ABSTRACT
Arctic habitats are changing rapidly and altering trophic webs and ecosystem functioning. Understanding how species' abundances and distributions differ among Arctic habitats is important in predicting future species shifts and trophic‐web consequences. We aimed to determine the habitat–abundance relationships for three small herbivores on the Seward Peninsula of Alaska, USA by fitting data from 983 point counts (collected during 2019, 2021, and 2022) with N‐mixture models that account for imperfect detection. These herbivore species, Willow Ptarmigan ( Lagopus lagopus ), Rock Ptarmigan ( L. muta ), and Arctic ground squirrels ( Urocitellus parryii) , are fundamental to tundra food webs, and primary prey for Arctic raptors including Gyrfalcons ( Falco rusticolus ). Second, we aimed to map herbivore densities within Gyrfalcon breeding territories. Third, we aimed to evaluate whether Gyrfalcons were more likely to occupy territories with higher prey densities using a multi‐season occupancy model coupled with occupancy observations from helicopter surveys conducted during 2016–2022 at 97 Gyrfalcon territories. We found that male Willow Ptarmigan were more abundant in areas with greater cover of tundra, tall shrubs, and tussock tundra. Conversely, male Rock Ptarmigan were more abundant in areas with greater cover of sparse vegetation and tundra. Arctic ground squirrels were more abundant at higher elevations with greater cover of sparse vegetation and low shrubs. Willow Ptarmigan were widespread within Gyrfalcon breeding territories, whereas Rock Ptarmigan and Arctic ground squirrels had patchier distributions with few areas of high abundance. Lastly, Gyrfalcons were more likely to occupy territories with higher densities of Willow Ptarmigan and Arctic ground squirrels. As the Artic continues to warm, Rock Ptarmigan and Arctic ground squirrels may be vulnerable to ongoing shrub encroachment, whereas Willow Ptarmigan may benefit. By tying abundances of three prey to Gyrfalcon occupancy, our results contribute to understanding potential impacts on higher levels of this Arctic trophic web.
Keywords: Arctic trophic web, habitat associations, predator–prey, prey abundance, raptors, territory occupancy
Arctic habitats are changing rapidly, impacting trophic webs and ecosystem functions. We examined the habitat–abundance relationships of three small herbivores on the Seward Peninsula, Alaska, and their distribution within Gyrfalcon breeding territories. We found that Gyrfalcons preferred territories with higher densities of Willow Ptarmigan and Arctic ground squirrels, and that changes in habitat may benefit some prey species, like Willow Ptarmigan, while making others, such as Rock Ptarmigan and Arctic ground squirrels, more vulnerable to warming and shrub encroachment.
1. Introduction
Arctic ecosystems are characterized by unique trophic webs with distinctive and dynamic ecological interactions (Meltofte, Josefson, and Payer 2013; Schmidt et al. 2017). However, the Arctic is experiencing accelerated climate change, with increased precipitation (Bintanja and Selten 2014; McCrystall et al. 2021) and average temperatures rising four times faster than those at lower latitudes (Rantanen et al. 2022). These altered weather patterns are expected to have strong ecological effects (Hassol and Corell 2006). For example, open and tree‐less tundra habitats that are characteristic of this region are being invaded by tall shrubs and trees (Tape, Sturm, and Racine 2006; Myers‐Smith et al. 2011, 2015). Such changes are predicted to affect Arctic animal communities and species population dynamics (Gilg et al. 2012). Understanding associations between Arctic wildlife and their habitats will be essential to monitoring ecological change and conserving sensitive species (Verberk 2011).
Habitat and climatic changes in the Arctic are expected to alter the demography of species, causing declines or disappearances for some species and increases for others via habitat and range shifts (Gilg et al. 2012). Over half of Arctic‐breeding wading birds (Charadriiformes) are reported to be declining (Smith et al. 2020). These declines are partially attributed to environmental changes in the Arctic such as habitat loss and phenological mismatches (Kwon et al. 2019; Smith et al. 2020). Conversely, climate‐induced environmental changes are facilitating the northward expansion of some species into the Arctic. The increase in woody shrubs in the Arctic has allowed for the North American beaver ( Castor canadensis ) to expand its range to areas of the Arctic tundra (Tape et al. 2022). The presence of the beaver and its landscape‐altering behaviors have cascading effects that could be intensifying the effects of climate change in the Arctic (Tape et al. 2018). Knowledge about demographic responses to environmental changes provides early insights potentially useful for predicting range contractions or expansions and ensuring that conservation strategies can be implemented in a timely manner, thus preserving trophic webs unique to the Arctic (Matthews and Whittaker 2015).
Recent studies suggest that Willow Ptarmigan ( Lagopus lagopus ), Rock Ptarmigan ( L. muta ) and Arctic ground squirrels ( Urocitellus parryii ) have declined in parts of the Arctic, potentially driven by changes in climate and habitat. Comprehensive reviews show declining trends in Willow Ptarmigan populations across Fennoscandia (Lehikoinen et al. 2014; Fuglei et al. 2020) and eastern Russia (Fuglei et al. 2020), whereas a dampening of population cycles has been observed in the Yukon territory of Canada (Mossop 2011). Willow Ptarmigan declines in Finland have been correlated with more snow‐free spring days (Melin et al. 2020), willow thicket fragmentation from ungulate browsing (Henden et al. 2011; Ims and Henden 2012), increased winter temperatures, and the collapse of small rodent populations (Kausrud et al. 2008). However, climate change is predicted to have conflicting effects on Willow Ptarmigan populations because changes in temperature and precipitation could reduce breeding success, whereas shrub encroachment may provide additional habitat (Scridel et al. 2021). Conversely, the expansion of woody shrubs into tundra habitats is already documented and is expected to reduce the extent of suitable habitat for Rock Ptarmigan and Arctic ground squirrels. Rock Ptarmigan populations exhibit negative trends in Iceland and Greenland (Fuglei et al. 2020) and mainland Europe (Revermann et al. 2012; Imperio et al. 2013; Lehikoinen et al. 2014; Furrer et al. 2016; Canonne et al. 2020) and are predicted to experience a major loss (> 50%) of their alpine habitat to tree‐line and shrub encroachment and increasing temperatures (Revermann et al. 2012; Pernollet, Korner‐Nievergelt, and Jenni 2015; Ferrarini, Alatalo, and Gustin 2017; Hotta et al. 2019; Scridel et al. 2021). Arctic ground squirrel populations have decreased rapidly at lower elevations and been extirpated from some boreal forest habitats of Canada (Werner et al. 2015). Tall and dense vegetation impacts squirrels' ability to detect and evade predators, reducing suitability of such habitats to support viable populations (Gillis et al. 2005; Donker and Krebs 2012; Wheeler and Hik 2014a; Flower et al. 2019). Consequently, shrub encroachment has been suggested as one of the mechanisms leading to squirrel ( Urocitellus parryii ) declines (Wheeler and Hik 2013, 2014b; Wheeler et al. 2015).
The Gyrfalcon is an Arctic apex predator likely to be affected by bottom‐up effects caused by changes in prey abundance (Barichello and Mossop 2011; Booms, Lindgren, and Huettmann 2011). The largest of the true falcons (Nielsen and Cade 2017), they nest on bluffs and cliffsides along the waterways and mountainous terrain of the Arctic tundra. On the Seward Peninsula, Alaska, these cliffs occur in a dynamic landscape containing few roads, areas of isolated human development, as well as a national reserve. The nesting success and productivity of Gyrfalcons in this landscape is variable (Anderson et al. 2019; Henderson, Booms, et al. 2021) and some Gyrfalcon territories are occupied consistently while others are used only sporadically (Bente 2011; Anderson et al. 2019). This varied use may be related to prey availability (Sergio and Newton 2003). For Gyrfalcons on the Seward Peninsula, Willow Ptarmigan, Rock Ptarmigan, and Arctic ground squirrels compose most of their diet (Robinson et al. 2019; Johnson et al. 2022). Because raptors, as apex predators, frequently function as important signalers of ecosystem change (Natsukawa and Sergio 2022; Sergio et al. 2008; Sergio, Newton, and Marchesi 2008), clarifying factors that underly patterns of predator and prey distribution helps fill information gaps fundamental to modeling systemic changes in Arctic habitats.
Small Arctic herbivores remain understudied across much of their range despite their importance as ecosystem engineers (ptarmigan, Tape et al. 2010; Arctic ground squirrels, Wheeler and Hik 2013) and as primary prey in trophic webs. We aimed to fill this knowledge gap by associating different habitat types with landscape‐level abundances of Willow Ptarmigan, Rock Ptarmigan, and Arctic ground squirrels on the Seward Peninsula. Secondly, we aimed to estimate their spatial abundance and distribution within historically occupied Gyrfalcon breeding territories. Lastly, we used a multi‐season occupancy model to examine the relationship between density estimates of these three species and Gyrfalcon occupancy. Understanding spatial patterns of prey abundance, rather than presence, will help determine key habitats for these important Arctic species. Further, knowledge of prey abundance is important for explaining demographic differences among raptor territories (Newton 1979; Steenhof et al. 1999; Nielsen and Cade 2017; Anderson et al. 2019). By tying prey species abundances to Gyrfalcon occupancy, our results should contribute to future studies aimed at understanding the potential impacts of a changing Arctic on higher levels of this Arctic trophic web.
2. Methods
2.1. Study Area
The Seward Peninsula in western Alaska, ancestral land of the Iñupiat (Inupiaq and Yupik) People, is characterized by rugged, mountainous terrain flanked by rolling hills of Arctic tundra crossed by numerous streams and rivers. Dispersed rock outcroppings, inland cliffs, and cliff‐lined river systems provide nesting substrates for cliff‐nesting raptors, including the Gyrfalcon (Booms et al. 2010; Anderson et al. 2019). The climate is harsh with long, cold, and typically dry winters and short summers. Temperature extremes during the Gyrfalcon breeding season can range from −43°C in March to 30°C in July (NOAA n.d.).
The most widespread habitat on the Seward Peninsula is dwarf shrub meadow dominated by tussock forming sedges ( Eriophorum vaginatum and Carex bigelowii ) interspersed with varying densities of dwarf shrub, predominately Betula nana , with Betula and heath‐dominated communities in drier parts of the landscape (Kessel 1989). Major river drainages and protected foothill slopes are dominated by dense thickets of willow (Salix spp.) and alder (Alnus spp.; Kessel 1989). More exposed and windblown sites among the disconnected ridges, domes, and flat‐topped mountains are often barren or sparsely vegetated by dwarf shrub mat: prostrate vegetative communities varying in amounts of mosses, lichens, xeric herbs and forbs, and dwarf shrub (Kessel 1989).
The study area comprised 14,150 km2 of the southern portion of the Seward Peninsula bounded by the Bering Sea along the south and west, Niukluk and Solomon rivers to the east, and the Bering Land Bridge National Preserve to the north (Anderson et al. 2019). We conducted point count surveys within three corridors following the three roads present in the southern portion of the Seward Peninsula. We buffered each road segment with an 8‐km polygon on either side to maximize surveyor safety and survey efficiency. For safety reasons, we removed sections in the buffered roads that were inaccessible, such as sheer cliffs, and areas at elevations greater than 500 m, although we recognize that prey species occur above such elevations. We then divided each road segment buffer into 5 units of roughly equal area and placed an 800 m x 800 m grid over the units and roads. Next, we generated points at the vertices of the grid, creating points that were 800 m apart. Straight‐line distance from survey points to the nearest road ranged from 2.5 m to 7.95 km with 63 points (6.4%) occurring less than 200 m from the nearest road. We did not expect a road effect on point count surveys from species avoidance or distribution of vegetative communities near the roads because the roads are primitive (dirt roads often in poor condition), remote, and lightly traveled (Hutto et al. 1995). There is minimal human development and disruption to native vegetation adjacent to roads compared to vegetation further from the roadside (Wellicome et al. 2014). Additionally, McCarthy et al. (2012) and Lituma and Buehler (2016) respectively found negligible roadside bias in abundance and distribution of multiple species 200 and 0–600 m from the roadside.
We mapped predictions from our models to an expanded study area based on methods outlined by Anderson et al. (2019). This approach involved merging 15‐km radius buffers around all historical Gyrfalcon territories occupied at least once between 1998 and 2016 with additional 4.5‐km radius buffers around all point count locations surveyed in 2019, 2021, and 2022. This merging ensured that point count survey locations were included in the expanded study area (see Figure 1).
FIGURE 1.
