Abstract
Osteoporosis is a degenerative bone disease that causes the weakening of bone structure. Since bone structure is dynamic throughout a person’s lifespan, bones are under constant growth and destruction in a process known as bone turnover or bone remodeling. Osteoporosis involves the disruption of this growth/destruction equilibrium towards the destructive side. An increase in bone turnover leads to a lower bone density and therefore a greater risk of fracture or injury of higher severity. Bone turnover markers serve as indicators of the process of bone turnover. These markers are split into two groups: formation (building up) markers and resorption (breaking down) markers. Using biochemical techniques and assays, these markers can be measured to monitor the activity of the markers as well as determine treatment options and efficacy based on this activity. The use of biomarkers in osteoporosis can pave the way for their use in other diseases such as cancer.
Keywords: osteoporosis, bone turnover, biomarkers, osteoclasts
Introduction
Osteoporosis is one of the most common bone disorders with over 200 million people currently estimated to be suffering from it.1 The disease is characterized by deteriorated bone structure which leads to a higher fracture or breakage risk, especially in older individuals. Due to the nature of the disease, these fractures can lead to more serious injury because of an already decreased density of the bone and malfunction of the bone repair process. Numerous case reports of elderly patients having minor falls that results in significant orthopedic trauma and even fatal hemorrhage have been published.2,3 Since the disease presents no immediate detectable symptoms, it is often unnoticed until it presents itself as a seemingly low trauma fracture of a major skeletal structure such as the spine, hip, or even the wrist with minor to no apparent cause.4 Current treatments involve both pharmaceutical and non-pharmaceutical preventive treatments. Pharmaceutical treatments take the form of oral medications that are categorized as either antiresorptive or anabolic.
Antiresorptive medications attempt to decrease the bone resorption/destruction rate while anabolic medications attempt to increase the bone formation rate and overtake the resorption rate. Non-pharmaceutical treatment involves dietary or lifestyle changes that could minimize the risk of future fractures. Adequate intake of essential vitamins and minerals such as calcium and vitamin D and exercise paired with cessation of smoking or alcohol consumption are all common treatments to reduce the severity and chances of future fractures.5
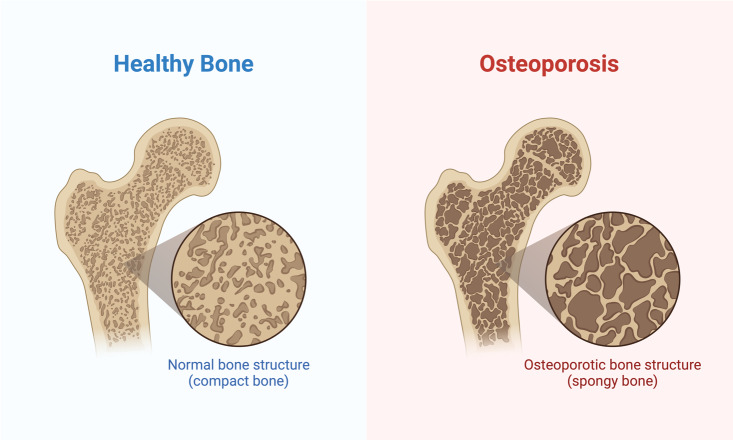
Osteoporosis itself is split into two categories: primary and secondary osteoporosis, each differentiated by what causes the disease. The apparent cause of primary osteoporosis is old age or sex hormone deficiency. The apparent cause of secondary osteoporosis is a pre-existing condition and sometimes a side effect of the medication for that treatment. For example, numerous inflammatory diseases, such as rheumatoid arthritis, involve treatment with glucocorticoid therapy which is known to accelerate bone loss5. The most prominent factor in osteoporosis, however, is hormonal in nature. Specifically, the decrease of sex hormones associated with aging and other treatments leads to an increase in bone resorption and therefore an increase in bone loss [Figure 1].
Figure 1. A comparison of a healthy bone with that of a bone with osteoporosis. The density of the healthy bone is much higher in healthy bone, making it much stronger. Created in https://BioRender.com.
