Abstract
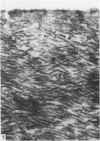
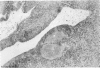
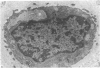
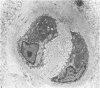
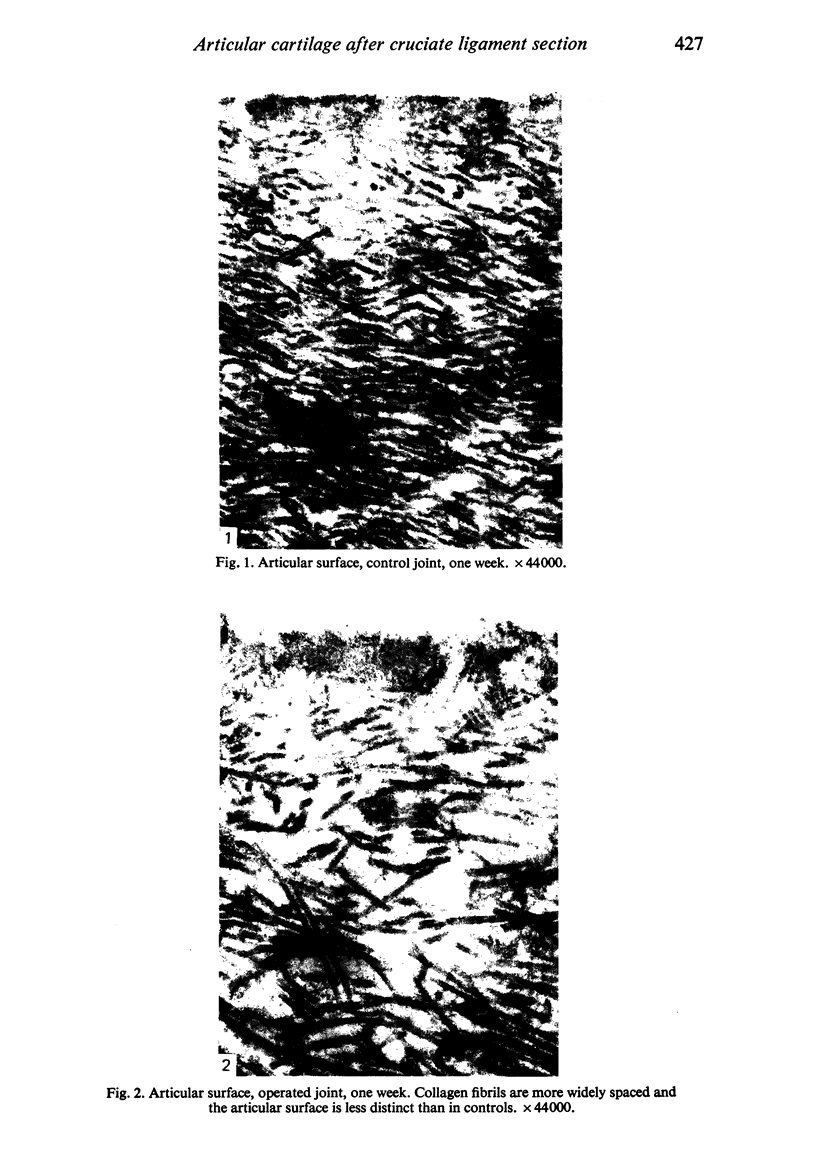
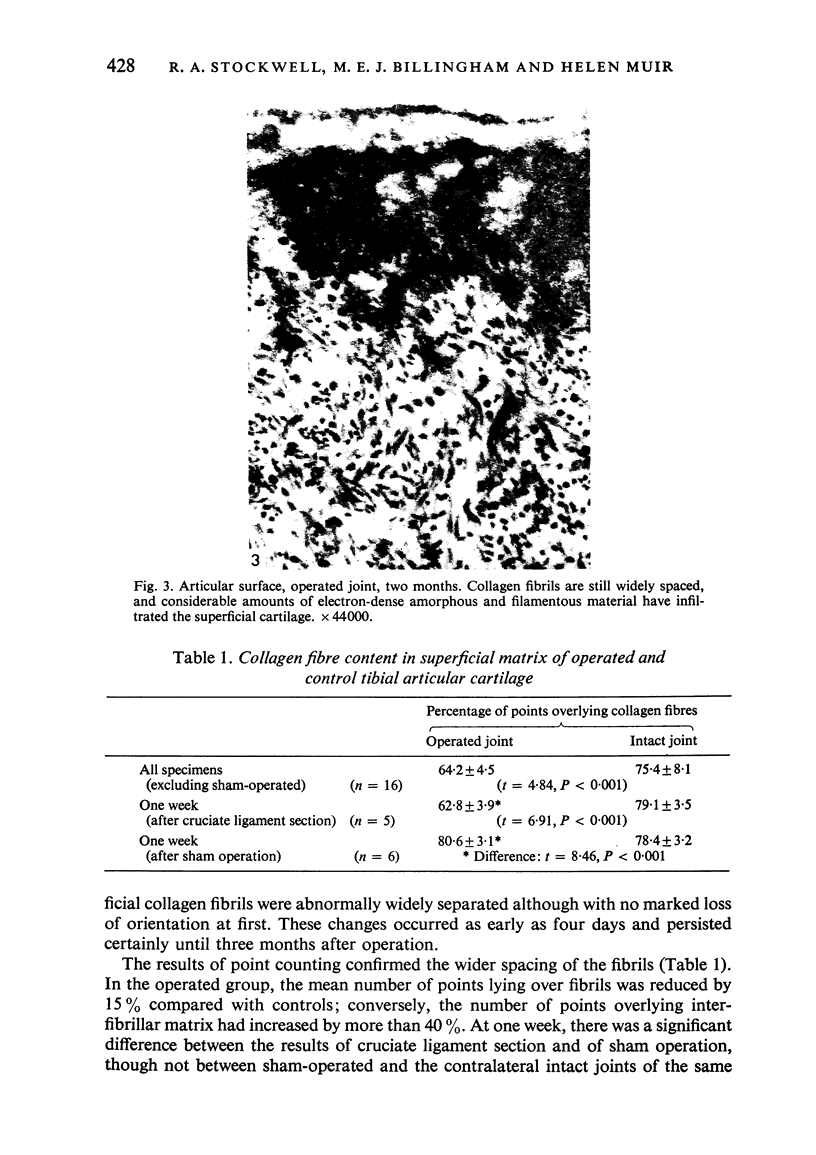
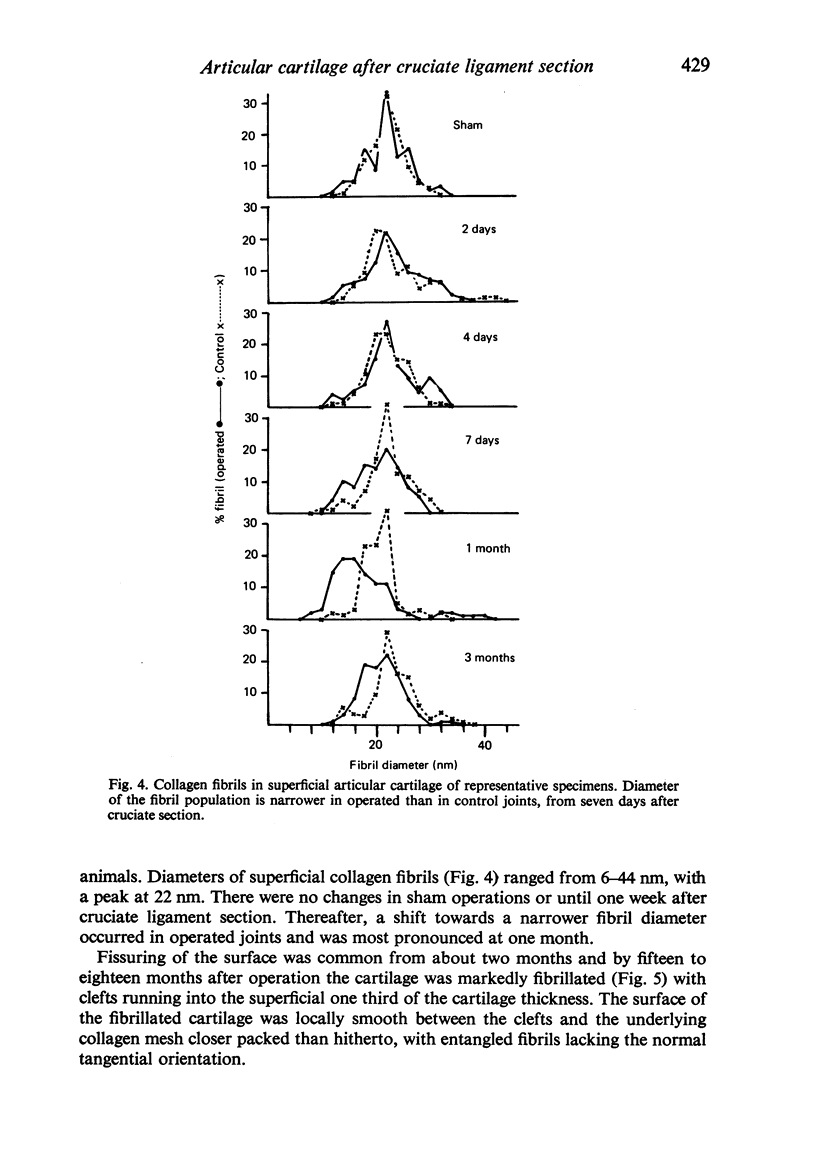
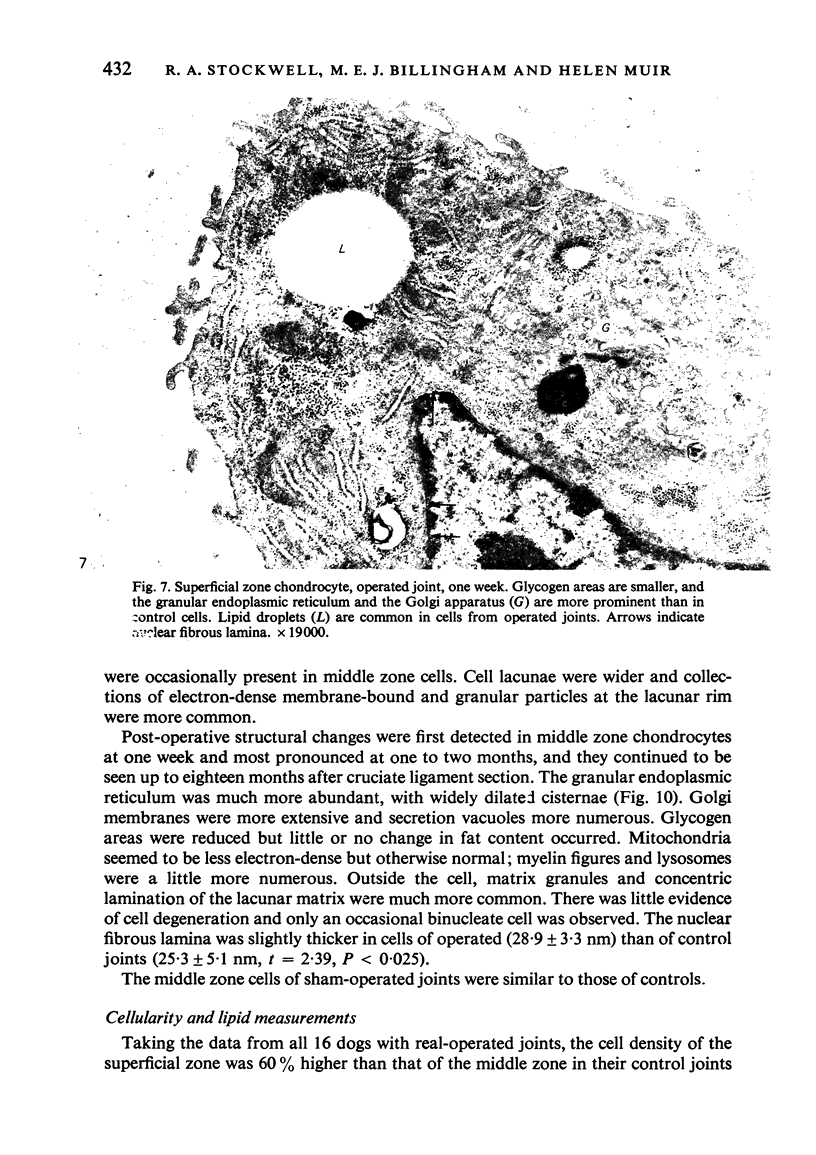
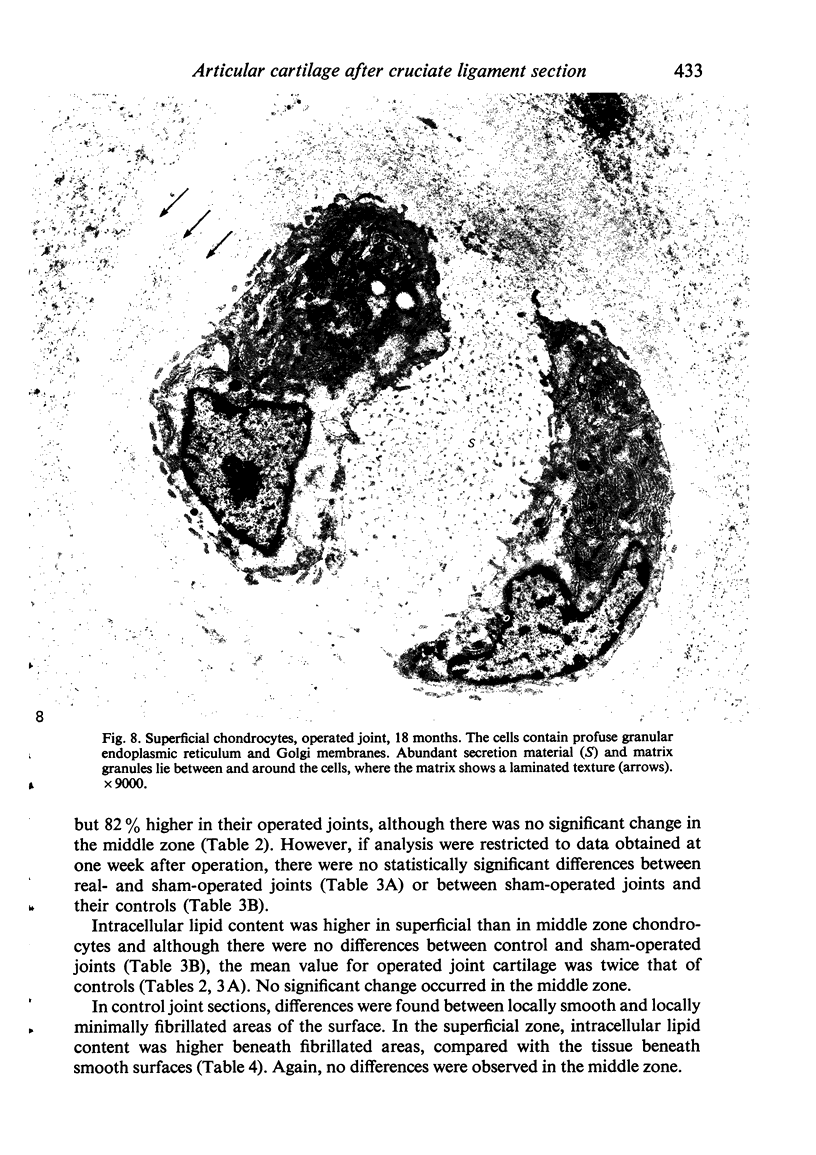
Ultrastructural changes in articular cartilage were studied in joint laxity induced by severing the anterior cruciate ligament of the right knee in sixteen mature dogs. The left knees provided controls; sham operations on six other dogs consisted of stab incision only, leaving the ligament intact. Cartilage from the medial tibial condyles was examined at intervals from two days to eighteen months later. In the superficial zone of the cartilage, collagen fibrils became abnormally widely spaced at four days, and narrower fibrils appeared from seven days after operation. Chondrocytes, particularly in the middle zone, became more active, with hypertrophy of cytoplasmic organelles detectable from four days. Superficial cells were initially healthy and became more numerous while their lipid content increased. The articular