ABSTRACT
Background:
No clinical trials have compared the efficacy and safety of beta-lactam antibiotics and fluoroquinolones in acute uncomplicated bacterial tonsillitis. This study aimed to compare the efficacy and safety of co-amoxiclav (amoxicillin/clavulanic acid), cefpodoxime proxetil, and levofloxacin monotherapy in patients with acute uncomplicated bacterial tonsillitis.
Methods:
This was a prospective, open-label, parallel-group study where 90 patients with acute uncomplicated bacterial tonsillitis were equally divided into three groups to receive either tablet co-amoxiclav 625 mg thrice daily, tablet cefpodoxime proxetil 200 mg twice daily, or tablet levofloxacin 500 mg once daily for five days. The efficacy was assessed by subjective clinical improvement and objective bacteriological cure at the end of treatment. Safety was assessed by monitoring adverse events during the study period.
Results:
Co-amoxiclav, cefpodoxime proxetil, and levofloxacin showed comparable clinical responses on days three and seven; however, on day five, levofloxacin showed a significantly reduced cure rate, but a higher improvement rate, than co-amoxiclav and cefpodoxime proxetil. Bacteriologically, the responses were similar in all three groups at week 1. All drugs were well tolerated with a few self-limiting adverse effects.
Conclusions:
Co-amoxiclav, cefpodoxime proxetil, and levofloxacin showed a comparable clinical and bacteriological cure in acute uncomplicated bacterial tonsillitis and showed a good safety profile.
Keywords: Acute uncomplicated bacterial tonsillitis, antibiotics, bacteriological, clinical, response
Introduction
Acute tonsillitis is a common bacterial infection in children and adults under 50 years of age and makes up approximately 1.3% of outpatient visits.[1] It results in frequent school and work absenteeism. Group A beta-hemolytic Streptococci are the most common organisms causing acute tonsillitis. The other causative bacteria include Staphylococcus aureus, Hemophilus influenza, and Pneumococcus.[2] Poor oro-dental hygiene, poor nutrition, and a congested environment are important predisposing factors for the disease.[3]
The predominant symptoms include sore throat, difficulty in swallowing, and fever with constitutional symptoms, such as headache, general body ache, malaise, and constipation.[4] The complications of acute tonsillitis include chronic tonsillitis, peritonsillar abscess, parapharyngeal abscess, acute otitis media, rheumatic fever, acute glomerulonephritis, and subacute bacterial endocarditis.[5] Therefore, it is appropriate to initiate treatment for acute tonsillitis at the earliest.
Depending upon the severity, the mainstay of management of acute tonsillitis includes antimicrobials, along with analgesic-antipyretics and other supportive measures. In severe cases, narrow-spectrum antimicrobials in high doses for a shorter duration are advocated.[6] Among antimicrobials, the most commonly preferred ones are beta-lactam antibiotics.[7] In individuals with penicillin hypersensitivity and drug resistance macrolides, fluoroquinolones, and azalides are preferred.[8] However, no clinical studies have compared the efficacy and safety of beta-lactam antibiotics and fluoroquinolones in acute uncomplicated bacterial tonsillitis. Hence, this study was performed to compare the efficacy and safety of co-amoxiclav (amoxicillin/clavulanic acid) (COA), cefpodoxime proxetil, (CEF), and levofloxacin (LEV) in patients with acute uncomplicated bacterial tonsillitis.
Patients and Methods
Ethics
This study was performed after obtaining institutional ethics committee approval. The study was registered in the Clinical Trials Registry – India (CTRI/2018/05/013756). Written informed consent/assent was obtained from all study patients participating in this study. All study procedures adhered to the Declaration of Helsinki, 1964, and its further amendments.
Patients
The inclusion criteria were patients of either gender between 10 and 55 years of age diagnosed with acute uncomplicated bacterial tonsillitis, as confirmed by throat swab test. Patients with suspected viral tonsillitis, complications (quinsy, parapharyngeal abscess, suppurative cervical adenitis, co-existing lower respiratory tract infections, and sinusitis), severe renal and/or hepatic dysfunction, pregnant and lactating women, history of any hypersensitivity to any study medications, history of antibiotic intake within the previous 48 hours, or those who had received long-acting penicillin within two weeks before enrolment were excluded.
