ABSTRACT
Background:
Pulmonary tuberculosis (PTB) accounts for 85% of all reported tuberculosis cases globally. Extrapulmonary involvement can occur in isolation or along with a pulmonary focus as in the case of patients with disseminated tuberculosis (TB). EPTB can occur through hematogenous, lymphatic, or localized bacillary dissemination from a primary source, such as PTB and affects the brain, eye, mouth, tongue, lymph nodes of neck, spine, bones, muscles, skin, pleura, pericardium, gastrointestinal, peritoneum and the genitourinary system as primary and/or disseminated disease. Although pulmonary TB is the most common presentation, EPTB is also an important problem clinically. Cartridge-based nucleic acid amplification (CBNAAT) test has a well-documented role in diagnosing pulmonary tuberculosis.
Aim:
To determine the distribution of EPTB in various samples received for CBNAAT testing in our Institution.
Methods:
In this retrospective cross-sectional study, data of suspected EPTB patients were retrieved and analyzed from January 2020 to December 2022.
Statistical Analysis:
All the statistical analyses were carried out using the Excel spreadsheet and Open-epi version 3.01 platform.
Results:
A total number of 1118(n) extrapulmonary samples were processed using GeneXpert MTB/RIF assay. Out of the 1118 received samples, (22%) were positive. Among the 249 positive samples, 55% samples were received from the female patients and 45% samples received from the male patients. We found that most MTB positive samples were from this age group (i.e. 21-30). Most common sample received for processing was Lymph node aspirate accounting to 37% followed by pleural fluid (30%), pus (8%) and gastric lavage amounting for 4% along with other miscellaneous samples making up the others to 21%. Out of 249 MTB detected samples, 47% were from lymph node aspirate, 13% from pleural fluid, 12% from pus, 3% from gastric lavage and 25% from other samples. We noted that the majority of the positive cases were rifampicin sensitive (97.68%).
Conclusion:
Results of our study suggest that younger age (third decade of life) and female gender may be independent risk factors for EPTB. In developing countries, the prevalence of EPTB is relatively lower than PTB but still it is an important cause of morbidity and mortality. Thus, early diagnosis and initiation of appropriate treatment are important for reducing the case load. Women especially should be investigated thoroughly for EPTB and BCG vaccination should be encouraged.
Keywords: Cartridge-based nucleic acid amplification test, extrapulmonary tuberculosis, GeneXpert MTB/RIF, rifampicin
Introduction
Tuberculosis (TB) is considered a major health concern globally till today, with a third of the total population being infected with Mycobacterium tuberculosis. According to the World Health Organization (WHO), India carries the highest TB burden in the world with 26% of the world’s total cases. Pulmonary TB is the most common presentation but extrapulmonary tuberculosis (EPTB) is also as important from a clinician’s perspective.[1] Statistically, EPTB accounts for ~16%[1] of the total reported cases. In India, EPTB constitutes 10-15% of total TB cases which primarily involve the pleura, lymph nodes, gastrointestinal tract and other organs with a significant case mortality rate (25 to 50%). Available evidence showed that an increase in the number of newly diagnosed EPTB cases were observed worldwide.[2,3,4] To curb this situation, the government has implemented the National Tuberculosis Elimination Program (NTEP); formerly known as Revised National TB Control Program (RNTCP).[5] TB primarily affects the lung parenchyma but also can cause various extrapulmonary complications, like TB meningitis, TB osteomyelitis, etc., There are many diagnostic techniques available for pulmonary TB but detection of Extrapulmonary TB (EPTB) is still a bit challenging as it is of paucibacillary nature and lacks proper diagnostic tools. Clinically, extrapulmonary tuberculosis (EPTB) does not exhibit the typical symptoms seen in pulmonary tuberculosis (PTB) patients, such as dyspnea, weight loss, cough, hemoptysis, and night sweats. Depending on the affected organ system, patients may experience abdominal pain, joint pain, headaches, diarrhea, or lymphadenopathy.[6,7] Additionally, patients might have a normal chest radiograph, which can obscure an important diagnosis. Therefore, invasive diagnostic methods are often required to diagnose EPTB.[6] Histological analysis is time-consuming and challenging for precise TB diagnosis.[8]
EPTB can affect almost any organ, resulting in a wide range of clinical manifestations and often involves sites that are difficult to access. The body fluids affected (mainly pleural and peritoneal) usually contain few bacteria, complicating effective disease diagnosis and treatment.[9] Patients frequently receive alternative diagnoses during their initial visits to primary health care facilities. Even in tertiary health care settings, many begin anti-TB therapy without bacteriologically confirmed results due to diagnostic difficulties. This leads to delays in diagnosis or misdiagnosis, underestimating the problem’s extent at the community level.[10]
Molecular diagnostic methods seem better for the diagnosis of EPTB. Among the different diagnostic modalities, CBNAAT is one of the fastest techniques available for a treating clinician or a microbiologist for reporting of TB cases.
