Abstract
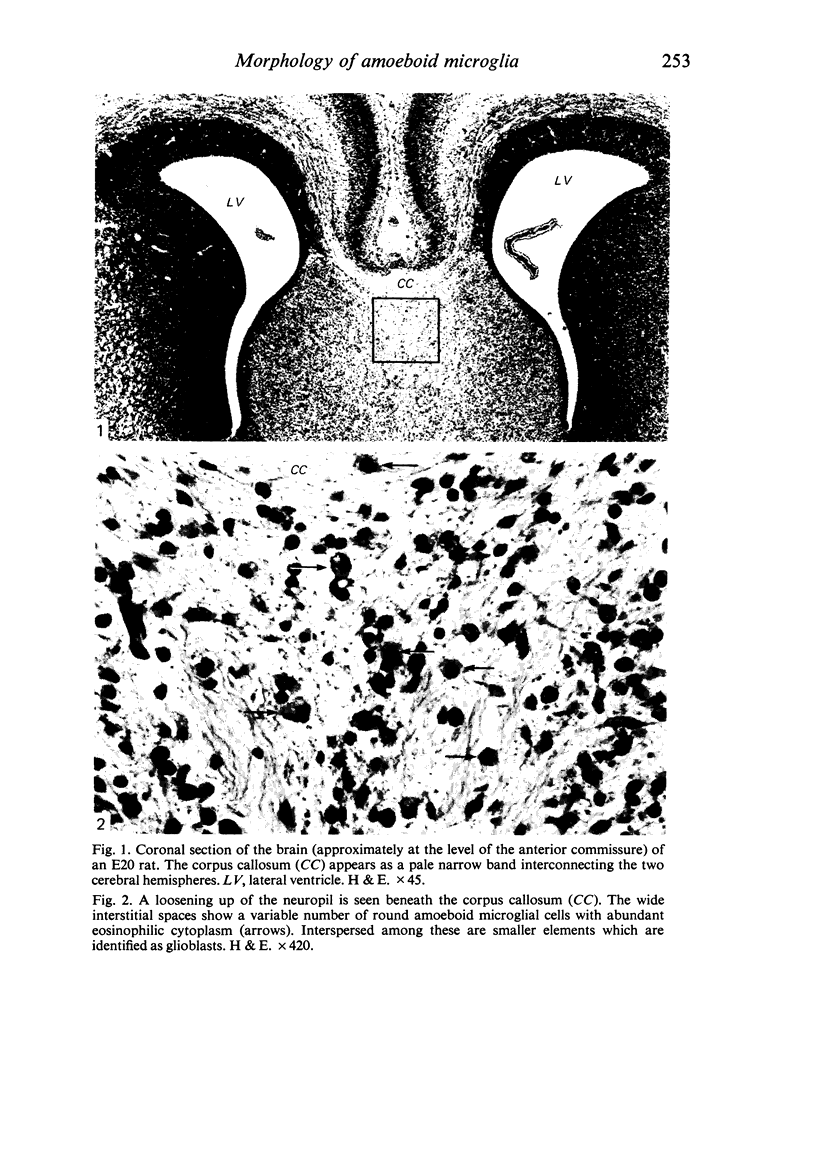
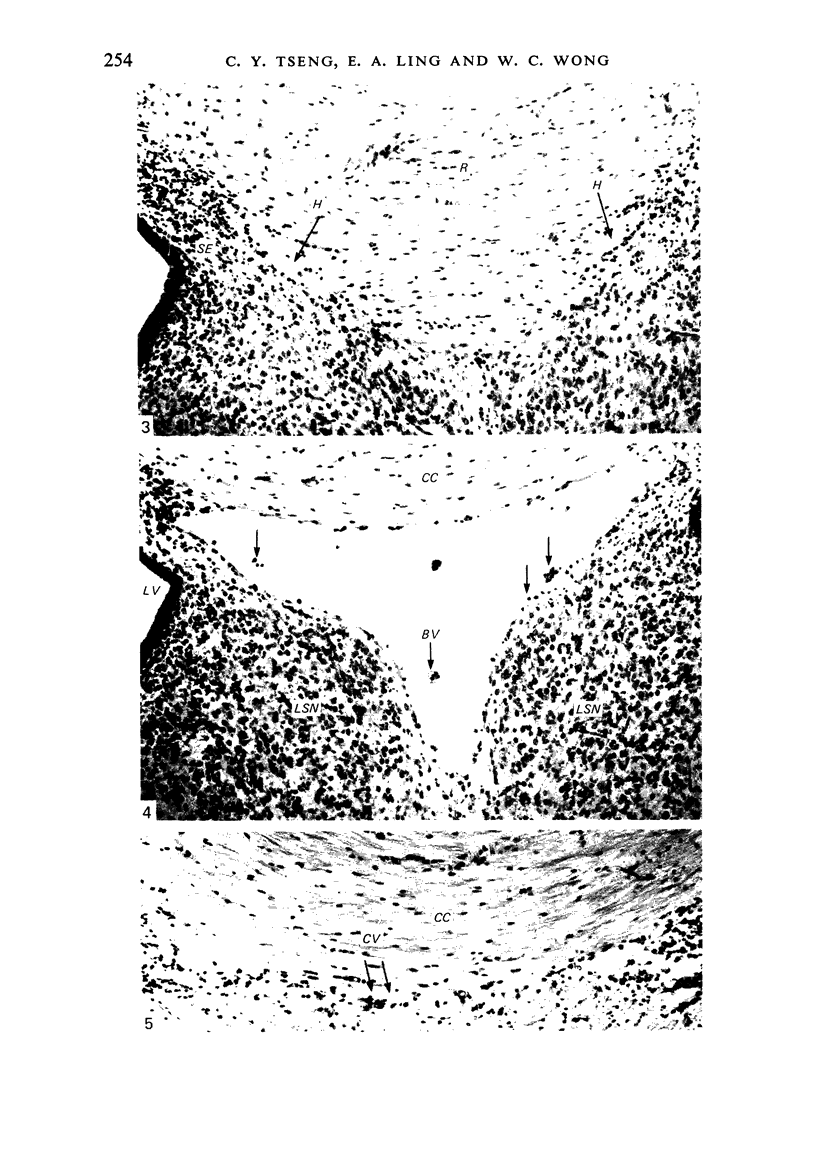
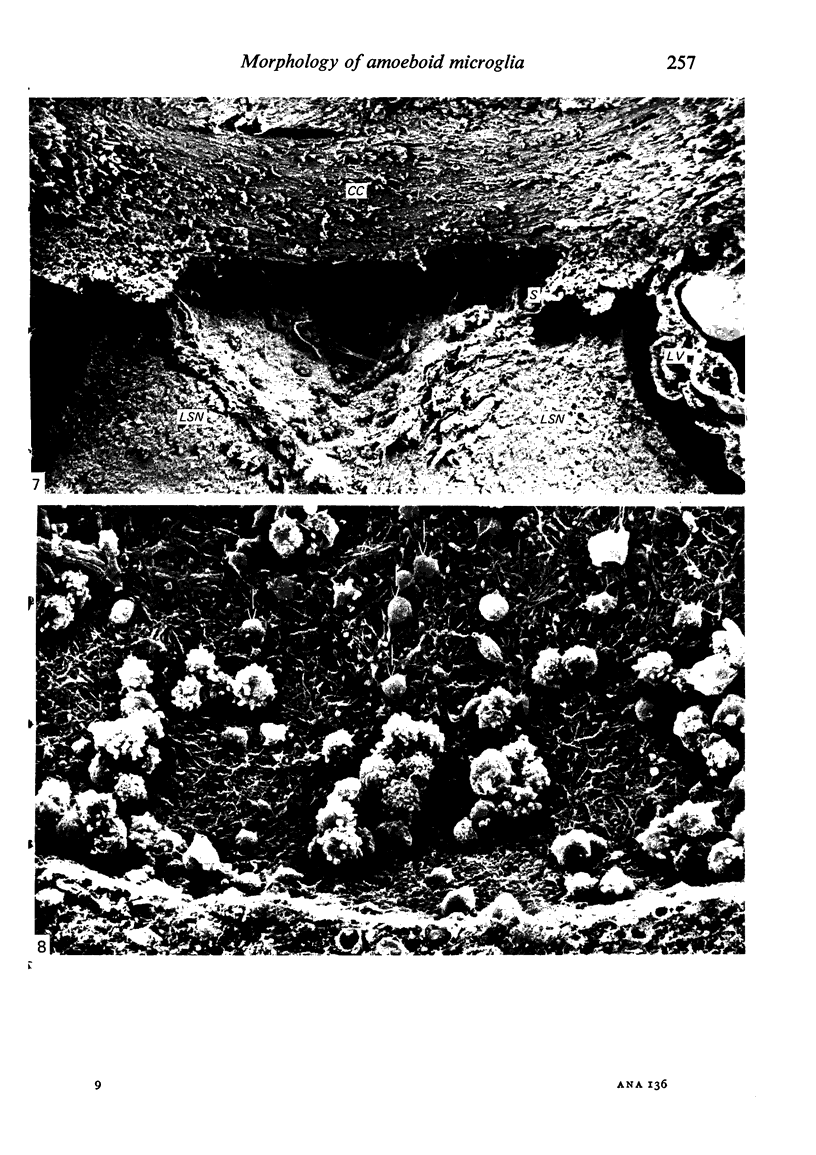
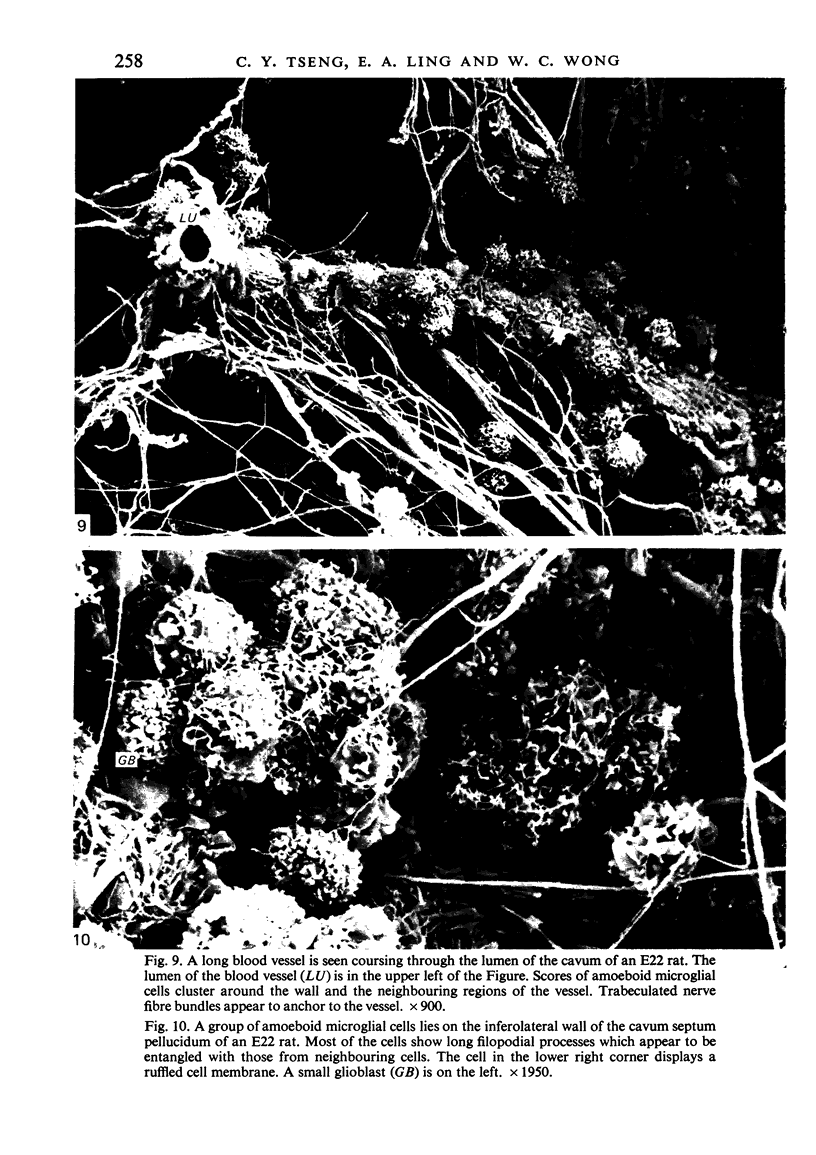
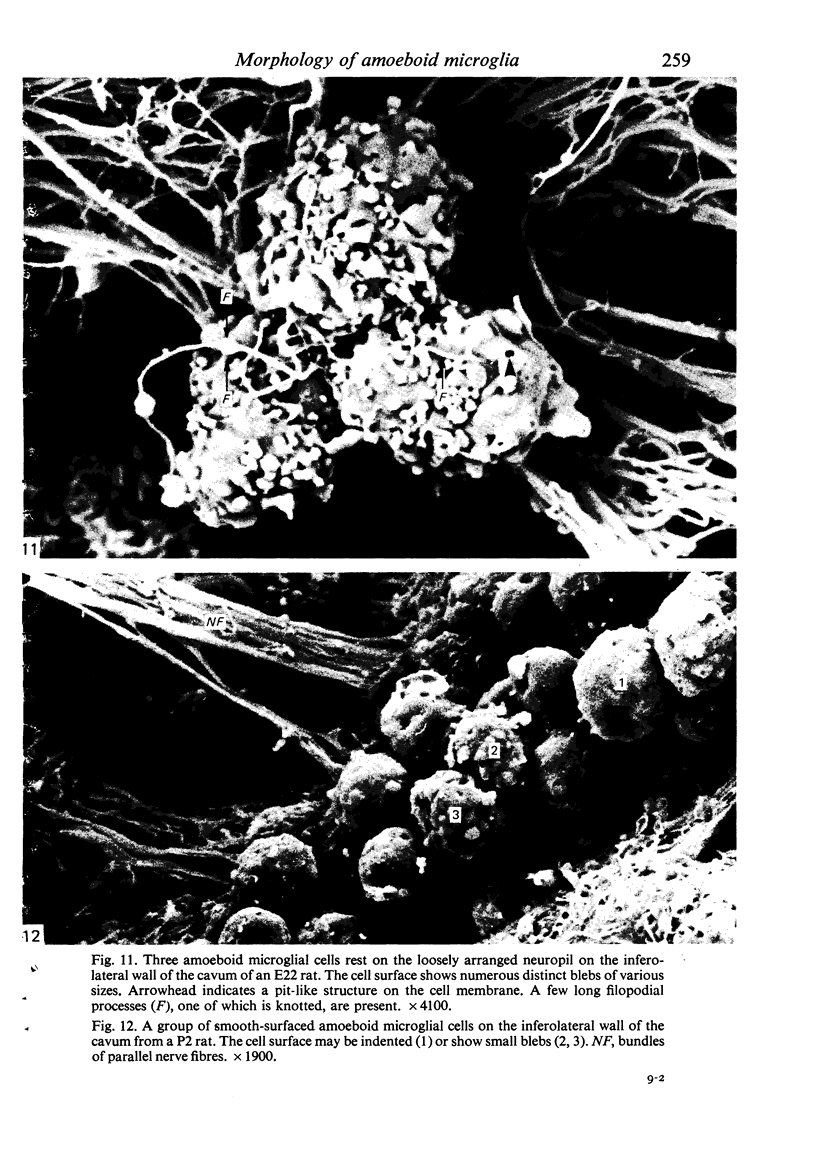
The cavum septum pellucidum in rats of different ages was studied by light and scanning electron microscopy. A reconstruction from serial paraffin sections showed that the cavum was a pyramidal shaped closed cavity which was bounded above by the corpus callosum and inferolaterally by the lateral septal nuclei. The first sign of the cavum formation was noted in the 20 days post conception rat where there was a loosening up of the neuropil beneath the corpus callosum deep to the longitudinal fissure. A variable number of amoeboid microglial cells, characterised by their abundant eosinophilic cytoplasm, was seen among the smaller immature cells in the wide interstitial spaces. A definitive cavity was formed in the 21 days post conception rat and it continued to grow until the fifth postnatal day when it gradually diminished in size to become slit-like by the fifteenth postnatal day. The scanning electron microscope showed that the wall of the cavum was composed of a feltwork of glial and nerve fibres. Two types