Abstract
Objective
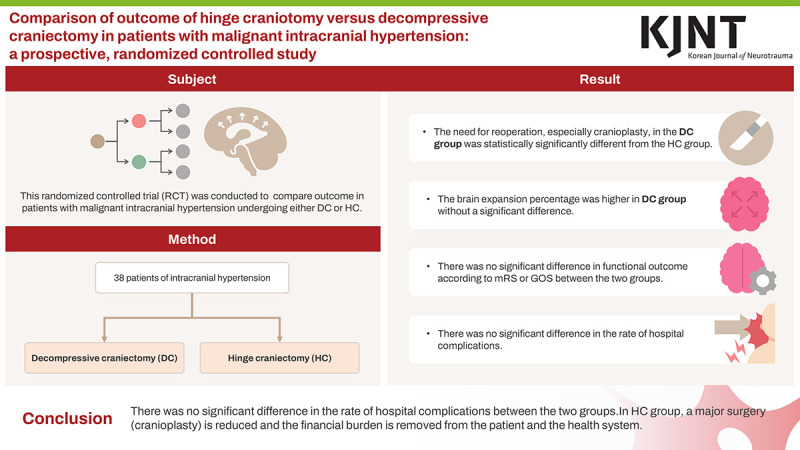
This randomized controlled trial (RCT) aimed to compare the short-, mid-, and long-term outcomes in patients with malignant intracranial hypertension undergoing either decompressive craniectomy (DC) or hinge craniotomy (HC).
Methods
In this prospective RCT, 38 patients diagnosed with malignant intracranial hypertension due to ischemic infarction, traumatic brain injury, or non-lesional spontaneous intracerebral hemorrhage, who required cranial decompression, were randomly allocated to the DC and HC groups.
Results
The need for reoperation, particularly cranioplasty, in the DC group was significantly different from that in the HC group. The percentage of brain expansion was higher in the DC group versus the HC group; however, the difference was not significant. There was no significant intergroup difference in the functional outcome according to the modified Rankin Scale or the Glasgow Outcome Scale. Additionally, no significant intergroup differences were observed in the rates of in-hospital complications.
Conclusion
The HC and DC groups did not significantly differ in the rate of hospital complications such as infection, need for reoperation owing to the lack of intracranial pressure control, wound healing problems, or bone infection. Our results suggest that by performing HC, the probability of the need for major surgery (cranioplasty) is reduced, thereby removing financial burden from the patient and the health system.
Trial Registration
Iranian Registry of Clinical Trials Identifier: IRCT20180515039678N1
Keywords: Elevated intracranial pressure, Decompressive craniectomy, Traumatic brain injury
GRAPHICAL ABSTRACT
INTRODUCTION
The main objective of cranial decompression is to reduce mortality and disability in patients with malignant intracranial hypertension caused by traumatic brain injury (TBI), ischemic infarction, or intra-cerebral hemorrhage.14) Improving blood flow, cerebral perfusion pressure (CPP) and preventing secondary brain injury after increased intracranial pressure (ICP) are the primary goals when medical treatment has been ineffective. Decompressive craniectomy (DC) is an established method for controlling refractory intracranial hypertension and is effective in controlling ICP and improving prognosis.13,14,26) Complications associated with DC include seizure, hydrocephalus, infection, cosmetic problems, and need for reoperation (cranioplasty).1,2,10,15,20) Hinge craniotomy (HC) has been introduced as an alternative in previous studies to resolve some of these limitations.26) This randomized controlled trial (RCT) compared short-, mid-, and long-term outcomes in patients with malignant intracranial hypertension undergoing either DC or HC.
MATERIALS AND METHODS
Study design
This RCT was registered in “name database” with registration number IRCT20180515039678N1. The study was approved by the ethics committee of the Tehran University of Medical Sciences and conducted in the neurosurgery department of Sina Hospital, Tehran, Iran and was conducted over a 36-month period, from March 2018 to April 2021. Study quality was assessed using the strengthening the reporting of observational studies in epidemiology template and the criteria for this RCT.
Patient characteristics
Thirty-eight patients diagnosed with malignant intracranial hypertension due to ischemic infarction, TBI, or non-lesional spontaneous intracerebral hemorrhage (SICH), who required cranial decompression, were randomly allocated to the DC and HC groups. Surgery was performed by five attending neurosurgeons practicing in a similar discipline at our hospital.
Patient selection
Inclusion criteria
Patients aged 18–80 years who required craniotomy for malignant intracranial hypertension (resulting from ischemic infarctions, TBI, or SICH) refractory to the maximum standard medical treatment were selected. ICP monitoring was not available at our center (owing to third-world problems and sanctions), and we used repeat imaging studies (computed tomography [CT] scans) and clinical examinations for decision-making and conversion to surgical intervention. The decision-making criteria differed based on the pathology.
Among the patients with ischemic infarctions, those with an infarction in the territory of the middle cerebral artery (MCA), cerebral herniation syndromes (defined as midline shift ≥5 mm and a compressed basal cistern), and reduced level of consciousness (Glasgow Coma Scale [GCS] score ≤9) despite maximum medical treatment (except those with barbiturate-induced coma) were selected for decompression. Among patients with TBI, those with cerebral herniation syndromes (i.e. midline shift ≥5 mm and at least compressed basal cistern) and reduced level of consciousness not due to the administration of sedatives (GCS score ≤9) were selected. Patients with cerebral herniation syndromes (with midline shift ≥5 mm and at least compressed basal cistern) and neurological deficits (GCS score ≤9) resulting from SICH in the MCA territory where hematoma required surgical treatment (hematoma volume >30 cc) were included in study.
Finally, patients with ischemic infarction, TBI, or SICH were included in the study, and the surgeon had to perform wide cranial decompression because of massive brain edema.
