ABSTRACT
Gastrointestinal stromal tumors (GISTs) are rare mesenchymal tumors that arise from interstitial cells of Cajal. Due to vague presentation, location and confusing imaging studies, they tend to mimic gynaecological tumors. They usually diagnosed intra-operative and histopathology followed by tumor specific receptors such as KIT, CD34, CD 117 and DOG 1 are mainstay of diagnosis of GIST. Prognosis of GISTs depends on mitotic rate, tumor size and organ of origin. Resection of mass with tumor free margins is the target of treatment. L ymphadenectomy is not routine. Chemotherapy with tyrosine kinase inhibitors such as Imatinib, Dasatinib, Sorafenib and follow- up depend upon risk category. In this case series, there were four cases with vague symptoms misdiagnosed as gynaecological tumors are reviewed. Preoperatively tumors assumed to be of gynaecological origin were found to be case of GISTs intra-operatively and confirmed by presence of cajal’s cells histopathology and mainly by DOG 1, CD117 and tyrosine kinase inhibitor receptors on immunohistochemistry. All belonged to high risk category of GISTs. Any abdomino-pelvic mass detected on ultrasonography and with unusual presentation presenting at primary health centre the possibility of non-gynecological tumors especially GISTs should be kept in mind and should be referred to higher centres for further investigation and proper management.
Keywords: Gastrointestinal tumors, interstitial cells of Cajal’s, gynaecological tumors, histopathology, immunohistochemistry, CD 117 and DOG 1, tyrosine kinase inhibitors
Gastrointestinal stromal tumors (GISTs) are the most frequent mesenchymal tumors of the gastrointestinal tract (GIT) but comprise less than 1% of all GIT tumors.[1] GISTs are rare variety tumors arising from gastrointestinal Cajal’s interstitial cells, known as pacemaker cells of GIT. They comprise three microscopic types—epithelioid, spindle cell, and mixed variety. They arise from the uterus, rectovaginal septum, vagina, retroperitoneum, omentum, and mesenteries along with GIT.[2] GISTs arise from stomach (60%), small gut (30%), colorectal (5 to 10%), and esophagus (5%).[3] These can present from benign tumors to sarcomas and its diagnosis can be deceptive as gynecological neoplasms. The mean age of presentation is 60 years, with male predominance.[4] GISTs present as abdominal mass, pain, and discomfort along with nausea, vomiting, and GIT bleeding due to mucosal ulceration.[5] Intraperitoneal GISTs can mimic ovarian mass.[6] Tyrosine kinase, CD34, CD117, and DOG1 are the GIST-specific receptors used for differentiating it from other mesenchymal tumors.[2] The prognosis of GIST depends on tumor size (>5 cm), mitotic rate (per 50 high-power field with 5 mm2), and organ of origin. Hence, the swifter is the diagnosis, the better the survival.
This case series describes GISTs misdiagnosed as gynecological tumors due to its common clinical presentation, location, and radiological findings and, finally, diagnosed intraoperatively and confirmed by histopathology and immunohistochemistry. This case series gives emphasis on key points which mislead the diagnosis. This will draw the attention of primary physicians for timely referral to higher center for better outcome and the importance of advanced modalities of imaging in atypical gynecological tumors. The patients were informed and consented for reporting due to the clinical importance of these cases.
