ABSTRACT
Objective:
Selecting the right medication for major depressive disorder (MDD) is challenging, and patients are often on several medications before an effective one is found. Using patient EEG patterns with computer models to select medications is a potential solution, however, it is not widely performed. Therefore, we evaluated a commercially available EEG data analysis system to help guide medication selection in a clinical setting.
Methods:
Patients with MDD were recruited, and their physicians used their own judgment to select medications (Control; n = 115) or relied on computer-guided selection (PEER n = 165) of medications. Quick Inventory of Depressive Symptomatology (QIDS SR-16) scores were obtained from patients, before the start of the study (day 0) and again at ~90 and ~180 d. Patients in the PEER arm were classified into one of 4 groups depending on if the report was followed throughout (RF/RF), the first 90 days only (RF/RNF), the second 90 days only (RNF/RF), or not at all (RNF/RNF). Outcomes were then compared with controls whose physician performed the EEG and submitted data but did not receive the PEER report.
Results:
Patients in the controls, RF/RF and RNF/RNF groups had fewer depressive symptoms at 90 and 180 days, but the response was significantly stronger for patients in the RF/RF group. Lower rates of suicidal ideation were also noted in the RF/RF group than the control group at 90 and 180 days of treatment.
Conclusion:
Computational analysis of EEG patterns may augment physicians’ skills at selecting medications for the patients.
Keywords: Computer-assisted treatment, depression, EEG, medication
Introduction
Major depressive disorder (MDD) is widespread and present in 11.0% of physician visits and 12.3% of emergency room visits.[1] Its prevalence varies with sex, age and race and is often untreated.[2,3] In 2020, ~21 million adults (8.4%) had at least one depressive episode[3] that was often comorbid with other medical conditions and disabilities, adversely affecting patient prognosis and quality of life. It is the second most common disability in terms of “years lost,”[4] and if untreated, as it is in nearly 49% of adults and 60% of youth, can lead to premature death by suicide.[4]
Suicide is a growing concern impacting families and communities nationwide. From 2011 to 2022, the suicide rate in the United States increased by 16%, making it the 11th leading cause of death.[5] Suicide rates are highest among American Indians (28.1 per 100,000), males (22.8 per 100,000), and those living in rural areas (20.2 per 100,000)-who often have limited access to psychiatric care.[6] This increase highlights the need for interventions to improve access to care for people with depression. Although many treatments exist, antidepressants are among the most effective and widely used. A major challenge in medicine, however, is selecting the right medication for a newly diagnosed MDD patient because of varying individual responses, often requiring weeks of experimentation to find an effective treatment.
Neurotransmitter levels and inflammatory indicators have been studied as biomarkers of medication response.[7,8,9] Peripheral blood levels of cytokines or neurotransmitters, however, poorly reflect brain levels of these biomarkers. genomics and MRI-based methods are not readily available at the onset of treatment. One alternative is using voltage data from electroencephalography (EEG) or electroretinography (ERG) as biomarkers. These methods are inexpensive, readily available, and produce standardized data that can be integrated into large clinical databases for statistical modeling.
Significant EEG pattern differences have been reported between patients with mood disorders and healthy individuals. Depressed patients exhibit increased EEG alpha asymmetry and left frontal region hypoactivity compared with nondepressed patients.[7,8] Patients with a history of depression show less left frontal and right posterior activation than those without.[9] Fluoxetine responders displayed increased EEG alpha amplitude and nonresponders to fluoxetine were similar to controls.[10] An early pilot study compared 6 patients treated from psychiatrists’ recommendations with 7 treated using an EEG-based classifier.[11] All but one in the EEG-guided group had significant improvements in the Hamilton rating scale for depression (HAM-D) and Beck Depression Inventory (BDI) scores compared with only one in the psychiatrist-guided group.[11] A single-blind, randomized clinical trial with 114 treatment-resistant subjects found significant improvements in QIDS SR16 and Quality of Life Enjoyment and Satisfaction Questionnaire-Short Form (Q-LES-Q-SF) for patients receiving EEG-guided treatments compared with those after the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study[12] recommendations.[13]
A recent meta-analysis reported that QEEG tests have some value for selecting effective antidepressants but noted small sample sizes and possible publication bias against weak or negative studies.[14] Another analysis found EEG-guided methods gave reasonably high accuracy (sensitivity = 83%, 95% CI: 74,89; specificity = 86%, 95% CI: 81,90) but cautioned that small sample sizes and lack of external validation may have inflated accuracy.[15] Advances in computing enabled the use of machine learning, neural networks, and deep learning to model high-dimensional data with little or no a priori knowledge. These techniques have developed algorithms such as PEER to identify likely successful medications based on a patient’s EEG patterns. These methods, however, are not widely used at the beginning of treatment. Therefore, we compared clinical outcomes of patients prescribed antidepressants based on physician preference alone vs. EEG and computer-based suggestions from the PEER algorithm.
Materials and Methods
This study is registered at www.clinicaltrials.gov as protocol NCT03328052 and was performed with the approval and oversight of the Western Institutional Review Board. All patients provided written informed consent and were free to withdraw at any time.