Exclusion criteria
Patients with a positive history of cerebrovascular accident (CVA), multiple or bilateral CVA, or a history of neurodegenerative diseases and cognitive disorders were not included in this study. Patients with TBI and intraventricular hemorrhage; GCS score of 3; bilateral mydriasis; injury to the abdomen, thorax, neck, and limbs; spinal injuries requiring surgical intervention; skull bone fractures for which bone flaps could not be used; and complicated skull base fractures were excluded.
Patients who died within the first 24 hours after operation were also excluded.
Technique of surgery and randomization
Surgery was performed as previously described by Schmidt et al.,22) under general anesthesia. To improve cerebral venous return and prevent intraoperative brain edema, 30–40° head elevations were performed. A standard reverse question mark incision starting from 1.5 cm superior to the zygomatic arc and 1 cm anterior to the tragus was selected, extending above the pinna to behind it up to the posterior mastoid line, and then rotated to the anterior at a distance of 1 cm lateral to the midline, terminating 5 mm behind the hairline. After reflecting on the incised scalp, the temporalis muscle was detached off the underlying bone using a Bovie cautery and reflected anteriorly based on the zygomatic arc. Four burr holes were left in the pterion, temporal, parietal boss, and frontal regions to reach the maximum diameter of the trauma flap. Optimal temporal fossa decompression is required to extend craniectomy to the floor of the middle cranial fossa using a rongeur. The cruciate pattern of dural splitting was fashioned considering gradual decompression in cases of subdural hematoma. Using suction along with continuous irrigation, the hematoma was evacuated, and hemostasis was achieved by applying hemostatic agents, bipolar electrocautery, and saline irrigation. Adjusted duraplasty with an autologous pericranial flap or artificial dura was performed in cases of paucity of the pericranium. At the time of surgery, each case was randomly selected from each arm for cranial decompression. Randomization was performed using sealed envelopes, each containing a piece of paper with DC or HC written on it, and randomly selected by one of the operating room staff. If the patient was assigned to the DC group, the bone flap was removed and saved in the abdominal subcutaneous fat to be replaced back 8–12 weeks later. If the patient was allocated to the HC group, three T-shaped titanium miniplates were applied: one in a region just posterior to the coronal suture that was fixed to the skull playing “the hinge role,” and the other 2 were fixed only on the bone flap in the region of the sphenoid wing and posterior temporal region to prevent the bone flap from moving inside, in the future. Temporalis muscle was reapproximated without suturing its fascia, followed by undermining the galea, to provide enough space for the “hinge effect.” The drain was placed under the scalp, and 2-layer water-tight closure of the scalp was ensured.
Postoperative care
Patients were transferred to intensive care unit (ICU) after surgery and were sedated with midazolam (20–100 mcg/kg/h) and remifentanil (0.05–0.2 mg/kg/min) infusions under the supervision of the anesthesiologist who was only aware that cranial decompression was performed for treating malignant intracranial hypertension and was blinded to the surgery type. The patients were examined by the chief resident of the neurosurgery department three times a day in the first week. In addition to a postoperative brain CT scan without contrast, patients underwent a brain CT scan on the third or fourth postoperative day. Moreover, patients underwent a complete neurological examination daily, and a brain CT scan was performed if necessary. Patients received routine ICU care during hospitalization and standard treatment according to the protocols if complications, such as infection, developed. We did not use other medical treatments, such as osmol therapy or hypothermia. The patients were referred to rehabilitation centers after discharge.
The patients were examined routinely at the end of 3, 6, and 12 months by the chief resident of neurosurgery, and functional neurological outcomes were assessed using the modified Rankin Scale (mRS) and Glasgow Outcome Scale (GOS). Cranioplasty was performed for patients in the DC group at 8–12 weeks post operation.
Patient demographics
All patient characteristics, including age, sex, GCS score, pupil examination findings, cranial decompression side, cranial decompression indication, use of anticoagulation and antiplatelet drugs, and comorbidities, were recorded in a data sheet before surgery.
Postoperative variables such as “ICP therapeutic index,” duration of mechanical ventilation, length of ICU stay, and length of hospital stay were included. In our study, the maximum treatment for controlling ICP was based on serial clinical examinations and imaging. There were no facilities to use instruments for ICP monitoring (owing to third-world problems).
Data on postoperative complications, such as infection, need for reoperation due to lack of ICP control, wound healing problems, and bone infection, were recorded in the same sheet for each case. Functional neurological outcomes were monitored using the mRS and GOS at 3, 6, and 12 months post operation, and evaluated by the same chief resident and attending neurosurgeon.
