Abstract
Background: Two-stage revision is known as the gold-standard method for knee prosthetic joint infection (PJI), but the most suitable treatment method remains controversial. Typically, weight-bearing is restricted during the interval between the stages. The aim of this study was to evaluate the clinical outcomes of unrestricted weight bearing with cement spacers fabricated using the Knee Articulating Spacer Mold (KASM®; Ortho Development Corporation, Draper, UT, USA) for knee PJI.
Methods: We retrospectively reviewed 16 patients who underwent two-stage revision surgery for knee PJI between April 2015 and March 2020. The procedure involved the removal of the infected prosthetic joints and the insertion of cement spacers made using KASM®. The evaluation focused on the possibility of full-weight bearing gait during the interval between the first and second stages, surgical time, blood loss, complications, and postoperative outcomes, including the Knee Society Score (KSS), Knee Society Function Score, and range of motion (ROM).
Results: All patients were able to walk with full weight-bearing. However, cement spacer dislocation occurred in one patient (6.3%). During the interval between stages, infection occurred in one patient (6.3%) and debridement was performed. Average interval between the stages was 92.7 days (range, 55-166 days). After the reimplantation, reinfection occurred in two patients (12.5%) out of the 16. Among the 14 patients with successful reimplantation, the average operative time was 116.1 min (range, 76-153 min) and the average perioperative blood loss was 476.1 mL (range, 89.5-859 mL). The KSS was 86.4 (range, 62-100), the Knee Society Function Score was 73.6 (range, 45-100), and flexion ROM was 111.8° (range, 95°-130°) at the latest follow-up. The mean follow-up period was 871 days (range, 117-1103 days).
Conclusions: Unrestricted weight-bearing gait using cement spacers during the waiting period for two-stage revision surgery for knee PJI led to favorable outcomes in this case series, which lacked a control group. Further studies are needed to assess whether the benefits of weight-bearing outweigh the risks and improve overall outcomes.
Keywords: articulating spacer, full weight-bearing, prosthetic joint infection, total knee arthroplasty, two-stage revision
Introduction
Prosthetic joint infection (PJI) is one of the most serious complications of knee arthroplasty, with a reported incidence of 0.5%-2.0% [1]. Although several studies have been conducted, there is still no consensus on the most suitable treatment method.
In the commonly used two-stage revision surgery for knee PJI, a cement spacer is generally implanted in the first stage. To date, there have been several reports on the therapeutic outcomes following two-stage revision using static spacers [2] and articulating spacers including cement-on-cement [3], cement-on-polyethylene [4], and metal-on-polyethylene spacers [5,6]. Articulating spacers confer mobility to the affected joint during the first stage of revision, which is their major advantage. Moderate range of motion (ROM) exercises are effective for maintaining the length and elasticity of the extensor mechanism, which is useful for preventing scarring of the soft tissue surrounding the joint, shortening of the quadriceps muscles, and hardening and contraction of the articular capsule [2,7,8]. However, weight-bearing during the interval period between stages is commonly restricted. This restriction poses a significant challenge, particularly for elderly or mobility-compromised patients, as prolonged weight-bearing restrictions can result in muscle weakness, deconditioning, and loss of independence. The stability is critical in enabling weight-bearing.
Various types of spacers are employed in two-stage revision surgery, including handcrafted spacers [3,9], those produced with metal molds [10], and those created using silicone molds [11,12]. We have used a type of cement-on-cement articulating antibiotic-loaded spacer (Knee Articulating Spacer Molds, KASM®; Ortho Development Corporation, Draper, UT, USA) for two-stage revision procedures since 2015. The KASM® spacer stands out due to its unique design and functional advantages. Unlike most femoral spacers, which typically feature a closed design, the KASM® spacer incorporates an open femoral mold that can be directly compressed onto the femur. This design facilitates the accommodation of irregularities or defects in the femoral bone, ensuring a secure fit and improved stability. The silicone-based tibial mold further enhances the spacer’s adaptability by allowing preoperative or intraoperative adjustments. Spacer thickness can be precisely tailored to match the joint gap, such as the extension or flexion gap, optimizing joint space and soft tissue balance. For patients with significant extension gaps, additional cement can be applied beneath the tibial mold to adjust joint alignment and improve stability. Furthermore, the KASM® includes three sizes for both femoral and tibial components, enabling it to accommodate a wide variety of knee joint anatomies. These features collectively support unrestricted weight-bearing between stages while maintaining ROM, proper joint space, and soft tissue balance.