Study design
Individual treating physicians were assigned to either the MYND Analytics PEER report group or the control group for managing patients with MDD. Patients presenting to these physicians were approached by study personnel (including sub-investigators) and offered an opportunity to participate. If agreeable, the patient was screened using the inclusion and exclusion criteria. Inclusion criteria: 1) a diagnosis of MDD by their physician based on their clinical judgment and/or screening tools and 2) 18 to 89 years of age. Exclusion criteria: 1) presence of a psychotic disorder, 2) history of craniotomy, cerebral metastases, cerebrovascular accident; current diagnosis of seizure disorder, schizophrenia, schizoaffective disorder, dementia, intellectual disability, or major depression with psychotic features, 3) use of neuroleptics in the last 12 months, 4) uncontrolled thyroid disorders (patients with controlled thyroid disorders were eligible), 5) chronic or acute pain requiring prescription pain medications, 6) patients who were pregnant, planning to become pregnant, or lactating, and 7) participating in any other therapeutic drug study within the previous 60 d. Written informed consent was then obtained. Patients taking stimulants or benzodiazepines were required to withdraw from these medications for 5 half-lives, lasting generally from 3-23 days, before participating in the study.
For all enrolled patients, a baseline EEG was performed using standard 21-lead digital EEG equipment while the patient was in a resting, but awake, state, for 30 to 60 min. Data were then forwarded to MYND analytics for analysis where they were Fourier transformed and submitted to the Psychiatric EEG Evaluation Registry (PEER) online platform[16] (MYND Analytics, Maynard, Massachusetts) that categorizes the patients based on 1142 variables calculated from the recording using an FDA-registered neuromeric system. Patients’ data were inputted into a model trained with data from ~11,000 EEGs taken from patients with known outcomes after being treated with medications (over 39,000 prescriptions) that were used to train the system. Study physicians in the intervention group received the PEER report that listed the probability of positive response to each class of medications. For patients in the intervention group, a PEER report was provided immediately but no report was given to physicians treating control group patients until the study was completed.
Although physicians in the intervention arm were encouraged to follow PEER report recommendations, all decisions were ultimately up to them. They could choose to follow the recommendations for all 180 days, for only the first 90 days, for only the second 90 days, or not at all. Patients were blind to their group assignment to preclude any placebo-type effects. Sub-investigators responsible for recruiting and managing patients included 9 psychiatrists and 29 primary care providers: 19 physicians, 9 nurse practitioners, and 1 physician assistant.
Evaluation scoring
The QIDS-SR16 survey instrument form was completed by each patient immediately before the EEG and again at ~3 and ~6 months during follow-up visits. Patients were classified as having: “No Depression” (QIDS score < 6), “Mild Depression” (QIDS score < 6-10), “Moderate Depression” (QIDS score 11-15), “Severe Depression” (16-20), or “Very Severe Depression” (QIDS score >20).
Data analysis
Data were entered into a secure database (REDCap™; Nashville, TN), locked by Mynd Analytics, and transferred to an external contract research organization that reviewed patient medication records and prepared the final datasets 6 months after the last patient’s enrollment or analysis by the research team. Patients were stratified into groups based on their initial assignment to the PEER intervention or control and their concordance with status with PEER recommendations at the first and second follow-ups at 90 and 180 days. Groups were: “Control” (EEG performed but no PEER report provided), Report Followed through 90 and 180 days (RF/RF), Report followed through 90 days but not through 180 days (RF/RNF), Report not followed through 90 but then followed through 180 days (RNF/RF), and report not followed throughout the study (RNF/RNF). Patients whose depression improved by 50% or more were also quantified. Suicidal ideation rates (anything but “no” to QIDS question 12 (death and suicide) were also compared. Total number of medications, adverse events, and whether a patient had one or more severe adverse events (defined as emergency room visits or hospitalizations) were quantified for each person, and numbers and rates compared between groups.
All statistical analyses were performed using the R statistical language (www.rproject.org). QIDS scores were analyzed using ordinal, generalized, or standard linear mixed effects models with patient effects considered random and all others (Group, Time, and Group by Time interaction) fixed. Counts of number of medications and adverse events were square-root transformed and compared between groups using a linear model. Rates of one or more severe adverse events for the patients were compared between groups by Fisher’s exact test and logistic regression. Rates of improvements in QIDS scores by 50% or more were analyzed using logistic regression, stratified by follow-up timepoint. Results are presented as odds ratios with 95% confidence intervals, fitted means with standard errors or differences from control with 95% confidence intervals. Multivariable models were also fit to confirm that observed associations were independent of confounding by patient sex, race-ethnicity, and age.
Results
Of the 165 patients whose physicians were provided the PEER report and for whom complete data on sex, race, and age was available, 94 (56.7%) followed it for both follow-up periods (Group RF/RF), 10 (6.1%) only followed it until the first time point (Group RF/RNF), 6 (3.7%) followed it during second follow-up period (Group RNF/RF), and the remaining 55 (33.5%) not following the suggestions for the entire time frame (Group RNF/RNF). QIDS data were available from 71 and 89 controls, 60 and 73 RF/RF patients, 4 and 5 RF/RNF patients, 4 and 3 RNF/RF patients, and 23 and 31 RNF/RNF patients at 90 and 180 days follow-up, respectively.