Radiographic analysis
All patients underwent preoperative and postoperative brain CT scan, and the Rotterdam score, including midline shift, basal cistern status (effaced or compressed), and subarachnoid hemorrhage (SAH), was calculated for each scan. The presence or absence of pre- and postoperative uncal herniation, subdural hemorrhage (SDH), and postoperative hematoma (requiring drainage) were also recorded. Other important variables evaluated on postoperative brain CT scan included the presence of a new hematoma or SAH and an increased volume of hematoma requiring surgical evacuation. The volume of hematoma in patients with SICH and the amount of cerebral parenchymal expansion after cranial decompression were estimated using Autodesk AutoCAD LT 2018 software®. The extent of brain expansion was calculated using the following formula and expressed as a percentage:
| Brain Expansion (%) = (Postoperative Volumetric Cerebral Volume/Preoperative Volumetric Cerebral Volume)−1*100 |
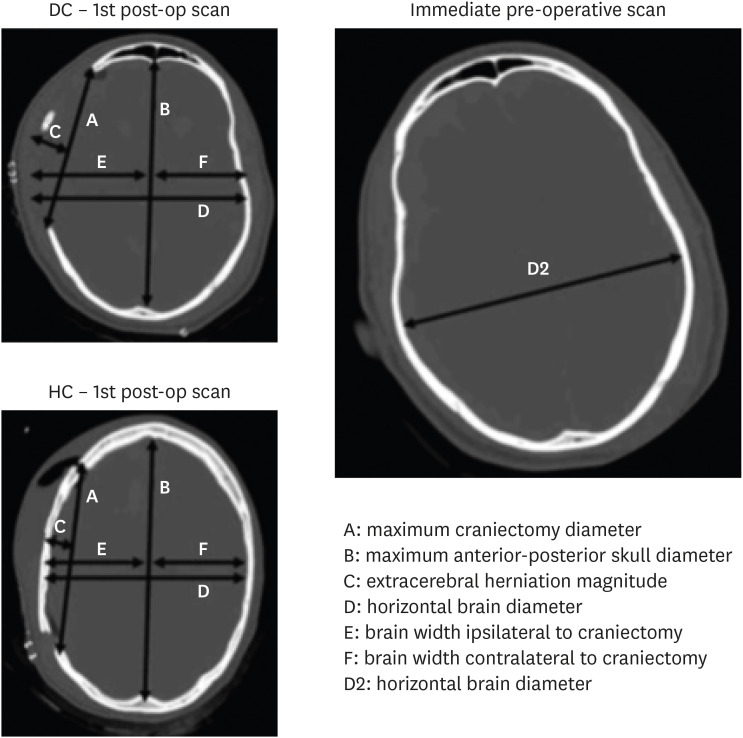
The techniques proposed by Flint et al.8) were used to compare the extent of cranial decompression between the 2 groups. In this study, the size of the craniotomy between the 2 groups were compared using the ratio of the maximum diameter of craniotomy to the maximum diameter of anterior-posterior skull in the same axial section on the CT scan (FIGURE 1).
FIGURE 1. The ratio of maximum diameter of craniotomy to maximum diameter of anterior-posterior skull in the same axial cut in CT scan was used.
CT: computed tomography, DC: decompressive craniectomy, HC: hinge craniotomy.
In addition to the percentage of brain expansion calculated using the software, the ratio of the magnitude of extra cerebral herniation to the maximum craniotomy diameter was also calculated.
Statistical analysis
The clinical, functional, and radiographic variables were compared between the DC and HC groups. Independent 2-sample t-test was used to compare variables with a normal distribution. The IBM SPSS software version 26.0 (IBM Corp., Armonk, NY, USA) was used for statistical analysis. Kaplan–Meier analysis was performed to compare the long-term outcomes of the patients. χ2 and Student’s t-tests were used to calculate the significance of the results.
RESULTS
Thirty-eight patients met the inclusion criteria, of whom 19 were randomly assigned to the DC group and 19 to the HC group. The reasons for increased ICP and the indications for surgery were: ischemic infarctions (n=6), TBI in (n=8), and SICH (n=5) in the DC group; and ischemic infarctions (n=5), TBI (n=8), and SICH in (n=6) in the HC group. All patients in the HC group were male, whereas, in the DC group, 17 patients were male and 2 were female. The mean age of the patients was 49.26±14.2 years in the DC group and 49.00±16.03 years in the HC group. The mean GCS and motor GCS scores were 6.63±1.06 and 4.21±0.78 in the DC and 6.95±1.17 and 4.42±0.76 in the HC group, respectively. Five patients in the DC group and four in the HC group had anisocoria. In both groups, cranial decompression was performed on the right side in 11 and on the left side in 8 patients. There were no significant differences in preoperative demographic variables between the 2 groups (TABLE 1).
TABLE 1. Patient characteristics.
| Characteristics | Decompressive craniectomy | Hinge craniotomy | Significance level | |
|---|---|---|---|---|
| Age (year) | 49.26±14.20 | 49.00±16.03 | Sig. (2-tailed)=0.95 | |
| Sex | χ2=0.14 | |||
| Female | 2 (10.5) | 0 (0.0) | ||
| Male | 17 (89.5) | 19 (100.0) | ||
| GCS | 6.63±1.06 | 6.95±1.17 | Sig. (2-tailed)=0.39 | |
| Motor GCS | 4.21±0.78 | 4.42±0.76 | Sig. (2-tailed)=0.41 | |
| Pupil | χ2=0.70 | |||
| Anisocoric | 5 (26.3) | 4 (21.1) | ||
| Equal | 14 (73.7) | 15 (78.9) | ||
| Use of anticoagulation/antiplatelet | 9 (47.4) | 6 (31.6) | χ2=0.78 | |
| Ischemic infarct | 6 (31.6) | 5 (26.3) | χ2=0.91 | |
| Traumatic brain injury | 8 (42.1) | 8 (42.1) | ||
| Non lesional spontaneous intracerebral hemorrhage | 5 (26.3) | 6 (31.6) | ||
| Side of decompression | χ2=1.00 | |||
| Right | 11 (57.9) | 11 (57.9) | ||
| Left | 8 (42.1) | 8 (42.1) | ||
Values are presented as mean ± standard deviation or number of patients (%).
GCS: Glasgow Coma Scale.
Postoperative results
The mean duration of mechanical ventilation, length of ICU stay, and length of hospital stay were 29.00±26.27, 32.79±26.30, and 47.16±27.28 days in the DC group and 24.21±30.21, 27.84±30.00, and 38.53±35.41 days in the HC group respectively, with no significant intergroup difference (TABLE 2).