The present study aimed to elucidate the achieving full weight-bearing gait and clinical outcomes with implantation of cement spacers constructed by using KASM during the waiting period of two-stage revision surgery for knee PJI.
Materials and methods
This retrospective study was approved by the local ethics committee, and all analyses were performed after obtaining permission from the Institutional Review Board of the authors' institution (Yamaguchi Prefectural Grand Medical Center, Approval No. 2020-J029). Informed consent was obtained from each patient. We investigated 23 patients who underwent surgery for knee PJI, involving the removal of their prosthetic joints and the insertion of cement spacers created using KASM® as part of a two-stage revision procedure. Data were obtained from the electronic medical records. Five cases with a postoperative follow-up period after reimplantation of less than three months were excluded. Two patients, deemed high-risk for infection due to advanced age and significant medical comorbidities that rendered highly invasive surgery unfavorable, underwent only the first stage of the two-stage revision with cement spacer retention and were excluded from the study. As a result, the final study group consisted of 16 patients. There were 15 total knee arthroplasty (TKA) infections and one unicompartmental knee arthroplasty (UKA) infection. The patients included seven males and nine females with a mean age of 73.1 years (range, 58-94). The mean heights, body weights, and body mass indices (BMI) were 155.5 cm (range, 138-180 cm), 61.5 kg (range, 47.1-90.5 kg), and 25.4 (range, 19.3-34.9), respectively. No patients with bilateral infections were included in the present study. Considering comorbidities, one patient had diabetes mellitus, and three patients had rheumatoid arthritis (Table 1).
Table 1. Patient Demographics and Clinical Characteristics.
The data represented as mean ± standard deviation
BMI: body mass index, TKA: total knee arthroplasty, UKA: unicompartmental knee arthroplasty
| Variables | Values |
| Mean Age (years) | 73.1 ± 9.4 |
| Sex | 7 males, 9 females |
| Mean Height (cm) | 155.5 ± 10.7 |
| Mean Body Weight (kg) | 61.5 ± 12.7 |
| Mean BMI (kg/m2) | 25.4 ± 4.1 |
| Comorbidities | 1 Diabetes mellitus, 3 Rheumatoid arthritis |
| Type of prothesis | 15 TKA, 1 UKA |
The duration from the initial knee surgery to diagnosis of PJI was 27.7 months (range, 27 days-105.6 months).
The organisms identified were as follows: methicillin-sensitive Staphylococcus aureus (MSSA) in eight cases, methicillin-resistant Staphylococcus aureus (MRSA) in three cases, coagulase-negative staphylococci (CNS) in two cases, Mycobacterium intracellulare in one case, negative cultures in one case, a combination of CNS and Candida glabrata in one case, and a combination of Staphylococcus capitis and S. epidermidis in one case.
Surgical technique for first-stage of revision surgery (cement spacer insertion)
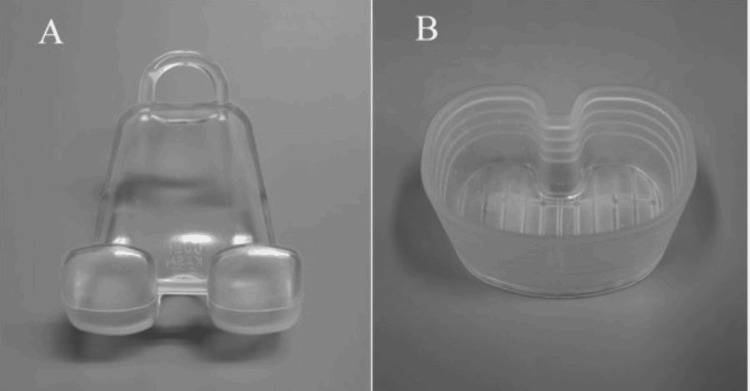
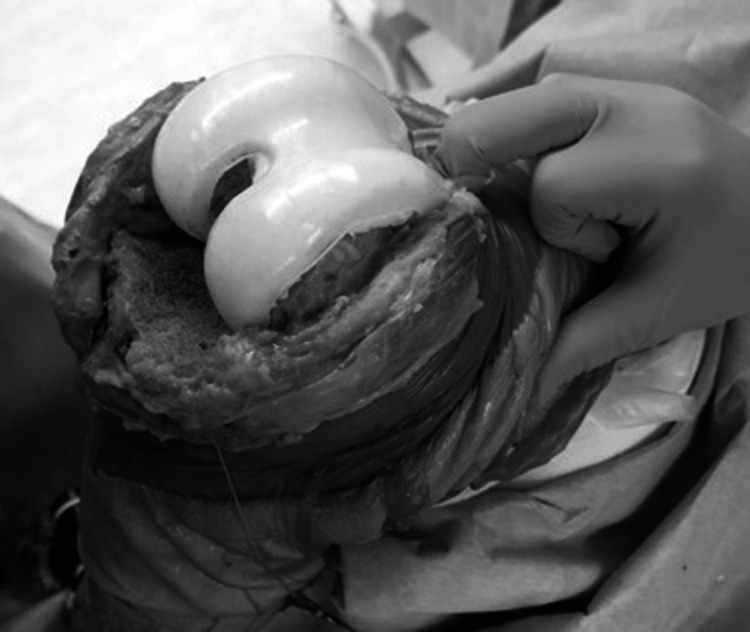
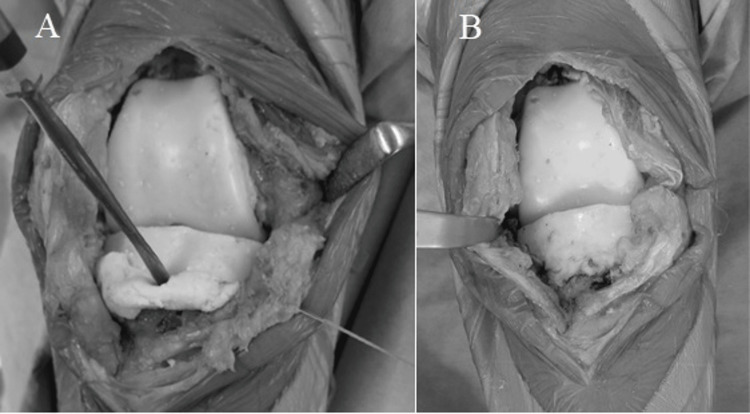