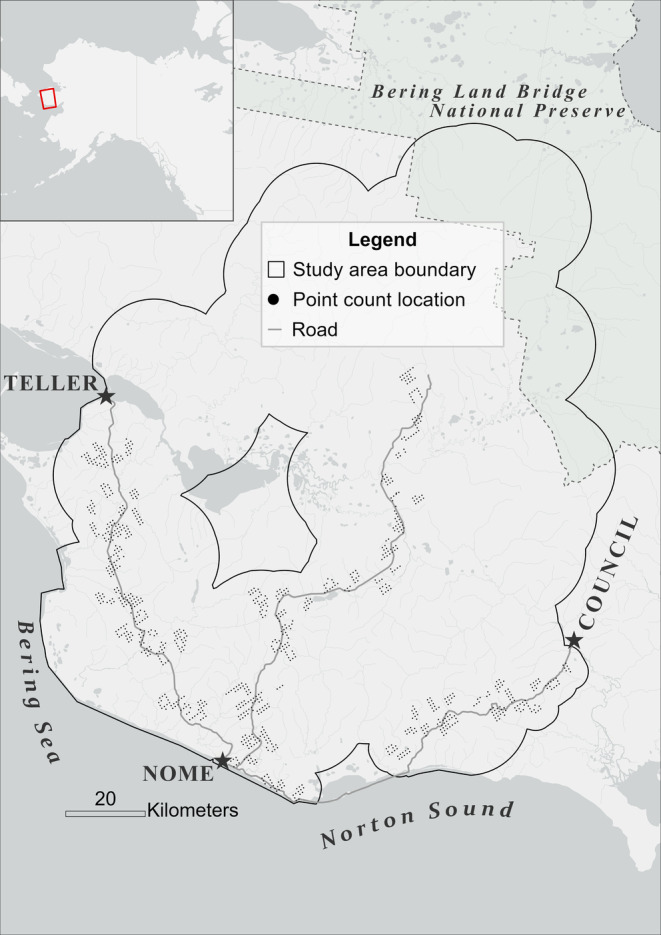
Map of the study area boundary on the southern portion of the Seward Peninsula, Alaska, USA. The inset map shows the location of the Seward Peninsula relative to Alaska (rectangle).
2.2. Point Count Surveys
We conducted multi‐species point count surveys (Bibby, Burgess, and Hill 2000; Ralph, Sauer, and Droege 1995) to detect all bird species and Arctic ground squirrels. We commenced surveys 30 min before astronomical sunrise, which is approximately the beginning of civil twilight, or dawn, and ended when at least 10 points were surveyed or ca. 12:00 each day (latest survey at 13:43). Each point was surveyed one time over the duration of 3 years. Surveys used a time‐removal approach where selected time intervals during the survey period act as replicate survey periods (Farnsworth et al. 2002). We conducted surveys for 10 min, preceded by a 2‐min quiet period, and recorded the first detection of an individual (auditory or visual) less than 400 m away (Savard and Hooper 1995; Farnsworth et al. 2002; Matsuoka et al. 2014). Subsequent detections of the same individual (e.g., if a bird continued calling) were not included (Farnsworth et al. 2002). Information collected for each detection included species, time, distance and bearing to individual(s), group size if multiple individuals (≥ 3) were within close proximity (i.e., flocks, squirrel colonies), and sex. Individuals that did not appear to be actively using the area or habitat (e.g., flyovers) were excluded from analysis (Bibby, Marsden, and Jones 1998; Bibby, Burgess, and Hill 2000). A primary observer dictated observations to a recorder.
We conducted point counts from May 10, 2019 to July 23, 2019 (467 points visited), May 5, 2021 to July 16, 2021 (449 points visited), and May 30, 2022 to June 29, 2022 (72 points visited). At each point (site), observers also measured temperature (°C) and wind speed (km/h) using a Kestrel 3000 Wind and Weather Meter and wind direction using a compass. We did not conduct surveys in sustained winds over 24 km/h, or in heavy fog or rain.
2.3. Prey Species
Willow Ptarmigan, Rock Ptarmigan, and Arctic ground squirrels are yearlong residents on the Seward Peninsula (Quay 1951; Kessel 1989) although Arctic ground squirrels undergo an extended hibernation period from August to May, depending on sex, age, and other physiological and environmental factors (Sheriff et al. 2012). Willow Ptarmigan are a medium‐sized ground‐dwelling bird in the family Tetraonidae occurring in Arctic, subarctic, and subalpine habitats (Hannon, Eason, and Martin 2020). During the breeding season (April—July), Willow Ptarmigan are typically found in low, moist habitats with dense vegetation, especially willow (Salix spp.) or birch (Betula spp.) shrub thickets of medium height (0.3–2.0 m) that provide food and shelter (Wilson and Martin 2008; Henden et al. 2011; Ehrich et al. 2012; Kvasnes, Pedersen, and Nilsen 2018; Hannon, Eason, and Martin 2020). They also occur in open tundra, hosting grasses, sedges, tussocks, and low‐growing shrubs (Schieck and Hannon 1993; Kastdalen et al. 2003; Hannon, Eason, and Martin 2020). Rock Ptarmigan share a portion of its range with Willow Ptarmigan but occur farther north than Willow Ptarmigan (Nielsen and Cade 2017; Montgomerie and Holder 2020). Rock Ptarmigan are typically found at higher elevations in dry and rocky habitats with sparse vegetation and dwarf shrubs, and well‐drained grassy tundra (Favaron et al. 2006; Wilson and Martin 2008; Revermann et al. 2012; Pedersen et al. 2014; Hotta et al. 2019; Montgomerie and Holder 2020). The Arctic ground squirrel is a rodent occurring in Arctic and subarctic regions of North America and northeast Russia that exhibit non‐cyclical temporal trends in abundance (ADFG n.d.‐a; Eddingsaas et al. 2004; Faerman et al. 2017). The Arctic ground squirrel inhabits boreal forest, and low‐ and high‐alpine tundra, as well as riverbanks and lakesides, but prefers open alpine meadows with sparse growing low shrubs (e.g., Dryas spp.), tall shrubs (e.g., Salix spp.), and rocky areas interspersed with forbs and lichens (Batzli and Sobaski 1980; Donker and Krebs 2011).
2.4. Habitat Covariates
We obtained land cover data describing vegetation on the Seward Peninsula from the Arctic Boreal Vulnerability Experiment project (hereafter ABoVE; Wang et al. 2019). The ABoVE project classified vegetation groups into 15 types based on the dominant vegetation derived from satellite imagery using a 30‐m grid cell resolution (described in Appendix, Table S1). We used the vegetation dataset from 2014, the most recent year available from the data published by the ABoVE project. Wang et al. (2019) provides further information on data processing, training, and assessments through the Oak Ridge National Laboratory (ORNL) Distributed Active Archive Center (DAAC).
For each species, we selected relevant vegetation types and habitat characteristics based on published descriptions of their preferred habitat described above under “Prey Species.” We selected habitats that would be used in each model to avoid correlated predictors and overparametization due to our low sample size for Rock Ptarmigan and Arctic ground squirrels. For Willow Ptarmigan, we selected herbaceous (hereafter referred to as “tundra”), tussock tundra (hereafter referred to as “tussock”), tall shrub, and elevation. For Rock Ptarmigan and Arctic ground squirrels, we selected low shrub, tundra, sparse vegetation, and elevation (examples in Figure 2). We extracted percent cover for each vegetation type within an 800 m × 800 m or 0.64‐km2 area surrounding each survey point. We obtained mean elevation within an 0.64‐km2 area surrounding survey point using a 5‐m resolution digital elevation model (DEM; U.S. Geological Survey 2017). We selected an area of 800 m × 800 m to closely replicate the area surveyed by point counts. We removed all cells with a mean elevation greater than 500 m as we did not have survey points above this elevation.
FIGURE 2.
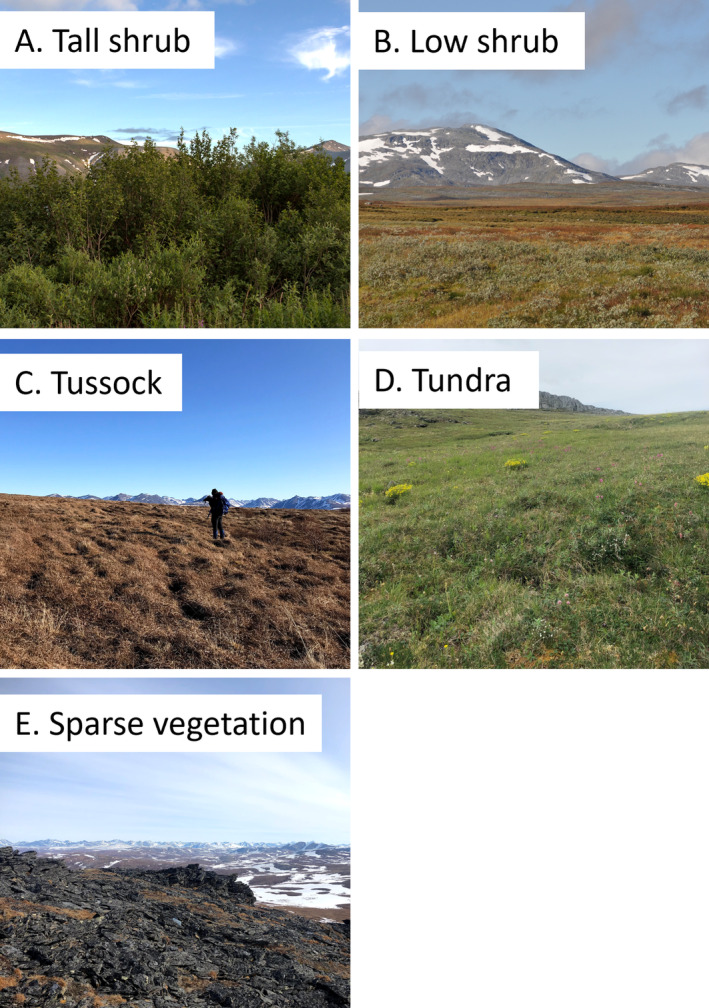
Example photos of vegetation types used in species abundance models. Photo credits: (A) Bill Saltzstein, (B) Mirja Lindberget, (C) Kari Williamson, (D and E) Michaela Gustafson.
Landscape predictors (tundra, tussock, sparse vegetation, low shrub, and tall shrub vegetation types and elevation) were not strongly correlated (Pearson's correlation coefficient, r| < |0.70; Akoglu 2018). We standardized landscape predictors by subtracting mean values and dividing by two standard deviations (Gelman 2008; Schielzeth 2010) before they were used in the species models described below. This allowed for direct comparisons of effect sizes and helped model convergence. All data manipulation and analyses were performed using the tidyverse (v2.0.0; Wickham et al. 2019) and unmarked (v1.3.2; Fiske and Chandler 2011) packages in R Statistical Software (v4.1.3; R Core Team 2023).
2.5. Gyrfalcon Territory Surveys
We surveyed nesting cliffs in our study area for occupied Gyrfalcon nests in May and June from 2016 to 2022 (except 2020 because of Covid‐19). We monitored 97 Gyrfalcon territories across two surveys per territory each year ranging from May 1 to July 2 during 2016–2022 (except 2020 because of Covid‐19). We conducted surveys from an R44 helicopter following protocols used in Bente (2011) and Anderson et al. (2019). We considered a territory to be potentially occupied if we observed a Gyrfalcon adult, egg, or nestling at a nest during each survey. A total of 60 surveys were not completed because of weather conditions and were assigned as missing values. Territory surveys also occurred every year from 2011 to 2015 but with different protocols. We used these prior surveys to confirm if a territory belonged to a Gyrfalcon during 2011–2015 since some were either unoccupied or occupied by other raptor species during 2016–2022. Our dataset thus, only included territories that were historical Gyrfalcon territories and were monitored during 2016–2022.