This study has been planned to determine the distribution of extrapulmonary tuberculosis in various samples received for CBNAAT in our Institution.
Materials and Methods
This study is a retrospective cross-sectional study conducted in our institution from 15th April 2023 to 15th May 2023. We had retrieved three years data, from 1st January 2020 to 31st December 2022 from our records. Data from all suspected extrapulmonary Tuberculosis patients’ samples sent to Microbiology department for processing were included in the study. All the samples were processed using a GeneXpert MTB/RIF assay. This instrument has a closed system consisting of disposable closed cartridges, thus preventing cross contamination. This system works on the principle of nucleic acid amplification to detect the presence of M. tuberculosis specific genes and also detects resistance towards rifampicin by checking for mutations in rpoB gene.
The collected samples included lymph node aspirate, pus, pleural fluid, gastric lavage, and other samples (ascitic fluid, sinus discharge, cold abscess).
The study has been conducted after getting clearance from the Ethics Committee (CMSDH/IEC/25/03-2023).
Statistical analysis
We included 3 years of data, from 1st January 2020 to 31st December 2022. Samples from all suspected patients sent to the Microbiology department for processing were included in the study. The statistical analyses were carried out using the Excel spreadsheet and Open-epi version 3.01 platform. Proportion will be expressed in percentages. The descriptive data will be expressed as mean ± SD, median, interquartile range, 95% CI of mean for numerical data; ratio proportion and percentage for categorical data.
Results
A total number of 6000 samples were processed from 1st January 2020 to 31st December 2022 by Genexpert MTB/RIF assay, among them 1118(n) were extrapulmonary samples.
Total 1200 samples were positive for M. tuberculosis, out of which 249 (20.75%) samples were extrapulmonary samples and the rest of the samples were pulmonary samples (79.25%).
Out of the 1118 extrapulmonary samples recived, 708 (63%) samples were from male patients and 410 (37%) samples were from female patients.
Among 1118 extrapulmonary samples, 249 were positive (22%) for M. tuberculosis.
Among the 249 positive samples, 138 (55%) samples were received from female patients and 111 (45%) samples were received from male patients.
According to our study, the mean age of an extrapulmonary TB-positive patient is 39.75 ± 5, ranging from 39.516 to 39.986 (where CI is 95%). We found that most MTB-positive samples are from this age group (i.e. 21-30) [Figure 1].
Figure 1.
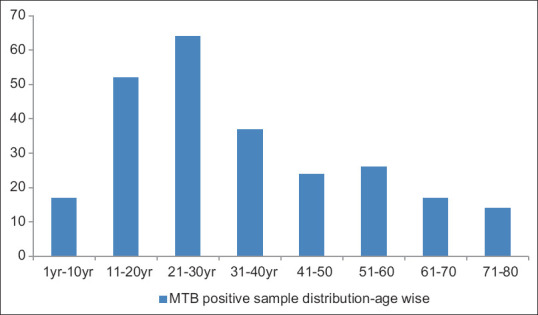
MTB positive sample distribution-age wise
The most common sample received for processing was Lymph node aspirate accounting for 37% (415) of the total samples followed by pleural fluid accounting for 30% (335 samples), pus accounting for 8% (89 samples), gastric lavage amounting for 4% (45 samples) and other miscellaneous samples (ascitic fluid, sinus discharge, cold abscess) making up the others to 21% (234 samples) [Figure 2].