In all cases, the surgery was performed with a tourniquet, with the patient in the supine position. All components of the prosthetic, cement, surrounding fragile soft tissue, and bone were removed as far as possible through a medial parapatellar approach. In case of UKA infection, femoral and tibial osteotomy was required to insert the cement spacers. The appropriate size of the spacer molds (Figure 1) was determined based on the removed components and the shape of the osteotomy site on the femoral and tibial sides. At least three 40 g bags of cement (Simplex P Bone Cement; Stryker, Limerick, Ireland), each containing 2.0 g of vancomycin, were prepared to restore the bone defect, minimize dead space, and maintain a functional joint. First, before the cement was fully cured (late dough phase), the mold was directly placed on the femur (Figure 2). Subsequently, the mold for the tibia side was hardened in advance (Figure 3), and cement was added below the space of the tibial mold to attain joint gap balance (Figure 4). After suture closure of the bursa with monofilament, 2.0 g tranexamic acid and 40 mg gentamicin were injected without leakage.
Figure 1. A photograph of a femoral mold (A) and a tibial mold (B) of Knee Articulating Spacer Mold (KASM®).
Figure 2. A photograph of the femoral mold being placed directly on the femur.
Figure 3. A photograph of the tibia side spacer, which was harden in advance with thickness adjusted according to the gap space.
Figure 4. A photograph of the tibial side cement spacer insertion technique.
Additional cement containing antibiotics was used below the tibial mold to fill the gap space at the knee extension position (A). The appearance after the cement spacer was placed, leaving minimal joint gap, is shown in (B).
Postoperative care between the stages
Postoperatively, wheelchair transfer training was initiated early. Weight-bearing was restricted for the first week to allow for wound rest. After that, all patients were allowed to walk with unrestricted weight-bearing gait with knee extension support.
Based on the causative organism and antibiotic sensitivity results, intravenous antibiotics were used for at least four weeks, followed by a minimum of two weeks of oral antibiotics. If no causative organism was detected, antibiotics were selected empirically. This regimen continued until the following conditions were met: the patients' body temperature returned to normal (< 37.0°C), local symptoms and signs had disappeared, and a progressive decline in C-reactive protein (CRP) levels was observed. Antibiotic therapy was discontinued once these criteria were satisfied, based on reports that implementing a drug holiday before reimplantation has a positive impact on the mid-term outcomes of PJI treatment [13]. For patients who did not meet these criteria, oral antibiotic therapy was continued without interruption until reimplantation surgery. Joint aspiration was performed in cases of joint effusion or a significant increase in CRP. In patients with persistently elevated CRP levels but without clinical signs of infection, including joint fluid analysis, and those requiring prolonged cement retention, reimplantation surgery was undertaken with consideration for surgical modifications based on intraoperative findings. If the synovial fluid analysis revealed an elevated cell count or bacterial presence, it was considered a recurrence of infection, and either debridement or replacement of the cement spacer was performed.