Case 1
A 74-year-old, postmenopausal woman presented with constipation and abdominal lump with continuous and non-radiating mild pain in the lower abdomen since 2–3 months which was relieved with analgesics, without fever, hematochezia/melena, weight loss or trauma to the abdomen, and relevant medical or surgical history or family history. She was normotensive and mildly pale. A mobile non-tender mass of 18 cms × 16 cms was occupying the flower abdomen up to the umbilicus, with well-defined margins, smooth surface, and variegated consistency. The uterus was atrophied, and groove was felt between the uterus and the mass. Complete blood count (CBC), liver function test (LFT), renal function test (RFT), thyroid function test (TFT) urinalysis with urine culture, and serum electrolytes were normal. Tumor markers—carcinoembryonic antigen (CEA), CA-125, beta-human chorionic gonadotropin (beta-HCG), and alpha-fetoprotein (AFP)—were normal. Ultrasonography (USG) revealed a large dermoid tumor of ovary with heterogeneous hyperechoic solid and cystic components of size 17.1 cm × 10.3 cm × 12.4 cm. Cone beam computed tomography (CECT) showed ill-defined heterogeneous, enhancing lesion of size 14.1 cm × 15 cm × 11 cm in the right adnexal region and solid with some fluid attenuation—both cystic and fat components suggestive of immature teratoma. Laparotomy revealed a mass of about 20 cm × 18 cm solid mass with irregular margins, arising 2 cm distal to duodenojejunal flexure with atrophied uterus, tubes, and ovaries. Resection of mass with end-to-end anastomosis of adjacent bowel was conducted. The postoperative period was uneventful. Histopathological examination (HPE) revealed spindle cells arranged in sheets and short fascicles without evidence of malignancy [Figure 1a and b].
Figure 1.
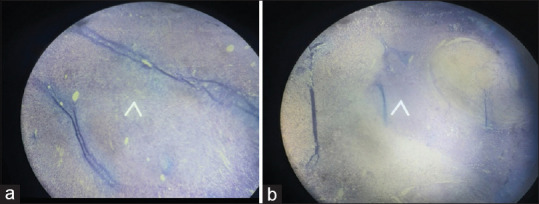
(a) Spindle cells arranged in sheets and short fascicles without evidence of malignancy. (b) Spindle cells arranged in sheets and short fascicles without evidence of malignancy
Confirmation was made by immunohistochemistry with positive CD117, DOG1, and CD34 markers (GIST markers). Smooth muscle actin (SMA) and H-caldesmon (smooth muscle markers) were focally positive. The patient advised follow-up and imatinib 800 mg daily for up to 3 years with computed tomography/magnetic resonance imaging (CT/MRI) quarterly.
Case 2
A 24-year-old woman, Parity1 + 1, Live1 cesarean delivery, presented with pain in the right lower abdomen and irregular menstruation since 6 months without nausea, vomiting, fever, gastrointestinal symptoms, or relevant past and family history. She had hypothyroidism with thyroxine supplement.
She was mildly pale. A suprapubic non-tender and mobile lump of 10 cm × 8 cm was found with smooth surface, regular margins, and firm consistency. The mass fell separately from the uterus through the right fornix and the left fornix was free. CBC, RFT, LFT, and TFT were normal. The tumor markers—CA-125, HE4, AFP, CA19-9, CEA, and HCG were normal. Lactate dehydrogenase (LDH) was mildly raised. USG revealed heterogeneous mass lesion of 79 mm × 69 mm in the right iliac fossa suggesting a pedunculated subserosal fibroid. CECT whole abdomen showed a soft tissue mass of 11 cm × 8 cm arising from the right adnexa, suggesting a right ovarian tumor or subserosal pedunculated fibroid. Laparotomy revealed a large cecal mass with stretched appendix sitting on it. Hence, a right hemicolectomy with side-to-side anastomosis and lymphadenectomy was conducted and sent for HPE [Figure 2a and b].
Figure 2.
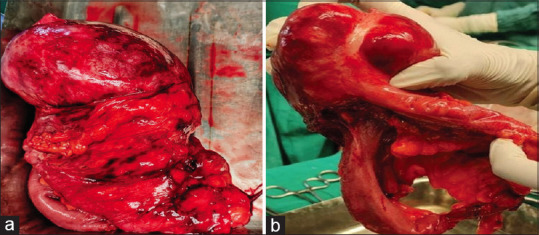
(a) Gross section of the tumor. (b) Gross section of the tumor with appendix embedded in it
The postoperative period was uneventful. HPE showed partly encapsulated and partly infiltrative benign appearing spindle cell neoplasm composed of mildly pleomorphic spindled tumor cells with elongated to plump nuclei. The appendix was embedded within the tumor. These features were suggestive of soft tissue myxoid variety gastrointestinal tumor.