Turnover process (Bone remodeling)
Bone structure is constantly dynamic and undergoes continuous remodeling throughout one’s lifespan. There are two main stages of bone turnover: bone resorption regulated by osteoclasts and bone formation regulated by osteoblasts. During adolescence, the turnover process favors the formation side until peak bone mass is attained. Once this is achieved, bones still remain active and the bone turnover process reaches equilibrium: bone formation and resorption occur at equal rates.6 Osteoblasts are mature bone cells responsible for bone formation and osteoclasts are responsible for bone resorption. These two cells work in tandem to regulate the bone turnover process. The most common regulation pathway is the RANKL/RANK pathway. RANKL is a protein produced by osteoblasts that binds to the RANK receptor of osteoclasts and initiates the bone resorption process. In order to initiate the formation process, the Wnt pathway is employed. The result of this pathway leads to the production of β-catenin which terminates osteoclasts and the resorption process which allows osteoblasts to bind to bone and initiate the formation process.7
Biochemical markers
Biochemical markers, or biomarkers in general, are molecules produced during a metabolic or disease process, either at the onset or during the progression of the disease. They can be used to track the progression of a disease and also its treatment efficacy of it. In terms of osteoporosis, these are labeled as bone turnover markers (BTMs) since the disease is characterized by a malfunction in the bone turnover process. Bone turnover markers are protein or protein derivatives that are released during the bone turnover process by either osteoclasts or osteoblasts. They provide insight as to whether resorption is occurring at a higher rate than formation. Osteoporosis involves either an underactive formation process or an overactive resorption process commonly caused by a malfunction in one of the communicative pathways between the two. Consequently, bone turnover markers take two forms: formation markers and resorption markers.6 Measuring these markers can give an insight as to which process is over or under-expressed.
Formation markers
Bone formation markers can be categorized as three different types: By-products of collagen synthesis, osteoblast enzymes, and matrix proteins. Collagen is an important component in the strength of the skeletal structure. A by-product of collagen synthesis by osteoblasts are 1 N-terminal propeptide (P1NP) and procollagen type 1 C-terminal propeptide (P1CP). These markers are the products of the posttranslational cleavage of type 1 procollagen molecules and originate mostly from proliferating osteoblasts when forming new bone tissue. In clinical use, P1NP is the preferred use as a marker for bone formation because P1CP is cleared out by the mannose receptor, and can also be influenced by the presence of growth and thyroid hormones and complicate the interpretation in patients that have pituitary or thyroid dysfunction.4 Both P1NP and P1CP can be measured in serum and can be analyzed by radioimmunoassay (RIA).6
Another marker is alkaline phosphatase (ALP), a membrane-bound enzyme of the osteoblast that is present in almost all body tissue. ALP plays a vital role in the regulation of mineralization by degrading pyrophosphate, which is an inhibitor of mineralization. ALP is one of the earliest BTMs that was used and has been phased out since it is also present in liver tissue. However, it is still useful in determining high levels of bone turnover in other bone diseases such as Paget’s disease.6 ALP can be measured in serum via immunoassays, electrophoresis, and high-performance liquid chromatography (HPLC). Out of these, immunoassays provide the most reproducible and precise results when compared to the other methods.8
The last common formation marker is osteocalcin, a small matrix protein synthesized by osteoblasts. Osteocalcin consists of three glutamic acid residues, which undergo vitamin
K-dependent carboxylation to form gamma-carboxyglutamate. This causes a conformational change that allows osteocalcin to bind with hydroxyapatite and minerals in the bone. Since it is a direct byproduct of osteoblasts, it serves as a better marker than ALP and has also been found to be a specifically useful BTM in primary osteoporosis.4 Available assays for measuring osteocalcin include radioimmunoassays, enzyme-linked immunosorbent assays (ELISA), and chemiluminescence immunoassays.9
Assaying the Markers (RIA)
Radioimmunoassays work via competitive binding and have contributed to the use of biomarkers greatly by acting as a way to quantify them. In RIA, a known quantity of the antigen of interest is radioactively labeled with 125I. This sample is then introduced into a set of wells with the antigen’s antibody fixed to the bottom of the well and the antibody is allowed to bind to the antibody. The sample of interest with an unknown concentration of the antigen is then added and allowed to competitively bind to the antibodies. This will cause the radiolabeled antigens to essentially be kicked off the antibodies and replaced with the antigens from the serum of interest. The radioactivity of the supernatant in the well starts off at zero since all of the radiolabeled antigens are bound to the antibody. However, since they have been kicked off by the serum antigens, they will populate the supernatant solution. The radioactivity of the supernatant is then directly proportional to the concentration of the serum antigen. The higher the radioactivity, the higher the concentration of the serum antigen. The radioimmunoassay is most commonly used when measuring both bone turnover markers as well as any other biochemical marker. For example, RIA is also used to measure the biochemical markers in cancer.
