Abstract
Bacteria, fungi, archaea, and viruses are reflective organisms that indicate soil health. Investigating the impact of crude oil pollution on the community structure and interactions among bacteria, fungi, archaea, and viruses in Calamagrostis epigejos soil can provide theoretical support for remediating crude oil pollution in Calamagrostis epigejos ecosystems. In this study, Calamagrostis epigejos was selected as the research subject and subjected to different levels of crude oil addition (0 kg/hm2, 10 kg/hm2, 40 kg/hm2). Metagenomic sequencing technology was employed to analyze the community structure and diversity of soil bacteria, fungi, archaea, and viruses. Additionally, molecular ecological network analysis was integrated to explore species interactions and ecosystem stability within these microbial communities. The functional profiles of soil microorganisms were elucidated based on data from the KEGG database. Results demonstrated a significant increase in petroleum hydrocarbon content, polyphenol oxidase activity, hydrogen peroxide enzyme activity, and acid phosphatase activity upon crude oil addition, while β-glucosidase content, fiber disaccharide hydrolase content, and tiller number decreased (P < 0.05). Proteobacteria and Actinobacteria were identified as dominant bacterial phyla; Ascomycota, Basidiomycota, and Mucoromycota were found to be dominant fungal phyla; Thaumarchaeota emerged as a dominant archaeal phylum; and Uroviricota represented a dominant viral phylum. The diversity of soil bacterial, fungal, archaeal, and viral communities increased with higher amounts of added crude oil. Ecological network analysis revealed a robust collaborative relationship among bacterial, fungal, archaeal, and viral community species in the control treatment (CK), while strong competitive relationships were observed among these species in the treatments with 10% (F10) and 40% (F40) crude oil concentrations. Structural equation modeling analysis indicated significant positive correlations between fungal community, viral community, enzyme activity, and plant growth; conversely, bacterial and archaeal communities showed significant negative correlations with plant growth (P < 0.05). Correlation analysis identified acid phosphatase as the primary environmental factor influencing soil microbial function. Acid phosphatase levels along with tiller number, aboveground biomass, and petroleum hydrocarbons significantly influenced the fungal community (P < 0.05), while underground biomass had a significant impact on the archaeal community (P < 0.05). Acid phosphatase levels along with cellulose-hydrolyzing enzymes, tiller number, and petroleum hydrocarbons exhibited significant effects on the viral community (P < 0.05). This study investigated variations in bacterial, fungal, archaeal, and viral communities under different crude oil concentrations as well as their driving factors, providing a theoretical foundation for evaluating Calamagrostis epigejos’ potential to remediate crude oil pollution.
Keywords: Calamagrostis epigejos, Soil microorganisms, Ecological network, Crude oil addition
Subject terms: Ecology, Microbiology, Environmental sciences
Introduction
Oil pollution has emerged as a pervasive global environmental issue, exerting profound ramifications on the vitality and equilibrium of ecosystems1. The discharge of petroleum hydrocarbons can impinge upon the physical and chemical attributes of soil, thereby engendering detrimental consequences for plant growth and soil microbial communities2. Soil microorganisms represent pivotal constituents within soil ecosystems, playing an unequivocal role in soil health and plant growth3. The configuration and functional diversity of microbial communities are indispensable for upholding the physical, chemical, and biological functionalities of soils4. They not only actively participate in organic matter decomposition, nutrient cycling, and formation of soil structure but also intricately interact with plants to influence their growth and well-being5. Soil microbial communities encompass diverse microorganisms such as bacteria, fungi, archaea, actinomycetes, viruses etc., which synergistically operate through intricate ecological relationships to facilitate nutrient transformation in soils while channeling energy flow6. For instance, bacteria and fungi serve as primary drivers behind organic matter degradation; they break down complex organic molecules into simpler compounds that can be assimilated by plants or other microorganisms7. Archaea play a pivotal role in the global carbon cycle by efficiently consuming methane, thereby potentially mitigating its atmospheric concentration8. Soil viruses exert significant influence on ecological equilibrium and biodiversity promotion through their infection of bacteria, archaea, and fungi in soil, profoundly impacting the structure and functionality of soil microbial communities9. Research has demonstrated that different microorganisms exhibit varying capacities to degrade petroleum hydrocarbons, with specific bacteria and fungi adept at utilizing these compounds as carbon sources10. Consequently, in soils contaminated with crude oil, the abundance of such microorganisms often escalates, leading to the formation of a distinct microbial community structure that indirectly influences the growth and health of Calamagrostis epigejos alterations in its rhizosphere environment.