Study design
This was a prospective, open-label, parallel-group study. The study was conducted from January 2016 to June 2017 in the outpatient department of Otorhinolaryngology. Purposive sampling was followed. The eligible patients were equally divided into three groups to receive either tablet co-amoxiclav 625 mg thrice daily (COA group), tablet cefpodoxime proxetil 200 mg twice daily (CEF group), or tablet levofloxacin 500 mg once daily (LEV group) after food for five days. In patients of <15 years of age, the doses were calculated based on body weight. The treatment adherence was also checked.
Outcomes
The demographic characteristics of the participants were recorded. The grade of tonsillitis according to the Brodsky scale,[9] symptoms and signs, and the type of tonsillitis were assessed. The efficacy was assessed by subjective clinical improvement for complete resolution of signs and symptoms on an outpatient basis or by telephonic interviews on days three, five, and seven. The bacteriological cure was assessed objectively based on a throat swab culture report one week after the study. The treatment response (clinical) was categorized as follows: (a) cured: complete resolution of signs and symptoms, (b) improved: significant but incomplete resolution of signs and symptoms, and (c) failure: worsened, persisted, or reappeared signs and symptoms. The treatment response was also assessed based on the results of throat swab culture.[10] Safety was assessed by monitoring adverse events during the study period.
Statistical analyses
The calculated total sample size was 90 (30 in each group). The continuous variables are represented by the means ± standard deviations, and the categorical variables are represented by numbers (percentages). The distribution of data was checked by the Kolmogorov–Smirnov test. Analysis of variance (ANOVA) was used to compare the continuous variables and the Chi-square test was used to compare the categorical variables among the three groups. All analyses were performed using SPSS version 18 (IBM, Armonk, NY, USA). A P value of < 0.05 was considered statistically significant.
Results
Out of the total 90 patients enrolled, 84 (93.3%) completed the study. The losses to follow-up in the three groups were: COA (n = 1), CEF (n = 3), and LEV (n = 2) [Figure 1]. The treatment adherence rate was 100%. There were no significant differences in terms of the demographic characteristics of the participants, symptoms and signs of tonsillitis, and the type of tonsillitis among the three groups [Table 1].
Figure 1.
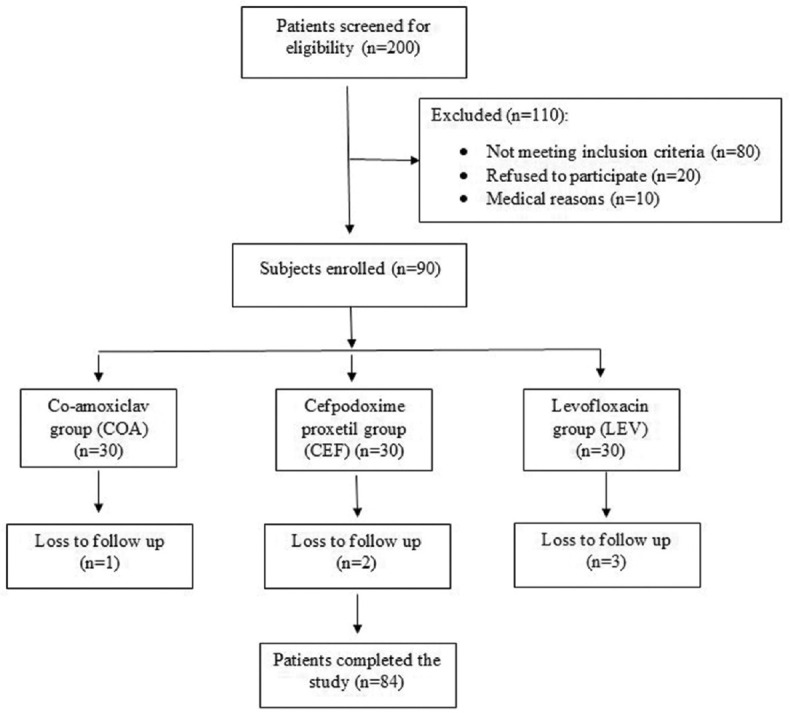
Study flow chart
Table 1.