TABLE 2. Postoperative clinical results.
| Results | Decompressive craniectomy | Hinge craniotomy | Significance level |
|---|---|---|---|
| Duration of mechanical ventilation (day) | 29.00±26.27 | 24.21±30.21 | Sig. (2-tailed)=0.60 |
| Duration of ICU admission (day) | 32.79±26.30 | 27.84±30.00 | Sig. (2-tailed)=0.59 |
| Duration of hospital stay (day) | 47.16±27.28 | 38.53±35.41 | Sig. (2-tailed)=0.40 |
| 30-day survival | 17 (89.5) | 15 (78.9) | χ2=0.37 |
Values are presented as mean ± standard deviation or number of patients (%).
ICU: intensive care unit.
Seventeen patients in the DC group and fifteen in the HC group survived their first hospitalization (TABLE 2). The 1-year mortality rate was 26.3% in both groups. According to the results of the clinical examination by a neurologist and electroencephalogram, 9 patients in the DC group and 6 in the HC group had postoperative seizures (TABLE 3).
TABLE 3. Postoperative complications.
| Complications | Decompressive craniectomy | Hinge craniotomy | Significance level |
|---|---|---|---|
| Subdural effusion | 6 (31.6) | 3 (15.8) | χ2=0.25 |
| Osteomyelitis | 1 (5.3) | 2 (10.5) | χ2=0.59 |
| Skin necrosis | 1 (5.3) | 1 (5.3) | |
| Bone flap resorption | 1 (5.3) | 0 (0.0) | |
| Infection of the bone flap storage site | 1 (5.3) | 0 (0.0) | |
| Trephine syndrome | 1 (5.3) | 0 (0.0) | |
| Evolution contralateral hematoma | 1 (5.3) | 0 (0.0) | |
| Reoperation due to uncontrolled intracranial pressure | 0 (0.0) | 1 (5.3) | χ2=1.02 |
| Seizure | 9 (47.4) | 6 (31.6) | χ2=0.31 |
| Hydrocephalus needed shunt | 2 (10.5) | 0 (0.0) | χ2=0.30 |
| Hydrocephalus needed external ventricular drainage | 1 (5.3) | 2 (10.5) | |
| Therapeutic lumbar puncture | 2 (10.5) | 2 (10.5) | χ2=1.00 |
| Deep vein thrombosis | 5 (26.3) | 2 (10.5) | χ2=0.20 |
| Infection (sepsis, pneumonia, …) | 12 (63.2) | 12 (63.2) | χ2=1.00 |
Values are presented as number of patients (%).
One patient each in both groups developed skin necrosis and underwent debridement and flap transfer by a plastic surgeon (TABLE 3).
Two patients in the HC group developed osteomyelitis (1 had skin necrosis), and underwent cranioplasty with mesh or bone cement after bone flap removal.
In the DC group, one patient developed osteomyelitis after cranioplasty and another patient developed a bone flap infection in the upper abdominal quadrant storage site. Both patients underwent a second cranioplasty after appropriate medical treatment.
In the DC group, two patients required shunt placement and one patient needed external ventricular drainage (EVD) to control hydrocephalus. In the HC group, one patient underwent reoperation and bone flap preservation in the abdomen considering the brain CT scan findings 1 day after the surgery, deterioration of the neurologic condition, and lack of improvement despite the administration of ICP-lowering agents. None of the postoperative variables were statistically significant (TABLE 3).
At the 30–90-day follow up, we observed moderate disability in 3 patients (15.7%) in the HC group and but not in the DC group (p=0.21). There was no difference in the outcomes of the patients according to the mRS between the 2 groups after cranioplasty at the 90–180-day follow up. At different time intervals during the year, there was no significant difference in functional outcomes according to the mRS or GOS between the 2 groups (TABLES 4 & 5).
TABLE 4. Functional outcomes of mRS.
| Groups | Slight disability | Moderate disability | Moderately sever disability | Sever disability | Dead | t-test | Sig. (2-tailed) | |
|---|---|---|---|---|---|---|---|---|
| mRS30–90 | 0.21 | |||||||
| DC | 0 | 0 | 8 | 8 | 1 | 4.59±0.61 | ||
| HC | 0 | 3 | 5 | 7 | 0 | 4.27±0.79 | ||
| mRS 90–180 | 0.47 | |||||||
| DC | 0 | 5 | 7 | 2 | 2 | 4.06±0.99 | ||
| HC | 1 | 5 | 6 | 2 | 1 | 3.80±1.01 | ||
| mRS 180–360 | 0.64 | |||||||
| DC | 2 | 7 | 4 | 1 | 0 | 3.29±0.82 | ||
| HC | 3 | 6 | 5 | 0 | 0 | 3.14±0.77 | ||
mRS: modified Rankin Scale, DC: decompressive craniectomy, HC: hinge craniotomy.
TABLE 5. Functional outcomes of GOS.
| Groups | Dead | Vegetative state | Sever disability | Moderate disability | Good recovery | t-test | Sig. (2-tailed) | |
|---|---|---|---|---|---|---|---|---|
| GOS 30–90 | 0.24 | |||||||
| DC | 1 | 1 | 13 | 2 | 0 | 2.94±0.65 | ||
| HC | 0 | 1 | 10 | 4 | 0 | 3.20±0.56 | ||
| GOS 90–180 | 0.26 | |||||||
| DC | 2 | 0 | 7 | 7 | 0 | 3.19±0.98 | ||
| HC | 1 | 0 | 6 | 5 | 3 | 3.60±1.05 | ||
| GOS 180–360 | 0.42 | |||||||
| DC | 0 | 0 | 4 | 7 | 3 | 3.93±0.73 | ||
| HC | 0 | 0 | 2 | 8 | 4 | 4.14±0.66 | ||
GOS: Glasgow Outcome Scale, DC: decompressive craniectomy, HC: hinge craniotomy.