Calamagrostis epigejos, a perennial herbaceous plant belonging to the Poaceae family, exhibits a wide distribution in temperate and tropical regions of Asia. It is particularly abundant in dry and semi-arid areas of China11. Due to its remarkable adaptability and drought tolerance, Calamagrostis epigejos plays a pivotal role not only in natural ecosystems but also in soil stabilization and ecological restoration initiatives12. Investigating the growth performance of Calamagrostis epigejos in crude oil-contaminated soil and its interaction with rhizosphere microorganisms is essential for comprehending the intricate relationships among plants, microorganisms, and pollutants. Rhizosphere microorganisms refer to microbial communities residing around plant roots that establish close ecological associations with root systems, thereby influencing their growth development and adaptation to environmental stressors13. In oil-polluted environments, rhizosphere microorganisms may actively participate in the decomposition and transformation of crude oil through diverse biochemical processes, consequently impacting the remediation of contaminated soils14. Previous studies have revealed that an increase in crude oil concentration significantly diminishes the height, biomass, leaf number, and root length of Calamagrostis epigejos15.
Plant-microbe interactions are highly complex. Plants regulate the composition of rhizosphere microorganisms through root exudates and their immune system, while rhizosphere microorganisms influence plant development, nutrient absorption, and stress responses through their metabolic activities. For example, plants can selectively promote the growth of beneficial microorganisms, such as nitrogen-fixing and phosphate-solubilizing bacteria, while inhibiting pathogenic microorganisms by modulating the composition of root exudates16,17. Although an increasing number of rhizosphere microorganisms are reported to have the potential to act as soil conditioners, the interaction mechanisms between microorganisms and plants in the soil remain unclear18. With the advancement of metagenomics, the molecular-level interaction mechanisms between rhizosphere microorganisms and plants have gradually been revealed. Rhizosphere microorganisms enhance the availability of phosphorus in the soil by secreting secondary metabolites that dissolve phosphate rock, converting it into plant-available phosphorus, which is crucial for promoting plant growth19. Rhizosphere microorganisms are capable of fixing nitrogen and converting it into ammonia, which plants can readily utilize. This process is a key mechanism by which plants acquire nitrogen. Additionally, microorganisms decompose organic matter, releasing nutrients that plants can absorb20. Furthermore, rhizosphere microorganisms produce plant hormones, such as indoleacetic acid (IAA) and cytokinins, which are crucial for plant growth and development21. In summary, the interaction between plants and rhizosphere microorganisms is multifaceted, involving the exchange of chemical signals, nutrient cycling, hormone synthesis, and immune system regulation. These interactions not only influence plant growth and development but also play a critical role in the health and stability of soil ecosystems.
The Xi’an Botanical Garden, situated in Xi’an City, Shaanxi Province, was chosen as the experimental site for this study. The objective is to investigate the influence of varying concentrations of crude oil pollution on the growth of Calamagrostis epigejos and alterations in the structure of rhizosphere microbial communities. By implementing different levels of crude oil pollution (0 g/kg, 10 g/kg, 40 g/kg) and employing techniques such as biomass measurement, analysis of rhizosphere soil enzyme activity, and metagenomic sequencing, we comprehensively assess the impact of crude oil pollution on Calamagrostis epigejos and its associated rhizosphere microorganisms. Furthermore, this study aims to explore the mechanisms underlying interactions between Calamagrostis epigejos and rhizosphere microorganisms—particularly how these microorganisms regulate plant physiological and biochemical responses under conditions of crude oil pollution—and how these processes affect soil remediation efficiency. Through extensive research endeavors, it is anticipated that this study will provide a theoretical foundation and technical support for ecological restoration efforts targeting crude oil-polluted soil while offering scientific guidance for utilizing Calamagrostis epigejos in ecological restoration.