2.6. Statistical Analysis
2.6.1. N‐Mixture Model for Prey Abundances
We collated detections of unique individuals of the three prey species as repeated counts using a time‐removal design (Farnsworth et al. 2002; Royle 2004). The method involves partitioning the total survey period into time intervals to mimic a removal design. We partitioned counts of unique detections for each species into five, 2‐min intervals. Counts were modeled separately for each species using an N‐mixture, time‐removal model (Kéry and Royle 2016; Chandler 2019) using the “unmarked” package (v1.3.2; Fiske and Chandler 2011; Kellner et al. 2023) in R (v4.1.3; R Core Team 2023). For both ptarmigan species, we retained counts of individuals identified as males in analyses because < 5% of counts for Willow Ptarmigan and < 7% of counts for Rock Ptarmigan were identified as female or unknown sex. We did not count juvenile ptarmigan. Therefore, results for both ptarmigan species are estimates of adult males only. We included all Arctic ground squirrel counts in analyses because sex and age classes are not readily distinguishable in the field. Each species Poisson N‐mixture model consisted of two submodels: (1) relating abundance to landscape predictors, and (2) relating the probability of detecting an individual of the species to predictors that may influence detection. For Willow Ptarmigan, we tested how abundance was related to tall shrub, tundra, tussock, and elevation. For Rock Ptarmigan and Arctic ground squirrel, we tested the relationship between abundance and tundra, low shrub, sparse vegetation, and elevation. Abundance submodels for the three species also included the area sampled by the point counts (assumed to be 0.5024 km2 with a 400‐m radius) as an offset, which converted the relative abundance estimated to density values per m2 and allowed density estimates to be made for regions of any area (Sillett et al. 2012; Chandler 2019). We did not include year as a factor in the abundance submodels because of low sample sizes. We assumed spatial differences in abundances to be constant and related to habitat. Detection submodels for the three species included ordinal date (recorded as sequential day of year), the number of minutes after civil twilight when the survey began (hereafter referred to as “time of day”), wind speed (kilometers per hour), and observer ID (included as a factor). These predictors have been shown to affect detectability in similar surveys methods (Best 1981; Ralph 1981; Richards 1981; Robbins 1981; Skirvin 1981; Bart and Herrick 1984; Schieck 1997; Simons et al. 2007; Farmer, Leonard, and Horn 2012). We evaluated the significance of predictors in each species model by examining the overlap of the 95% confidence interval with zero. Those predictors having 95% confidence intervals that did not overlap with zero were deemed significant. We also plotted marginalized effect plots for significant predictors. Marginalized effect plots assess the partial relationship between a predictor and the response, while keeping other predictors in the model at a constant value (i.e., the mean).
Lastly, we evaluated model fit for each species model using parametric bootstrapping and Pearson's Chi‐squared statistic (χ 2) to compare between abundances estimated from the model versus those observed in the data. p‐values greater than 0.05 suggest that the observed and estimated abundances are not significantly different from each other and thus indicate that the models are reliable (Kéry and Royle 2016). We also evaluated the dispersion measure ĉ where values > 1 indicate more variance in the observed data than expected by the model and much higher than one (i.e., > 4) poor model fit. A dispersion measure < 1 suggests there may have been more empty sites than what would be predicted by a Poisson model (MacKenzie and Bailey 2004).
2.6.2. Mapping Prey Population Density
We used our estimated abundance models to make predictions of species density on an 800 m × 800 m grid overlapping our study area using the ‘predict’ function from the “unmarked” package (v1.3.2). We excluded cells with mean elevations > 500 m and with covariate values outside the range considered in our prey models. We aggregated the 5‐m digital elevation model using bilinear interpolation to calculate the mean. We standardized each predictor layer (habitat and elevation) with its corresponding mean and two standard deviations from the predictor values used to fit the models.
To estimate prey density inside each Gyrfalcon territory, we excluded cells within each territory with vegetation percentage values that fell outside the ranges used in our prey abundance models, and with elevations exceeding 500 m. We calculated the area size of each territory correcting for these removed cells. We summed expected density of each prey from the grid cells inside each Gyrfalcon territory and divided by the corrected territory area. For Willow Ptarmigan and Rock Ptarmigan, densities were expressed as males per square kilometer, whereas for Arctic Ground Squirrels, densities were reported as squirrels per square kilometer.
2.6.3. Gyrfalcon Occupancy Model
We employed a multi‐season occupancy model that accounts for imperfect detection (MacKenzie et al. 2002) to assess the relationship between annual Gyrfalcon occupancy (during 2016–2019, and 2021–2022) and densities of Willow Ptarmigan, Rock Ptarmigan and Arctic Ground Squirrels inside each territory. The model featured a hierarchical structure with two main components: an ecological submodel that linked occupancy to prey densities and a random intercept for year to allow for annual variability in occupancy, and an observation submodel that related detection probability to the day of the year the survey occurred as well as territory as a random intercept, to account for variability in detection among territories, as well as the repeated measures of territories over multiple years. The short time series and non‐temporal measures of prey density made this model structure more suitable than a dynamic occupancy approach.
We fitted the model using the “unmarked” package (v1.4.1, Fiske and Chandler 2011; Kellner et al. 2023) in R (v4.4.1, R Core Team 2024). We evaluated the significance of predictors based on non‐overlap of 95% confidence intervals with zero. We plotted marginalized effect plots for significant predictors. We evaluated model fit using parametric bootstrapping of Pearson's chi‐squared statistic to compare the tally of observed capture histories against those predicted in the model following MacKenzie and Bailey (2004). This was done using package AICcmodavg (v2.3.3, Mazerolle 2023).
3. Results
3.1. Summary Statistics
We retained 983 survey points in the analysis after removing points with a mean elevation greater than 500 m.a.s.l. within the surrounding 0.64 km2 area. We counted 485 male Willow Ptarmigan at 250 points, 55 male Rock Ptarmigan at 43 points, and 80 Arctic ground squirrels at 42 points (see Table 1 for yearly distribution of counts and how many points for each species).
TABLE 1.
Yearly distribution of counts for male Willow Ptarmigan, male Rock Ptarmigan, and Arctic ground squirrels and number of points where each species was detected.
| Willow Ptarmigan | ||
| Year | Individuals counted | Number of points |
| 2019 | 239 | 118 |
| 2021 | 237 | 127 |
| 2022 | 9 | 5 |
| 485 | 250 | |
| Rock Ptarmigan | ||
| Year | Individuals counted | Number of points |
| 2019 | 31 | 24 |
| 2021 | 14 | 13 |
| 2022 | 10 | 6 |
| 55 | 43 | |
| Arctic ground squirrel | ||
| Year | Individuals counted | Number of points |
| 2019 | 42 | 17 |
| 2021 | 35 | 23 |
| 2022 | 3 | 2 |
| 80 | 42 | |
Note: Prey surveys took place on the Seward Peninsula, Alaska, USA from May through July of 2019, 2021, and 2022.
3.2. Prey Distribution and Abundance
We found weak evidence of overdispersion for all three species as indicated by ĉ values that were not significantly higher than 1 (Willow Ptarmigan = 1.01, Rock Ptarmigan = 0.90, Arctic ground squirrel = 0.74), although the Arctic ground squirrel data were underdispersed.
The Pearson's chi‐squared statistic (χ 2) suggested reasonable fit for the Willow Ptarmigan (p = 0.35), Rock Ptarmigan (p = 0.62), and Arctic ground squirrel (p = 0.60) models.
The probability of detection for Willow Ptarmigan was related primarily to the day of year when surveys were conducted (Figure 3B), with detection declining sharply as the season progressed (Figure 4A). Time of day and wind speed were statistically significant in the detection submodel (Figure 3B) but had weak negative effects on Willow Ptarmigan detection (Figure 4B,C). We found considerable observer variation in detection probability (Figure 3C). Observer detection probabilities are based on a 2‐min temporal replicate within a full 10‐min survey conducted at a given site. Day of year was also important for the probability of detection for Rock Ptarmigan with a negative effect as the season progressed (Figures 3B and 4E). Time of day, wind speed and observer ID had non‐significant effects on the probability of detection for Rock Ptarmigan. Time of day had the strongest relationship with the probability of detection for Arctic ground squirrels (Figure 3B) with detection increasing later in the day (Figure 3G). Day of year and wind speed had significant, but weak, negative effects on detection probability (Figures 3B and 4F,H). Observer ID had non‐significant effects on detection of Arctic ground squirrels.
FIGURE 3.
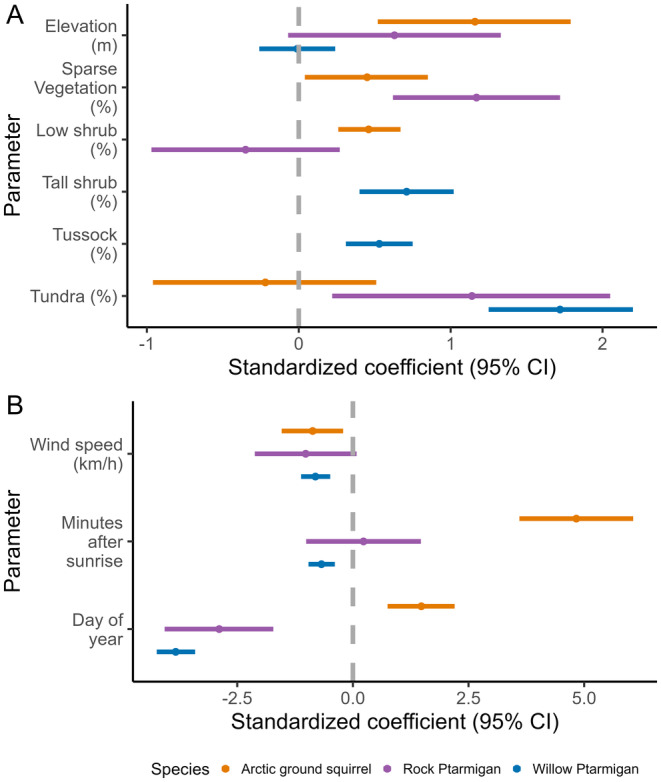
Averaged parameter estimates (standardized regression coefficients) of continuous model predictors are shown as points with lower and upper 95% confidence intervals as horizontal lines for all three species' time‐removal models with abundance (A) and detection (B) submodels. Values that do not overlap with zero (vertical gray dashed line) are considered significant.
FIGURE 4.
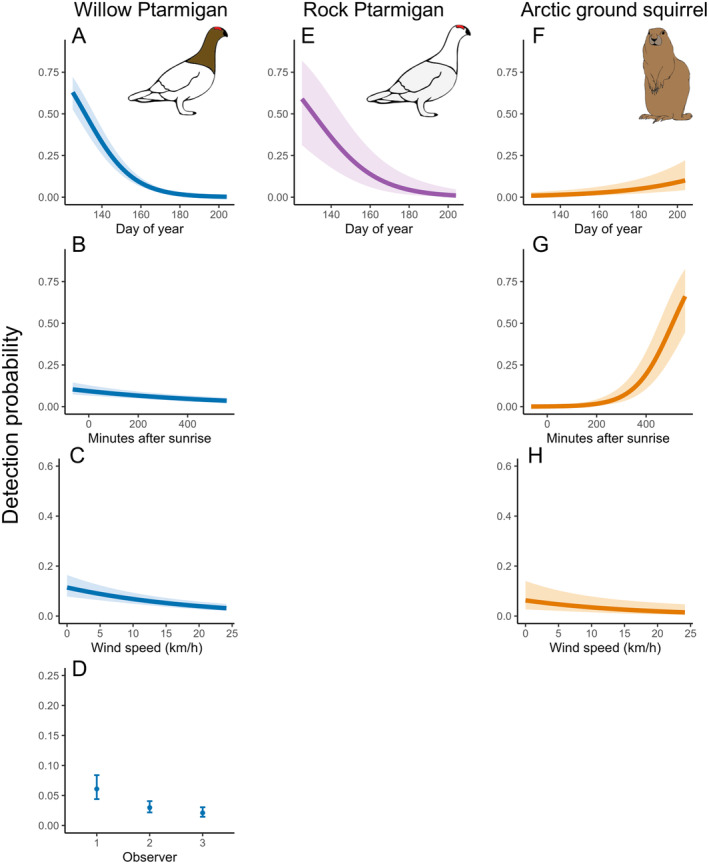
Marginalized effects plot of significant predictors, based on 95% CI, of Willow Ptarmigan (A–D), Rock Ptarmigan (E), and Arctic ground squirrel (F–H) detection on the Seward Peninsula, Alaska, USA from May through July of 2019, 2021, and 2022. Means and 95% confidence intervals are shown by the solid lines and light‐colored bands, respectively. Additional model parameters were held constant.
Willow Ptarmigan density was significantly and positively correlated to percent cover of tundra (%), followed by the percent cover of tall shrubs and tussock tundra (Figures 3A and 5A–C). Rock Ptarmigan density was significantly and positively correlated with the percent cover of sparse vegetation and tundra (Figures 3A and 5D). Arctic ground squirrel density was significant and positively correlated to cover of sparse vegetation, low shrub, and elevation (Figures 3A and 5F–H).
FIGURE 5.