Patients in the RF/RF (P = 0.042) and RF/RNF groups (P = 0.041) tended to be slightly younger than those in the other groups. Most patients in each group were female and of white race-ethnicity. Some differences in the of sex and race-ethnicity [Table 1] were found at baseline. Further analysis with linear models and Woolf tests suggested that the distribution of age (P = 0.998), sex (P = 0.971), and race-ethnicity (P = 0.989) observed between groups at baseline remained consistent at day 90 and day 180. This suggests that data are missing at random and would have minimal impact -tending to bias results towards the null. Therefore, no imputation strategy was applied.
Table 1.
Demographics of patients in the 5 groups with number of medication changes, adverse events, and severe adverse events at the conclusion of the study
| Parameter | Group | P | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Control | RF/RF | RF/RNF | RNF/RF | RNF/RNF | ||
| Patients | 115 | 94 | 10 | 6 | 55 | |
| Age1 | 45.9±17.3 | 41.3±14.4 | 35.2±19.2 | 45.0±15.5 | 46.6±16.6 | 0.0023 |
| Sex2 | 0.0484 | |||||
| Female | 76 (66%) | 76 (81%) | 8 (80) | 5 (83) | 38 (69) | |
| Male | 39 (34%) | 18 (19%) | 2 (20) | 1 (17) | 17 (31) | |
| Race-Ethnicity2 | <0.0024 | |||||
| Asian | 1 (1%) | 1 (1%) | 0 (0%) | 0 (0%) | 0 (0%) | |
| Black/African | 4 (3%) | 2 (2%) | 0 (0%) | 0 (%) | 2 (4%) | |
| White/Caucasian | 86 (75%) | 53 (56%) | 6 (60%) | 3 (50%) | 28 (51%) | |
| Multiracial | 6 (5%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (2%) | |
| Other | 1 (1%) | 1 (1%) | 0 (0%) | 0 (0%) | 0 (0%) | |
| Unknown/Declined | 17 (15%) | 37 (39%) | 4 (40%) | 3 (50%) | 24 (44%) | |
| Medication changes1 | 5.3±3.4 | 9.5±6.47 | 7.2±5.2 | 10.8±6.1 | 6.9±5.6 | <0.0013 |
| Adverse events1 | 1.1±0.3 | 1.5±0.9 | 1.5±0.8 | 1.3±0.52 | 1.3±0.7 | <0.0013 |
| Patents with 1 or More severe adverse events2 | 3 (3%) | 10 (11%) | 3 (30%) | 0 (0%) | 7 (13%) | <0.0014 |
1Mean (sd), 2n (%), 3ANOVA, 4Chi-Square Test
QIDS scores did not significantly differ between groups at baseline, suggesting that all patients had similar levels of depression at the start of the study. By 90 and 180 days; however, QIDS scores significantly declined for the RF/RF, and RNF/RNF groups [Figure 1a]. Patients in the RF/RNF or RNF/RF groups had no change in QIDS scores from baseline [Figure 1a]. These findings suggest that there were significant improvements in depressive symptoms during the period for which the report was followed. Since fewer depressive symptoms were also observed in control patients [Figure 1a], we normalized responses to the degree that depressive symptoms were observed to improve in the control patients at day 90 and 180. When this was performed, the decline in QIDS scores remained statistically significant for the RF/RF groups but not the RNF/RNF group [Figure 1b]. These results suggest that the improvement was significantly greater for the RF/RF group than it was for the controls or the RNF/RNF groups at both time points. In a similar fashion, patients in the RF/RNF group had significant improvements in QIDS scores at the first, but not the second, follow-up when they may have been on less effective medications. No improvements in QIDS scores were observed for patients whose report was followed only during the second interval (RNF/RF group). These results remained significant after adjustment for possible confounding because of differences between groups regarding sex, age, and race-ethnicity [Figure 1c and 1d], suggesting that our observed findings are independent of these factors.
Figure 1.