Results
Soil physicochemical factors
The addition of crude oil did not significantly affect the physicochemical properties of the soil, including pH, available potassium content, and alkaline nitrogen content, as shown in Fig. 1. However, it markedly increased the levels of petroleum hydrocarbons, polyphenol oxidase activity, hydrogen peroxide enzyme activity, acid phosphatase (ACP), N-acetylglucosaminidase (NAG), as well as the aboveground to belowground biomass ratio in the soil. Conversely, it led to a decrease in available phosphorus levels and impacted β-glucosidase (BG), cellulose-hydrolyzing enzymes (CBH), and tiller number in Calamagrostis epigejos.
Fig. 1.
Physical and chemical properties of soil. Note: CK-0 g/kg, F10-10 g/kg, F40-40 g/kg. Different lowercase letters indicate significant treatment differences following LSD test adjustment for the addition of crude oil wastewater (P < 0.05). These values are presented as mean ± SE. The same applies below.
Composition of soil microbial community structure
Through metagenomic sequencing of microorganisms (Fig. 2), it was determined that there were thirteen bacterial phyla, primarily including Proteobacteria, Actinobacteria, Acidobacteria, Chloroflexi, Candidatus, Verrucomicrobia and Gemmatimonadetes. Among these phyla, the bacteria from the first three were dominant with total relative abundances of 84.33% (CK), 85.33% (F10) and 87.33% (F40) at different levels of crude oil addition. The relative abundance of Actinobacteria was lowest in the F10 treatment at 55.96%, while it reached its highest level in the F40 treatment at 40.00%. On the other hand, Proteobacteria exhibited its lowest relative abundance in the CK treatment at 27.00%, but showed higher levels in both F10 and F40 treatments at 39.33% and 40.00%, respectively. Bacteroidetes, Cyanobacteria, Firmicutes, Nitrospirae, and Elusimicrobia had a relative abundance below 1% (< 1%), indicating their rarity; whereas the remaining five bacterial phyla displayed a relative abundance ranging from one to ten% (1-10%). Moreover, the differences observed among Proteobacteria, Acidibactera, Chloroflexi, and Verrucomicrobia between treatments were statistically significant (P < 0 0.05). The fungal kingdom comprises eight phyla, namely Ascomycota, Basidiomycota, Mucoromycota, Blastocladiomycota, Chytridiomycota, Cryptomycota, Microsporidia and Zoopagomycota. Among these phyla, the fungi belonging to the first three are predominant with relative abundances of 99.50% (CK), 97.44% (F10) and 98.09% (F40) respectively under different levels of crude oil addition. Basidiomycota exhibits the lowest relative abundance at 37.88% in CK treatment and the highest at 50.03% in F40 treatment; whereas Ascomycota shows the lowest relative abundance at 6.40% in CK treatment and the highest at 43.61% in F40 treatment. The remaining five phyla have a relative abundance below 1%, indicating their rarity. Furthermore, statistically significant differences (P < 0.05) were observed among the treatments for Ascomycota, Mucoromycota, and Blastocladiomycota phyla. The archaea domain comprises five main phyla: Thaumarchaeota, Euryarchaeota, Candidatus, Crenarchaeota, and Nanoarchaeota. Thaumarchaeota emerges as the predominant archaeal phylum with total relative abundances of 91.24% (CK), 87.99% (F10), and 83.77% (F40) under varying levels of crude oil addition. Nanoarchaeota exhibits a rare occurrence with a relative abundance below 1%, while the remaining three phyla range from 1 to 10%. Additionally, there are seven viral phyla including Uroviricota, Preplasmiviricota, Phixviricota, Nucleocytoviricota, lenarviricota, hofneiviricota, and cossaviricota.The Uroviricotaspecies dominates the viral community with relative abundances of 92.31%(CK), 92.52%(F10), and 92.59%(F40). The other six viralphyla have relative abundances below 1%, indicating their rarity. Significant differences in Phixviricota were also observed among the treatments(P < 0.05).
Fig. 2.
Composition of soil microbial communities (%). Note: * indicates a significant difference between treatments (P < 0.05).
The study investigated the differences in soil microbial community composition after adding different crude oils using PCA analysis (Fig. 3). The total explanatory power of the differences in archaeal, viral, bacterial, and fungal communities was all above 80%, with R > 0, indicating variations in soil microbial community structure under different levels of crude oil addition. ANOSIM test revealed that crude oil addition significantly influenced viral, bacterial, and fungal communities.
Fig. 3.