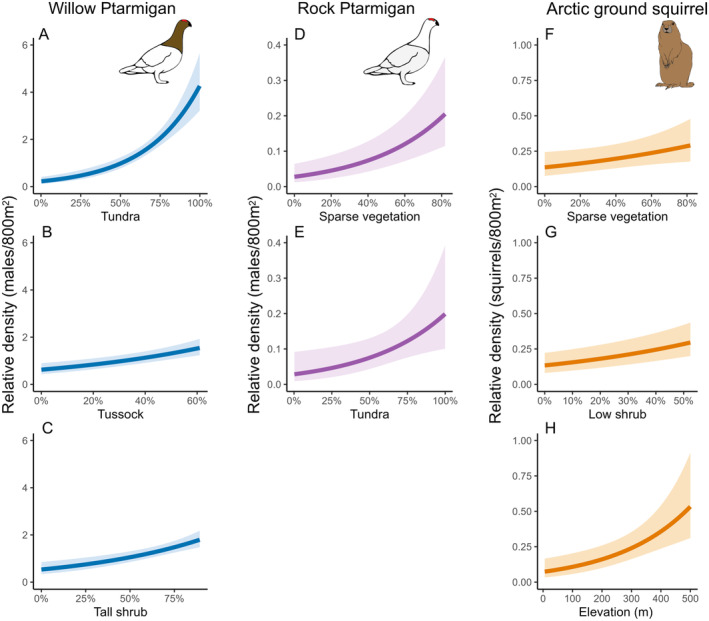
Marginalized effects plots showing the relationship between important (based on 95% CIs non‐overlapping zero) predictors and abundance for (A–C) Willow Ptarmigan, (D–E) Rock Ptarmigan, and (F–H) Arctic ground squirrel. Means and 95% credible intervals are shown by the solid lines and light‐colored shaded area, respectively. Additional model parameters were held at mean values.
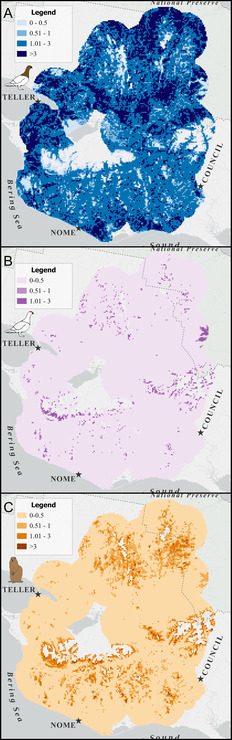
Willow Ptarmigan, Rock Ptarmigan, and Arctic ground squirrels differed widely in their spatial abundance and distribution (Figure 6). Willow Ptarmigan were widespread within the study area, with higher densities (> 3 males per 0.64 km2) found in the northern portion of the study area, but also more patchily in the west near Teller and in the south near Nome and extending southeast along the Norton Sound (see Figure 6A). The remainder of the study area had densities from 1 to 3 Willow Ptarmigan males per 0.64 km2 grid cell making Willow Ptarmigan the most abundant of the three species studied. Willow Ptarmigan had a mean density of 2.13 males per 0.64 km2 (95% CI, 1.66–2.76). Minimum and maximum Willow Ptarmigan densities were 0.26 and 5.08 males per 0.64 km2 (0.4–7.9 males/km2), respectively. Total estimated abundance for the study area was 55,431 males (95% CI, 43,187—71,712).
FIGURE 6.
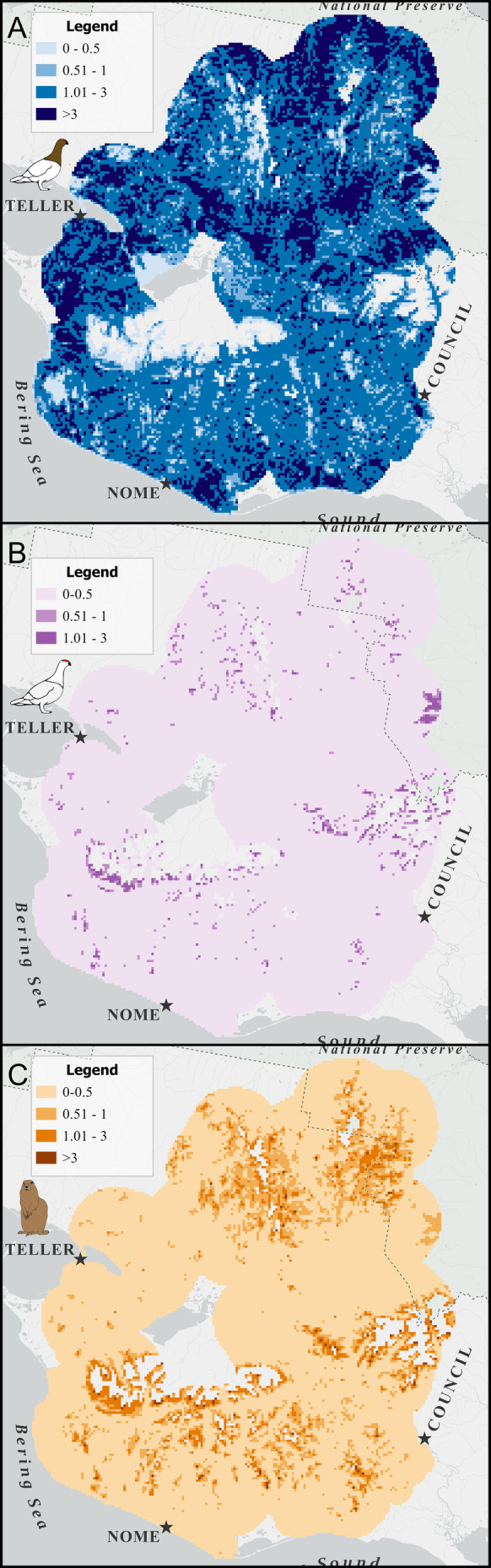
Maps showing the density (males/800 m2 for ptarmigan and individuals/800 m2 for squirrels) and distribution of three key prey species for Gyrfalcons: (A) Willow Ptarmigan, (B) Rock Ptarmigan, and (C) Arctic ground squirrel, within the study area on the Seward Peninsula, Alaska, USA from May through July of 2019, 2021, and 2022. No densities > 3 were observed for Rock Ptarmigan and that category was not displayed.
Rock Ptarmigan were the most sparsely distributed of the three species, with patchy populations scattered across the peninsula, appearing more concentrated in the north and central portions of the study area (see Figure 6B). In areas where Rock Ptarmigan did occur, they were primarily present at densities of < 0.5 males per 0.64 km2. Areas with densities > 1 male within 0.64 km2 appeared patchily in the north and south, with two areas of higher concentration along the mountains south of Teller and in the northeast within the Bering Land Bridge National Preserve. These high‐density areas were confined to high‐elevation sites within the study area, although elevation was not an important predictor in the model. Rock Ptarmigan had a mean density of 0.15 males per 0.64 km2 (95% CI, 0.07–0.33). Minimum and maximum Rock Ptarmigan densities were 0.003 and 2.94 males per 0.64 km2 (0.004–4.6 males/km2), respectively. Total estimated abundance for the study area was 4041 males (95% CI, 1973—8568).
Arctic ground squirrels were more widespread than Rock Ptarmigan but less abundant than Willow Ptarmigan (see Figure 6C). Areas of high densities occurred in the northern portion of the study area and within the Bering Land Bridge National Preserve and along high elevation areas of the mountains south of Teller extending east to north of Council. Arctic ground squirrels had a mean density of 0.36 squirrels per 0.64 km2 (95% CI, 0.21–0.65). Minimum and maximum Arctic ground squirrel densities were 0.07 and 6.65 individuals per 0.64 km2 (0.1–10.4 squirrels/km2), respectively. Total estimated abundance for the study area was 9512 squirrels (95% CI, 5426—16,965).
3.3. Gyrfalcon Occupancy
The Gyrfalcon occupancy model was not over‐dispersed (c‐hat = 1.2) and had a reasonable fit based on the χ 2 statistic (p = 0.27). The mean occupancy probability was 0.53 and mean detection probability was 0.53. Gyrfalcon detection probability was higher earlier in the season (Figure 7). Detection also varied largely among territories (variance = 7.46), which reflects the difference in visibility among cliffs. Gyrfalcon occupancy was positively associated with densities of Willow Ptarmigan and Arctic Ground Squirrels (Figure 7A). Rock Ptarmigan density had a marginally significant positive effect on Gyrfalcon occupancy (Figure 7A). Mean Gyrfalcon occupancy probability was ~70% when Willow Ptarmigan density reached 4 males per square kilometer (Figure 7B). Mean Gyrfalcon occupancy probability was ~60% when Arctic ground squirrels reached about 1 individual per square kilometer (Figure 7C). Occupancy did not vary significantly across years (variance = 0.00).
FIGURE 7.
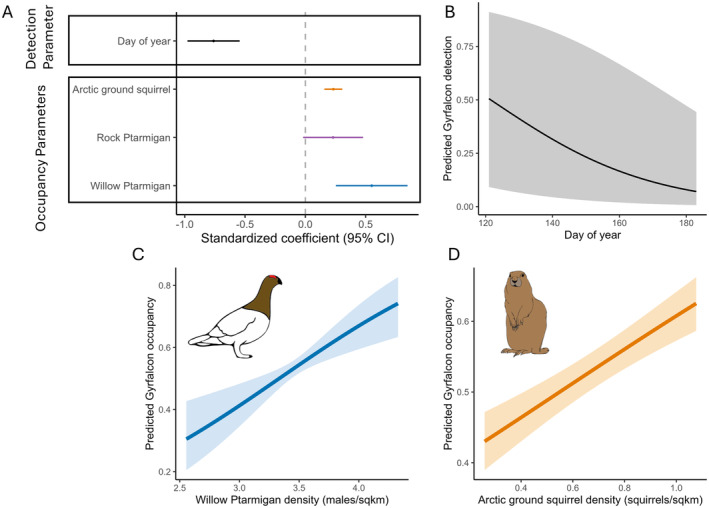
(A, top left) Averaged standardized regression coefficients with 95% confidence intervals for Gyrfalcon occupancy and detection submodels. (B, top right) Marginalized effects plot showing the relationship between day of the year and Gyrfalcon occupancy probability, with mean and 95% credible intervals. (C, bottom left, and D, bottom right) Marginalized effects plots showing the relationship between Willow Ptarmigan and Arctic ground squirrel density and Gyrfalcon occupancy probability, with mean and 95% credible intervals. Other model parameters were held at mean values.
4. Discussion
Understanding relationships between Arctic habitats and the abundance and distribution of keystone species or species of conservation concern is a crucial first step in predicting individual responses to habitat shifts resulting from ongoing climate change. We provide the first assessments of habitat‐related abundances of three small herbivore species and one of their top predators on the Seward Peninsula, Alaska, considered fundamental elements of tundra food webs and ecosystem function. We also assessed whether Gyrfalcons were more likely to occupy territories with higher densities these three important prey species. We found Willow Ptarmigan were most abundant in areas with a high proportion of tundra cover, followed by areas with tall shrub and tussock tundra cover. Rock Ptarmigan were most common in areas of tundra and sparse vegetation whereas Arctic ground squirrels were most abundant in higher elevations with sparse vegetation and low shrubs. Rock Ptarmigan and Arctic ground squirrels had patchy distributions, with few pockets of high abundance. Gyrfalcons, in turn, were more likely to occupy territories with higher densities of Willow Ptarmigan and Arctic ground squirrels. Gyrfalcon occupancy was also positively associated with Rock Ptarmigan density, but not significantly.
The habitat relationships for the three species supported previous findings. We found that Willow Ptarmigan were most abundant in areas with a higher percentage cover of open tundra. While tall shrubs and tussock grasses were important, their effect was less pronounced than that of tundra. This supports previous studies suggesting that Willow Ptarmigan rely on a mix of open tundra and shrubs for food, nesting sites, and refugia from predation (Wilson and Martin 2008; Kvasnes, Pedersen, and Nilsen 2018; Hannon, Eason, and Martin 2020). Rock Ptarmigan were most abundant in areas with higher percent cover of open tundra and sparse vegetation, which they likely use for foraging and nesting (Favaron et al. 2006; Wilson and Martin 2008; Sawa, Takeuchi, and Nakamura 2011; Revermann et al. 2012; Hotta et al. 2019; Montgomerie and Holder 2020). Artic ground squirrels were most abundant at higher elevations and with higher percent cover of sparse vegetation and low shrubs, complementing previous studies suggesting that Arctic ground squirrels prefer open habitats for better predator detection (Wheeler and Hik 2014a; Wheeler et al. 2015) and burrowing (Karels and Boonstra 1999).