Comparison of the demographic and clinical characteristics of the three groups
| Parameters | Co-amoxclav group (n=30) | Cefpodoxime group (n=30) | Levofloxacin group (n=30) |
|---|---|---|---|
| Demographic characteristics | |||
| Age (years) | 23.90±10.62 | 22.63±10.17 | 23.07±9.80 |
| Weight (kg) | 60.83±13.35 | 58.90±10.43 | 65.83±12.82 |
| Height (cm) | 163.77±8.19 | 161.47±7.18 | 164.53±7.60 |
| Body mass index (kg/m2) | 22.44±3.45 | 22.83±3.30 | 24.05±3.13 |
| Tonsillitis grade | 1.40±0.62 | 1.37±0.61 | 1.43±0.68 |
| Symptoms and signs | |||
| Odynophagia | 22 (73.3%) | 24 (80%) | 24 (80%) |
| Fever or chills | 13 (43.3%) | 12 (40%) | 17 (56.7%) |
| Strawberry tongue | 1 (3.3%) | 0 (0%) | 2 (6.7%) |
| Tender lymph nodes | 4 (13.3%) | 5 (16.7%) | 1 (3.3%) |
| Loss of appetite | 2 (6.7%) | 6 (20%) | 4 (13.3%) |
| Headache | 2 (6.7%) | 6 (20%) | 4 (13.3%) |
| Cough | 2 (6.7%) | 1 (3.3%) | 0 (0%) |
| Cold | 0 (0%) | 0 (0%) | 1 (3.3%) |
| Earache | 1 (3.3%) | 0 (0%) | 0 (0%) |
| Voice change | 0 (0%) | 0 (0%) | 1 (3.3%) |
| Types of tonsillitis | |||
| Acute follicular | 23 (76.7%) | 20 (66.7%) | 19 (63.3%) |
| Acute parenchymatous | 7 (23.3%) | 9 (30%) | 11 (36.7%) |
| Acute membranous | 0 (0%) | 1 (3.3%) | 0 (0%) |
The continuous variables are represented by the means±standard deviations, and the categorical variables are represented by numbers (percentages)
The COA, CEF, and LEV groups showed comparable clinical responses on days three and seven; however, on day five, the LEV group showed a significantly reduced cure rate, but a higher improvement rate, than the COA and CEF groups [Table 2]. Bacteriologically, the responses were similar in all three groups at week one [Table 3]. All drugs were well tolerated with a few self-limiting adverse effects.
Table 2.
Clinical response to antimicrobial agents in the three groups
| Clinical response | Co-amoxclav group (n=30) | Cefpodoxime group (n=30) | Levofloxacin group (n=30) | P |
|---|---|---|---|---|
| Day 3 | ||||
| Improved | 29 (96.7%) | 28 (93.3%) | 30 (100%) | 0.770 |
| Lost to follow-up | 1 (3.3%) | 2 (6.7%) | 0 (0%) | |
| Day 5 | ||||
| Cured | 24 (80%) | 24 (80%) | 18 (60%) | 0.056 |
| Improved | 5 (16.7%) | 4 (13.3%) | 12 (40%) | |
| Lost to follow-up | 1 (3.3%) | 2 (6.7%) | 0 (0%) | |
| Day 7 | ||||
| Cured | 29 (96.7%) | 27 (90%) | 28 (93.3%) | 0.561 |
| Treatment failure | 0 (0%) | 1 (3.3%) | 2 (6.7%) | |
| Lost to follow-up | 1 (3.3%) | 2 (6.7%) | 0 (0%) |
The results are represented by numbers (percentages)
Table 3.
Results of culture in the three groups
| Results of culture | Co-amoxclav group (n=30) | Cefpodoxime group (n=30) | Levofloxacin group (n=30) | P |
|---|---|---|---|---|
| Baseline | ||||
| Beta-hemolytic Streptococcus | 20 (66.7%) | 15 (50%) | 14 (46.7%) | 0.282 |
| Staphylococcus aureus | 7 (23.3%) | 14 (46.7%) | 13 (43.3%) | |
| Gram-positive cocci and Gram-negative bacilli | 3 (10%) | 1 (3.3%) | 3 (10%) | |
| 1 week | ||||
| No growth | 30 (100%) | 29 (96.7%) | 26 (86.7%) | 0.171 |
| Gram-positive cocci | 0 (0%) | 0 (0%) | 1 (3.3%) | |
| Staphylococcus aureus | 0 (0%) | 0 (0%) | 1 (3.3%) | |
| Lost to follow-up | 0 (0%) | 1 (3.3%) | 2 (6.7%) |
The results are represented by numbers (percentages)
Discussion
In this study, the efficacy and safety of COA were compared with those of CEF and LEV in acute uncomplicated bacterial tonsillitis. There were improvements in both clinical and bacteriological parameters in all the groups. The majority of patients showed clinical improvements on day three of the treatment. All three groups had comparable clinical responses on days three and seven. However, on day five, the LEV group showed a significantly reduced cure rate, but a higher improvement rate. Also, CEF and LEV showed comparable efficacy to COA in terms of the results of the culture.