PCA analysis of soil microbial communities.
Soil microbial community diversity
The diversity indices of soil archaea, viruses, bacteria, and fungi (Fig. 4) exhibited significant differences among the treatments (P < 0.05). Moreover, there was an upward trend in the Shannon and Simpson indices for soil archaea, viruses, bacteria, and fungi as the amount of crude oil increased. The F40 treatment showed significantly higher diversity indices compared to other treatments (P < 0.05).
Fig. 4.
Index of soil microbial community diversity. Note: P < 0.05 indicates significant differences between treatments, while P > 0.05 indicates no significant differences between treatments. The same applies below.
Co-occurrence patterns of soil microbial communities
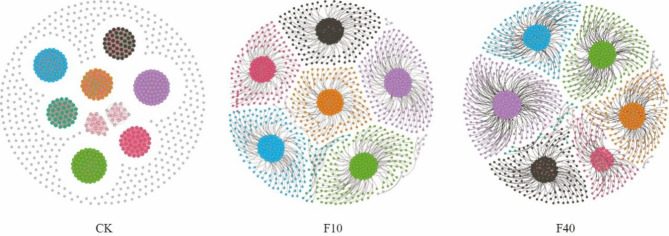
Different additions of crude oil exhibited significant variations in the co-occurrence network characteristics of soil microorganisms (Fig. 5; Table 1). Compared to the control group (CK), crude oil addition significantly increased the number of edges and nodes in the co-occurrence network, indicating a higher complexity of the microbial community’s co-line network and more intricate interactions among different species. However, there was a significant reduction in average weighted degree, graph density, and modularity, suggesting a transition from a highly organized and tightly connected state to a looser and more evenly distributed state within the co-line network. Furthermore, compared to other treatments, CK had a higher proportion of positive correlation edges while exhibiting lower proportions of negative correlation edges. This indicates that there is strong cooperation among microbial community species in CK treatment but stronger competition among microbial community species in crude oil addition treatment.
Fig. 5.
Co-occurrence network analysis of soil microbial communities. Note: the size of the nodes in the network diagram corresponds to their degree, which represents the number of connections they have. Positive connections are depicted by red lines, while negative connections are represented by green lines. Additionally, nodes are color-coded based on distinct categories.
Table 1.
Topological parameters of soil microbial co-occurrence network.
| Topological parameters | CK | F10 | F40 |
|---|---|---|---|
| Node | 776 | 1194 | 1191 |
| Edge | 9721 | 12,700 | 13,429 |
| Positive edge | 60.96% | 54.03% | 56.18% |
| Negative edge | 39.04% | 45.97% | 43.82% |
| Average weighting | 12.527 | 10.637 | 11.275 |
| Graph density | 0.016 | 0.009 | 0.009 |
| Modularity | 0.83 | 0.83 | 0.796 |
KEGG functional annotation and analysis
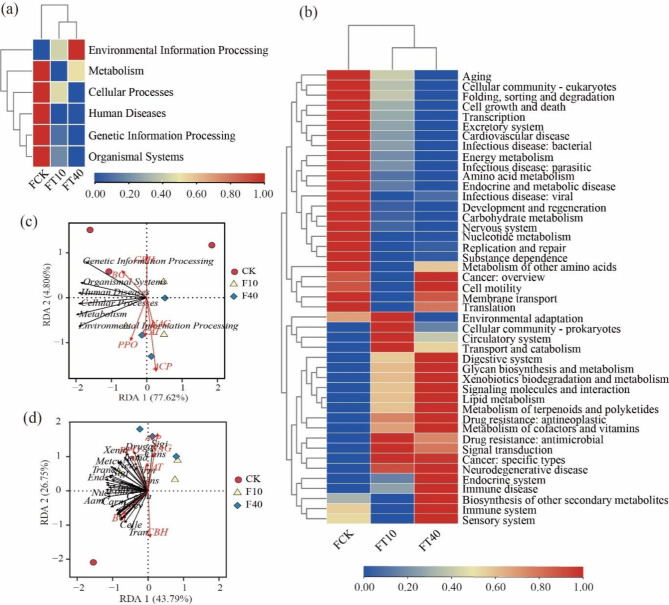
The soil microbial functional genes were annotated using the KEGG database (http://www.genome.jp/kegg/)22. According to Fig. 6a, it can be observed that Metabolism-related genes had the highest abundance (49.99%) among the six subsystems corresponding to KEGG primary pathways, followed by Environmental information processing (13.31%) and Genetic information processing (15.92%). Other highly abundant categories included Cellular processes (10.15%) and Human Diseases (5.61%), among others. With an increase in crude oil addition, there was an upward trend in Environmental information processing while a downward trend was observed for Metabolism, Genetic information processing, Cellular processes, and Human Diseases. Further annotation of KEGG secondary functional genes (Fig. 6b) showed similar results across all treatments with mainly annotated pathways such as Carbohydrate metabolism, Amino acid metabolism, Energy metabolism, and Metabolism of cofactors and vitamins. Additionally, with an increase in crude oil addition, Aging, Carbohydrate metabolism, Amino acid metabolism, and Energy metabolism exhibited a decreasing trend while Cell motility, Digestive system, Lipid metabolism, Endocrine system, Immune system, and Sensory system showed an increasing trend.