Panel a: Impact of treatment on QIDS scores. Shown are changes from baseline (with 95% confidence intervals) for patients who received no PEER report (Controls), Patients whose physicians followed the PEER report recommendations during the entire 180-day periods (RF/RF) or only the first 90 days (RF/RNF), or the second 90 days only (RNF/RF) or not at all (RNF/RNF). Points where the error bars cross 0 are not statistically different from control. Panel b: Impact of following the PEER report on QIDS scores relative to control patients. Shown are changes from baseline (with 95% confidence intervals) for patients who received no PEER report (Controls), Patients whose physicians followed the PEER report recommendations during the entire 180-day periods (RF/RF) or only the first 90 days (RF/RNF), or the second 90 days only (RNF/RF) or not at all (RNF/RNF). Points are adjusted for the improvement observed in controls and when the error bars cross 0, the results are not statistically different from control. Panels c and d same analyses as in panels A and B but with adjustments for age, sex, and race-ethnicity
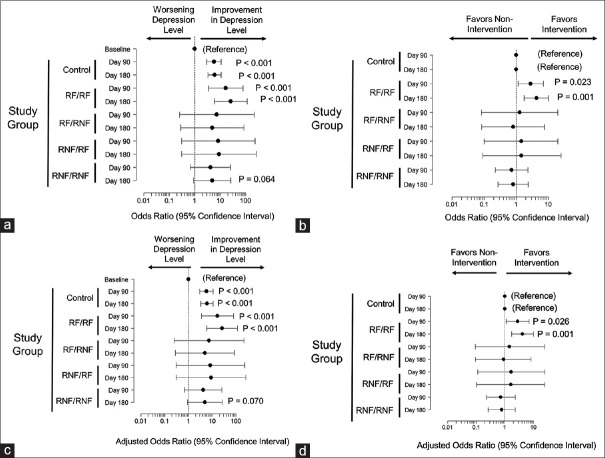
Because QIDS scores are quantitative variables, they could overstate patient improvement. To address this, we compared the impact of following the PEER report on clinically meaningful levels of depression in the patients that are often classified by QIDS scores. We found that depression levels generally improved for the control group and those who followed the PEER report throughout (RF/RF). Both groups had increased odds of being in the next improved category at day 90 and 180 compared with baseline [Figure 2a]. No impact was observed for the remaining groups although there was a trend towards significance for the RNF/RNF group at 180 days. When controlling for time-related improvements observed in the controls, the RF/RF group still showed increased odds improvement in depression levels at 90 and 180 days [Figure 2b]. This suggests that the degree of improvement in the RF/RF group was significantly greater than it was for the control patients at both time points. Similarly the RF/RNF group showed improvement during the first period, when the report was being followed, but not in the second, when it was not. Results were unchanged after adjustment for possible confounding because of the patient’s age, race, or sex [Figure 2c and 2d] suggesting that following the PEER report correlates with clinically relevant improvements in depression.
Figure 2.
Panel a. Impact of treatment on depression level as determined by QIDS scores. Shown is the odds ratio with 95% CI for moving to the next level of improvement in depression level for patients who received no PEER report (Controls), Patients whose physicians followed the PEER report recommendations during the entire 180-day period (RF/RF) or only the first 90 days (RF/RNF), or the second 90 days only (RNF/RF) or not at all (RNF/RNF). Points where the error bars cross 1 are not statistically different from control. Panel b, Impact of following the PEER report on depression level relative to control patients. Shown are changes from baseline (with 95% confidence intervals) for patients who received no PEER report (Controls), Patients whose physicians followed the PEER report recommendations during the entire 180-day period (RF/RF) or only the first 90 days (RF/RNF), or the second 90 days only (RNF/RF) or not at all (RNF/RNF). Points are adjusted for the improvement observed in controls and when the error bars cross 0, the results are not statistically different from control. Panels c and d have, same analyses as panels A and B but with adjustments for patient sex, race, and age
We also examined the proportions of patients who reduced their QIDS scores by 50% or more. Almost twice as many patients had reduced their QIDS scores by 50% or more in the RF/RF group than in the controls (43.3% vs. 25.3%; P = 0.031) or the other groups (< =25%). By 180 days, 48% of the RF/RF patients reduced their QIDS scores by 50% or more, compared with 30.3% in the controls (P = 0.009). This suggests that one in every 5 to 6 patients treated (NNT = 5.6), has a 50% reduction in QIDS scores from the PEER protocol.
At baseline, there were no differences in rates of suicidal ideation (as judged by answering anything but “no” to QIDS question 12). In controls, rates declined from 36.8% at baseline to 32.4% at day 90 (OR = 0.56, 95% CI: 0.23,1.37) and then to 23.6% at day 180, where results reached statistical significance (OR = 0.32; 95% CI: 0.14, 0.78). RF/RF patients, rates declined from 44.1% at baseline to 23.3% at 90 days (OR = 0.13; 95% CI: 0.04, 0.41) and again to 15.1% at 180 days (OR = 0.07; 95% CI: 0.02, 0.22). The magnitude of the decline was greater for the RF/RF group at both day 90 (OR = 0.24, 95% CI: 0.06,0.90) and 180 (OR = 0.20, 95% CI: 0.05, 0.84). No other changes were detected for the remaining groups and results held after adjustment for patient age, sex, and race-ethnicity. These results suggest that after the PEER report correlated with significant improvements in patients’ depressive symptoms and rates of suicidal ideation.
Patients in the RF/RF and RNF/RF groups had more medication changes than control (4.2 ± 0.7, mean difference ± SEM; P < 0.001) and [5.5 ± 2.2, P = 0.018; Table 1]. RF/RF patients also had more adverse events than control (P < 0.001), although the difference was small (1.5 ± 0.1 vs. 1.1 ± 0.1 events per person). RF/RNF patients also had more adverse events than controls (1.5 ± 0.3 vs. 1.1 ± 0.1; P = 0.073) but results did not reach statistical significance. Number of adverse events correlated with number of medication changes (rho = 0.28, P < 0.001) but RF/RF patients had more adverse events than controls after adjusting for medication changes (P = 0.011).