COA is commonly preferred for the treatment of acute uncomplicated bacterial tonsillitis due to its well-established safety, efficacy, narrow spectrum of activity, and fewer reports of resistance.[11] Although Group A beta-hemolytic Streptococci are always susceptible to penicillin, bacteriologic failure occurs in up to 20% of the patients treated with penicillin, and half of these cases are also a clinical failure. The absence of interfering aerobic and anaerobic organisms may also lead to the failure of penicillin therapy. Further, some patients have hypersensitivity to penicillin. All these conditions warrant alternative antibiotic usage.[8]
Short-course (five days) antimicrobial treatment of acute uncomplicated bacterial tonsillitis has several advantages in terms of adherence, adverse effects, cost, and patient satisfaction.[11] A recent study has shown that short-course antibiotics are as effective as longer courses for most common infections managed in ambulatory care.[12] Our results suggest that CEF and LEV can be considered effective and safe alternatives to COA for the management of acute uncomplicated bacterial tonsillitis. In a recent meta-analysis, the authors have declared that there is uncertainty in terms of clinically relevant differences in symptom resolution when comparing cephalosporins and macrolides with penicillin in the treatment of tonsillopharyngitis caused by Group A beta-hemolytic Streptococci.[13] This is consistent with our results. Another meta-analysis has inferred that clindamycin and amoxicillin with clavulanate are superior to penicillin with preferable effects on the microbiological flora and the number of future attacks in patients with recurrent acute.[14] Several earlier studies have also shown the superiority of cephalosporins over penicillins in tonsillopharyngitis.[15] However, the clinical and bacteriological efficacy of quinolones in acute uncomplicated bacterial tonsillitis has not been demonstrated yet.[16]
It is pertinent here to discuss the relevant guidelines on the management of sore throat. The American Academy of Family Physicians recommends that in patients with sinus infections, acute bacterial rhinosinusitis should be diagnosed and treated with antibiotics only if symptoms have not improved after 10 days or have worsened after five to seven days.[17] According to the Infectious Diseases Society of America, penicillin or amoxicillin remains the treatment of choice for pharyngitis caused by Group A beta-hemolytic Streptococci.[18] According to the European Society for Clinical Microbiology and Infectious Diseases, penicillin V is the first-line drug for the management of acute sore throat.[19]
There are certain limitations to this study. First, this was an open-label non-randomized study, thereby inviting several biases. The sample size was also small. Also, we did not evaluate the cost-effectiveness of the three antibiotics used. Further large-scale randomized clinical trials are required to evaluate the efficacy and safety of different antimicrobials in acute tonsillitis.
Conclusions
COA, CEF, and LEV showed comparable clinical and bacteriological cure efficacy in acute uncomplicated bacterial tonsillitis and showed a good safety profile.
Conflicts of interest
There are no conflicts of interest.