HPE and immunohistochemistry with positive CD117 and DOG1 receptors confirmed GIST. The patient advised imatinib 800 mg daily, quarterly CT/MRI, and follow-up for 3 years.
Case 3
A 55-year-old postmenopausal woman came with complaints of abdominal lump and generalized pain in the abdomen since 3–4 months, associated with nausea, vomiting, and bloating sensation, but no fever, haematochezia, constipation, weight loss or trauma to the abdomen, and relevant past or family history. She was mildly pale and normotensive. A suprapubic mobile lump of about 10 × 10 cm with variegated consistency, smooth surface, and well-defined margins was felt, almost extending into the right lumbar region. The uterus was found normal, separately from the mass which was high up. CBC, RFT, urinalysis, and serum electrolytes were normal. However, her LFT was mildly deranged, with serum glutamate pyruvate transaminase (SGPT) of 124 U/L, serum glutamic-oxaloacetic transaminase (SGOT) of 145 U/L, and alkaline phosphatase (ALP), and tumor markers, CEA, CA-125, beta-HCG, and AFP, were normal.
CECT showed well-defined heterogeneous enhancing 95 × 69 × 103 mm intraperitoneal exophytic soft tissue mass lesion with central areas of hypoenhancement in the lower abdomen suggesting mature teratoma.
On laparotomy, a pedunculated hard mass of 12 × 10 cm, from the ascending colon, was found. The uterus, adnexa, and ovaries were normal. Resection of the mass was conducted and sent for HPE.
HPE showed round cells, clear to eosinophilic cytoplasm in sheets or nests, and nuclear crowding with pleomorphism and conspicuous nucleoli, suggesting epithelioid type of gastrointestinal tumor the from ascending colon. DOG1 and CD117 receptor positivity confirmed diagnosis. Chemotherapy with follow-up was conducted.
Case 4
A 40-year-old women had complained of dull aching, and continuous and moderate intensity pain in the lower abdomen since 2 years. She had no fever or GI symptoms. A 10 cms × 10 cms lump, variegated consistency, well-defined margins with restricted mobility, separate from the uterus, was felt in the left iliac fossa. The uterus and adnexa were normal. CBC, LFT, RFT, TFT, coagulation profile, blood sugar, serology, and urine analysis were normal. CECT showed 15.2 × 12.6 cms, well-defined, round to oval cystic lesion with multiple internal septations and heterogeneously enhancing internal solid components with necrotic areas from the left adnexa causing left-sided grade II hydroureteronephrosis, nodular deposit, and vascular involvement suggestive of ovarian cancer.
On exploratory laparotomy, a large mass of 11 × 10 cms from the pelvis adherent to the sigmoid colon was found. The uterus and ovaries appeared normal. Removal of mass along with colectomy, bowel repair, and lymphadenectomy was performed. HPE showed spindle cell tumor-GIST high-risk category with pericolic node invasion and parietal wall margins free of tumor [Figure 3].
Figure 3.
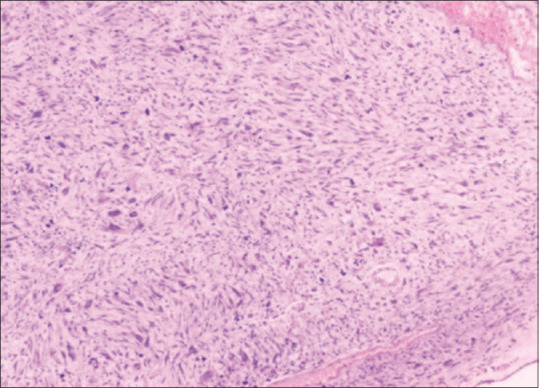
Spindle cell variety GIST
Immunohistochemistry showed positive CD117 and DOG1 receptors. She advised tablet dasatinib and follow-up for 3 years.