Surgical technique for second-stage of revision surgery (reimplantation)
In all cases, an air tourniquet was applied to the femur to minimize intraoperative bleeding. The femoral and tibial cement spacers were meticulously removed. Bone resections were performed using implant guides to achieve a mechanical alignment of 90° to the mechanical axis in the frontal plane for both the femur and tibia. Depending on the extent of bone defects, augments and long stems were employed, and the implants were securely fixed using bone cement. After suturing the bursa with monofilament, 2.0 g of tranexamic acid and 40 mg of gentamicin were injected.
Postoperative management after reimplantation
Postoperatively, the antibiotics that were effective during the first stage were administered intravenously for one week, followed by oral antibiotics for an additional three weeks. Range-of-motion and gait training were initiated in the early postoperative period. In cases of severe localized swelling with blister formation or delayed wound healing, a brief period of rest lasting several days was required; however, no additional specific restrictions were imposed.
Evaluation parameters
Walking ability after cement spacer insertion during the interval between stages was assessed. We considered full weight-bearing achieved if patients could walk unaided, or if they were able to maintain a standing position without assistance, even if they used a cane to prevent falls. Conversely, if walking remained unstable even with a cane, if a walker was required, or if walking was not possible, weight-bearing was classified as partial.
Further, the following items were evaluated in both cement spacer insertion and reimplantation surgeries: occurrence of mechanical complications such as cement spacer or implant fractures, dislocations, loosening, and bone fractures; occurrence of postoperative infections; surgical time; and hidden blood loss (HBL), which was indirectly estimates using formulas based on anthropometric data and laboratory parameters [14,15]. HBL is an indicator used not only to assess intraoperative blood loss but also to evaluate the total blood loss during the perioperative period.
Knee Society Knee Score (KSS), Knee Society Function Score, and knee range of motion (ROM) were evaluated in patients who underwent reimplantation and were followed up for more than six months postoperatively.
Results
All 16 patients were able to achieve full weight-bearing gait during the interval between the first and second stages. Out of 16 patients, one (6.3%) patient (female, 50.6 kg, and BMI 22.3) experienced the complication of cement spacer dislocation due to instability, but no weight-bearing-related complications were observed in other patients. The operative time for the first stage of cement insertion was 106.3 min (range, 62-184 min) and the amount of HBL was 309.8 mL (range, 21.2-1106 mL).
During the interval between the stages, infection was observed in one of 16 (6.3%) patients at 11 days after the cement spacer insertion (Case 4: Table 2). In that patient, cement spacer replacement was not performed. Instead, debridement of the surrounding tissue was carried out, and reimplantation surgery was performed 80 days later. The mean interval between first and second stages of intervention was 92.7 days (range, 55-130 days).
Table 2. Patient characteristics and outcomes in two-stage revision for knee prosthetic joint infection.
M: male, F: female, BMI: body mass index, ROM: range of motion, KSS: Knee Society Score, KS function score: Knee Society function score, MRSA: methicillin-resistant Staphylococcus aureus, CNS: coagulase negative Staphylococcus, MSSA: methicillin-sensitive Staphylococcus aureus, M. intracell: Mycobacterium intracellulare, S. capitis: Staphylococcus capitis, S. epidemidis: Staphylococcus epidermidis