Results in imaging
There was no significant difference in the preoperative brain CT findings between the 2 groups. The mean Rotterdam score was 4.53±0.84 and 4.58±0.6 in the DC and HC group respectively, indicating no significant difference. The ratio of the largest craniotomy diameter to the largest anterior-posterior diameter of the skull was 0.76±0.05 in the DC group and 0.76±0.04 in the HC group. The brain expansion percentage was 10.22%±3.54% in DC group and 8.69%±2.15% in HC group, with no significant difference. Six patients in the DC group and three in the HC group had postsurgical subdural effusions, with no significant difference (TABLE 6).
TABLE 6. Radiographic results.
| Variables | DC | HC | Significance level | |
|---|---|---|---|---|
| Rotterdam score (preop) | 4.53±0.84 | 4.58±0.60 | Sig. (2-tailed)=0.82 | |
| Rotterdam score (postop) | 2.68±0.74 | 2.53±0.84 | Sig. (2-tailed)=0.37 | |
| Preop cistern | χ2=0.28 | |||
| Present | 1 (5.3) | 0 (0.0) | ||
| Compressed | 13 (68.4) | 10 (52.6) | ||
| Effaced | 5 (26.3) | 9 (47.4) | ||
| Postop cistern | χ2=0.67 | |||
| Present | 15 (78.9) | 16 (84.2) | ||
| Compressed | 4 (21.1) | 3 (15.8) | ||
| Effaced | 0 (0.0) | 1 (5.3) | ||
| Preop uncal herniation | 9 (47.4) | 8 (42.1) | χ2=0.74 | |
| Postop uncal herniation | 1 (5.3) | 1 (5.3) | χ2=1.00 | |
| Postop midline shift | χ2=0.42 | |||
| <5 mm | 16 (84.2) | 14 (73.7) | ||
| ≥5 mm and <10 mm | 3 (15.8) | 5 (26.3) | ||
| New SAH present | 6 (31.6) | 1 (5.3) | χ2=0.036 | |
| New hematoma formation or hematoma expansion | 3 (15.8) | 3 (15.8) | χ2=1.00 | |
| Volume of ICH in SICH group | 43.40±7.16 | 44.67±5.46 | Sig. (2-tailed)=0.74 | |
| Percent of brain volume expansion | 10.22±3.54 | 8.69±2.15 | Sig. (2-tailed)=0.11 | |
| Ratio* | 0.76±0.05 | 0.76±0.04 | Sig. (2-tailed)=0.75 | |
| ECH index† | 0.18±0.03 | 0.14±0.03 | Sig. (2 tailed)=0.001 | |
Values are presented as mean ± standard deviation or number of patients (%).
DC: decompressive craniectomy, HC: Hinge craniotomy, preop: pre-operative, postop: post-operative; SAH: subarachnoid hemorrhage, ICH: intracerebral hemorrhage, SICH: spontaneous intracerebral hemorrhage, ECH: extracerebral herniation.
*Ratio: maximum craniectomy or craniotomy diameter/maximum anteroposterior skull diameter.
†ECH index: ECH magnitude/maximum craniectomy or craniotomy diameter.
In the DC group, 12 patients underwent cranioplasty using autologous bone flaps, 5 patients died before cranioplasty, 1 patient needed titanium mesh to supplement the patient's own resorbed bone, and 1 patient underwent cranioplasty with titanium mesh due to infection at the storage site (TABLE 7).
TABLE 7. Cases in the DC group underwent cranioplasty with bone flap which had a significant difference with the HC group.
| Groups | Hinge cranioplasty | Cranioplasty with autologous bone in DC group | Expired without cranioplasty | Cranioplasty with mesh and bone cement due to osteomyelitis | Cranioplasty with autologous bone and mesh | χ2 |
|---|---|---|---|---|---|---|
| DC | 0 (0.0) | 12 (63.2) | 5 (26.3) | 1 (5.3) | 1 (5.3) | 0.000 |
| HC | 16 (84.2) | 0 (0.0) | 1 (5.3) | 2 (10.5) | 0 (0.0) |
Values are presented as number of patients (%).
DC: decompressive craniectomy, HC: hinge craniotomy.
In the HC group, one patient underwent reoperation for ICP control and his bone flap was removed; however, this patient died before cranioplasty. Cranioplasty with titanium mesh and bone cement was performed in two patients with osteomyelitis (TABLE 7).
DISCUSSION
In patients with ischemic infarction, TBI, or SICH presenting with malignant intracranial hypertension, the main objectives of treatment are to improve blood flow and oxygenation, followed by decreased ICP and increased CPP.16) The patient's prognosis will improve if the pre-insult physiological condition is reached and secondary insults are avoided. Although the results of DC are promising,13,14,26) controversies regarding this method have increased in terms of the complications and burden of this method on the patient, health system, and society.19)
HC was introduced as a method of cranial decompression in 2007, and its effectiveness has been assessed in several retrospective studies.9,17,18,22,27) We attempted to confirm or rule out the hypothesis that HC is more effective in candidates for cranial decompression by evaluating short-, mid-, and long-term outcomes, compared with DC.
Short-term outcomes
Unfortunately, 21% of the patients in the HC group and 10.5% of the patients in the DC group died within 30 days after surgery due to pneumonia, severe sepsis, or multi-organ damage, such as myocardial infarction, and other causes. One patient in the HC group underwent reoperation; temporal lobectomy was performed, and the bone flap was placed in the abdominal subcutaneous fat (crossover). This patient expired after 7 days due because of severe sepsis and diabetic ketoacidosis.
The mortality rate was 26.3% in both groups during the 1-year follow-up period, indicating no significant differences. The high mean duration of mechanical ventilation, length of ICU stay, and length of hospital stay were due to complications, pointing out the aggressive nature and high burden of illness on the patient and the health system. Approximately 73% of the patients developed complications requiring long-term hospitalization, of whom 85% developed various infections (pneumonia, sepsis, etc.). However, there was no significant difference in the rate of in-hospital complications between the 2 groups (63% in each). The differences in the mean duration of mechanical ventilation, length of ICU stay, and length of hospital stay between the HC and DC groups were not significant. There was no significant intergroup difference in the duration of the first hospital stay (TABLE 3).