The three herbivore species are likely to respond differently to shrub encroachment, given their current habitat associations. Increased shrub growth and expansion in Arctic tundra biomes (Tape, Sturm, and Racine 2006; Myers‐Smith et al. 2011; Vuorinen et al. 2017) has largely been attributed to increasing air temperature (Elmendorf et al. 2012; Myers‐Smith and Hik 2018). However, shrub expansion has been heterogeneous and may to be limited by a number of factors such as soil characteristics, topography (Tape, Sturm, and Racine 2006; Myers‐Smith et al. 2011; Tape et al. 2012; Swanson 2015; Ackerman et al. 2017; Liljedahl et al. 2020; Schore et al. 2023), and herbivory (Christie et al. 2015). If shrub expansion remains limited to hillslopes and incised topography such as water tracks, then Willow Ptarmigan will likely benefit. Open tundra and more sparsely vegetated habitats at higher elevations, further from floodplains and riparian zones may be initially resistant to shrub expansion, thereby providing refugia for Rock Ptarmigan and Arctic ground squirrels. However, increased fire frequency and permafrost degradation along with increasing temperatures and summer precipitation may facilitate future shrub growth in areas currently unsuitable for them (Wahren, Walker, and Bret‐Harte 2005; Chen, Hu, and Lara 2021; Liu et al. 2022). Nonetheless, the impacts of Arctic warming and shrub expansion over long time scales remain uncertain and complex, highlighting the need for ongoing monitoring of how animals are responding as these changes occur.
Densities of Willow Ptarmigan (3.32 males/km2), Rock Ptarmigan (0.23 males/km2), and Arctic ground squirrels (0.56 squirrels/km2) on the Seward Peninsula were lower than densities reported elsewhere. Willow Ptarmigan densities ranged between 6 and 229 birds/km2 in Norway (Holmstad, Hudson, and Skorping 2005; Kvasnes et al. 2017; Breisjøberget et al. 2018), 9 birds/km2 in western Canada, and 7.45–30 birds/km2 in Alaska, (Bart et al. 2011). Rock Ptarmigan densities were 2–17 males/km2 in Iceland (Nielsen 2011), 8–64 adults/km2 in Scotland (Zohmann and Wöss 2008), 0.47–6.4 males/km2 in the European Alps (Favaron et al. 2006), and 0.86–5.57 birds/km2 in Alaska (Bart et al. 2011). Arctic ground squirrel densities were 50–150 squirrels/km2 in northern Alaska (Batzli and Sobaski 1980) 38–610 squirrels/km2 in southwest Canada (Donker and Krebs 2011), and 40–270 squirrels/km2 in northwestern Canada (Donker and Krebs 2011). Differences in densities across regions may be related to resource availability. Ptarmigan populations in other locations cycle, or go through regular, repeating periods of population increase followed by declines (Fuglei et al. 2020). It is possible our surveys captured populations during a regular period of decline, however, without long‐term population data, we cannot say if the ptarmigan populations on the Seward Peninsula cycle.
Population differences in abundance among regions may also be due to top‐down effects including hunting pressure from humans and predation (Sandercock et al. 2011). In Alaska, Willow and Rock Ptarmigan are managed through hunting regulations, with bag limits of 20 birds per day and seasonal closures (ADFG n.d.‐b). There is no closed season and no bag limits in place for the hunting of Arctic ground squirrels (ADFG n.d.‐a), but Arctic ground squirrels are not a popular or frequently hunted species (Bacon et al. 2009). Rock Ptarmigan populations were highest in Iceland, where major predators include Gyrfalcons, Common Ravens ( Corvus corax ), owls ( Bubo scandiacus , Asio otus , Asio flammeus ), Arctic foxes ( Vulpes lagopus ), and mink ( Neovison vison ). Whereas the Seward Peninsula is host to many avian predators during the breeding season, as well as bears (Urus arctos), lynx ( Lynx canadensis ), foxes ( Vulpes vulpes , Vulpes lagopus ), wolverines ( Gulo gulo ), ermine or short‐tailed weasel ( Mustela erminea ), and least weasels ( Mustela nivalis ) that may depredate all three species at various life stages (ADFG n.d.‐c).
Future survey protocols could be modified to maximize detection. Arctic ground squirrels hid in their burrows and Rock Ptarmigan flushed outside the 400‐m boundary as we walked toward the survey points. Aleix‐Mata et al. (2020) show that plot‐sampling methods underestimated Rock Ptarmigan densities by 87%. Thus, alternative survey protocols could include distance sampling or repeated counts from walking transects for Arctic ground squirrels and Rock Ptarmigan (Amundson, Royle, and Handel 2014; Kukka et al. 2021). Further, results from the detection submodels suggest that ptarmigan surveys should continue starting before sunrise and early in the breeding season (April–May), whereas the ground squirrel surveys should shift to starting mid‐morning and surveying throughout the afternoon, later in the season (e.g., July). Due to our low sample sizes for Rock Ptarmigan and Arctic ground squirrels, we caution that our models may not be applicable outside of our study area.
We found evidence that the spatial differences in abundance of the three primary prey species resulted in differences in occupancy among Gyrfalcon territories across the study area. Gyrfalcons were more likely to occupy territories with higher densities of all three species with two being statistically significant. At the population level, Gyrfalcons consume more ptarmigan and squirrels than any other prey item during the breeding season but show shifts in ptarmigan or squirrels being the dominant prey type consumed at different times during the breeding season and from one breeding season to the next (Robinson et al. 2019). However, Gyrfalcons also show individual preferences in their diet with some individuals on the Seward Peninsula having more specialized diets, whereas others ate more diverse diets (Johnson et al. 2022). As shrubs expand throughout the region, the spatial distribution of prey is likely to change. Declines in their primary prey may force predators to shift to alternative species that may not meet their energetic or nutritional requirements (Resano‐Mayor et al. 2016) or may expose them to novel diseases (Radcliffe and Henderson 2023). Overall, changes in habitat are expected to affect prey abundances in different ways. Those changes are expected to scale up the trophic web, impacting the behavior, diet, and demography of the predators that rely on them.
Understanding species abundances and distributions provides a foundation to explore diverse aspects of Arctic ecology, from predator–prey relationships to predicting future spatial changes in habitats and associated species. Recognizing the importance of this insight for conservation and ecosystem functioning, we emphasize the key role of jointly investigating prey abundances, the habitats that they rely on, and the predators that they support. This not only enhances our understanding of Arctic raptor resilience but also contributes to unraveling the complexities of Arctic trophic webs. Our findings extend beyond current knowledge, offering a comprehensive view of habitat dynamics and multi‐trophic web relationships in the Arctic.
Author Contributions
Michaela Gustafson: data curation (equal), formal analysis (lead), investigation (lead), methodology (supporting), supervision (equal), validation (equal), visualization (lead), writing – original draft (lead), writing – review and editing (equal). Jennifer D. McCabe: conceptualization (equal), formal analysis (supporting), methodology (equal), writing – review and editing (equal). Brian W. Rolek: conceptualization (equal), formal analysis (supporting), methodology (equal), writing – review and editing (equal). Travis L. Booms: conceptualization (equal), data curation (equal), funding acquisition (equal), investigation (equal), methodology (equal), project administration (equal), writing – review and editing (equal). Michael T. Henderson: data curation (equal), funding acquisition (equal), investigation (equal), methodology (supporting), project administration (equal), supervision (equal), writing – review and editing (equal). Leah Dunn: conceptualization (equal), methodology (equal), writing – review and editing (equal). David L. Anderson: conceptualization (equal), funding acquisition (equal), methodology (equal), project administration (equal), writing – review and editing (equal). Jennyffer Cruz: data curation (equal), formal analysis (supporting), methodology (supporting), supervision (equal), validation (equal), visualization (supporting), writing – original draft (supporting), writing – review and editing (equal).
Conflicts of Interest
The authors declare no conflicts of interest.
Declaration of Generative AI and AI‐Assisted Technologies in the Writing Process
During the preparation of this work, the authors used GPT 4.0 (OpenAI) to improve the clarity and readability of this work. After using this tool, the authors reviewed and edited the content as needed and take full responsibility for the content of the publication.
Supporting information
Appendix S1.
Acknowledgments
We thank Peter Bente for his decades of hard work surveying Gyrfalcons and delineating territories that facilitated this research. We thank Kari Williamson and Derek Sauer for their hard work in the field and John Earthman for providing logistical support for the project. We also thank Chris McClure for his guidance in developing point count methodology and Jesse Barber for his comments on earlier drafts. We thank the Associate Editor and anonymous reviewers for their valuable comments that improved the quality of the paper.
Funding: This study was supported by Alaska Department of Fish and Game, The Peregrine Fund, Lynn and Jack Loacker, The Eppley Foundation, the Mohamed bin Zayed Species Conservation Fund, the Robert Wood Johnson Foundation, and Bill and Rae Saltzstein.
Data Availability Statement
The data that support the findings of this study are openly available on Dryad. https://doi.org/10.5061/dryad.djh9w0w94.
References
- Ackerman, D. , Griffin D., Hobbie S. E., and Finlay J. C.. 2017. “Arctic Shrub Growth Trajectories Differ Across Soil Moisture Levels.” Global Change Biology 23: 4294–4302. 10.1111/gcb.13677. [DOI] [PubMed] [Google Scholar]
- Akoglu, H. 2018. “User's Guide to Correlation Coefficients.” Turkish Journal of Emergency Medicine 18, no. 3: 91–93. 10.1016/j.tjem.2018.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alaska Department of Fish and Game . n.d.‐a. “Arctic Ground Squirrel (Spermophilus parryii).” State of Alaska. Retrieved March 25, 2024. https://www.adfg.alaska.gov/index.cfm?adfg=arcticgroundsquirrel.main.
- Alaska Department of Fish and Game . n.d.‐b. “Small Game Hunting in Alaska.” State of Alaska. Retrieved March 25, 2024. https://www.adfg.alaska.gov/index.cfm?adfg=smallgamehunting.species.
- Alaska Department of Fish and Game . n.d.‐c. “Willow Ptarmigan (Lagopus lagopus).” State of Alaska. Retrieved March 25, 2024. https://www.adfg.alaska.gov/index.cfm?adfg=willowptarmigan.main.
- Aleix‐Mata, G. , Mossoll‐Torres M., Marty E., Boos M., Sánchez A., and Pérez J. M.. 2020. “Distance Sampling Vs. Plot Sampling for Monitoring Population Abundances of the Pyrenean Rock Ptarmigan.” European Journal of Wildlife Research 66, no. 4: 68. 10.1007/s10344-020-01407-9. [DOI] [Google Scholar]
- Amundson, C. L. , Royle J. A., and Handel C. M.. 2014. “A Hierarchical Model Combining Distance Sampling and Time Removal to Estimate Detection Probability During Avian Point Counts.” Auk 131, no. 4: 476–494. 10.1642/AUK-14-11.1. [DOI] [Google Scholar]
- Anderson, D. L. , Bente P. J., Booms T. L., Dunn L., and McClure C. J. W.. 2019. “Nonrandom Territory Occupancy by Nesting Gyrfalcons ( Falco rusticolus ).” Arctic Science 5, no. 3: 148–160. 10.1139/as-2018-0024. [DOI] [Google Scholar]
- Bacon, J. J. , Hepa T. R., Brower H. K. Jr., et al. 2009. “Estimates of Subsistence Harvest for Villages on the North Slope of Alaska, 1994–2003.” Barrow, Alaska: North Slope Borough Department of Wildlife Management.