† Follow-up after reimplantation
‡ average flex ROM after reimplantation
| Case no. | Age (year) | Sex | BMI (kg/m2) | Onset of postoperative infection (weeks) | Organism | Interval between stages (days) | Complication during the waiting period | Complication after reimplantation | Follow-up (days)† | Latest outcome | ||
| Flex ROM‡ (degree) | KSS | KS function score | ||||||||||
| 1 | 77 | F | 25.7 | 251.4 | MRSA | 121 | - | Infection | - | - | - | - |
| 2 | 82 | M | 22.6 | 137.6 | MRSA | 166 | - | Infection | - | - | - | - |
| 3 | 58 | M | 23.7 | 149.3 | CNS | 87 | - | - | 879 | 100 | 85 | 65 |
| 4 | 94 | F | 22.3 | 452.6 | Negative | - | Spacer dislocation | - | - | - | - | - |
| - | Negative | 101 | - | - | 1103 | 120 | 89 | 45 | ||||
| 5 | 59 | M | 33.4 | 27 | MSSA | 109 | - | - | 861 | 100 | 80 | 75 |
| 6 | 70 | M | 25.5 | 539 | MSSA | 130 | - | - | 641 | 110 | 82 | 90 |
| 7 | 73 | M | 29.9 | 2.7 | MSSA | 100 | - | - | 449 | 130 | 91 | 70 |
| 8 | 82 | F | 24.3 | 10.6 | MSSA | 91 | - | - | 450 | 100 | 95 | 95 |
| 9 | 83 | F | 23.4 | 20.1 | MSSA | 55 | - | - | 623 | 95 | 84 | 55 |
| 10 | 78 | M | 25.5 | 10.9 | MSSA | 84 | - | - | 357 | 130 | 81 | 100 |
| 11 | 71 | M | 24 | 29.9 | MSSA | 72 | - | - | 153 | 125 | 100 | 70 |
| 12 | 72 | F | 19.3 | 3.9 | MRSA | 91 | Infection | - | 272 | 110 | 92 | 80 |
| 13 | 70 | F | 25.1 | 19.4 | CNS, Candida glabrata | 114 | - | - | 187 | 100 | 62 | 55 |
| 14 | 69 | F | 23.2 | 206 | M.intracell | 80 | - | - | 191 | 110 | 90 | 65 |
| 15 | 63 | F | 34.9 | 18 | S.capitis, S.epidermidis | 85 | - | - | 197 | 125 | 90 | 90 |
| 16 | 68 | F | 23.1 | 23.3 | MSSA | 99 | - | - | 117 | 110 | 88 | 75 |
| Mean ± SD | 73.1 ± 9.4 | - | 25.4 ± 4.1 | 108.0 ± 175.4 | - | 92.7 ± 18.5 | - | - | 871 ± 188.8 | 111.8 ± 11.7 | 86.4 ± 8.6 | 73.6 ± 15.6 |
Before reimplantation, antibiotic use was stopped preoperatively for 48.1 days (range, 7-112 days) in nine patients, whereas oral antibiotics were continued in seven patients. The mean final CRP level before reimplantation was 0.7 mg/dL (range, 0.07-2.62 mg/dL).
In two out of 16 (12.5%) patients, infection occurred after reimplantation (Cases 1 and 2: Table 2). In these cases, the implant was removed, and cement spacer insertion was performed. In the other 14 patients who underwent successful reimplantation after the first stage surgery of cement spacer insertion, the mean flexion ROM was 110.1° (range, 95°-130°), the mean KSS was 86.4 (range, 62-100), and the mean Knee Society Function Score was 73.6 (range, 45-100) at the latest follow-up. The mean follow-up period was 871 days (range, 117-1103 days) after reimplantation. The operative time for the second stage of reimplantation was 116.1 min (range, 76-153 min) and the amount of HBL was 476.1 mL (range, 89.5-859) for the second stage. The general clinical data are presented in Table 2.
Discussion
Insall et al. [16] reported that a recommended interval between first and second stages of intervention of six weeks. However, this period may be further prolonged, which is sufficient to compromise knee joint function and reduce patient activity levels. Given this risk, strategies to optimize patient outcomes during this waiting period are critical. Elderly patients, in particular, are likely to manage better with full weight-bearing gait while awaiting reimplantation [12]. In our series, management with unrestricted weight-bearing and moderate ROM exercises might result in better postoperative outcomes in our series compared to the previously reported outcomes [3,9-12,17-22], despite the high average age and relatively short-term follow-up (Table 3).
Table 3. Recent studies of single / two-stage revision outcome for knee PJI.