Fig. 6.
KEGG functional annotation of different treatment communities. Note: (a) KEGG primary pathway function heat map, (b) KEGG primary pathway function heat map, (c) KEGG primary pathway function and soil enzyme activity redundancy analysis, (d) KEGG secondary pathway function and soil enzyme activity redundancy analysis. PPO: polyphenol oxidase activity, CAT: catalase activity, ACP: acid phosphatase, NAG: N-acetylglucosaminidase, CBH: cellobiohydrolase, BG: β-glucosidase, Aam: Amino acid metabolism, Bio: Biosynthesis of other secondary metabolites, Cano: Cancer overview, Cans: Cancer specific types, Carm: Carbohydrate metabolism, Card: Cardiovascular disease, Celld: Cell growth and death, Cellm: Cell motility, Cellp: Cellular community – prokaryotes, Cirm: Circulatory system, Dever: Development and regeneration, Druga: Drug resistance: antimicrobial, Ends: Endocrine system, Enva: Environmental adaptation, Excs: Excretory system, Glym: Glycan biosynthesis and metabolism, Immd: Immune disease, Infeb: Infectious disease: bacterial, Infev: Infectious disease viral, Lipm: Lipid metabolism, Met: Membrane transport, Metaa: Metabolism of other amino acids, Mettp: Metabolism of terpenoids and polyketides, Ners: Nervous system, Neu: Neurodegenerative disease, Nuc: Nucleotide metabolism, Repr: Replication and repair, Sens: Sensory system, Sigt: Signal transduction, Sigi: Signaling molecules and interaction, Subd: Substance dependence, Tranc: Transport and catabolism, Xenm: Xenobiotics biodegradation and metabolism.
The redundancy analysis of KEGG primary pathway functions and soil enzyme activities (Fig. 6c) revealed significant positive correlations between Genetic Information Processing, Organismal Systems, and Human Diseases with CBH and BG. Additionally, Metabolism and Environmental Information Processing were significantly positively correlated with PPO, CAT, NAG, and ACP. Among these, soil ACP emerged as the primary environmental factor influencing soil microbial function. Similarly, the redundancy analysis of KEGG secondary pathway functions and soil enzyme activities (Fig. 6d) indicated significant positive correlations between Signaling Molecules and Interaction, Cancer: Specific Types, Drug Resistance: Antineoplastic, Xenobiotics Biodegradation and Metabolism, Circulatory System, and Sensory System with PPO, ACP, NAG, and CAT. Moreover, Transcription, Cellular Community - Eukaryotes, Cellular Community - Prokaryotes, and Carbohydrate Metabolism were significantly positively correlated with BG and CBH. Once more, soil ACP was identified as the key environmental factor affecting soil microbial function.
Relationship between soil microbial community and environmental factors
Through correlation analysis (Fig. 7a), it was observed that tiller number exhibited a significant negative correlation with aboveground carbon pool (ACP) and a significant positive correlation with circumference at breast height (CBH) (P < 0.05). Additionally, aboveground biomass displayed a significant negative correlation with ACP, while exhibiting significant positive correlations with tiller number, CBH, and belowground biomass (BG) (P < 0.05). Furthermore, belowground biomass demonstrated a significant positive correlation with ACP and nitrogen accumulation in the grain (NGA), but showed a significant negative correlation with tiller number (P < 0.05). Moreover, potassium availability (AK) exhibited a noteworthy negative association with peroxidase activity (PPO) and catalase activity (CAT) levels(P < 0.05). Similarly, phosphorus availability (AP) displayed a notable positive relationship with tiller number and aboveground biomass; however, it revealed a significant negative association with ACP and belowground biomass(P < 0.05). Lastly, petroleum hydrocarbons exhibited substantial positive correlations with ACP, NGA, and belowground biomass; nevertheless, it presented an evident inverse relationship to tiller number(P < 0.05).