- Barichello, N. , and Mossop D.. 2011. “The Overwhelming Influence of Ptarmigan Abundance on Gyrfalcon Reproductive Success in the Central Yukon, Canada.” In Gyrfalcons and Ptarmigan in a Changing World, Volume I, edited by Watson R. T., Cade T. J., Fuller M., Hunt G., and Potapov E., 307–322. Boise, ID: Peregrine Fund. 10.4080/gpcw.2011.0205. [DOI] [Google Scholar]
- Bart, J. , Fuller M., Smith P., and Dunn L.. 2011. “Use of Large‐Scale, Multi‐Species Surveys to Monitor Gyrfalcon and Ptarmigan Populations.” In Gyrfalcons and Ptarmigan in a Changing World, Volume I, edited by Watson R. T., Cade T. J., Fuller M., Hunt G., and Potapov E., 263–272. Boise, ID: Peregrine Fund. 10.4080/gpcw.2011.0126. [DOI] [Google Scholar]
- Bart, J. , and Herrick J.. 1984. “Diurnal Timing of Bird Surveys.” Auk 101: 384–387. [Google Scholar]
- Batzli, G. O. , and Sobaski S. T.. 1980. “Distribution, Abundance, and Foraging Patterns of Ground Squirrels Near Atkasook, Alaska.” Arctic and Alpine Research 12, no. 4: 501–510. [Google Scholar]
- Bente, P. J. 2011. “Abundance and Multi‐Year Occupancy of Gyrfalcons Falco rusticolus on the Seward Peninsula, Alaska.” In Gyrfalcons and Ptarmigan in a Changing World, edited by Watson R. T., Cade T. J., Fuller M., Hunt G., and Potapov E., 295–306. Boise, ID: Peregrine Fund. 10.4080/gpcw.2011.0204. [DOI] [Google Scholar]
- Best, L. 1981. “Seasonal Changes in Detection of Individual Bird Species.” Studies in Avian Biology 6: 252–261. [Google Scholar]
- Bibby, C. J. , Burgess N. D., and Hill D. A.. 2000. Bird Census Techniques. 2nd ed. London, UK: Academic Press. [Google Scholar]
- Bibby, C. J. , Marsden S., and Jones M.. 1998. Bird Surveys: Expedition Field Techniques. London, UK: Expedition Advisory Centre. [Google Scholar]
- Bintanja, R. , and Selten F. M.. 2014. “Future Increases in Arctic Precipitation Linked to Local Evaporation and Sea‐Ice Retreat.” Nature 509, no. 7501: 479–482. 10.1038/nature13259. [DOI] [PubMed] [Google Scholar]
- Booms, T. , Lindgren M., and Huettmann F.. 2011. “Linking Alaska's Predicted Climate, Gyrfalcon, and Ptarmigan Distributions in Space and Time: A Unique 200‐Year Perspective.” In Gyrfalcons and Ptarmigan in a Changing World, Volume I, edited by Watson R. T., Cade T. J., Fuller M., Hunt G., and Potapov E., 177–190. Boise, ID: Peregrine Fund. 10.4080/gpcw.2011.0116. [DOI] [Google Scholar]
- Booms, T. L. , Schempf P. F., McCaffery B. J., Lindberg M. S., and Fuller M. R.. 2010. “Detection Probability of Cliff‐Nesting Raptors During Helicopter and Fixed‐Wing Aircraft Surveys in Western Alaska.” Journal of Raptor Research 44, no. 3: 175–187. 10.3356/JRR-09-70.1. [DOI] [Google Scholar]
- Breisjøberget, J. I. , Odden M., Wegge P., Zimmermann B., and Andreassen H.. 2018. “The Alternative Prey Hypothesis Revisited: Still Valid for Willow Ptarmigan Population Dynamics.” PLoS One 13, no. 6: 1–14. 10.1371/journal.pone.0197289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canonne, C. , Novoa C., Muffat‐Joly B., et al. 2020. “Life on the Edge: Common Slow Pace of Life but Contrasted Trajectories of Alpine Rock Ptarmigan Populations at Their Southern Margin.” Wildlife Biology 2020, no. 2: 1–11. 10.2981/wlb.00628. [DOI] [Google Scholar]
- Chandler, R. 2019. “Modeling and Mapping Species Distributions.” https://cran.rstudio.com/web/packages/unmarked/vignettes/spp‐dist.html.
- Chen, Y. , Hu F. S., and Lara M. J.. 2021. “Divergent Shrub‐Cover Responses Driven by Climate, Wildfire, and Permafrost Interactions in Arctic Tundra Ecosystems.” Global Change Biology 27, no. 3: 652–663. 10.1111/gcb.15451. [DOI] [PubMed] [Google Scholar]
- Christie, K. S. , Bryant J. P., Gough L., Ravolainen V. T., Ruess R. W., and Tape K. D.. 2015. “The Role of Vertebrate Herbivores in Regulating Shrub Expansion in the Arctic: A Synthesis.” Bioscience 65, no. 12: 1123–1133. 10.1093/biosci/biv137. [DOI] [Google Scholar]
- Donker, S. A. , and Krebs C. J.. 2011. “Habitat‐Specific Distribution and Abundance of Arctic Ground Squirrels ( Urocitellus parryii plesius ) in Southwest Yukon.” Canadian Journal of Zoology 89, no. 6: 570–576. 10.1139/z11-041. [DOI] [Google Scholar]
- Donker, S. A. , and Krebs C. J.. 2012. “Evidence for Source–Sink Dynamics in a Regional Population of Arctic Ground Squirrels ( Urocitellus parryii plesius ).” Wildlife Research 39, no. 2: 163–170. [Google Scholar]
- Eddingsaas, A. A. , Jacobsen B. K., Lessa E. P., and Cook J. A.. 2004. “Evolutionary History of the Arctic Ground Squirrel ( Spermophilus parryii ) in Nearctic Beringia.” Journal of Mammalogy 85, no. 4: 601–610. 10.1644/BRB-204. [DOI] [Google Scholar]
- Ehrich, D. , Henden J.‐A., Ims R. A., et al. 2012. “The Importance of Willow Thickets for Ptarmigan and Hares in Shrub Tundra: The More the Better?” Oecologia 168, no. 1: 141–151. 10.1007/s00442-011-2059-0. [DOI] [PubMed] [Google Scholar]
- Elmendorf, S. , Henry G., Hollister R., et al. 2012. “Plot‐Scale Evidence of Tundra Vegetation Change and Links to Recent Summer Warming.” Nature Climate Change 2: 453–457. 10.1038/nclimate1465. [DOI] [Google Scholar]
- Faerman, M. , Bar‐Gal G. K., Boaretto E., et al. 2017. “DNA Analysis of a 30,000‐Year‐Old Urocitellus Glacialis From Northeastern Siberia Reveals Phylogenetic Relationships Between Ancient and Present‐Day Arctic Ground Squirrels.” Scientific Reports 7, no. 1: 42639. 10.1038/srep42639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer, R. G. , Leonard M. L., and Horn A. G.. 2012. “Observer Effects and Avian‐Call‐Count Survey Quality: Rare‐Species Biases and Overconfidence.” Auk 129, no. 1: 76–86. 10.1525/auk.2012.11129. [DOI] [Google Scholar]
- Farnsworth, G. L. , Pollock K. H., Nichols J. D., Simons T. R., Hines J. E., and Sauer J. R.. 2002. “A Removal Model for Estimating Detection Probabilities From Point‐Count Surveys.” Auk 119, no. 2: 414–425. 10.1093/auk/119.2.414. [DOI] [Google Scholar]
- Favaron, M. , Scherini G. C., Preatoni D., Tosi G., and Wauters L. A.. 2006. “Spacing Behaviour and Habitat Use of Rock Ptarmigan ( Lagopus mutus ) at Low Density in the Italian Alps.” Journal of Ornithology 147, no. 4: 618–628. 10.1007/s10336-006-0087-z. [DOI] [Google Scholar]
- Ferrarini, A. , Alatalo J. M., and Gustin M.. 2017. “Climate Change Will Seriously Impact Bird Species Dwelling Above the Treeline: A Prospective Study for the Italian Alps.” Science of the Total Environment 590–591: 686–694. 10.1016/j.scitotenv.2017.03.027. [DOI] [PubMed] [Google Scholar]
- Fiske, I. , and Chandler R.. 2011. “Unmarked: An R Package for Fitting Hierarchical Models of Wildlife Occurrence and Abundance.” Journal of Statistical Software 43, no. 10: 1–23. [Google Scholar]
- Flower, C. E. , Dalton J. E., Whelan C. J., Brown J. S., and Gonzalez‐Meler M. A.. 2019. “Patch Use in the Arctic Ground Squirrel: Effects of Micro‐Topography and Shrub Encroachment in the Arctic Circle.” Oecologia 190, no. 1: 243–254. 10.1007/s00442-019-04400-5. [DOI] [PubMed] [Google Scholar]
- Fuglei, E. , Henden J.‐A., Callahan C. T., et al. 2020. “Circumpolar Status of Arctic Ptarmigan: Population Dynamics and Trends.” Ambio 49, no. 3: 749–761. 10.1007/s13280-019-01191-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furrer, R. , Schaub M., Bossert A., et al. 2016. “Variable Decline of Alpine Rock Ptarmigan ( Lagopus muta helvetica ) in Switzerland Between Regions and Sites.” Journal of Ornithology 157, no. 3: 787–796. 10.1007/s10336-016-1324-8. [DOI] [Google Scholar]
- Gelman, A. 2008. “Scaling Regression Inputs by Dividing by Two Standard Deviations.” Statistics in Medicine 27, no. 15: 2865–2873. 10.1002/sim.3107. [DOI] [PubMed] [Google Scholar]
- Gilg, O. , Kovacs K. M., Aars J., et al. 2012. “Climate Change and the Ecology and Evolution of Arctic Vertebrates.” Annals of the New York Academy of Sciences 1249, no. 1: 166–190. 10.1111/j.1749-6632.2011.06412.x. [DOI] [PubMed] [Google Scholar]
- Gillis, E. A. , Hik D. S., Boonstra R., Karels T. J., and Krebs C. J.. 2005. “Being High is Better: Effects of Elevation and Habitat on Arctic Ground Squirrel Demography.” Oikos 108, no. 2: 231–240. 10.1111/j.0030-1299.2005.13535.x. [DOI] [Google Scholar]
- Hannon, S. J. , Eason P. K., and Martin K.. 2020. “Willow Ptarmigan ( Lagopus lagopus ).” In Birds of the World, edited by Billerman S. M., Keeney B. K., Rodewald P. G., and Schulenberg T. S.. Ithaca, NY: Cornell Lab of Ornithology. 10.2173/bow.wilpta.01. [DOI] [Google Scholar]
- Hassol, S. J. , and Corell R. W.. 2006. “Arctic Climate Impact Assessment.” In Avoiding Dangerous Climate Change, 205–214. Cambridge, UK: Cambridge University Press. [Google Scholar]
- Henden, J.‐A. , Ims R. A., Yoccoz N. G., and Killengreen S. T.. 2011. “Declining Willow Ptarmigan Populations: The Role of Habitat Structure and Community Dynamics.” Basic and Applied Ecology 12, no. 5: 413–422. 10.1016/j.baae.2011.05.006. [DOI] [Google Scholar]
- Henderson, M. T. , Booms T. L., Robinson B. W., Johnson D. L., and Anderson D. L.. 2021. “Direct and Indirect Effects of Nesting Site Characteristics for a Cliff‐Nesting Raptor in Western Alaska.” Journal of Raptor Research 55, no. 1: 17–32. 10.3356/0892-1016-55.1.17. [DOI] [Google Scholar]
- Holmstad, P. R. , Hudson P. J., and Skorping A.. 2005. “The Influence of a Parasite Community on the Dynamics of a Host Population: A Longitudinal Study on Willow Ptarmigan and Their Parasites.” Oikos 111, no. 2: 377–391. 10.1111/j.0030-1299.2005.13640.x. [DOI] [Google Scholar]
- Hotta, M. , Tsuyama I., Nakao K., et al. 2019. “Modeling Future Wildlife Habitat Suitability: Serious Climate Change Impacts on the Potential Distribution of the Rock Ptarmigan Lagopus muta japonica in Japan's Northern Alps.” BMC Ecology 19, no. 1: 23. 10.1186/s12898-019-0238-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutto, R. L. , Hejl S. J., Kelly J. F., and Pletschet S. M.. 1995. “A Comparison of Bird Detection Rates Derived From On‐Road vs. off‐Road Point Counts in Northern Montana.” In Monitoring Bird Populations by Point Counts. Gen. Tech. Rep. PSW‐GTR‐149, edited by Ralph C. J., Sauer J. R., and Droege S., 103–110. Albany, CA: U.S. Department of Agriculture, Forest Service, Pacific Southwest Research Station. https://research.fs.usda.gov/treesearch/31746. [Google Scholar]
- Imperio, S. , Bionda R., Viterbi R., and Provenzale A.. 2013. “Climate Change and Human Disturbance Can Lead to Local Extinction of Alpine Rock Ptarmigan: New Insight From the Western Italian Alps.” PLoS One 8, no. 11: e81598. 10.1371/journal.pone.0081598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ims, R. A. , and Henden J.‐A.. 2012. “Collapse of an Arctic Bird Community Resulting From Ungulate‐Induced Loss of Erect Shrubs.” Biological Conservation 149, no. 1: 2–5. 10.1016/j.biocon.2012.02.008. [DOI] [Google Scholar]
- Johnson, D. L. , Henderson M. T., Anderson D. L., Booms T. L., and Williams C. T.. 2022. “Isotopic Niche Partitioning and Individual Specialization in an Arctic Raptor Guild.” Oecologia 198, no. 4: 1073–1084. 10.1007/s00442-022-05154-3. [DOI] [PubMed] [Google Scholar]
- Karels, T. J. , and Boonstra R.. 1999. “The Impact of Predation on Burrow Use by Arctic Ground Squirrels in the Boreal Forest.” Proceedings of the Royal Society of London. Series B: Biological Sciences 266, no. 1433: 2117–2123. 10.1098/rspb.1999.0896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastdalen, L. , Pedersen H., Fjone G., and Andreassen H.. 2003. “Combining Resource Selection Functions and Distance Sampling: An Example with Willow Ptarmigan.” Resource Selection Methods and Application. Western EcoSystems Technology, Cheyenne, Wyoming, USA, 52–59.