surface was fissured from two months and cell degeneration was rarely seen until several months after operation. These findings correlate with previous biochemical studies and are similar to early changes noted in degeneration of human articular cartilage.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOLE G. G. Synovial fluid lipids in normal individuals and patients with rheumatoid arthritis. Arthritis Rheum. 1962 Dec;5:589–601. doi: 10.1002/art.1780050606. [DOI] [PubMed] [Google Scholar]
- Eyre D. R., McDevitt C. A., Billingham M. E., Muir H. Biosynthesis of collagen and other matrix proteins by articular cartilage in experimental osteoarthrosis. Biochem J. 1980 Jun 15;188(3):823–837. doi: 10.1042/bj1880823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fell H. B., Jubb R. W. The effect of synovial tissue on the breakdown of articular cartilage in organ culture. Arthritis Rheum. 1977 Sep-Oct;20(7):1359–1371. doi: 10.1002/art.1780200710. [DOI] [PubMed] [Google Scholar]
- Ghadially F. N., Dick C. E., Lalonde J. M. Thickening of the nuclear fibrous lamina in injured human semilunar cartilages. J Anat. 1980 Dec;131(Pt 4):717–722. [PMC free article] [PubMed] [Google Scholar]
- Ghadially F. N., Mehta P. N., Kirkaldy-Willis W. H. Ultrastructure of articular cartilage in experimentally produced lipoarthrosis. J Bone Joint Surg Am. 1970 Sep;52(6):1147–1158. [PubMed] [Google Scholar]
- Gilbertson E. M. Development of periarticular osteophytes in experimentally induced osteoarthritis in the dog. A study using microradiographic, microangiographic, and fluorescent bone-labelling techniques. Ann Rheum Dis. 1975 Feb;34(1):12–25. doi: 10.1136/ard.34.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ITO S., WINCHESTER R. J. The fine structure of the gastric mucosa in the bat. J Cell Biol. 1963 Mar;16:541–577. doi: 10.1083/jcb.16.3.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson S. F. Environmental control of macromolecular synthesis in cartilage and bone: morphogenetic response to hyaluronidase. Proc R Soc Lond B Biol Sci. 1970 Sep 29;175(1041):405–453. doi: 10.1098/rspb.1970.0029. [DOI] [PubMed] [Google Scholar]
- Light N. D., Bailey A. J. Polymeric C-terminal cross-linked material from type-I collagen. A modified method for purification, anomalous behaviour on gel filtration, molecular weight estimation, carbohydrate content and lipid content. Biochem