PJI: prosthetic joint infection, Single: single stage revision, Two-stage: two-stage revision, KSS: Knee Society Score, ROM: range of motion, NR: not reported, A: articulating spacer, S: static spacer, FWB: full-weight bearing, PWB: partial-weight bearing, NWB: non-weight bearing
| Study | Year | Number of knees | Age (years) | Follow-up (years) | Single/ Two-stage | Spacer (Articulating / Static) | Weight-bearing | Awaiting time for revision | KSS | Knee Society Function score | flex ROM (degree) | Mechanical Complication (%) | Infection (%) |
| Shen et al. [3] | 2010 | 10 | 68.9 | 2.5 | Two | A | PWB | 7.8 months | 83.6 | 75.5 | 96.7 | 2 (20%) | 0% |
| Westrich et al. [9] | 2010 | 75 | 72 | 4.4 | Two | A | NWB | 3.8 months | 90.1 | 90 | NR | NR | 9.30% |
| Tian et al. [10] | 2018 | 25 | 64.9 | 5.4 | Two | A | NWB | 11.5 weeks | 83 | 78 | 94 | 5 (20%) | 0 |
| Van Thiel et al. [11] | 2011 | 60 | 66 | 2.9 | Two | A | 39 (65%); PWB, 16 (26%); FWB 5 (8.3%); NWB | 75 days | 78.6 | NR | 101.3 | 1 (1.7%) | 7 (12%) |
| Tsai et al. [12] | 2019 | 32 | 70.4 | 3.1 | Two | A | PWB | 8.8 months | NR | NR | 102 | 2 (6.3%) | 4 (12.5%) |
| Emerson et al. [17] | 2002 | 22 26 | 65.1 65.7 | 3.8 7.5 | Two | A S | PWB | 6 to 12 weeks | NR | NR | 107.8 93.7 | 1 (3.8%) | 2 (9.0%) 2 (7.6%) |
| Bauer et al. [18] | 2006 | 30 77 | 71.8 68.3 | 4.5 4.5 | Single Two | - Both | NR | NR | 75.5 74.8 | 62.5 62.5 | 92.5 93 | NR | 33% 33% |
| Haddad et al. [19] | 2015 | 28 74 | 63 68 | 2 | Single Two | - A | - PWB | - 62days | 88 76 | NR | NR | NR | 0 5 (7%) |
| Lichstein et al. [20] | 2016 | 109 | 67 | 3.7 | Two | S | NR | ≧6 weeks | 86 | 85 | 100 | NR | 6% |
| Current study | − | 16 | 73.1 | 2.4 | Two | A | FWB | 92.7 days | 86.4 | 73.6 | 110.1 | 1 (6.4%) | 2 (12.5%) |
Weight-bearing helps maintain flexibility [23], which is believed to influence both the operative time and postoperative range of motion. Blood loss and transfusion are risk factors for reinfection [24], but shorter operative time also contributes to a reduction in blood loss. The operative time for reimplantation in our series was 116.1 minutes (range, 76-153 minutes), which was shorter than the 141 ± 66.7 minutes reported in previous studies [25], and none of the patients required blood transfusions.
However, the spacer must be able to withstand weight bearing because an interim spacer exchange in two-stage revision is associated with worse patient outcomes [22]. Additionally, the stress from weight-bearing cannot be excluded as having a negative impact on local rest necessary for infection resolution. Thiel et al. [11] reported cement-on-cement spacers, in which a minimum of 4.0 g of antibiotics per package of cement was used; in this study, 16 patients were allowed to perform weight bearing as tolerated, and there was one (6.3%) spacer breakage in total. In the present study, even the patient who was the most overweight (male, 90.5 kg, and BMI 33.4) was able to walk with full weight-bearing gait during the 109-day interval until reimplantation without complication of cement spacer damage in the present study. In this study, we used at least three packs (120 g) of cement, which was sufficient to fill the bone defect and achieve a stable extension gap; further, the spacer had a high cement concentration (2.0 g of vancomycin per 40 g of cement), which may have contributed to the low incidence of cement spacer damage. It is recommended that the spacer contain 3.6 g of antibiotics for 40 g of cement [2,25], while Nodzo et al. [26] reported that there was no relationship between raw quantities of vancomycin and aminoglycoside in the spacer and successful outcomes. One of the complications we encountered during weight-bearing management was dislocation of the cement spacer. This dislocation resulted from a technical error, specifically the insufficient filling of the intra-articular gap, which suggests that appropriate measures could improve and prevent such occurrences.
The present study has several limitations. First, this study has a small sample size and the lack of a control group. Second, as this study only includes patients who completed the two-stage revision, selection bias may exist in the data regarding treatment outcomes and complication rates with unrestricted weight-bearing. Therefore, it is not yet clear whether performing weight-bearing actually contributed to improved postoperative outcomes. Further randomized controlled trials with a larger cohort are needed to support the findings presented here. Third, it was a limited evaluation of full weight-bearing gait in a group of patients with low body weight compared to that of Caucasians. Fourth, the protocol of antibiotic treatment before and after the surgery was not strict. The type of antibiotics, duration of use, and antibiotic-free periods were adjusted for each patient based on their physical findings and blood test results, including CRP level, white blood cell count, and renal function results. Fifth, the follow-up period at the final observation was relatively short, so the risk of developing a late infection was low, and the actual infection rate may have been underestimated.
Conclusions
Management with unrestricted weight-bearing gait using cement spacers is a useful treatment option during the waiting period for two-stage revision surgery for knee PJI, because the postoperative outcomes after reimplantation were favorable even with a relatively short follow-up period. However, since complications related to weight-bearing may arise, the placement of stable cement spacers is essential. Further studies are needed to confirm whether the benefits of performing weight-bearing outweigh the risk of complications and lead to improved postoperative outcomes.