Patients had 0, (n = 257); 1 (n = 3), 2 (n = 15), 3 (n = 3), or 4 (n = 2) severe adverse events (SAE) reported. For analysis, data were dichotomized to 0 or at least one SAE. SAE rates were 3/115 (2.6%) for controls but significantly greater for RF/RF patients (10/94, 10.6%; P = 0.027), RF/RNF patients 3/10 (30.0%; P = 0.002) and RNF/RNF (7/55, 12/7%, P = 0.018). No effect, SAEs were reported for the RNF/RF patients (0/6, 0%, P = 0.989). These associations remained significant after adjustment for patient age, sex, and race.
Discussion
Rationale for use of EEG
Finding the optimum intervention that is tailored to a patient’s needs is a hallmark of personalized medicine that is particularly important to psychiatry. Although antidepressants are effective, only a subset of patients with similar clinical symptoms respond adequately to a given antidepressant, and finding the right medication can take months. Technologies such as genome-wide association studies, brain structure (eg. hippocampus and anterior cingulate cortex), and peripheral concentrations of neurotrophic factors such as BDNF have been widely explored to find biomarkers for classifying psychiatric patients, assessing their prognosis, and monitoring their progress during treatment.[7,8,9] EEG on patients who are resting, but awake, can be easily performed in outpatient settings and provide data that machine learning algorithms can use to identify medications that will maximize the probability of clinical improvement while minimizing side effects. Furthermore, the affordability of EEG system makes the PEER system usable for most practices.
Interpretation of findings
We found that the PEER protocol, an algorithm using EEG for optimizing depression medications, was highly beneficial for medication selection. Patients whose physicians used the PEER report had significantly greater improvements in QIDS scores at 90 and 180 days compared with those whose physicians did not follow the recommendations. In addition, a larger proportion of these patients experienced over 50% reductions in QIDS scores. Although there were higher numbers of medication changes and adverse events in the groups where the PEER report was followed, this could be partially because of the physicians were aware of the treatments, leading to more rapid medication changes, and overreporting of adverse events. The Number Needed to Harm (NNH) for the RF/RF group was 12.45- suggesting a low absolute risk from using the PEER report. Furthermore, no suicides or suicide attempts were observed. Although 30% of the patients in the RF/RNF group had at least one severe adverse event, these events may have resulted in the report not being followed in the 2nd 90-day interval. Therefore, the benefits seem to outweigh the risks to PEER report for the patients.
Prior research in QEEG for antidepressant selection
Our findings align with previous chart series where the PEER report improved clinical outcomes for patients with depression and anxiety improving outcomes and reducing the number of prescribed medications.[17] Although we observed increases medications and adverse events, this may be because of the non-double-blinded design of our study. Our finding of improved QIDS scores is consistent with an earlier randomized clinical trial using referenced EEG-guided pharmacotherapy, where significant improvements were observed compared with the standard protocol.[13] Other studies using QEEG demonstrated that early changes in the frontal and prefrontal theta values changed with the onset of SSRI treatment in depressed patients but could not predict medication responses.[18] A recent meta-analysis suggested that QEEG could reasonably predict the antidepressant response reasonably well but highlighted this may be an artifact of study design, small studies, or publication bias[14] Our study’s large and diverse patient population used for the current study may have overcome these limitations. Smaller studies, however, sometimes benefit from less patient-to-patient variation. One reported that some QEEG parameters could predict the patient response to antidepressants with 92.3% positive predictive value when performed before the initiation of treatment.[19]
One study of 122 patients used baseline QEEG features such as alpha peak frequency and frontal alpha asymmetry to guide medication selection between venlafaxine, escitalopram, and sertraline.[20] The QEEG-guided group had significantly more improvement in depression symptoms compared to patients receiving treatment as usual as usual group. (36.8% change in BDI scale vs 23.9%). This aligns with our results, where 43.3% of patients in the RF/RF reduced their QIDS scores by 50% or more as compared to 25.3% in the control group. In the previous study, 65% of EEG-informed clinicians followed recommendations, which compares closely to the 56.7% of clinicians who followed the PEER report at both 90- and 180-day points in our study. Although patients in our study were blinded to the use of PEER, those in the van der Vinne et al.[20] 2021 knew if their clinician used EEG-informed decisions, which may have biased results towards greater reduction in BDI scores in the EEG-informed group.
Several studies support the utility of EEG characteristics such as alpha, delta, and theta power to guide medication selection. One study used a support vector machine classifier trained on a database of baseline EEGs from 181 patients (Canadian Biomarker Integration Network in Depression (CAN-BIND-1) to predict which patients with the major depressive disorder would respond to Escitalopram.[21] The classifier identified Escitalopram responders with an accuracy of 79.2% (sensitivity, 67.3%; specificity, 91.0%). This study applied similar techniques to the PEER model but focused on predicting the efficacy of one specific medication.