- Kausrud, K. L. , Mysterud A., Steen H., et al. 2008. “Linking Climate Change to Lemming Cycles.” Nature 456, no. 7218: 93–97. 10.1038/nature07442. [DOI] [PubMed] [Google Scholar]
- Kellner, K. F. , Smith A. D., Royle J. A., Kéry M., Belant J. L., and Chandler R. B.. 2023. “The Unmarked R Package: Twelve Years of Advances in Occurrence and Abundance Modelling in Ecology.” Methods in Ecology and Evolution 14, no. 6: 1408–1415. 10.1111/2041-210X.14123. [DOI] [Google Scholar]
- Kéry, M. , and Royle J. A.. 2016. Applied Hierarchical Modeling in Ecology: Analysis of Distribution, Abundance and Species Richness in R and BUGS. London, UK: Elsevier/AP, Academic Press is an imprint of Elsevier. [Google Scholar]
- Kessel, B. 1989. Birds of the Seward Peninsula, Alaska: Their Biogeography, Seasonality, and Natural History. Alaska: University of Alaska Press. [Google Scholar]
- Kukka, P. M. , Werner J. R., Andresen L. M., Krebs C. J., and Jung T. S.. 2021. “Landscape Drivers of Site Occupancy by Remnant Populations of Arctic Ground Squirrels ( Urocitellus parryii ).” European Journal of Wildlife Research 67, no. 6: 93. 10.1007/s10344-021-01534-x. [DOI] [Google Scholar]
- Kvasnes, M. A. J. , Pedersen H. C., and Nilsen E. B.. 2018. “Quantifying Suitable Late Summer Brood Habitats for Willow Ptarmigan in Norway.” BMC Ecology 18, no. 1: 41. 10.1186/s12898-018-0196-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvasnes, M. A. J. , Pedersen H. C., Storaas T., and Nilsen E. B.. 2017. “Vegetation Type and Demography of Low Density Willow Ptarmigan Populations.” Journal of Wildlife Management 81, no. 1: 174–181. 10.1002/jwmg.21180. [DOI] [Google Scholar]
- Kwon, E. , Weiser E. L., Lanctot R. B., et al. 2019. “Geographic Variation in the Intensity of Warming and Phenological Mismatch Between Arctic Shorebirds and Invertebrates.” Ecological Monographs 89, no. 4: e01383. 10.1002/ecm.1383. [DOI] [Google Scholar]
- Lehikoinen, A. , Green M., Husby M., Kålås J. A., and Lindström Å.. 2014. “Common Montane Birds Are Declining in Northern Europe.” Journal of Avian Biology 45, no. 1: 3–14. 10.1111/j.1600-048X.2013.00177.x. [DOI] [Google Scholar]
- Lituma, C. M. , and Buehler D. A.. 2016. “Minimal Bias in Surveys of Grassland Birds From Roadsides.” Condor 118, no. 4: 715–727. 10.1650/condor-16-73.1. [DOI] [Google Scholar]
- Liljedahl, A. K. , Timling I., Frost G. V., et al. 2020. “Arctic Riparian Shrub Expansion Indicates a Shift From Streams Gaining Water to Those That Lose Flow.” Communications Earth & Environment 1: 50. 10.1038/s43247-020-00050-1. [DOI] [Google Scholar]
- Liu, Y. , Riley W. J., Keenan T. F., et al. 2022. “Dispersal and Fire Limit Arctic Shrub Expansion.” Nature Communications 13, no. 1: 3843. 10.1038/s41467-022-31597-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKenzie, D. I. , and Bailey L. L.. 2004. “Assessing the Fit of Site‐Occupancy Models.” Journal of Agricultural, Biological, and Environmental Statistics 9, no. 3: 300–318. 10.1198/108571104X3361. [DOI] [Google Scholar]
- MacKenzie, D. I. , Nichols J. D., Lachman G. B., Droege S., Andrew Royle J., and Langtimm C. A.. 2002. “Estimating Site Occupancy Rates When Detection Probabilities Are Less Than One.” Ecology 83, no. 8: 2248–2255. 10.1890/0012-9658(2002)083[2248:ESORWD]2.0.CO;2. [DOI] [Google Scholar]
- Matsuoka, S. M. , Mahon C. L., Handel C. M., et al. 2014. “Reviving Common Standards in Point‐Count Surveys for Broad Inference Across Studies.” Condor 116, no. 4: 599–608. 10.1650/CONDOR-14-108.1. [DOI] [Google Scholar]
- Matthews, T. J. , and Whittaker R. J.. 2015. “REVIEW: On the Species Abundance Distribution in Applied Ecology and Biodiversity Management.” Journal of Applied Ecology 52, no. 2: 443–454. 10.1111/1365-2664.12380. [DOI] [Google Scholar]
- Mazerolle, M. J. 2023. “AICcmodavg: Model Selection and Multimodel Inference Based on (Q)AIC(c).” R Package Version 2.3.3. https://cran.r‐project.org/package=AICcmodavg.
- McCarthy, K. P. , Fletcher R. J. Jr., Rota C. T., and Hutto R. L.. 2012. “Predicting Species Distributions From Samples Collected Along Roadsides.” Conservation Biology 26: 68–77. 10.1111/j.1523-1739.2011.01754.x. [DOI] [PubMed] [Google Scholar]
- McCrystall, M. R. , Stroeve J., Serreze M., Forbes B. C., and Screen J. A.. 2021. “New Climate Models Reveal Faster and Larger Increases in Arctic Precipitation Than Previously Projected.” Nature Communications 12, no. 1: 6765. 10.1038/s41467-021-27031-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melin, M. , Mehtätalo L., Helle P., Ikonen K., and Packalen T.. 2020. “Decline of the Boreal Willow Grouse ( Lagopus lagopus ) has Been Accelerated by More Frequent Snow‐Free Springs.” Scientific Reports 10, no. 1: 6987. 10.1038/s41598-020-63993-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltofte, H. , Josefson A. B., and Payer D., eds. 2013. “Arctic Biodiversity Assessment: Status and Trends in Arctic Biodiversity. Synthesis.” Conservation of Arctic Flora and Fauna; Arctic Council.
- Montgomerie, R. , and Holder K.. 2020. “Rock Ptarmigan ( Lagopus muta ).” In Birds of the World, edited by Billerman S. M., Keeney B. K., Rodewald P. G., and Schulenberg T. S.. Ithaca, NY: Cornell Lab of Ornithology. 10.2173/bow.rocpta1.01. [DOI] [Google Scholar]
- Mossop, D. H. 2011. “Long‐Term Studies of Willow Ptarmigan and Gyrfalcon in the Yukon Territory: A Collapsing 10‐Year Cycle and Its Apparent Effect on the Top Predator.” In Gyrfalcons and Ptarmigan in a Changing World, Volume I, edited by Watson R. T., Cade T. J., Fuller M., Hunt G., and Potapov E., 323–336. Boise, ID: Peregrine Fund. 10.4080/gpcw.2011.0206. [DOI] [Google Scholar]
- Myers‐Smith, I. H. , Elmendorf S. C., Beck P. S. A., et al. 2015. “Climate Sensitivity of Shrub Growth Across the Tundra Biome.” Nature Climate Change 5, no. 9: 887–891. 10.1038/nclimate2697. [DOI] [Google Scholar]
- Myers‐Smith, I. H. , Forbes B. C., Wilmking M., et al. 2011. “Shrub Expansion in Tundra Ecosystems: Dynamics, Impacts and Research Priorities.” Environmental Research Letters 6, no. 4: 045509. 10.1088/1748-9326/6/4/045509. [DOI] [Google Scholar]
- Myers‐Smith, I. H. , and Hik D. S.. 2018. “Climate Warming as a Driver of Tundra Shrubline Advance.” Journal of Ecology 106: 547–560. 10.1111/1365-2745.12817. [DOI] [Google Scholar]
- Natsukawa, H. , and Sergio F.. 2022. “Top Predators as Biodiversity Indicators: A Meta‐Analysis.” Ecology Letters 25, no. 9: 2062–2075. 10.1111/ele.14077. [DOI] [PubMed] [Google Scholar]
- Newton, I. 1979. Population Ecology of Raptors. Vermillion, SD: Buteo Books. [Google Scholar]
- Nielsen, Ó. K. 2011. “Gyrfalcon Population and Reproduction in Relation to Rock Ptarmigan Numbers in Iceland.” In Gyrfalcons and Ptarmigan in a Changing World, Volume II, edited by Watson R. T., Cade T. J., Fuller M., Hunt G., and Potapov E., 21–48. Boise, ID: Peregrine Fund. 10.4080/gpcw.2011.0210. [DOI] [Google Scholar]
- Nielsen, Ó. K. , and Cade T. J.. 2017. “Gyrfalcon and Ptarmigan Predator‐Prey Relationship.” In Applied Raptor Ecology: Essentials From Gyrfalcon Research, edited by Anderson D. L., McClure C. J. W., and Franke A., 43–74. Boise, ID: Peregrine Fund. 10.4080/are.2017/003. [DOI] [Google Scholar]
- NOAA . n.d. “National Weather Service.” Retrieved May 26, 2024. https://www.weather.gov/.
- Pedersen, Å. Ø. , Blanchet M.‐A., Hörnell‐Willebrand M., Jepsen J. U., Biuw M., and Fuglei E.. 2014. “Rock Ptarmigan ( Lagopus muta ) Breeding Habitat Use in Northern Sweden.” Journal of Ornithology 155, no. 1: 195–209. 10.1007/s10336-013-1001-0. [DOI] [Google Scholar]
- Pernollet, C. A. , Korner‐Nievergelt F., and Jenni L.. 2015. “Regional Changes in the Elevational Distribution of the Alpine Rock Ptarmigan Lagopus muta helvetica in Switzerland.” Ibis 157, no. 4: 823–836. 10.1111/ibi.12298. [DOI] [Google Scholar]
- Quay, W. B. 1951. “Observations on Mammals of the Seward Peninsula, Alaska.” Journal of Mammalogy 32, no. 1: 88–99. 10.2307/1375417. [DOI] [Google Scholar]
- R Core Team . 2023. “R: A Language and Environment for Statistical Computing.” R Foundation for Statistical Computing. https://www.R‐project.org/.
- R Core Team . 2024. “R: A Language and Environment for Statistical Computing.” R Foundation for Statistical Computing, Vienna, Austria. https://www.R‐project.org/.