Figure 2.
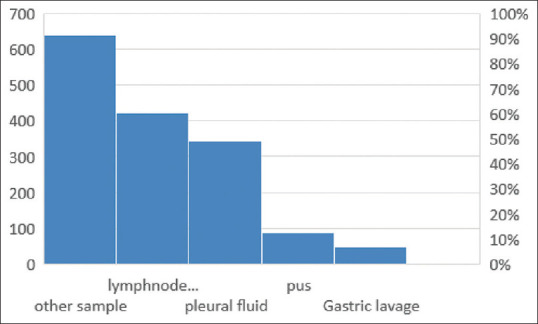
Distribution of different extrapulmonary samples received for CBNAAT
Out of 249 MTB detected samples, 47% is positive for lymph node aspirate (117 samples), 13% is positive for pleural fluid (32 samples), 12% is for pus (30 samples), 3% for gastric lavage (7 samples), and 25% (63 samples) for other samples [Figure 3].
Figure 3.
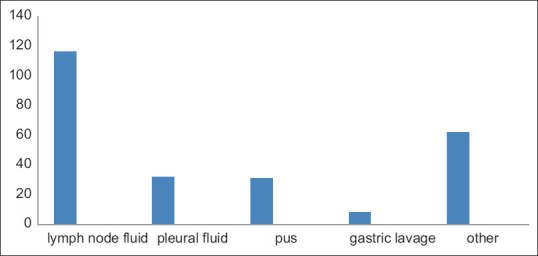
Distribution of different MTB-positive samples
We noted that the majority of the positive cases were rifampicin sensitive (97.68%) with 6 (2.32%) being rifampicin resistant [Figure 4].
Figure 4.
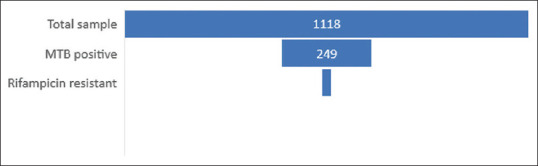
Proportion of rifampicin-resistant samples among all the MTB-positive extrapulmonary and total number of extrapulmonary samples
Discussion
EPTB accounts for around 20.75% of all TB cases in our study. This finding correlates well with some previous studies.[11,12] Among the MTB detected specimen, the majority of the samples were that of lymph node aspirate (47%), pleural fluid (13%), pus (12%), gastric aspirate (3%), and other samples (25%) were seen among the extrapulmonary samples similar to previous studies.[13,14,15,16] MTB positivity rate is higher in female patients (55%) than the male patients in our study.[17,18] We found that the MTB positivity rate is highest in this (21-30) age group (i.e. 25.70%) like some previous studies done in India.[19,20] The highest positivity rate was found in lymph node aspirate (47%), this trend is similar to some previous studies.[21,22,23] The diagnosis of EPTB is challenging because of the paucibacillary nature of this disease. The serous cavity fluids also have a low positivity rate and low sensitivity of ascitic fluid, pericardial fluid is seen in some previous studies also.[24] We did not find any immunosuppressive condition as a predisposing factor in the case of EPTB, which is contrary in the case of PTB. In our study, we found that the majority of the positive cases were rifampicin sensitive 97.68% with only 2.32% being rifampicin resistant. This trend in rifampicin resistance has also been noted in another study.[25] All these rifampicin-resistant cases had not responded to first-line ATT drugs so they were initiated on other drugs as per the sensitivity report of the Xpert MTB/RIF assay. Although culture and sensitivity testing are gold standard tests, Xpert MTB/RIF assay allows rapid detection of MTB DNA along with the rpoB gene and it needs minimal technical skill.[26]
Conclusion
Results of our study suggest that younger age (3rd decade of life) and female gender may be independent risk factors for EPTB. In developing countries, the prevalence of EPTB is relatively lower than PTB but still, it remains an important cause of morbidity and mortality. Thus, early diagnosis and initiation of appropriate treatment are important for reducing the caseload. Women, especially, should be investigated thoroughly for EPTB, and BCG vaccination should be encouraged.