Two patients in the HC group and one in the DC group developed osteomyelitis requiring bone flap removal, wound debridement, and cranioplasty after antibiotic therapy. Excluding these patients and the patients who expired before cranioplasty, 63% of the patients in the DC group underwent cranioplasty with bone flap, which was significantly different from the proportion in the HC group (TABLE 7). Reducing the need for major surgery and subsequently reducing the length of hospital and ICU stays to 9 and 5 days, respectively, can be considered as an advantage for reducing the financial burden. The patients required a second cranioplasty after DC. Cranioplasty is associated with additional complications, such as infection, bone resorption, and hematoma. These complications led to reoperation. All of these possible operations increase the financial burden on families and health systems.
Seizures were reported in 47% of the patients in the DC group and 31% of the patients in the HC group, showing no significant difference (TABLE 3). The severity of the initial insult appears to play a prominent role in the occurrence of seizures. Two patients in the DC group developed seizures after cranioplasty, whereas they had no seizures before the procedure. Seizures are one of the most important complications of cranioplasty.24)
Approximately 10% of the patients in the DC group required shunt placement, and 5% required an EVD to control hydrocephalus. Moreover, 31% of the patients in the DC group developed subdural effusion, and one third of them improved with multiple lumbar punctures (TABLE 3). The dura and skull play important roles in cerebrospinal fluid (CSF) hydrodynamics, and DC may facilitate ventricular enlargement.7) Several studies have suggested that DC is a risk factor for the development of hydrocephalus in TBIs,5,12) and some researchers have argued that DC and hydrocephalus are both correlated with the extent of craniotomy.15) In our study, patients with hydrocephalus did not undergo a larger craniotomy. Some studies found that the distance of craniotomy from the midline (atmospheric pressure on the draining veins) and age are risk factors for hydrocephalus in patients undergoing DC.6,12,25) In our study, patients who required shunt placement in the DC group (10%) were young and the distance of craniotomy from midline was about 3 cm. Other probable factors are implicated in CSF hydrodynamic impairment and development of hydrocephalus in DC. Some authors have denied the role of DC as determinants of hydrocephalus.21,25,28)
In our study, 10% of the patients in the HC group needed an EVD for controlling hydrocephalus, 15% showed the evidence of subdural effusion, two thirds of them underwent multiple therapeutic lumbar punctures (TABLE 3). The Waziri’s theory of the mechanism of hydrocephalus following DC suggests that28) DC may play a flattening role in the normally dicrotic CSF pulse wave in patients who undergo DC because of pressure pulse transmission throughout the cranial defect. Moreover, the function of arachnoid granulations depends on the pressure difference between the subarachnoid space and draining veins; therefore, it is possible that disturbances in pulsatile ICP dynamics secondary to cranial defects reduce CSF outflow and absorption, resulting in increased odds of hydrocephalus.3,4,11) Patients who undergo HC also experience changes in CSF dynamics.
Long-term outcomes
Evaluation of the functional neurological outcomes at the end of 3, 6, and 12 months using the mRS and GOS showed no significant differences between the DC and HC groups. Although the number of patients with moderate disability in the short-term was higher in the HC group versus the DC group, there were no significant intergroup differences in the long-term (TABLES 4 & 5).
Hussain et al. studied the effect of cranioplasty on cerebral blood perfusion and neurological outcomes in patients with DC and found marked improvements in neurological outcome, cognition, and cerebral blood perfusion following cranioplasty.23) In our study, the mRS score improved from 5 to 4 in 5 patients and from 4 to 3 in 5 patients in the DC group. Considering the short- and long-term outcome, it may be concluded that the HC may be a good alternative to DC in patients with malignant intracranial hypertension.
Radiographic evaluation
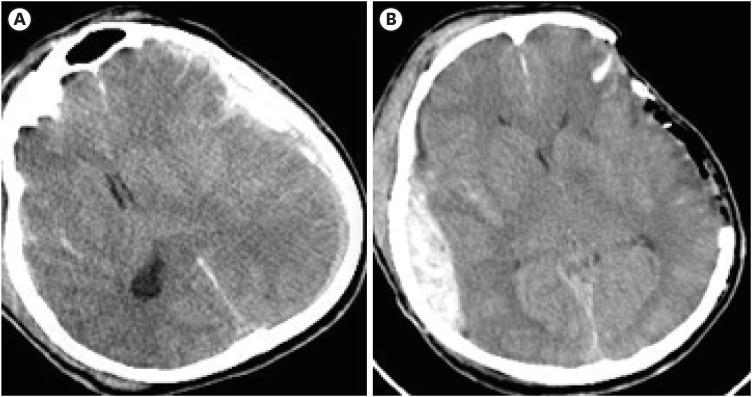
Although the percentage of cerebral parenchymal expansion was higher in patients in the DC group versus those in the HC group (10.22% vs. 8.69%), the intergroup difference was not significant (TABLE 6). The higher percentage and unlimited parenchymal expansion in patients who undergo DC may increase the volume of the parenchymal hematoma and result in hemorrhage on the contralateral side.27) This happened in 31% of the patients with expansion of hematoma or new SAH postoperatively. There was a significant difference in new SAH after DC between 2 groups (31% in DC vs. 5% in HC, p=0.036) (TABLE 6). This could be due to the greater brain expansion in the DC group than in the HC group. One patient in the DC group developed contralateral SDH after DC, requiring evacuation (FIGURE 2). The unlimited expansion in the DC group removed the hemostatic effect caused by packing in the parenchyma on the contralateral side and increased the risk of hematoma.