- Radcliffe, R. W. , and Henderson M. T.. 2023. “21 Gyrfalcons as Sentinels for Changing Disease Ecology in the Arctic.” In Wildlife Disease and Health in Conservation, p. 365, edited by Jessup D. A. and Radcliffe R. W.. Baltimore, MD: JHU Press. [Google Scholar]
- Ralph, C. 1981. “An Investigation of the Effect of Seasonal Activity Levels on Avian Censusing.” Studies in Avian Biology 6: 265–270. [Google Scholar]
- Ralph, C. J. , Sauer J. R., and Droege S.. 1995. “Monitoring Bird Populations by Point Counts (PSW‐GTR‐149; p. PSW‐GTR‐149).” U.S. Department of Agriculture, Forest Service, Pacific Southwest Research Station. 10.2737/PSW-GTR-149. [DOI]
- Rantanen, M. , Karpechko A. Y., Lipponen A., et al. 2022. “The Arctic Has Warmed Nearly Four Times Faster Than the Globe Since 1979.” Communications Earth & Environment 3, no. 1: 168. 10.1038/s43247-022-00498-3. [DOI] [Google Scholar]
- Resano‐Mayor, J. , Real J., Moleón M., Sánchez‐Zapata J. A., Palma L., and Hernández‐Matías A.. 2016. “Diet‐Demography Relationships in a Long‐Lived Predator: From Territories to Populations.” Oikos 125, no. 2: 262–270. 10.1111/oik.02468. [DOI] [Google Scholar]
- Revermann, R. , Schmid H., Zbinden N., Spaar R., and Schröder B.. 2012. “Habitat at the Mountain Tops: How Long Can Rock Ptarmigan ( Lagopus muta helvetica ) Survive Rapid Climate Change in the Swiss Alps? A Multi‐Scale Approach.” Journal of Ornithology 153, no. 3: 891–905. 10.1007/s10336-012-0819-1. [DOI] [Google Scholar]
- Richards, D. G. 1981. “Environmental Acoustics and Censuses of Singing Birds.” Studies in Avian Biology 6: 297–300. [Google Scholar]
- Robbins, C. S. 1981. “Effect of Time of Day on Bird Activity.” Studies in Avian Biology 6, no. 3: 275–286. [Google Scholar]
- Robinson, B. W. , Booms T. L., Bechard M. J., and Anderson D. L.. 2019. “Dietary Plasticity in a Specialist Predator, the Gyrfalcon ( Falco rusticolus ): New Insights Into Diet During Brood Rearing.” Journal of Raptor Research 53, no. 2: 115–126. 10.3356/JRR-15-58. [DOI] [Google Scholar]
- Royle, J. A. 2004. “N ‐Mixture Models for Estimating Population Size From Spatially Replicated Counts.” Biometrics 60, no. 1: 108–115. 10.1111/j.0006-341X.2004.00142.x. [DOI] [PubMed] [Google Scholar]
- Sandercock, B. K. , Nilsen E. B., Brøseth H., and Pedersen H. C.. 2011. “Is Hunting Mortality Additive or Compensatory to Natural Mortality? Effects of Experimental Harvest on the Survival and Cause‐Specific Mortality of Willow Ptarmigan: Harvest and Ptarmigan Survival.” Journal of Animal Ecology 80, no. 1: 244–258. 10.1111/j.1365-2656.2010.01769.x. [DOI] [PubMed] [Google Scholar]
- Savard, J.‐P. L. , and Hooper T. D.. 1995. “Influence of Survey Length and Radius Size on Grassland Bird Surveys by Point Counts at Williams Lake, British Columbia.” Monitoring Bird Populations by Point Counts. USDA Forest Service General Technical Report PSW‐149, 49–62.
- Sawa, Y. , Takeuchi Y., and Nakamura H.. 2011. “Nest Site Selection and Nesting Biology of Rock Ptarmigan Lagopus muta Japonicus in Japan.” Bird Study 58, no. 2: 200–207. 10.1080/00063657.2011.559532. [DOI] [Google Scholar]
- Schieck, J. 1997. “Biased Detection of Bird Vocalizations Affects Comparisons of Bird Abundance Among Forested Habitats.” Condor 99, no. 1: 179–190. [Google Scholar]
- Schieck, J. O. , and Hannon S. J.. 1993. “Clutch Predation, Cover, and the Overdispersion of Nests of the Willow Ptarmigan.” Ecology 74, no. 3: 743–750. 10.2307/1940802. [DOI] [Google Scholar]
- Schielzeth, H. 2010. “Simple Means to Improve the Interpretability of Regression Coefficients.” Methods in Ecology and Evolution 1, no. 2: 103–113. 10.1111/j.2041-210X.2010.00012.x. [DOI] [Google Scholar]
- Schmidt, N. M. , Hardwick B., Gilg O., et al. 2017. “Interaction Webs in Arctic Ecosystems: Determinants of Arctic Change?” Ambio 46, no. Suppl 1: 12–25. 10.1007/s13280-016-0862-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schore, A. I. G. , Fraterrigo J. M., Salmon V. G., Yang D., and Lara M. J.. 2023. “Nitrogen Fixing Shrubs Advance the Pace of Tall‐Shrub Expansion in Low‐Arctic Tundra.” Communications Earth & Environment 4, no. 1: 421. 10.1038/s43247-023-01098-5. [DOI] [Google Scholar]
- Scridel, D. , Brambilla M., de Zwaan D. R., et al. 2021. “A Genus at Risk: Predicted Current and Future Distribution of all Three Lagopus Species Reveal Sensitivity to Climate Change and Efficacy of Protected Areas.” Diversity and Distributions 27, no. 9: 1759–1774. 10.1111/ddi.13366. [DOI] [Google Scholar]
- Sergio, F. , Caro T., Brown D., et al. 2008. “Top Predators as Conservation Tools: Ecological Rationale, Assumptions, and Efficacy.” Annual Review of Ecology, Evolution, and Systematics 39, no. 1: 1–19. 10.1146/annurev.ecolsys.39.110707.173545. [DOI] [Google Scholar]
- Sergio, F. , and Newton I.. 2003. “Occupancy as a Measure of Territory Quality.” Journal of Animal Ecology 72, no. 5: 857–865. 10.1046/j.1365-2656.2003.00758.x. [DOI] [Google Scholar]
- Sergio, F. , Newton I., and Marchesi L.. 2008. “Top Predators and Biodiversity: Much Debate, Few Data.” Journal of Applied Ecology 45, no. 3: 992–999. 10.1111/j.1365-2664.2008.01484.x. [DOI] [Google Scholar]
- Sheriff, M. J. , Williams C. T., Kenagy G. J., Buck C. L., and Barnes B. M.. 2012. “Thermoregulatory Changes Anticipate Hibernation Onset by 45 Days: Data From Free‐Living Arctic Ground Squirrels.” Journal of Comparative Physiology B 182, no. 6: 841–847. 10.1007/s00360-012-0661-z. [DOI] [PubMed] [Google Scholar]
- Sillett, T. S. , Chandler R. B., Royle J. A., Kéry M., and Morrison S. A.. 2012. “Hierarchical Distance‐Sampling Models to Estimate Population Size and Habitat‐Specific Abundance of an Island Endemic.” Ecological Applications 22, no. 7: 1997–2006. 10.1890/11-1400.1. [DOI] [PubMed] [Google Scholar]
- Simons, T. R. , Alldredge M. W., Pollock K. H., and Wettroth J. M.. 2007. “Experimental Analysis of the Auditory Detection Process on Avian Point Counts.” Auk 124, no. 3: 986–999. 10.1093/auk/124.3.986. [DOI] [Google Scholar]
- Skirvin, A. A. 1981. “Effect of Time of Day and Time of Season on the Number of Observations and Density Estimates of Breeding Birds.” Studies in Avian Biology 6: 271–274. [Google Scholar]
- Smith, P. A. , McKinnon L., Meltofte H., et al. 2020. “Status and Trends of Tundra Birds Across the Circumpolar Arctic.” Ambio 49, no. 3: 732–748. 10.1007/s13280-019-01308-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenhof, K. , Kochert M. N., Carpenter L. B., and Lehman R. N.. 1999. “Long‐Term Prairie Falcon Population Changes in Relation to Prey Abundance, Weather, Land Uses, and Habitat Conditions.” Condor 101, no. 1: 28–41. 10.2307/1370443. [DOI] [Google Scholar]
- Swanson, D. K. 2015. “Environmental Limits of Tall Shrubs in Alaska's Arctic National Parks.” PLoS One 10, no. 9: e0138387. 10.1371/journal.pone.0138387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tape, K. , Sturm M., and Racine C.. 2006. “The Evidence for Shrub Expansion in Northern Alaska and the Pan‐Arctic.” Global Change Biology 12, no. 4: 686–702. 10.1111/j.1365-2486.2006.01128.x. [DOI] [Google Scholar]
- Tape, K. D. , Clark J. A., Jones B. M., et al. 2022. “Expanding Beaver Pond Distribution in Arctic Alaska, 1949 to 2019.” Scientific Reports 12, no. 1: 7123. 10.1038/s41598-022-09330-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tape, K. D. , Hallinger M., Welker J. M., and Ruess R. W.. 2012. “Landscape Heterogeneity of Shrub Expansion in Arctic Alaska.” Ecosystems 15, no. 5: 711–724. 10.1007/s10021-012-9540-4. [DOI] [Google Scholar]
- Tape, K. D. , Jones B. M., Arp C. D., Nitze I., and Grosse G.. 2018. “Tundra Be Dammed: Beaver Colonization of the Arctic.” Global Change Biology 24, no. 10: 4478–4488. 10.1111/gcb.14332. [DOI] [PubMed] [Google Scholar]
- Tape, K. D. , Lord R., Marshall H.‐P., and Ruess R. W.. 2010. “Snow‐Mediated Ptarmigan Browsing and Shrub Expansion in Arctic Alaska.” Écoscience 17, no. 2: 186–193. 10.2980/17-2-3323. [DOI] [Google Scholar]
- [dataset] U.S. Geological Survey . 2017. “5 Meter Alaska Digital Elevation Models (DEMs)—USGS National Maps 2DEP Downloadable Data Collection: U.S. Geological Survey [dataset].”
- Verberk, W. 2011. “Explaining General Patterns in Species Abundance and Distributions.” Nature Education Knowledge 3: 38. [Google Scholar]
- Vuorinen, K. E. M. , Oksanen L., Oksanen T., Pyykönen A., Olofsson J., and Virtanen R.. 2017. “Open Tundra Persist, But Arctic Features Decline—Vegetation Changes in the Warming Fennoscandian Tundra.” Global Change Biology 23, no. 9: 3794–3807. 10.1111/gcb.13710. [DOI] [PubMed] [Google Scholar]
- Wahren, C.‐H. A. , Walker M. D., and Bret‐Harte M. S.. 2005. “Vegetation Responses in Alaskan Arctic Tundra After 8 Years of a Summer Warming and Winter Snow Manipulation Experiment.” Global Change Biology 11, no. 4: 537–552. 10.1111/j.1365-2486.2005.00927.x. [DOI] [Google Scholar]
- [dataset] Wang, J. , Sulla‐Menashe D., Woodcock C., Sonnentag O., Keeling R., and Friedl M.. 2019. “ABoVE: Landsat‐Derived Annual Dominant Land Cover Across ABoVE Core Domain, 1984–2014.” ORNL DAAC.
- Wellicome, T. I. , Kardynal K. J., Franken R. J., and Gillies C. S.. 2014. “Off‐Road Sampling Reveals a Different Grassland Bird Community Than Roadside Sampling: Implications for Survey Design and Estimates to Guide Conservation.” Avian Conservation and Ecology 9, no. 1: 4. 10.5751/ACE-00624-090104. [DOI] [Google Scholar]
- Werner, J. R. , Krebs C. J., Donker S. A., Boonstra R., and Sheriff M. J.. 2015. “Arctic Ground Squirrel Population Collapse in the Boreal Forests of the Southern Yukon.” Wildlife Research 42, no. 2: 176–184. [Google Scholar]
- Wheeler, H. C. , Chipperfield J. D., Roland C., and Svenning J.‐C.. 2015. “How Will the Greening of the Arctic Affect an Important Prey Species and Disturbance Agent? Vegetation Effects on Arctic Ground Squirrels.” Oecologia 178, no. 3: 915–929. 10.1007/s00442-015-3240-7. [DOI] [PubMed] [Google Scholar]
- Wheeler, H. C. , and Hik D. S.. 2013. “Arctic Ground Squirrels Urocitellus parryii as Drivers and Indicators of Change in Northern Ecosystems.” Mammal Review 43, no. 3: 238–255. 10.1111/j.1365-2907.2012.00220.x. [DOI] [Google Scholar]
- Wheeler, H. C. , and Hik D. S.. 2014a. “Giving‐Up Densities and Foraging Behaviour Indicate Possible Effects of Shrub Encroachment on Arctic Ground Squirrels.” Animal Behaviour 95: 1–8. 10.1016/j.anbehav.2014.06.005. [DOI] [Google Scholar]
- Wheeler, H. C. , and Hik D. S.. 2014b. “Influence of Shrub Canopies on Growth Rate and Pre‐Hibernation Mass of Juvenile Arctic Ground Squirrels.” Wildlife Biology 20, no. 4: 253–258. 10.2981/wlb.00038. [DOI] [Google Scholar]
- Wickham, H. , Averick M., Bryan J., et al. 2019. “Welcome to the Tidyverse.” Journal of Open Source Software 4, no. 43: 1686. 10.21105/joss.01686. [DOI] [Google Scholar]
- Wilson, S. , and Martin K.. 2008. “Breeding Habitat Selection of Sympatric White‐Tailed, Rock and Willow Ptarmigan in the Southern Yukon Territory, Canada.” Journal of Ornithology 149, no. 4: 629–637. 10.1007/s10336-008-0308-8. [DOI] [Google Scholar]
- Zohmann, M. , and Wöss M.. 2008. “Spring Density and Summer Habitat Use of Alpine Rock Ptarmigan Lagopus muta helvetica in the Southeastern Alps.” European Journal of Wildlife Research 54, no. 2: 379–383. 10.1007/s10344-007-0149-4. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1.
Data Availability Statement
The data that support the findings of this study are openly available on Dryad. https://doi.org/10.5061/dryad.djh9w0w94.