A 2021 study used EEG to predict response to three antidepressant combinations (tricyclic antidepressants, fluoxetine, or fluoxetine augmented with magnesium).[22] The study found a correlation between a remission in depressive symptoms (measured with the Hamilton Depression Rating Scale, HDRS) and a positive pharmaco-EEG profile taken 6 hours after the first dose of fluoxetine plus magnesium (P = 0.035). A positive pharmaco-EEG profile was characterized by reduced alpha oscillation power, slower alpha rhythms, and increased beta, delta, and theta oscillation power.
QEEG for prediction of responsiveness to non-antidepressants
One randomized, double-blinded study examined the use of QEEG to predict responses to ketamine in 55 outpatients with treatment-resistant depression using a wearable headband EEG device.[23] Responders to ketamine showed significantly weaker EEG power in the theta and low alpha bands compared with nonresponders. The study predicted the effect of ketamine with 81.3 ± 9.5% accuracy, 82.1 ± 8.6% sensitivity, and 91.9 ± 7.4% specificity based on a support vector machine with a radial basis function predictor.
A study of 46 patients treated with rTMS for 7 weeks identified several EEG characteristics predicting response to treatment.[24] This study observed higher pretreatment alpha power in responders than nonresponders and reduced delta power in rTMS responders compared with nonresponders. Interestingly, the relationship of alpha power to treatment response was opposite with rTMS treatment compared with antidepressants, where patients with reduced alpha power had greater remission in symptoms.[21] Another study of 123 patients with depression reported no significant difference in alpha power or theta connectivity between responders and nonresponders to rTMS, however.[25]
QEEG for medication selection in schizophrenia and ADHD
QEEG may also be effectively applied to predict responses to medication trials in ADHD and schizophrenia. One study of 51 children with ADHD reported that specific baseline EEG characteristics predicted methylphenidate responsiveness.[26] Another study recorded baseline EEGs in 47 patients with schizophrenia or schizoaffective disorder to identify a Transfer Entropy (TE) parameter[27] that was used to develop a random forest classifier to predict response to electroconvulsive therapy (ECT). TE was higher, particularly in frontal areas, in patients who responded to ECT.
Strengths and limitations
The large population of patients and providers, including primary care physicians is a strength of our study. Long waits for psychiatry appointments, because of a shortage of psychiatrists often forces primary care physicians to diagnose and manage psychiatric medications. Two-thirds of primary care physicians were unable to find outpatient mental healthcare for their patients[28] and only 28% of Americans live in areas with enough psychiatrists. Furthermore, 51% of U.S. counties have no practicing psychiatrists.[29] These shortages require that primary care physicians manage mental health problems until a specialist is available.[30,31]
Using PEER technology would provide primary care physicians without extensive psychopharmacology training a tool to guide treatment based on evidence from thousands of patient EEGs. Psychiatrists could also use the PEER report to support their decision-making. In our study, providers were randomized to either the PEER or control group. Providers in the PEER group could determine whether to follow, reflecting real-world, clinical settings where physicians have guidelines but to make individual patient-based decisions. Long-term follow-up at 3 and 6 months is another advantages. Patients were unaware of their group, reducing risk of placebo effects, although physicians’ knowledge of the PEER protocol might have had an indirect placebo-type effect.
Limitations of the study include the nonrandomiz treatment allocations and strict use of the QIDS SR-16 score to evaluate outcomes. Randomization would have controlled for all sources of bias and confounding but may not reflect clinical settings where physicians adjust treatments based on patient needs. In primary care, diagnostic tools such as the PHQ-9 and HAM-D are routinely used, although the QIDS SR-16 provides a more comprehensive assessment and is better at assessing pharmacological treatment response.[32,33] Future studies should compare PHQ-9 and HAM-D scores in patients treated according to PEER recommendations vs. controls. Our study also did not account for patients concurrently receiving psychotherapy, so its impact on treatment responses could not be evaluated. Participants on antidepressants and other medications at baseline could have affected EEG waveforms and faulty recommendations but this would have biased results towards the null. Larger and longer-term studies are needed to assess how the PEER report may reduce depression’s consequences and comorbidities such as substance abuse, suicide, problematic gambling, anhedonia, and anxiety.
Conclusions
Treatment with medications suggested by the PEER protocol was associated with greater clinical improvements and less suicidal ideation. This suggests that the application of QEEG and PEER could improve outcomes in patients with clinical depression in the outpatient setting.
Ethics
This study was performed with the approval and oversight of the WCG IRB as Protocols Mynd 001 and Mynd 002 with written informed consent from all study participants.
Author contributions
Conceptualization and Methodology: RS, JF, MV; Funding acquisition: JF; Investigation: RS, JF, MV, VH and KP; Data Curation and Formal Analysis: MRP, RS; Supervision: RS; Project administration: JF, MV; Visualization: MRP; Writing-Original Draft: MRP, ET, RS; Writing-Review and Editing: RS, JF, MV, ET, VH, KP, and MRP.