of cells were present in the cavum: cells identified as glioblasts, and amoeboid microglial cells. The glioblasts were were characterised by having a smooth cell body with radiating long processes. The amoeboid microglial cells showed diverse forms of surface protrusions: blebs, filopodia and membrane ruffles similar to other tissue macrophages. They were either adherent to the walls of the cavum, clustered around the blood vessel which traversed the cavum, or floating freely in the lumen. It was suggested that the amoeboid microglial cells were probably derived from extravasated blood monocytes in response to the physical damage resulting from the formation of the cavum septum pellucidum in the developing brain.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen D. J., Low F. N. Scanning electron microscopy of the subarachnoid space in the dog. III. Cranial levels. J Comp Neurol. 1975 Jun 15;161(4):515–539. doi: 10.1002/cne.901610404. [DOI] [PubMed] [Google Scholar]
- Boyd J. D. The occurrence of subependymal cysts during the development of the human cerebellum. Acta Anat Suppl (Basel) 1969;56:80–94. doi: 10.1159/000143325. [DOI] [PubMed] [Google Scholar]
- Choi B. H. Hematogenous cells in the central nervous system of developing human embryos and fetuses. J Comp Neurol. 1981 Mar 10;196(4):683–694. doi: 10.1002/cne.901960412. [DOI] [PubMed] [Google Scholar]
- Cloyd M. W., Low F. N. Scanning electron microscopy of the subarachnoid space in the dog. I. Spinal cord levels. J Comp Neurol. 1974 Feb 15;153(4):325–368. doi: 10.1002/cne.901530402. [DOI] [PubMed] [Google Scholar]
- Coates P. W. Supraependymal cells: light and transmission electron microscopy extends scanning electron microscopic demonstration. Brain Res. 1973 Jul 27;57(2):502–507. doi: 10.1016/0006-8993(73)90157-1. [DOI] [PubMed] [Google Scholar]
- Deimann W., Fahimi H. D. The appearance of transition forms between monocytes and Kupffer cells in the liver of rats treated with glucan. A cytochemical and ultrastructural study. J Exp Med. 1979 Apr 1;149(4):883–897. doi: 10.1084/jem.149.4.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson C. A., Trinkaus J. P. Microvilli and blebs as sources of reserve surface membrane during cell spreading. Exp Cell Res. 1976 May;99(2):375–384. doi: 10.1016/0014-4827(76)90595-4. [DOI] [PubMed] [Google Scholar]
- Imamoto K., Leblond C. P. Radioautographic investigation of gliogenesis in the corpus callosum of young rats. II. Origin of microglial cells. J Comp Neurol. 1978 Jul 1;180(1):139–163. doi: 10.1002/cne.901800109. [DOI] [PubMed] [Google Scholar]