Disclosures
Human subjects: Consent for treatment and open access publication was obtained or waived by all participants in this study. Institutional Review Board of Yamaguchi Prefectural Grand Medical Center issued approval 2020-J029. This study was conducted in accordance with the principles outlined in the Declaration of Helsinki.
Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue.
Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following:
Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work.
Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work.
Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Author Contributions
Concept and design: Kazuya Uehara, Eiichi Shiigi, Kazushige Seki
Acquisition, analysis, or interpretation of data: Kazuya Uehara, Takashi Sakai, Eiichi Shiigi, Toshihiro Seki, Takashi Imagama, Hiroshi Tanaka
Drafting of the manuscript: Kazuya Uehara, Takashi Sakai
Critical review of the manuscript for important intellectual content: Kazuya Uehara, Takashi Sakai, Eiichi Shiigi, Toshihiro Seki, Kazushige Seki, Takashi Imagama, Hiroshi Tanaka
References
- 1.Outcome of single-stage versus two-stage exchange for revision knee arthroplasty for chronic periprosthetic infection. Pangaud C, Ollivier M, Argenson JN. EFORT Open Rev. 2019;4:495–502. doi: 10.1302/2058-5241.4.190003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Infection after total knee arthroplasty and its gold standard surgical treatment: spacers used in two-stage revision arthroplasty. Lu J, Han J, Zhang C, Yang Y, Yao Z. Intractable Rare Dis Res. 2017;6:256–261. doi: 10.5582/irdr.2017.01049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Intraoperatively-made cement-on-cement antibiotic-loaded articulating spacer for infected total knee arthroplasty. Shen H, Zhang X, Jiang Y, Wang Q, Chen Y, Wang Q, Shao J. Knee. 2010;17:407–411. doi: 10.1016/j.knee.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 4.Successful treatment of total hip and knee infection with articulating antibiotic components: a modified treatment method. Evans RP. Clin Orthop Relat Res. 2004:37–46. doi: 10.1097/01.blo.0000143739.07632.7c. [DOI] [PubMed] [Google Scholar]
- 5.Treatment of infected total knee arthroplasty using an articulating spacer. Hofmann AA, Kane KR, Tkach TK, Plaster RL, Camargo MP. Clin Orthop Relat Res. 1995;321:45–54. [PubMed] [Google Scholar]
- 6.Spacer prostheses in two-stage revision of infected knee arthroplasty. Jämsen E, Sheng P, Halonen P, et al. Int Orthop. 2006;30:257–261. doi: 10.1007/s00264-006-0102-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Are prosthetic spacers safe to use in 2-stage treatment for infected total knee arthroplasty? Choi HR, Malchau H, Bedair H. J Arthroplasty. 2012;27:1474–1479. doi: 10.1016/j.arth.2012.02.023. [DOI] [PubMed] [Google Scholar]
- 8.Antibiotic-loaded cement articulating spacer for 2-stage reimplantation in infected total knee arthroplasty: a simple and economic method. Hsu YC, Cheng HC, Ng TP, Chiu KY. J Arthroplasty. 2007;22:1060–1066. doi: 10.1016/j.arth.2007.04.028. [DOI] [PubMed] [Google Scholar]
- 9.Prediction of blood volume in normal human adults. Nadler SB, Hidalgo JU, Bloch T. https://www.surgjournal.com/article/0039-6060(62)90166-6/abstract. Surgery. 1962;51:224–232. [PubMed] [Google Scholar]
- 10.How much blood is really lost in total knee arthroplasty? Correct blood loss management should take hidden loss into account. Sehat KR, Evans R, Newman JH. Knee. 2000;7:151–155. doi: 10.1016/s0968-0160(00)00047-8. [DOI] [PubMed] [Google Scholar]
- 11.Two-stage reimplantation for the salvage of infected total knee arthroplasty. Insall JN, Thompson FM, Brause BD. J Bone Jt Surg - Ser A. 1983;65:1087–1098. [PubMed] [Google Scholar]
- 12.A preliminary study of the novel antibiotic-loaded cement computer-aided design-articulating spacer for the treatment of periprosthetic knee infection. Tsai CH, Hsu HC, Chen HY, et al. J Orthop Surg Res. 2019;14:136. doi: 10.1186/s13018-019-1175-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Modern treatment of infected total knee arthroplasty with a 2-stage reimplantation protocol. Westrich GH, Walcott-Sapp S, Bornstein LJ, Bostrom MP, Windsor RE, Brause BD. J Arthroplasty. 2010;25:1015-21, 1021.e1-2. doi: 10.1016/j.arth.2009.07.017. [DOI] [PubMed] [Google Scholar]