Conflicts of interest
Justin Feintuch and Mabel Vasquez, MD are employees and stockholders of Telemynd, Inc. Neither of these individuals were involved in the statistical analyses of the data nor were they able to influence the outcomes or results of the study. The remaining authors have no personal or professional interest in the outcome of the study.
Funding Statement
Funds for this project were acquired from Telemynd, Inc. and Horizon Healthcare Services, Inc.
References
- 1.CDC. FastStats-Depression. [[Last accessed on 2023 Oct 18]]. Available from: https://www.cdc.gov/nchs/fastats/depression.htm .
- 2.ADAA. Depression Facts & Statstics. Anxiety and Depression Association of America. 2009. Available from: https://adaa.org/understanding-anxiety/depression/facts-statistics .
- 3.NIMH. Major Depression. National Institute of Mental Health. 2023. Available from: https://www.nimh.nih.gov/health/statistics/major-depression .
- 4.Global Burden of Disease Study C. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990-2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386:743–800. doi: 10.1016/S0140-6736(15)60692-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.SAVE. Suicide Statistics. Suicide Awareness Voices of Education. Available from: www.save.org. [Google Scholar]
- 6.Bommersbach TJ, Rosenheck RA, Rhee TG. National trends of mental health care among US adults who attempted suicide in the past 12 months. JAMA Psychiatry. 2022;79:219–31. doi: 10.1001/jamapsychiatry.2021.3958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gotlib IH. EEG alpha asymmetry, depression and cognitive functioning. Cogn Emot. 1998;12:449–78. [Google Scholar]
- 8.Gollan JK, Hoxha D, Chihade D, Pflieger ME, Rosebrock L, Cacioppo J. Frontal alpha EEG asymmetry before and after behavioral activation treatment for depression. Biol Psychol. 2014;99:198–208. doi: 10.1016/j.biopsycho.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Henriques JB, Davidson RJ. Left frontal hypoactivation in depression. J Abnorm Psychol. 1991;100:535–45. doi: 10.1037//0021-843x.100.4.535. [DOI] [PubMed] [Google Scholar]
- 10.Bruder GE, Sedoruk JP, Stewart JW, McGrath PJ, Quitkin FM, Tenke CE. Electroencephalographic alpha measures predict therapeutic response to a selective serotonin reuptake inhibitor antidepressant: Pre- and post-treatment findings. Biol Psychiatry. 2008;63:1171–7. doi: 10.1016/j.biopsych.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suffin SC, Emory WH, Gutierrez N, Arora GS, Schiller MJ, Kling A. A QEEG database method for predicting pharmacotherapeutic outcome in refractory major depressive disorders. J Am Phys Surg. 2007;124:104–9. [Google Scholar]
- 12.Rush AJ, Fava M, Wisniewski SR, Lavori PW, Trivedi MH, Sackeim HA, et al. Sequenced treatment alternatives to relieve depression (STAR*D): Rationale and design. Control Clin Trials. 2004;25:119–42. doi: 10.1016/s0197-2456(03)00112-0. [DOI] [PubMed] [Google Scholar]
- 13.DeBattista C, Kinrys G, Hoffman D, Goldstein C, Zajecka J, Kocsis J, et al. The use of referenced-EEG (rEEG) in assisting medication selection for the treatment of depression. J Psychiatr Res. 2011;45:64–75. doi: 10.1016/j.jpsychires.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 14.Widge AS, Bilge MT, Montana R, Chang W, Rodriguez CI, Deckersbach T, et al. Electroencephalographic biomarkers for treatment response prediction in major depressive illness: A meta-analysis. Am J Psychiatry. 2019;176:44–56. doi: 10.1176/appi.ajp.2018.17121358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cohen SE, Zantvoord JB, Wezenberg BN, Daams JG, Bockting CLH, Denys D, et al. Electroencephalography for predicting antidepressant treatment success: A systematic review and meta-analysis. J Affect Disord. 2023;321:201–7. doi: 10.1016/j.jad.2022.10.042. [DOI] [PubMed] [Google Scholar]
- 16.Iosifescu DV, Neborsky RJ, Valuck RJ. The use of the Psychiatric Electroencephalography Evaluation Registry (PEER) to personalize pharmacotherapy. Neuropsychiatr Dis Treat. 2016;12:2131–42. doi: 10.2147/NDT.S113712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoffman DA, Debattista C, Valuck RJ, Iosifescu DV. Measuring severe adverse events and medication selection using a “PEER Report” for nonpsychotic patients: A retrospective chart review. Neuropsychiatr Dis Treat. 2012;8:277–84. doi: 10.2147/NDT.S31665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singh A, Arun P, Singh GP, Kaur D, Kaur S. QEEG predictors of treatment response in major depressive disorder- A replication study from Northwest India. Clin EEG Neurosci. 2024;55:176–84. doi: 10.1177/15500594221142396. [DOI] [PubMed] [Google Scholar]