- Ling E. A., Kaur C., Wong W. C. Light and electron microscopic demonstration of non-specific esterase in amoeboid microglial cells in the corpus callosum in postnatal rats: a cytochemical link to monocytes. J Anat. 1982 Sep;135(Pt 2):385–394. [PMC free article] [PubMed] [Google Scholar]
- Ling E. A. Light and electron microscopic demonstration of some lysosomal enzymes in the amoeboid microglia in neonatal rat brain. J Anat. 1977 Jul;123(Pt 3):637–648. [PMC free article] [PubMed] [Google Scholar]
- Ling E. A., Penney D., Leblond C. P. Use of carbon labeling to demonstrate the role of blood monocytes as precursors of the 'ameboid cells' present in the corpus callosum of postnatal rats. J Comp Neurol. 1980 Oct 1;193(3):631–657. doi: 10.1002/cne.901930304. [DOI] [PubMed] [Google Scholar]
- Ling E. A. Some aspects of amoeboid microglia in the corpus callosum and neighbouring regions of neonatal rats. J Anat. 1976 Feb;121(Pt 1):29–45. [PMC free article] [PubMed] [Google Scholar]
- Malloy J. J., Low F. N. Scanning electron microscopy of the subarachnoid space in the dog. IV. Subarachnoid macrophages. J Comp Neurol. 1976 Jun 1;167(3):257–283. doi: 10.1002/cne.901670302. [DOI] [PubMed] [Google Scholar]