J. 1980 Jul 1;189(1):111–124. doi: 10.1042/bj1890111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCELLIGOTT T. F., POTTER J. L. Increased fixation of sulfur-35 by cartilage in vitro following depletion of the matrix by intravenous papain. J Exp Med. 1960 Nov 1;112:743–750. doi: 10.1084/jem.112.5.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mankin H. J., Lippiello L. Biochemical and metabolic abnormalities in articular cartilage from osteo-arthritic human hips. J Bone Joint Surg Am. 1970 Apr;52(3):424–434. [PubMed] [Google Scholar]
- Maroudas A. I. Balance between swelling pressure and collagen tension in normal and degenerate cartilage. Nature. 1976 Apr 29;260(5554):808–809. doi: 10.1038/260808a0. [DOI] [PubMed] [Google Scholar]
- McDevitt C. A., Muir H. Biochemical changes in the cartilage of the knee in experimental and natural osteoarthritis in the dog. J Bone Joint Surg Br. 1976 Feb;58(1):94–101. doi: 10.1302/0301-620X.58B1.131804. [DOI] [PubMed] [Google Scholar]
- McDevitt C., Gilbertson E., Muir H. An experimental model of osteoarthritis; early morphological and biochemical changes. J Bone Joint Surg Br. 1977 Feb;59(1):24–35. doi: 10.1302/0301-620X.59B1.576611. [DOI] [PubMed] [Google Scholar]