Discussion
Initially, GISTs were referred to as sarcomas till immunohistochemistry and electron microscopy revealed its origin from Cajal’s cells.[5] Marked discrepancy between preoperative and intraoperative findings, its rarity, diverse mode of presentation, and lack of distinguishing characteristics with gynecological tumor on imaging cause dilemma in diagnosis.[7] In this series, every tumor was mimicking gynecological tumors due to its location and confusing non-distinguishing features on imaging. As suggested by Belics et al.,[8] the presence of ante-uterine pelvic mass should have clinical suspicion of GIST which is usually found in mature cystic teratoma which was shared by cases 1 and 4 in this series.
In this case series, cases 1 and 3 were operated on with a preliminary diagnosis of cystic teratoma according to imaging and clinical findings which is in coherence with the study by Belic et al.[8] All the cases presented with vague and mild symptoms of abdominal discomfort and pain as reported by Eisenberg BL and Pipas, 2012.[9] Irrespective of origin, GISTs have wide spectrum of presentation in terms of malignant potential, that is, from benign to malignancy. Tumors with low mitotic rate are mostly benign, some of which do metastasize.[10] Small tumors less than 5 centimeters with low mitotic rate (<5 mitoses/50 high-power fields) are designated as low risk.[11] Large tumors more than 5 centimeters in size and more than 5 mitoses per 50 high-power fields are considered higher risk. In our series, all cases were considered high risk.
The GISTs comprise three subtypes depending upon the microscopic features—spindle (70%), epithelioid (20%), and mixed (10%).[10] We found three cases of histological type of spindle cells and one of epithelioid variety. Central myxoid degeneration and microcystic changes inside the tumor as hyperechoic areas are due to imbalance of tumor growth and angiogenesis as seen in case 2. The imbalance depends upon the size of the tumor and is more severe in larger sizes and associated with confluence of areas of hemorrhagic necrosis and colliquative changes.
Zighelboim et al.[12] reported the presence of trace-free fluid in the abdomen along with GIST. Although marked ascites are very uncommon, none of our cases had similar findings.
Usually, sonographic diagnosis of GIST is very difficult but CT scan and MRI are useful for characterization of tumor component especially with contrast[13] for recording mitotic index, which is a measure of malignant potential in GIST.[14]
Immunohistochemistry brought remarkable changes in the diagnosis of GISTs by expression of diagnostic markers, such as CD117, KIT, CD34, DOG1, SMA, and desmin, and mainly, CD117, CD34, DOG1, and KIT were positive in this series.
Resection of tumor with pseudo-capsule along with negative microscopic border is gold standard treatment for GIST and is typically followed by chemotherapy with tyrosine kinase inhibitors, such as imatinib and dasatinib.[1] Also, patients need follow-up.[15] As lymph node metastasis is a rare occurrence, routine lymphadenectomy is not indicated.[16] We followed the same protocol and have not witness any case of recurrence till date.
We reported cases visited to the gynecology department only, which lead to lesser number of cases in this case series, which is a limitation of our study.
We reported only gynecological tumors in contrast to previous studies.
Conclusion
Due to common symptomatology and imaging features of GISTs with gynecological tumors, diagnosis can be misleading. So, for any abdominopelvic mass detected on ultrasonography and with unusual presentation, the possibility of non-gynecological tumors especially GISTs should be kept in mind. There are therapeutic and prognostic implications of misdiagnoses of GISTs as gynecological tumors.
Conflicts of interest
There are no conflicts of interest.