Fig. 7.
Correlation analysis between soil physicochemical factors and microorganisms. (a) correlation heatmap analysis; (b) Mantel heatmap analysis; (c) structural equation model diagram; (d) archaea redundancy analysis; (e) virus redundancy analysis; (f) bacteria redundancy analysis; g-fungi redundancy analysis. In the structural equation model, solid red line arrows represent statistically significant positive paths, dashed green line arrows indicate negative paths, and the numbers on the arrows denote standardized path coefficients. Additionally, X2 = 127.74, df = 11, GFI = 0.39, RMSEA = 1.152 were obtained as evaluation metrics for model fit. PPO refers to polyphenol oxidase activity; CAT represents catalase activity; ACP denotes acid phosphatase activity; NAG stands for N-acetylglucosaminidase activity; CBH indicates cellulose hydrolysis enzyme activity; BG signifies β-glucosidase activity; TIN corresponds to tiller number of Calamagrostis epigejos; AB represents aboveground biomass; BB refers to belowground biomass; AK denotes available potassium in soil; AN represents alkaline nitrogen in soil; AP signifies available phosphorus in soil; PEH stands for petroleum hydrocarbon.
Through Mantel analysis (Fig. 7b), it was observed that ACP, tiller number, aboveground biomass, and petroleum hydrocarbons exerted significant influences on the fungal community (P < 0.05). Belowground biomass significantly impacted the archaeal community (P < 0.05). ACP, CBH, tiller number, and petroleum hydrocarbons exhibited a significant impact on the viral community (P < 0.05). However, crude oil addition did not yield a significant effect on the bacterial community, suggesting that soil bacterial communities are influenced by multiple factors in concert. Further analysis employing structural equation modeling revealed a significant influence of soil enzyme activity on the bacterial community (P < 0.05) (Fig. 7c). Additionally, there existed a noteworthy positive correlation between fungal community composition, viral community composition, soil enzyme activity and plant growth; conversely, there was a notable negative correlation between bacterial and archaeal communities with respect to plant growth (P < 0.05).
Through redundancy analysis of archaea (Fig. 7d), Nanoarchaeota and Candidatus were positively correlated with BG, NAG, PPO, CAT, and ACP. Thaumarchaeota showed a positive correlation with AP and CBH, while Crenarchaeota and Euryarchaeota were positively correlated with pH. Redundancy analysis of viruses (Fig. 7e) revealed that Nucleocytoviricota, Phixviricota, and Preplasmiviricota were positively correlated with pH, ACP, NAG, and PPO. Uroviricota and Hofneiviricota showed a positive correlation with CAT, AP, and BG. Cossaviricota and Lenarviricota exhibited a positive correlation with CBH. Redundancy analysis of bacteria (Fig. 7f) indicated that Candidatus Planctomycetes and Verrucomicrobia were positively correlated with ACP, NAG, CAT, and PPO. Gemmatimonadetes, Nitrospirae, and Acidobacteria showed a positive correlation with BG, AP, and CBH. Chloroflexi, Firmicutes, Elusimicrobia, and Bacteroidetes were positively correlated with pH. Redundancy analysis of fungi (Fig. 7g) revealed that Cryptomycota was positively correlated with BG, AP, and CBH. Ascomycot and Chytridiomycota showed a positive correlation with ACP, NAG, CAT, and PPO. Basidiomycot, Zoopagomycot and Blastocladiomycota were positively correlated with pH.