- Merchant R. E., Low F. N. Scanning electron microscopy of the subarachnoid space in the dog. V. Macrophages challenged by bacillus Calmette-Guerin. J Comp Neurol. 1977 Apr 1;172(3):381–407. doi: 10.1002/cne.901720302. [DOI] [PubMed] [Google Scholar]
- Parakkal P., Pinto J., Hanifin J. M. Surface morphology of human mononuclear phagocytes during maturation and phagocytosis. J Ultrastruct Res. 1974 Aug;48(2):216–226. doi: 10.1016/s0022-5320(74)80078-x. [DOI] [PubMed] [Google Scholar]
- Peters A. The surface fine structure of the choroid plexus and ependymal lining of the rat lateral ventricle. J Neurocytol. 1974 Mar;3(1):99–108. doi: 10.1007/BF01111935. [DOI] [PubMed] [Google Scholar]
- Polliack A., Gordon S. Scanning electron microscopy of murine macrophages. Surface characteristics during maturation, activation, and phagocytosis. Lab Invest. 1975 Nov;33(5):469–477. [PubMed] [Google Scholar]
- Polliack A. The contribution of scanning electron microscopy in haematology: its role in defining leucocyte and erythrocyte disorders. J Microsc. 1981 Aug;123(Pt 2):177–187. doi: 10.1111/j.1365-2818.1981.tb01293.x. [DOI] [PubMed] [Google Scholar]