Funding Statement
Nil
References
- 1.Karaca N, Akpak YK, Tatar Z, Batmaz G, Erken A. Gastrointestinal stromal tumor: May mimic adnexal mass. Glob J Health Sci. 2016;8:20–6. doi: 10.5539/gjhs.v8n2p20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van der Zwan SM, DeMatteo RP. Gastrointestinal stromal tumor:5 years later. Cancer. 2005;104:1781–8. doi: 10.1002/cncr.21419. [DOI] [PubMed] [Google Scholar]
- 3.Boldorini R, Tosoni A, Leutner M, Ribaldone R, Surico N, Comello E, et al. Multiple small intestinal stromal tumours in a patient with previously unrecognised neurofibromatosis type 1: Immunohistochemical and ultrastructural evaluation. Pathology. 2001;33:390–5. [PubMed] [Google Scholar]
- 4.Pierie JP, Choudry U, Muzikansky A, Yeap BY, Souba WW, Ott MJ. The effect of surgery and grade on outcome of gastrointestinal stromal tumors. Arch Surg. 2001;136:383–9. doi: 10.1001/archsurg.136.4.383. [DOI] [PubMed] [Google Scholar]
- 5.D'amato G, Steinert DM, McAuliffe JC, Trent JC. Update on the biology and therapy of gastrointestinal stromal tumors. Cancer Control. 2005;12:44–56. doi: 10.1177/107327480501200106. [DOI] [PubMed] [Google Scholar]
- 6.Gupta P, Tewari M, Shukla HS. Gastrointestinal stromal tumor. Surg Oncol. 2008;17:129–38. doi: 10.1016/j.suronc.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 7.Hsu S, Chen SS, Chen YZ. Gastrointestinal stromal tumors presenting as gynecological tumors. Eur J Obstet Gynecol Reprod Biol. 2006;125:139–40. doi: 10.1016/j.ejogrb.2005.07.027. [DOI] [PubMed] [Google Scholar]
- 8.Belics Z, Csapó Z, Szabó I, Pápay J, Szabó J, Papp Z. Large gastrointestinal stromal tumor presenting as an ovarian tumor. A case report. J Reprod Med. 2003;48:655–8. [PubMed] [Google Scholar]
- 9.Eisenberg BL, Pipas JM. Gastrointestinal stromal tumor—background, pathology, treatment. Hematol Oncol Clin. 2012;26:1239–59. doi: 10.1016/j.hoc.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 10.Fletcher CD, Berman JJ, Corless C, Gorstein F, Lasota J, Longley BJ, et al. Diagnosis of gastrointestinal stromal tumors: A consensus approach. Int J Surg Pathol. 2002;10:81–9. doi: 10.1177/106689690201000201. [DOI] [PubMed] [Google Scholar]
- 11.Miettinen M, Lasota J. Gastrointestinal stromal tumors–definition, clinical, histological, immunohistochemical, and molecular genetic features and differential diagnosis. Virchows Archiv. 2001;438:1–12. doi: 10.1007/s004280000338. [DOI] [PubMed] [Google Scholar]
- 12.Zighelboim I, Henao G, Kunda A, Gutierrez C, Edwards C. Gastrointestinal stromal tumor presenting as a pelvic mass. Gynecol Oncol. 2003;91:630–5. doi: 10.1016/j.ygyno.2003.08.025. [DOI] [PubMed] [Google Scholar]
- 13.Ghanem N, Altehoefer C, Furtwängler A, Winterer J, Schäfer O, Springer O, et al. Computed tomography in gastrointestinal stromal tumors. Eur Radiol. 2003;13:1669–78. doi: 10.1007/s00330-002-1803-6. [DOI] [PubMed] [Google Scholar]
- 14.Amano M, Okuda T, Amano Y, Tajiri T, Kumazaki T. Magnetic resonance imaging of gastrointestinal stromal tumor in the abdomen and pelvis. Clin Imaging. 2006;30:127–31. doi: 10.1016/j.clinimag.2005.09.025. [DOI] [PubMed] [Google Scholar]
- 15.Enodien B, Hendie D, Müller T, Taha-Mehlitz S, Frey DM, Taha A. Gastrointestinal stromal tumor (GIST): A remarkable case report and literature review. Cureus. 2023;15:e35931. doi: 10.7759/cureus.35931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Demetri GD, Benjamin RS, Blanke CD, Blay JY, Casali, Choi H, et al. NCCN Task Force report: Management of patients with gastrointestinal stromal tumor (GIST)—update of the NCCN clinical practice guidelines. J Natl Compr Cancer Netw. 2007;5:S1–29. [PubMed] [Google Scholar]