Discussion
Effects of crude oil addition on soil environmental factors and microbial community characteristics in Calamagrostis epigejos
Soil is a crucial component of the ecosystem, and its physicochemical properties have significant impacts on plant growth and microbial activity. In the context of soil pollution, the impact of adding crude oil to soil physicochemical properties has received extensive attention23,24. The findings from this study indicate that the addition of crude oil does not significantly alter soil pH, available potassium levels, and alkaline nitrogen content. This observation may be attributed to the relatively limited effect of crude oil contamination on soil acid-base balance and potassium-nitrogen content25. However, it is noteworthy that adding crude oil substantially increases petroleum hydrocarbon concentrations in the soil. This phenomenon could be attributed to the inhibitory effect of adding crude oil on the biodegradation process of petroleum hydrocarbons in the soil, leading to their accumulation26. Furthermore, it is worth mentioning that adding crude oil significantly enhances polyphenol oxidase and hydrogen peroxide enzyme activities in the soil, which play pivotal roles in organic matter decomposition and plant growth processes. This may have triggered oxidation reactions of organic matter in the soil due to crude oil pollution27. However, there was a significant decrease in the content of -glucosidase and cellulase, indicating that crude oil pollution had a detrimental impact on soil enzyme activity. -glucosidase and cellulase are vital enzymes in the soil, and their reduced activity may suggest an inhibitory effect of adding crude oil on microbial metabolism in the soil28. In summary, the influence of adding crude oil on soil physical and chemical properties is complex, encompassing both promoting and inhibiting effects. This influence could be attributed to the properties and quantity of added crude oil as well as specific characteristics unique to the soil. Further research is warranted to investigate how adding crude oil affects microbial community structure in soils and elucidate the role played by these microorganisms during biodegradation processes.
Soil microorganisms are small in size, widely distributed, relatively short-lived, diverse in species, and exhibit a rapid response to environmental disturbances. They possess the ability to quickly adapt to changes in the environment and maintain ecosystem stability. Consequently, they serve as important indicators for assessing soil quality and indicating changes within the soil environment29,30. The results of this study revealed that Proteobacteria and Actinobacteria were the dominant bacterial phyla which showed an increasing trend with higher levels of crude oil addition. In contrast, Acidobacteria and Chloroflexi exhibited a decreasing trend with increasing concentrations of crude oil. This observation can be attributed to the superior crude oil degradation capabilities typically possessed by Proteobacteria and Actinobacteria in their natural habitat. The addition of crude oil may provide these bacteria with additional carbon sources and energy, thereby promoting their growth and reproduction31. On the other hand, Acidobacteria and Chloroflexi may not possess similar advantages in crude oil degradation processes; hence their relative abundance decreases under conditions where crude oil is added32. As for fungi, Ascomycota, Basidiomycota, and Mucoromycota were identified as the dominant phyla. Ascomycota demonstrates an increasing trend with the elevation of crude oil addition levels, while Mucoromycota exhibits a declining trend. This phenomenon may be attributed to Ascomycota’s robust capacity for decomposing organic matter, including complex polycyclic aromatic hydrocarbons (PAHs) and petroleum hydrocarbons. Consequently, these fungi are likely to exhibit an upward trend as they can effectively utilize carbon sources present in crude oil33. Conversely, Mucoromycota might display sensitivity towards specific chemical components in crude oil and possess weaker adaptability to environments contaminated by it, thereby limiting their growth in the presence of added crude oil34. Archaea are predominantly represented by Thaumarchaeota and manifest a decreasing trend with the escalation of crude oil addition levels. This decline could be attributed to Thaumarchaeota generating substantial amounts of reactive oxygen species during ammonia oxidation process that would typically be counteracted by intracellular antioxidant systems. However, the introduction of crude oil might induce heightened oxidative stress surpassing Thaumarchaeota’s antioxidant capacity35. Additionally, Thaumarchaeota may rely on symbiotic relationships with algae for energy acquisition; hence, the inclusion of crude oil could disrupt these symbiotic associations leading to constraints on its growth36. The dominant viral phylum is Uroviricota and its relative abundance remains relatively stable across different levels of added crude oil. This observation suggests that Uroviricota may possess a certain degree of tolerance towards crude oil pollution or have the ability to colonize suitable hosts and sustain their survival in environments contaminated with crude oil37. Through multiple comparative analyses, we identified differences in microbial communities, including Proteobacteria, Acidobacteria, Chloroflexi, Ascomycota, Mucoromycota, Candidatus Phixviricota etc., most of which are known to play crucial roles in enhancing soil health and promoting plant growth38,39. The observed variations in microbial phyla under the influence of crude oil imply that different levels of crude oil addition could potentially serve as an effective strategy for enhancing plant productivity, reducing diseases, and improving the soil biotic environment.