Resorption markers
Bone resorption markers can be categorized as collagen degradation products, non-collagenous proteins, osteoclastic enzymes, and osteocyte activity markers. A collagen degradation product is carboxy-terminal cross-linked telopeptides of type 1 collagen (CTX). CTX undergoes isomerization during bone digestion to produce α and β forms. These are then isomerized again to form D and L forms.4 CTX is then excreted from the body through urine and is consequently analyzed in urine samples. Since CTX displays circadian variation, where the concentration of a molecule produced in the body is dependent on the time of day, the timing of sample collection is important. Specifically, fasting morning samples give optimal results. CTX is also currently the best resorption marker since it is a direct product of bone digestion. As a result, measuring this marker can provide direct insight into the rate of bone digestion. Analytical methods for CTX include RIA, ELISA, and electrochemiluminescence assay6 with RIA being the most common.
A non-collagenous protein used as a resorption marker is bone sialoprotein (BSP) which is a glycoprotein found in the bone matrix. BSP contributes to cell-matrix adhesion of bone minerals and constitutes a large portion of bone structure; subsequently, BSP levels can serve as an indication of how much bone is being digested. BSP is commonly measured in serum by RIA. BSP is not commonly used as a hallmark marker since it is one of the harder markers to assay since RIA does not pick up all of the available BSP in a sample.6
Tartrate-resistant acid phosphatase isoform 5b (TRAP5b) is an isoform of acid phosphatase that is resistant to tartrate degradation. During bone resorption, TRAP5b cleaves type 1 collagen into fragments and is mostly found in the border of osteoclasts. Osteoclasts secrete TRAP5b during bone resorption, which makes it another excellent gauge of resorption activity.4 While CTX is currently the most used resorption marker, TRAP5b shows promise since it is analyzed in serum and is therefore not affected by any renal dysfunctions.
Also, TRAP5b inactivates a protein called osteopontin, which allows osteoclasts to bind to the bone. Therefore, low levels of TRAP5b are associated with prolonged digestion of bone tissue by osteoclasts. TRAP5b can be assayed using enzymatic and immunoassay techniques. However, immunoassays are more effective since there are other forms of TRAP enzymes in serum that may come from other types of cells, specifically immune cells which have TRAP5a6 [Table 1].
Table 1. A summary of the turnover markers that were previously explored.
| Formation | Resorption |
|---|---|
| P1NP/P1CP: Result from posttranslational cleavage of procollagen | CTX: Direct product of bone digestion commonly found in urine samples |
| ALP: Membrane-bound enzyme in osteoblasts that plays a vital role in mineralization regulation | BSP: Glycoprotein in the bone matrix that plays a role in cellular adhesion of minerals |
| Osteocalcin: By product of osteoblasts during mineralization of bones | TRAP5b: Cleaves collagen into fragments and is secreted during bone resorption |
Evaluation of markers
Bone turnover markers can be used to predict the risk of osteoporotic fractures or injuries. Increased bone resorption leads to lower bone density due to the deterioration of the bone structure and lack of supplemental formation. Bone turnover markers can measure this deterioration and serve as a supplement to bone mineral density tests, which only assess fracture risk. Increased levels of bone turnover markers indicate decreased bone integrity since newly synthesized bone tissue is less mineralized and has fewer posttranslational modifications.4 A study observed 435 healthy postmenopausal women aged 50-89 years. In 55 cases with reported fractures against a control group of 380, there was an increased level of bone turnover markers P1CP, P1NP, and CTX. The increased levels of formation markers P1CP and P1NP is normal because of the fracture. During a fracture, bone formation is heightened in order to facilitate the repair of the damaged bone. However, increased levels of CTX are not normal. This means that the fracture is most likely caused due to osteoporosis and will result in a decreased repair of the damaged bone. As shown in table 2, serum P1CP and P1NP, which are bone formation markers, were present in higher concentrations. Additionally, CTX, a bone resorption marker, was also present in higher concentrations than those of the control group. The higher concentrations of bone resorption markers indicate that the fractures were most likely due to a lower bone density caused by increased bone resorption and signaling osteoporosis.10 The presence of these resorption markers in higher concentrations during a fracture is also a concern for inadequate bone repair, which will only further weaken bone strength and displays how damaging osteoporosis can be [Table 2].