Our data indicate that EPTB is common and affects various anatomical sites. The study underscores the need for healthcare workers nationwide to recognize the varied presentations of EPTB. Further research is necessary to better define clinical predictors and improve microbiological tests for early detection of EPTB. Additionally, a prospective study is needed to assess the treatment response of EPTB cases that began anti-TB therapy based on a presumptive diagnosis, which is important for exploring misdiagnosis.[27]
Limitations
Study samples were not correlated with histology or culture but we are planning to do so in our future studies.
Conflicts of interest
There are no conflicts of interest.
Funding Statement
Nil
References
- 1.Global Tuberculosis Report 2020. Licence: CC BY-NCSA 3.0 IGO. Geneva: World Health Organization; 2020. [[Last accessed on 2024 Oct 24]]. Available from: https://www.who.int/publications/i/item/9789240013131 . [Google Scholar]
- 2.World Health Organization. Global Tuberculosis Report 2017; 2017. Available from: http://www.who.int/tb/publications/global_report/en/
- 3.World Health Organization. Global Tuberculosis Report 2012; 2012. Available from: http://apps.who.int/iris/bitstream/10665/75938/1/9789241564502_eng.pdf .
- 4.World Health Organization. Global Tuberculosis Report 2015; 2015. [[Last accessed on 2024 Oct 24]]. Available from: https://www.who.int/publications/i/item/9789241565059 .
- 5.India TB Report; 2020. [[Last accessed on 2024 Oct 24]]. Available from: https://tbcindia.mohfw.gov.in/wp-content/uploads/2023/05/India-TBReport-2020.pdf .
- 6.Sanford CA, Jong EC. The travel and tropical medicine manual E-book. Elsevier; 2022. [Google Scholar]
- 7.Rodriguez-Takeuchi SY, Renjifo ME, Medina FJ. Extrapulmonary tuberculosis: Pathophysiology and imaging findings. Radiographics. 2019;39:2023–37. doi: 10.1148/rg.2019190109. [DOI] [PubMed] [Google Scholar]
- 8.Djannah F, Massi MN, Hatta M, Bukhari A, Hasanah I. Profile and histopathology features of top three cases of extra pulmonary tuberculosis (EPTB) in West Nusa Tenggara: A retrospective cross-sectional study. Ann Med Surg (Lond) 2022;75:103318. doi: 10.1016/j.amsu.2022.103318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gan SY, Senthil V, Salahudin B, Fitjerald H. Abdominal tuberculosis, a surgeon's diagnostic dilemma: Case report. 2020 [doi:10.13140/RG.2.2.12873.88162] [Google Scholar]
- 10.Bahar Moni AS, Johnson TA, McGarvey CR, Ivy RS, Yean TJ, Ahmad MZ. Diagnostic dilemma in disseminated TB could be fatal:lesson learned through a bitter encounter. Egypt J Intern Med. 2023;35:76. [Google Scholar]
- 11.Sachdeva K, Shrivastava T. CBNAAT: A boon for early diagnosis of tuberculosis-head and neck. Indian J Otolaryngol Head Neck Surg. 2018;70:572–7. doi: 10.1007/s12070-018-1364-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yadhav K, Veena M. Role of GeneXpert in rapid molecular detection of extrapulmonary tuberculosis in tertiary care hospital. Int J Med Res Rev. 2018;6:271–6. [Google Scholar]
- 13.Rolo M, González-Blanco B, Reyes CA, Rosillo N, López-Roa P. Epidemiology and factors associated with extra-pulmonary tuberculosis in a low-prevalence area. J Clin Tuberc Other Mycobact Dis. 2023;32:100377. doi: 10.1016/j.jctube.2023.100377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alwani H, Subhankar S, Rao CM, Dash DP. Role of CBNAAT in extrapulmonary tuberculosis –an ongoing pilot study. Eur Respir J. 2019;54:PA3007. [Google Scholar]
