biochem. Biophys. Res. Commun, 2008, 365, 814–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Girijashanker K, He L, Soleimani M, Reed JM, Li H, Liu Z, Wang B, Dalton TP and Nebert DW, Slc39a14 gene encodes ZIP14, a metal/bicarbonate symporter: similarities to the ZIP8 transporter, Mol. Pharmacol, 2008, 73, 1413–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liuzzi JP, Aydemir F, Nam H, Knutson MD and Cousins RJ, ZIP14 (SLC39A14) mediates non-transferrin-bound iron uptake into cells, Proc. Natl. Acad. Sci. U. S. A, 2006, 103, 13612–13617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li HC, Szigligeti P, Worrell RT, Matthews JB, Conforti L and Soleimani M, Missense mutations in Na+/HCO3− cotransporter NBC1 show abnormal trafficking in polarized kidney cells: a basis for renal proximal tubular acidosis, Am. J. Physiol, 2005, 289, F61–F71. [DOI] [PubMed] [Google Scholar]
- 13.Bergeron M and Gougoux A, The renal Fanconi syndrome, in The Metabolic Basis of Inherited Disease, ed. Scriver CR, Beaudet AL, Sly WS and Valle D, McGraw-Hill, New York, 1989, pp. 2569–2580. [Google Scholar]
- 14.Okubo M, Yamada K, Hosoyamada M, Shibasaki T and Endou H, Cadmium transport by human NRAMP2 expressed in Xenopus laevis oocytes, Toxicol. Appl. Pharmacol, 2003, 187, 162–167. [DOI] [PubMed] [Google Scholar]
- 15.Olivi L, Sisk J and Bressler J, Involvement of DMT1 in uptake of Cd in MDCK cells: role of protein kinase C, Am. J. Physiol, 2001, 281, C793–C800. [DOI] [PubMed] [Google Scholar]
- 16.Park JD, Cherrington NJ and Klaassen CD, Intestinal absorption of cadmium is associated with divalent metal transporter-1 in rats, Toxicol. Sci, 2002, 68, 288–294. [DOI] [PubMed] [Google Scholar]
- 17.Bannon DI, Abounader R, Lees PS and Bressler JP, Effect of DMT1 knockdown on iron, cadmium, and lead uptake in Caco-2 cells, Am. J. Physiol, 2003, 284, C44–C50. [DOI] [PubMed] [Google Scholar]
- 18.Bressler JP, Olivi L, Cheong JH, Kim Y and Bannona D, Divalent metal transporter-1 in lead and cadmium transport, Ann. N. Y. Acad. Sci, 2004, 1012, 142–152. [DOI] [PubMed] [Google Scholar]
- 19.Hinkle PM and Osborne ME, Cadmium toxicity in rat pheochromocytoma cells: studies on the mechanism of uptake, Toxicol. Appl. Pharmacol, 1994, 124, 91–98. [DOI] [PubMed] [Google Scholar]
- 20.Olivi L and Bressler J, Maitotoxin stimulates Cd influx in Madin-Darby kidney cells by activating Ca-permeable cation channels, Cell Calcium, 2000, 27, 187–193. [DOI] [PubMed] [Google Scholar]
- 21.Bergeron PM and Jumarie C, Reciprocal inhibition of Cd2+ and Ca2+ uptake in human intestinal crypt cells for voltage-independent Zn-activated pathways, Biochim. Biophys. Acta, 2006, 1758, 702–712. [DOI] [PubMed] [Google Scholar]
- 22.Fujishiro H, Yano Y, Takada Y, Tanihara M and Himeno S, Roles of ZIP8, ZIP14, and DMT1 in transport of cadmium and manganese in mouse kidney proximal tubule cells, Metallomics, 2012, 4, 700–708. [DOI] [PubMed] [Google Scholar]
- 23.Pinilla-Tenas JJ, Sparkman BK, Shawki A, Illing AC, Mitchell CJ, Zhao N, Liuzzi JP, Cousins RJ, Knutson MD and Mackenzie B, ZIP14 is a complex broad-scope metal-ion transporter whose functional properties support roles in cellular uptake of zinc and nontransferrin-bound iron, Am. J. Physiol, 2011, 301, C862–C871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang B, He L, Dong H and Dalton TP, Nebert DW. Generation of a Slc39a8 hypomorph mouse: markedly decreased ZIP8 Zn2+/(HCO3−)2 transporter expression, Biochem. Biophys. Res. Commun, 2011, 410, 289–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gálvez-Peralta M, He L, Jorge-Nebert LF, Wang B, Miller ML, Eppert BL, Afton S and Nebert DW, ZIP8 zinc transporter: indispensable role for both multiple-organ organogenesis and hematopoiesis in utero, PLoS ONE, 2012, 7, e36055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang CY, Jenkitkasemwong S, Duarte S, Sparkman B, Shawki A, Mackenzie B and Knutson MD, ZIP8 is an iron and zinc transporter whose cell-surface expression is up-regulated by cellular iron loading, J. Biol. Chem, 2012, 287, 34032–34043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Antala S and Dempski RE, The human ZIP4 transporter has two distinct binding affinities and mediates transport of multiple transition metals, Biochemistry, 2012, 51, 963–973. [DOI] [PubMed] [Google Scholar]
- 28.Perazella MA and Moeckel GW, Nephrotoxicity from chemotherapeutic agents: clinical manifestations, pathobiology, and prevention/therapy, Semin. Nephrol., 2010, 30, 570–581. [DOI] [PubMed] [Google Scholar]
- 29.Falcone D and Andrews DW, Both the 5′ untranslated region and the sequences surrounding the start site contribute to efficient initiation of translation in vitro, Mol. Cell. Biol, 1991, 11, 2656–2664. [DOI] [PMC free article] [PubMed] [Google Scholar]