Table 2. Comparison of biochemical markers of women who experienced osteoporotic fractures to a control group that does not have osteoporosis. Formation markers P1CP/P1NP were found in higher concentrations, which is normal. CTX was also found to be in higher concentrations which is indicative of an osteoporotic fracture.
| Turnover marker | Concentration in Women with Fracture | Concentration in Control |
|---|---|---|
| P1CP | 100 ± 30 ng/mL | 96 ± 33 ng/mL |
| P1NP | 56 ± 23 ng/mL | 53 ± 21 ng/mL |
| CTX | 4.0 ± 1.9 nmol/L | 3.6 ± 1.6 nmol/L |
Treatment monitoring
Turnover markers can also provide an insight on the therapeutic efficacy of osteoporosis treatment. This allows for the optimization of therapy techniques and is useful for determining a patient’s compliance to medication. One of the most common pharmaceutical treatments involves the use of oral bisphosphonates which inhibit osteoclasts and slowing down the bone resorption process. Turnover markers can determine if the treatment is ineffective at suppressing the resorption process, in which case the treatment can be adjusted accordingly by increasing the dosage or trying another treatment option.4 Another effective treatment is Denosumab, a monoclonal antibody that works by inhibiting RANKL and therefore osteoclast initiation. An IMPACT study, a study done to analyze the result of a specific change, was done on 2302 postmenopausal women in which CTX and NTX levels were assessed at baseline, 10, and 22 weeks of treatment with 5 mg/day of risedronate, a bisphosphonate. As shown in table 3, a decrease of more than 30% of the resorption markers was found and the number of patients with a fracture reduced by 1.6% compared to those that did not show a significant decrease after 22 weeks.11 This provides evidence that the medication is both working and viable for the treatment of osteoporosis. Companies developing new treatments and medications can use similar methods of tracking the turnover markers to see whether or not the treatment is effective. The efficacy of a treatment is important because every drug has a side effect. How well a treatment works, and the severity of the side effect are important factors in drug production.
Optimally, a drug would present optimal efficacy with minimal side effects. In terms of osteoporosis, a side effect of bisphosphonates is that it can actually make bones weaker and cause atypical fractures. Bisphosphonates work by inhibiting bone resorption by osteoclasts, this includes the resorption of old bone tissue that may not be as strong. This means that older and weaker bone tissue will remain which has an increased risk of fracture. Hence, close monitoring of the turnover markers can keep track of whether or not the bisphosphonates are doing their job correctly or if they are causing more harm.
Limitations
The main limitation in the use of turnover markers, like other biochemical markers, is their variability and ability to be assayed. Generally, resorption markers have a higher variability than formation markers and urine samples have a higher variability than serum samples. Serum samples are more easily assayed than urine samples since urine samples are easily subject to variation due to renal dysfunctions, especially in older individuals. One of the important technical factors is the sampling procedure. For example, urinary samples must ideally be taken in the morning due to circadian cycles and must account for creatinine. Blood samples contain anticoagulants which can affect osteocalcin counts. However, these are circumvented by the advancement of immunoassays. By using an antibody specific to the antigen of interest, the other antigens in the sample will not bind and therefore not affect the measurements. There are other factors such as age, gender, ethnicity, diet, and other biological factors that must also be taken into account. For example, one study found that bone markers were found to have a lower concentration in black children than in white children.12
Conclusion
Bone turnover markers serve as important biological tools in the treatment and monitoring of osteoporosis that are becoming more popular in the clinical world. These various markers can be used to determine fracture risk as well as treatment determination and efficacy. Despite the variability of their use, these markers serve as baseline indicators of the bone turnover process and osteoporosis. If utilized, these markers can catch diseases like osteoporosis early and therefore have a better prognosis. However, some markers are better than others. In the example of osteoporosis, the resorption marker P1CP is the most critical because it is a direct product of bone construction. Resorption marker CTX is the most critical for a similar reason, it is a direct product of bone digestion. These two markers are also the most easily sampled and assayed when compared to the other markers, making them more reliable. The use of biomarkers in osteoporosis has set the stage for biomarkers’ potential in use for other diseases such as cancer. The efficacy of a treatment for a disease can be analyzed by the use of these biomarkers and can be used to develop new treatments for current diseases.