FIGURE 2. In decompressive craniectomy group developed a contralateral subdural hemorrhage after decompressive craniectomy requiring evacuation.
Limitation
The limitations of this study were as follows: 1) inhomogeneous causes of malignant intracranial hypertension, such as ischemic infarctions, TBI, and SICH; 2) lack of access to ICP monitoring, (patients were monitored using repeated neurological examinations and CT scans); 3) small sample size, which was due to the strict inclusion and exclusion criteria and single-center nature of the study; 4) operator-dependent nature of measurements on CT scans (however, the operators were blinded to the study results); and 5) the surgery being performed by five different neurosurgeons, which could have led to bias (this bias was reduced by selecting neurosurgeons from similar disciplines who were blinded to the type of surgery).
We propose a future study to increase the sample size, specify the study on one cause of malignant intracranial hypertension (e.g., ischemic infarctions) and qualify the study using single-photon emission CT or magnetic resonance perfusion for evaluating cerebral blood flow and CPP in both the HC and DC groups.
CONCLUSIONS
The need for reoperation, particularly cranioplasty, in the DC group was significantly different from that in the HC group. The percentage of brain expansion was higher in the DC group versus the HC group; however, the difference was not significant. There was no significant intergroup difference in the functional outcomes according to the mRS or GOS. Additionally, there was no significant difference between the groups in the rate of hospital complications such as infection, need for reoperation due to lack of ICP control, wound healing problems, or bone infection. Therefore, HC can be a suitable alternative to DC in patients with malignant intracranial hypertension as the in-hospital complication rate and short- and long-term outcomes of the patients are comparable in the DC and HC groups. Moreover, by performing HC, the probability of the need for undergoing a major surgery (cranioplasty) can be significantly reduced, and the financial burden can be removed from the patient and the health system.
Footnotes
Funding: No funding was obtained for this study.
Conflict of Interest: The authors have no financial conflicts of interest.
Informed Consent: Informed consent was obtained from all participants.
Ethics Approval: The tenets of the Declaration of Helsinki were considered in all stages of the study. The protocol of the study was approved by Tehran University of Medical Sciences (IR.TUMS.MEDICINE.REC.1396.4230).
References
- 1.Aarabi B, Hesdorffer DC, Ahn ES, Aresco C, Scalea TM, Eisenberg HM. Outcome following decompressive craniectomy for malignant swelling due to severe head injury. J Neurosurg. 2006;104:469–479. doi: 10.3171/jns.2006.104.4.469. [DOI] [PubMed] [Google Scholar]
- 2.Albanèse J, Leone M, Alliez JR, Kaya JM, Antonini F, Alliez B, et al. Decompressive craniectomy for severe traumatic brain injury: evaluation of the effects at one year. Crit Care Med. 2003;31:2535–2538. doi: 10.1097/01.CCM.0000089927.67396.F3. [DOI] [PubMed] [Google Scholar]
- 3.Black PM, Tzouras A, Foley L. Cerebrospinal fluid dynamics and hydrocephalus after experimental subarachnoid hemorrhage. Neurosurgery. 1985;17:57–62. doi: 10.1227/00006123-198507000-00009. [DOI] [PubMed] [Google Scholar]
- 4.Blasberg R, Johnson D, Fenstermacher J. Absorption resistance of cerebrospinal fluid after subarachnoid hemorrhage in the monkey; effects of heparin. Neurosurgery. 1981;9:686–691. doi: 10.1227/00006123-198112000-00012. [DOI] [PubMed] [Google Scholar]
- 5.Choi I, Park HK, Chang JC, Cho SJ, Choi SK, Byun BJ. Clinical factors for the development of posttraumatic hydrocephalus after decompressive craniectomy. J Korean Neurosurg Soc. 2008;43:227–231. doi: 10.3340/jkns.2008.43.5.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Bonis P, Pompucci A, Mangiola A, Rigante L, Anile C. Post-traumatic hydrocephalus after decompressive craniectomy: an underestimated risk factor. J Neurotrauma. 2010;27:1965–1970. doi: 10.1089/neu.2010.1425. [DOI] [PubMed] [Google Scholar]
- 7.Ding J, Guo Y, Tian H. The influence of decompressive craniectomy on the development of hydrocephalus: a review. Arq Neuropsiquiatr. 2014;72:715–720. doi: 10.1590/0004-282x20140106. [DOI] [PubMed] [Google Scholar]
- 8.Flint AC, Manley GT, Gean AD, Hemphill JC, 3rd, Rosenthal G. Post-operative expansion of hemorrhagic contusions after unilateral decompressive hemicraniectomy in severe traumatic brain injury. J Neurotrauma. 2008;25:503–512. doi: 10.1089/neu.2007.0442. [DOI] [PubMed] [Google Scholar]
- 9.Goettler CE, Tucci KA. Decreasing the morbidity of decompressive craniectomy: the Tucci flap. J Trauma. 2007;62:777–778. doi: 10.1097/TA.0b013e31802ee55e. [DOI] [PubMed] [Google Scholar]