- 15.Li T, Yan X, Du X, Huang F, Wang N, Ni N, et al. Extrapulmonary tuberculosis in China: A national survey. Int J Infect Dis. 2023;128:69–77. doi: 10.1016/j.ijid.2022.12.005. [DOI] [PubMed] [Google Scholar]
- 16.Rai DK, Pandey S. A hospital based cross sectional study on clinicodemographic characteristic of extrapulmonary tuberculosis cases coming to a tertiary hospital of Bihar. Indian J Community Med. 2018;43:122–3. doi: 10.4103/ijcm.IJCM_308_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Min J, Park JS, Kim HW, Ko Y, Oh JY, Jeong Y-J, et al. Differential effects of sex on tuberculosis location and severity across the lifespan. Sci Rep. 2023;13:6023. doi: 10.1038/s41598-023-33245-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shirzad-Aski H, Hamidi N, Sohrabi A, Abbasi A, Golsha R, Movahedi J. Incidence, risk factors and clinical characteristics of extra-pulmonary tuberculosis patients: A ten-year study in the North of Iran. Trop Med Int Health. 2020;25:1131–9. doi: 10.1111/tmi.13452. [DOI] [PubMed] [Google Scholar]
- 19.Wang D-M, Li Q-f, Zhu M, Xu Y-H, Liao Y. Clinical characteristics, common sites and drug resistance profile in culture-confirmed extrapulmonary TB/HIV co-infection patients, Southwest China. J Glob Antimicrob Resist. 2022;28:1–7. doi: 10.1016/j.jgar.2021.10.028. [DOI] [PubMed] [Google Scholar]
- 20.Zangpo T, Tsheten, Tenzin P, Dorji C, Nima G, Dorjee S, et al. Demographic risk factors for extra-pulmonary tuberculosis: A rising public health threat in Bhutan. Indian J Tuberc. 2024;71:137–46. doi: 10.1016/j.ijtb.2023.04.016. [DOI] [PubMed] [Google Scholar]
- 21.Zürcher K, Ballif M, Kiertiburanakul S, Chenal H, Yotebieng M, Grinsztejn B, et al. Diagnosis and clinical outcomes of extrapulmonary tuberculosis in antiretroviral therapy programmes in low- and middle-income countries: A multicohort study. J Int AIDS Soc. 2019;22:e25392. doi: 10.1002/jia2.25392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shetty D, Vyas D. Combination method for the diagnosis of tuberculous lymphadenitis in high burden settings. Surg Exp Pathol. 2022;5:11. [Google Scholar]
- 23.Gopalaswamy R, Dusthackeer VNA, Kannayan S, Subbian S. Extrapulmonary tuberculosis—An update on the diagnosis, treatment and drug resistance. J Respir. 2021;1:141–64. [Google Scholar]
- 24.INDEX-TB Guidelines. Evidence Summaries and Guideline Panel Decision Tables Core Group Guideline Meeting, 14-18 July. 2015;2016 [Google Scholar]
- 25.Reddy R, Alvarez-Uria G. Molecular epidemiology of rifampicin resistance in Mycobacterium tuberculosis using the GeneXpert MTB/RIF assay from a rural setting in India. J Pathog. 2017;2017:6738095. doi: 10.1155/2017/6738095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kasat S, Biradar M, Deshmukh A, Jadhav S, Deshmukh H. Effectiveness of CBNAAT in the diagnosis of extrapulmonary tuberculosis. Int J Res Med Sci. 2018;6:3925–8. [Google Scholar]
- 27.Arega B, Mersha A, Minda A, Getachew Y, Sitotaw A, Gebeyehu T, et al. Epidemiology and the diagnostic challenge of extra-pulmonary tuberculosis in a teaching hospital in Ethiopia. PLoS One. 2020;15:e0243945. doi: 10.1371/journal.pone.0243945. [DOI] [PMC free article] [PubMed] [Google Scholar]