References
- An overview and management of osteoporosis. Sözen T., Özışık L., Başaran N. Ç. 2017European journal of rheumatology. 4(1):46–56. doi: 10.5152/eurjrheum.2016.048. https://doi.org/10.5152/eurjrheum.2016.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isolated sternal fracture after low-energy trauma in a geriatric patient: a case report. Sairanen J. J., Arponen O. 2022Int J Emerg Med. 15:34. doi: 10.1186/s12245-022-00437-1. https://doi.org/10.1186/s12245-022-00437-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- How a broken vertebra can lead to a fatal hemorrhage: a case report. Ploumen R. A. W., van Wezenbeek M. R., Willems P. C. P. H.., et al. 2024Int J Emerg Med. 17:24. doi: 10.1186/s12245-024-00594-5. https://doi.org/10.1186/s12245-024-00594-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bone turnover markers: Emerging tool in the management of osteoporosis. Shetty S., Kapoor N., Bondu J. D., Thomas N., Paul T. V. 2016Indian journal of endocrinology and metabolism. 20(6):846–852. doi: 10.4103/2230-8210.192914. https://doi.org/10.4103/2230-8210.192914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutrition and Osteoporosis Prevention. Rizzoli R., Chevalley T. Dec;2024 Curr Osteoporos Rep. 22(6):515–522. doi: 10.1007/s11914-024-00892-0. https://doi.org/10.1007/s11914-024-00892-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biochemical markers of bone turnover – uses and limitations. Hlaing T. T., Compston J. E. 2014Annals of Clinical Biochemistry. 51(2):189–202. doi: 10.1177/0004563213515190. https://doi.org/10.1177/0004563213515190 [DOI] [PubMed] [Google Scholar]
- Osteoblast-osteoclast interactions. Chen X., Wang Z., Duan N., Zhu G., Schwarz E. M., Xie C. 2018Connective tissue research. 59(2):99–107. doi: 10.1080/03008207.2017.1290085. https://doi.org/10.1080/03008207.2017.1290085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burtis C. A., Ashwood E. A., Burns D. E. Teitz textbook of clinical chemistry and molecular diagnostics. Vol. 4 Elsevier; St Louis: [Google Scholar]
- Osteocalcin: diagnostic methods and clinical applications. Power M. J., Fottrell P. F. 1991Crit Rev Clin Lab Sci. 28:287–335. doi: 10.3109/10408369109106867. [DOI] [PubMed] [Google Scholar]
- Biochemical Markers of Bone Turnover, Endogenous Hormones and the Risk of Fractures in Postmenopausal Women: The OFELY Study. Garnero P., Sornay-Rendu E., Claustrat B., Delmas P. D. 2000J Bone Miner Res. 15:1526–1536. doi: 10.1359/jbmr.2000.15.8.1526. https://doi.org/10.1359/jbmr.2000.15.8.1526 [DOI] [PubMed] [Google Scholar]
- Bone turnover markers and bone mineral density response with risedronate therapy: Relationship with fracture risk and patient adherence. Eastell R., Vrijens B., Cahall D. L., Ringe J. D., Garnero P., Watts N. B. 2011J Bone Miner Res. 26:1662–1669. doi: 10.1002/jbmr.342. https://doi.org/10.1002/jbmr.342 [DOI] [PubMed] [Google Scholar]
- A comparison of the urinary excretion of bone resorptive products in white and black children. Pratt J. H., Manatunga A. K., Peacock M. Jan;1996 J Lab Clin Med. 127(1):67–70. doi: 10.1016/s0022-2143(96)90167-5. https://doi.org/10.1016/s0022-2143(96)90167-5 [DOI] [PubMed] [Google Scholar]