- 10.Guerra WK, Gaab MR, Dietz H, Mueller JU, Piek J, Fritsch MJ. Surgical decompression for traumatic brain swelling: indications and results. J Neurosurg. 1999;90:187–196. doi: 10.3171/jns.1999.90.2.0187. [DOI] [PubMed] [Google Scholar]
- 11.Hasan D, Tanghe HL. Distribution of cisternal blood in patients with acute hydrocephalus after subarachnoid hemorrhage. Ann Neurol. 1992;31:374–378. doi: 10.1002/ana.410310405. [DOI] [PubMed] [Google Scholar]
- 12.Jiao QF, Liu Z, Li S, Zhou LX, Li SZ, Tian W, et al. Influencing factors for posttraumatic hydrocephalus in patients suffering from severe traumatic brain injuries. Chin J Traumatol. 2007;10:159–162. [PubMed] [Google Scholar]
- 13.Jüttler E, Schwab S, Schmiedek P, Unterberg A, Hennerici M, Woitzik J, et al. Decompressive surgery for the treatment of malignant infarction of the middle cerebral artery (destiny): a randomized, controlled trial. Stroke. 2007;38:2518–2525. doi: 10.1161/STROKEAHA.107.485649. [DOI] [PubMed] [Google Scholar]
- 14.Jüttler E, Unterberg A, Woitzik J, Bösel J, Amiri H, Sakowitz OW, et al. Hemicraniectomy in older patients with extensive middle-cerebral-artery stroke. N Engl J Med. 2014;370:1091–1100. doi: 10.1056/NEJMoa1311367. [DOI] [PubMed] [Google Scholar]
- 15.Kan P, Amini A, Hansen K, White GL, Jr, Brockmeyer DL, Walker ML, et al. Outcomes after decompressive craniectomy for severe traumatic brain injury in children. J Neurosurg. 2006;105:337–342. doi: 10.3171/ped.2006.105.5.337. [DOI] [PubMed] [Google Scholar]
- 16.Kenning TJ, Gandhi RH, German JW. A comparison of hinge craniotomy and decompressive craniectomy for the treatment of malignant intracranial hypertension: early clinical and radiographic analysis. Neurosurg Focus. 2009;26:E6. doi: 10.3171/2009.4.FOCUS0960. [DOI] [PubMed] [Google Scholar]
- 17.Kenning TJ, Gooch MR, Gandhi RH, Shaikh MP, Boulos AS, German JW. Cranial decompression for the treatment of malignant intracranial hypertension after ischemic cerebral infarction: decompressive craniectomy and hinge craniotomy. J Neurosurg. 2012;116:1289–1298. doi: 10.3171/2012.2.JNS111772. [DOI] [PubMed] [Google Scholar]
- 18.Ko K, Segan S. In situ hinge craniectomy. Neurosurgery. 2007;60:255–258. doi: 10.1227/01.NEU.0000255380.64969.81. [DOI] [PubMed] [Google Scholar]
- 19.Kontopoulos V, Foroglou N, Patsalas J, Magras J, Foroglou G, Yiannakou-Pephtoulidou M, et al. Decompressive craniectomy for the management of patients with refractory hypertension: should it be reconsidered? Acta Neurochir (Wien) 2002;144:791–796. doi: 10.1007/s00701-002-0948-z. [DOI] [PubMed] [Google Scholar]
- 20.Pillai A, Menon SK, Kumar S, Rajeev K, Kumar A, Panikar D. Decompressive hemicraniectomy in malignant middle cerebral artery infarction: an analysis of long-term outcome and factors in patient selection. J Neurosurg. 2007;106:59–65. doi: 10.3171/jns.2007.106.1.59. [DOI] [PubMed] [Google Scholar]
- 21.Rahme R, Weil AG, Sabbagh M, Moumdjian R, Bouthillier A, Bojanowski MW. Decompressive craniectomy is not an independent risk factor for communicating hydrocephalus in patients with increased intracranial pressure. Neurosurgery. 2010;67:675–678. doi: 10.1227/01.NEU.0000383142.10103.0B. [DOI] [PubMed] [Google Scholar]
- 22.Schmidt JH, 3rd, Reyes BJ, Fischer R, Flaherty SK. Use of hinge craniotomy for cerebral decompression. Technical note. J Neurosurg. 2007;107:678–682. doi: 10.3171/JNS-07/09/0678. [DOI] [PubMed] [Google Scholar]
- 23.Shahid AH, Mohanty M, Singla N, Mittal BR, Gupta SK. The effect of cranioplasty following decompressive craniectomy on cerebral blood perfusion, neurological, and cognitive outcome. J Neurosurg. 2018;128:229–235. doi: 10.3171/2016.10.JNS16678. [DOI] [PubMed] [Google Scholar]
- 24.Shih FY, Lin CC, Wang HC, Ho JT, Lin CH, Lu YT, et al. Risk factors for seizures after cranioplasty. Seizure. 2019;66:15–21. doi: 10.1016/j.seizure.2018.12.016. [DOI] [PubMed] [Google Scholar]
- 25.Tian HL, Xu T, Hu J, Cui YH, Chen H, Zhou LF. Risk factors related to hydrocephalus after traumatic subarachnoid hemorrhage. Surg Neurol. 2008;69:241–246. doi: 10.1016/j.surneu.2007.02.032. [DOI] [PubMed] [Google Scholar]
- 26.Vahedi K, Hofmeijer J, Juettler E, Vicaut E, George B, Algra A, et al. Early decompressive surgery in malignant infarction of the middle cerebral artery: a pooled analysis of three randomised controlled trials. Lancet Neurol. 2007;6:215–222. doi: 10.1016/S1474-4422(07)70036-4. [DOI] [PubMed] [Google Scholar]
- 27.Vahedi K, Vicaut E, Mateo J, Kurtz A, Orabi M, Guichard JP, et al. Sequential-design, multicenter, randomized, controlled trial of early decompressive craniectomy in malignant middle cerebral artery infarction (DECIMAL Trial) Stroke. 2007;38:2506–2517. doi: 10.1161/STROKEAHA.107.485235. [DOI] [PubMed] [Google Scholar]
- 28.Waziri A, Fusco D, Mayer SA, McKhann GM, 2nd, Connolly ES., Jr Postoperative hydrocephalus in patients undergoing decompressive hemicraniectomy for ischemic or hemorrhagic stroke. Neurosurgery. 2007;61:489–493. doi: 10.1227/01.NEU.0000290894.85072.37. [DOI] [PubMed] [Google Scholar]