Funding Statement
Nil
References
- 1.Pearce S, Bowen AC, Engel ME, de la Lande M, Barth DD. The incidence of sore throat and group A streptococcal pharyngitis in children at high risk of developing acute rheumatic fever: A systematic review and meta-analysis. PLoS One. 2020;15:e0242107. doi: 10.1371/journal.pone.0242107. doi:10.1371/journal.pone.0242107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bisno AL, Gerber MA, Gwaltney JM, Kaplan EL, Schwartz RH Infectious Diseases Society of America. Practice guidelines for the diagnosis and management of group A streptococcal pharyngitis. Infectious Diseases Society of America. Clin Infect Dis. 2002;35:113–25. doi: 10.1086/340949. [DOI] [PubMed] [Google Scholar]
- 3.Hayes CS, Williamson H., Jr Management of group A beta-hemolytic streptococcal pharyngitis. Am Fam Physician. 2001;63:1557–64. [PubMed] [Google Scholar]
- 4.Georgalas CC, Tolley NS, Narula PA. Tonsillitis. BMJ Clin Evid. 2014;2014:0503. [PMC free article] [PubMed] [Google Scholar]
- 5.Johnson BC, Alvi A. Cost-effective workup for tonsillitis. Testing, treatment, and potential complications. Postgrad Med. 2003;113:115–8. doi: 10.3810/pgm.2003.03.1391. 121. [DOI] [PubMed] [Google Scholar]
- 6.Stelter K. Tonsillitis and sore throat in children. GMS Curr Top Otorhinolaryngol Head Neck Surg. 2014;13:Doc07. doi: 10.3205/cto000110. doi:10.3205/cto000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Skoog Ståhlgren G, Tyrstrup M, Edlund C, et al. Penicillin V four times daily for five days versus three times daily for 10 days in patients with pharyngotonsillitis caused by group A streptococci: Randomised controlled, open label, non-inferiority study. BMJ. 2019;367:l5337. doi: 10.1136/bmj.l5337. doi:10.1136/bmj.l5337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brook I. Failure of penicillin to eradicate group A beta-hemolytic streptococci tonsillitis: Causes and management. J Otolaryngol. 2001;30:324–9. doi: 10.2310/7070.2001.19359. [DOI] [PubMed] [Google Scholar]
- 9.Ng SK, Lee DL, Li AM, Wing YK, Tong MC. Reproducibility of clinical grading of tonsillar size. Arch Otolaryngol Head Neck Surg. 2010;136:159–62. doi: 10.1001/archoto.2009.170. [DOI] [PubMed] [Google Scholar]
- 10.Wakode PT, Gawarle SH, Joshi SV, Bajoriya R. Throat swab culture and sensitivity reports-An overview. Indian J Otolaryngol Head Neck Surg. 2003;55:76–80. doi: 10.1007/BF02974607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holm AE, Llor C, Bjerrum L, Cordoba G. Short- vs. Long-course antibiotic treatment for acute streptococcal pharyngitis: Systematic review and meta-analysis of randomized controlled trials. Antibiotics (Basel) 2020;9:733. doi: 10.3390/antibiotics9110733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dawson-Hahn EE, Mickan S, Onakpoya I, Roberts N, Kronman M, Butler CC, et al. Short-course versus long-course oral antibiotic treatment for infections treated in outpatient settings: A review of systematic reviews. Fam Pract. 2017;34:511–9. doi: 10.1093/fampra/cmx037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Driel ML, De Sutter AI, Thorning S, Christiaens T. Different antibiotic treatments for group A streptococcal pharyngitis. Cochrane Database Syst Rev. 2021;3:CD004406. doi: 10.1002/14651858.CD004406.pub5. doi:10.1002/14651858.CD004406.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Munck H, Jørgensen AW, Klug TE. Antibiotics for recurrent acute pharyngo-tonsillitis: Systematic review. Eur J Clin Microbiol Infect Dis. 2018;37:1221–30. doi: 10.1007/s10096-018-3245-3. [DOI] [PubMed] [Google Scholar]
- 15.Bisno AL. Are cephalosporins superior to penicillin for treatment of acute streptococcal pharyngitis? Clin Infect Dis. 2004;38:1535–7. doi: 10.1086/392520. [DOI] [PubMed] [Google Scholar]
- 16.Spinks A, Glasziou PP, Del Mar CB. Antibiotics for sore throat. Cochrane Database Syst Rev 2013. 2013:CD000023. doi: 10.1002/14651858.CD000023.pub4. doi:10.1002/14651858.CD000023.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wong DM, Blumberg DA, Lowe LG. Guidelines for the use of antibiotics in acute upper respiratory tract infections. Am Fam Physician. 2006;74:956–66. [PubMed] [Google Scholar]
- 18.Shulman ST, Bisno AL, Clegg HW, Gerber MA, Kaplan EL, Lee G, et al. Clinical practice guideline for the diagnosis and management of group A streptococcal pharyngitis: 2012 update by the Infectious Diseases Society of America. Clin Infect Dis. 2012;55:1279–82. doi: 10.1093/cid/cis847. [DOI] [PubMed] [Google Scholar]
- 19.ESCMID Sore Throat Guideline Group. Pelucchi C, Grigoryan L, Galeone C, Esposito S, Huovinen P, et al. Guideline for the management of acute sore throat. Clin Microbiol Infect. 2012;18(Suppl 1):1–28. doi: 10.1111/j.1469-0691.2012.03766.x. [DOI] [PubMed] [Google Scholar]