- 19.Kandilarova SS, Terziyski KV, Draganova AI, Stoyanov DS, Akabaliev VH, Kostianev SS. Response to pharmacological treatment in major depression predicted by electroencephalographic Alpha power-A pilot naturalistic study. Folia Med (Plovdiv) 2017;59:318–25. doi: 10.1515/folmed-2017-0040. [DOI] [PubMed] [Google Scholar]
- 20.van der Vinne N, Vollebregt MA, Rush AJ, Eebes M, van Putten M, Arns M. EEG biomarker informed prescription of antidepressants in MDD: A feasibility trial. Eur Neuropsychopharmacol. 2021;44:14–22. doi: 10.1016/j.euroneuro.2020.12.005. [DOI] [PubMed] [Google Scholar]
- 21.Zhdanov A, Atluri S, Wong W, Vaghei Y, Daskalakis ZJ, Blumberger DM, et al. Use of machine learning for predicting escitalopram treatment outcome from electroencephalography recordings in adult patients with depression. JAMA Netw Open. 2020;3:e1918377. doi: 10.1001/jamanetworkopen.2019.18377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Skalski M, Mach A, Januszko P, Ryszewska-Pokrasniewicz B, Biernacka A, Nowak G, et al. Pharmaco-electroencephalography-based assessment of antidepressant drug efficacy-the use of magnesium ions in the treatment of depression. J Clin Med. 2021;10:3135. doi: 10.3390/jcm10143135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zehong C, Chin-Teng L, Weiping D, Mu-Hong C, Cheng-Ta L, Tung-Ping S. Identifying ketamine responses in treatment-resistant depression using a wearable forehead EEG. IEEE Trans Biomed Eng. 2019;66:1668–79. doi: 10.1109/TBME.2018.2877651. [DOI] [PubMed] [Google Scholar]
- 24.Hasanzadeh F, Mohebbi M, Rostami R. Prediction of rTMS treatment response in major depressive disorder using machine learning techniques and nonlinear features of EEG signal. J Affect Disord. 2019;256:132–42. doi: 10.1016/j.jad.2019.05.070. [DOI] [PubMed] [Google Scholar]
- 25.Bailey NW, Krepel N, van Dijk H, Leuchter AF, Vila-Rodriguez F, Blumberger DM, et al. Resting EEG theta connectivity and alpha power to predict repetitive transcranial magnetic stimulation response in depression: A non-replication from the ICON-DB consortium. Clin Neurophysiol. 2021;132:650–9. doi: 10.1016/j.clinph.2020.10.018. [DOI] [PubMed] [Google Scholar]
- 26.Sari Gokten E, Tulay EE, Beser B, Elagoz Yuksel M, Arikan K, Tarhan N, et al. Predictive value of slow and fast EEG oscillations for methylphenidate response in ADHD. Clin EEG Neurosci. 2019;50:332–8. doi: 10.1177/1550059419863206. [DOI] [PubMed] [Google Scholar]
- 27.Min B, Kim M, Lee J, Byun JI, Chu K, Jung KY, et al. Prediction of individual responses to electroconvulsive therapy in patients with schizophrenia: Machine learning analysis of resting-state electroencephalography. Schizophr Res. 2020;216:147–53. doi: 10.1016/j.schres.2019.12.012. [DOI] [PubMed] [Google Scholar]
- 28.Cunningham PJ. Beyond parity: Primary care physicians'perspectives on access to mental health care. Health Aff (Millwood) 2009;28:w490–501. doi: 10.1377/hlthaff.28.3.w490. [DOI] [PubMed] [Google Scholar]
- 29.KFF. Mental Health Care HEalth Professional Shortage Areas. 2023 [Google Scholar]
- 30.Marcus SC, Olfson M. National trends in the treatment for depression from 1998 to 2007. Arch Gen Psychiatry. 2010;67:1265–73. doi: 10.1001/archgenpsychiatry.2010.151. [DOI] [PubMed] [Google Scholar]
- 31.Reeves WC, Strine TW, Pratt LA, Thompson W, Ahluwalia I, Dhingra SS, et al. Mental illness surveillance among adults in the United States. MMWR Suppl. 2011;60:1–29. [PubMed] [Google Scholar]
- 32.Rush AJ, Trivedi MH, Ibrahim HM, Carmody TJ, Arnow B, Klein DN, et al. The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): A psychometric evaluation in patients with chronic major depression. Biol Psychiatry. 2003;54:573–83. doi: 10.1016/s0006-3223(02)01866-8. [DOI] [PubMed] [Google Scholar]
- 33.Trivedi MH, Rush AJ, Ibrahim HM, Carmody TJ, Biggs MM, Suppes T, et al. The Inventory of Depressive Symptomatology, Clinician Rating (IDS-C) and Self-Report (IDS-SR), and the Quick Inventory of Depressive Symptomatology, Clinician Rating (QIDS-C) and Self-Report (QIDS-SR) in public sector patients with mood disorders: A psychometric evaluation. Psychol Med. 2004;34:73–82. doi: 10.1017/s0033291703001107. [DOI] [PubMed] [Google Scholar]