- Meachim G., Sheffield S. R. Surface ultrastructure of mature adult human articular cartilage. J Bone Joint Surg Br. 1969 Aug;51(3):529–539. [PubMed] [Google Scholar]
- Meats J. E., McGuire M. B., Russell R. G. Human synovium releases a factor which stimulates chondrocyte production of PGE and plasminogen activator. Nature. 1980 Aug 28;286(5776):891–892. doi: 10.1038/286891a0. [DOI] [PubMed] [Google Scholar]
- Muir H. Heberden Oration, 1976. Molecular approach to the understanding of osteoarthrosis. Ann Rheum Dis. 1977 Jun;36(3):199–208. doi: 10.1136/ard.36.3.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevo Z., Dorfman A. Stimulation of chondromucoprotein synthesis in chondrocytes by extracellular chondromucoprotein. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2069–2072. doi: 10.1073/pnas.69.8.2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pond M. J., Nuki G. Experimentally-induced osteoarthritis in the dog. Ann Rheum Dis. 1973 Jul;32(4):387–388. doi: 10.1136/ard.32.4.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprinz R., Stockwell R. A. Changes in articular cartilage following intraarticular injection of tritiated glyceryl trioleate. J Anat. 1976 Sep;122(Pt 1):91–112. [PMC free article] [PubMed] [Google Scholar]
- Stockwell R. A., Sprinz R. Glycosaminoglycan content and cell density of rabbit articular cartilage in experimental lipoarthrosis. J Anat. 1981 Sep;133(Pt 2):309–315. [PMC free article] [PubMed] [Google Scholar]
- Stockwell R. A. The interrelationship of cell density and cartilage thickness in mammalian articular cartilage. J Anat. 1971 Sep;109(Pt 3):411–421. [PMC free article] [PubMed] [Google Scholar]
- Weibel E. R., Kistler G. S., Scherle W. F. Practical stereological methods for morphometric cytology. J Cell Biol. 1966 Jul;30(1):23–38. doi: 10.1083/jcb.30.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss C., Mirow S. An ultrastructural study of osteoarthritis changes in the articular cartilage of human knees. J Bone Joint Surg Am. 1972 Jul;54(5):954–972. [PubMed] [Google Scholar]
- Wiebkin O. W., Muir H. Influence of the cells on the pericellular environment. The effect of hyaluronic acid on proteoglycan synthesis and secretion by chondrocytes of adult cartilage. Philos Trans R Soc Lond B Biol Sci. 1975 Jul 17;271(912):283–291. doi: 10.1098/rstb.1975.0053. [DOI] [PubMed] [Google Scholar]