- 14.Intraoperative molds to create an articulating spacer for the infected knee arthroplasty. Van Thiel GS, Berend KR, Klein GR, Gordon AC, Lombardi AV, Della Valle CJ. Clin Orthop Relat Res. 2011;469:994–1001. doi: 10.1007/s11999-010-1644-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Comparison of a static with a mobile spacer in total knee infection. Emerson RH Jr, Muncie M, Tarbox TR, Higgins LL. Clin Orthop Relat Res. 2002:132–138. doi: 10.1097/00003086-200211000-00023. [DOI] [PubMed] [Google Scholar]
- 16.[Résultats des changements de prothèse de genou pour infection] Bauer T, Piriou P, Lhotellier L, Leclerc P, Mamoudy P, Lortat-Jacob A. Rev Chir Orthop Reparatrice Appar Mot. 2006;92:692–700. doi: 10.1016/s0035-1040(06)75930-x. [DOI] [PubMed] [Google Scholar]
- 17.Is single-stage revision according to a strict protocol effective in treatment of chronic knee arthroplasty infections? Haddad FS, Sukeik M, Alazzawi S. Clin Orthop Relat Res. 2015;473:8–14. doi: 10.1007/s11999-014-3721-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Treatment of periprosthetic knee infection with a two-stage protocol using static spacers. Lichstein P, Su S, Hedlund H, Suh G, Maloney WJ, Goodman SB, Huddleston JI 3rd. Clin Orthop Relat Res. 2016;474:120–125. doi: 10.1007/s11999-015-4443-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Short-term follow-up of antibiotic-loaded articulating cement spacers in two-stage revision of infected total knee arthroplasty: a case series. Tian MQ, Yang XT, Tian XB, Sun YB, Duan YH, Sun L. Orthop Surg. 2018;10:128–133. doi: 10.1111/os.12381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Re-infection outcomes following one- and two-stage surgical revision of infected knee prosthesis: a systematic review and meta-analysis. Kunutsor SK, Whitehouse MR, Lenguerrand E, Blom AW, Beswick AD. PLoS One. 2016;11:0. doi: 10.1371/journal.pone.0151537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Outcomes and risk factors associated with 2-stage reimplantation requiring an interim spacer exchange for periprosthetic joint infection. Klemt C, Smith EJ, Tirumala V, Bounajem G, van den Kieboom J, Kwon YM. J Arthroplasty. 2021;36:1094–1100. doi: 10.1016/j.arth.2020.09.012. [DOI] [PubMed] [Google Scholar]
- 22.Effect of early weight bearing program with conventional physiotherapy on functional outcomes in surgically treated proximal tibia fracture: a pilot randomized controlled trial. Kadam A, Wani S, Gadekar G, Katage G, Garg K, Mungikar S. Arch Orthop Trauma Surg. 2024;144:2481–2489. doi: 10.1007/s00402-024-05347-w. [DOI] [PubMed] [Google Scholar]
- 23.Preoperative blood transfusion associated with increased length of stay and increased postoperative complications after revision total knee arthroplasty. Gu A, Maybee CM, Wei C, Probasco WV, Ast MP, Sculco PK. J Orthop. 2019;16:265–268. doi: 10.1016/j.jor.2019.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Revision total knee arthroplasty for periprosthetic joint infection is associated with increased postoperative morbidity and mortality relative to noninfectious revisions. Boddapati V, Fu MC, Mayman DJ, Su EP, Sculco PK, McLawhorn AS. J Arthroplasty. 2018;33:521–526. doi: 10.1016/j.arth.2017.09.021. [DOI] [PubMed] [Google Scholar]
- 25.Static and mobile antibiotic-impregnated cement spacers for the management of prosthetic joint infection. Jacobs C, Christensen CP, Berend ME. J Am Acad Orthop Surg. 2009;17:356–368. doi: 10.5435/00124635-200906000-00004. [DOI] [PubMed] [Google Scholar]
- 26.Success rates, characteristics, and costs of articulating antibiotic spacers for total knee periprosthetic joint infection. Nodzo SR, Boyle KK, Spiro S, Nocon AA, Miller AO, Westrich GH. Knee. 2017;24:1175–1181. doi: 10.1016/j.knee.2017.05.016. [DOI] [PubMed] [Google Scholar]