- Roessmann U., Friede R. L. Entry of labeled monocytic cells into the central nervous system. Acta Neuropathol. 1968 Jun 7;10(4):359–362. doi: 10.1007/BF00690711. [DOI] [PubMed] [Google Scholar]
- Sievers J., Abele D., Mangold U. Transitory subependymal cysts in the developing rat rhombencephalon. Anat Embryol (Berl) 1981;161(4):433–451. doi: 10.1007/BF00316053. [DOI] [PubMed] [Google Scholar]
- Stensaas L. J., Reichert W. H. Round and amoeboid microglial cells in the neonatal rabbit brain. Z Zellforsch Mikrosk Anat. 1971;119(2):147–163. doi: 10.1007/BF00324517. [DOI] [PubMed] [Google Scholar]
- Sturrock R. R. A semithin light microscopic, transmission electron microscopic and scanning electron microscopic study of macrophages in the lateral ventricle of mice from embryonic to adult life. J Anat. 1979 Aug;129(Pt 1):31–44. [PMC free article] [PubMed] [Google Scholar]
- Thompson I. M. On the Cavum Septi Pellucidi. J Anat. 1932 Oct;67(Pt 1):59–77. [PMC free article] [PubMed] [Google Scholar]
- Warfel A. H., Elberg S. S. Macrophage membranes viewed through a scanning electron microscope. Science. 1970 Oct 23;170(3956):446–447. doi: 10.1126/science.170.3956.446. [DOI] [PubMed] [Google Scholar]