With an increase in the amount of crude oil addition, the Shannon and Simpson indices of soil archaea, viruses, bacteria, and fungi exhibit an upward trend, indicating that crude oil addition enhances community diversity. This can be attributed to alterations in soil environmental conditions caused by crude oil addition which enable previously unable microorganisms to thrive and increase microbial community diversity40. Crude oil addition significantly impacts soil microbial community diversity with an optimal amount promoting microbial community health. These findings are significant for guiding ecological restoration efforts in soils contaminated with crude oil as they suggest considering the impact of crude oil on microbial communities when conducting soil remediation for optimal restoration results. PCA analysis visually displays differences between sample points while inferring possible relationships between grouping categories and actual sample distributions41. The results indicate a clear separation among soil bacterial, viral, and fungal communities regarding different treatments. Previous studies on soybean and rapeseed oil additions have also found distinct composition differences between microbial communities from soils with or without added crude oil42.
The impact of crude oil addition on the interaction between soil microorganisms and Calamagrostis epigejos
In the study of soil microbial co-occurrence networks, the number of edges and nodes in the network serves as crucial indicators of network complexity43. The introduction of crude oil enhances both the frequency and complexity of species interactions within the microbial community. This can be attributed to crude oil acting as an exogenous carbon and energy source, attracting a greater diversity of microbial species involved in degradation processes, thereby promoting increased microorganism interactions44. Average weighted degree, graph density, and modularity are metrics used to assess node significance, network connectivity tightness, and structural differentiation within co-occurrence networks45. The addition of crude oil results in a significant reduction in these parameters, indicating that the co-occurrence network undergoes structural changes following exposure to crude oil from a highly organized and tightly connected state to a more loosely distributed state. This alteration may arise due to crude oil disrupting energy and nutrient balance within the microbial community, intensifying competition among microorganisms and consequently leading to modifications in network structure46. Compared to other treatments, the CK treatment demonstrates a higher proportion of positive correlations and a lower proportion of negative correlations among microbial community species. This observation suggests a robust cooperative relationship among microorganisms in the CK treatment, potentially facilitated by the provision of more stable and conducive environmental conditions. Consequently, microorganisms are able to collaborate effectively and optimize resource utilization. In contrast, the addition of crude oil treatment exhibits a pronounced competitive relationship among microbial community species. This can be attributed to alterations in resource availability caused by crude oil addition, thereby intensifying competition for limited resources among microorganisms47.
From an ecological perspective, the addition of crude oil to soil can have significant implications for the stability and functionality of soil ecosystems. On one hand, it has the potential to enhance microbial diversity and activity in the soil, thereby facilitating the biodegradation process of crude oil and mitigating its impact on soil pollution48. On the other hand, it may induce alterations in both structure and functionality of microbial communities, potentially leading to a decline in sensitive or beneficial microorganisms that could negatively affect the health and sustainability of soil ecosystems49. In practical applications, comprehending and harnessing dynamic changes within microbial co-occurrence networks is crucial for developing effective bioremediation strategies. By carefully adjusting both quantity and timing of crude oil addition, it becomes possible to optimize both structure and functionality of microbial communities, thus enhancing efficiency in crude oil degradation as well as effectiveness in soil remediation efforts. Furthermore, investigating shifts within microbial co-occurrence networks can provide valuable theoretical foundations for elucidating underlying mechanisms governing soil microbial ecology while contributing towards improved protection and restoration practices for soil environments.
The impact of crude oil addition on the functional diversity of soil microbial communities in Calamagrostis epigejos
Microorganisms play crucial roles in soil ecosystems and exhibit diverse functions, including nutrient cycling and metabolism50. In this study, the introduction of crude oil significantly influenced the metabolic pathways of soil microorganisms. Genes associated with metabolism exhibited the highest abundance, suggesting that microbial communities actively adjust their metabolic pathways to adapt to environments contaminated with crude oil51. As the amount of crude oil increased, genes related to environmental information processing, cell motility, and digestive system pathways showed an increase in abundance. This response may be attributed to microorganisms adapting to external environmental changes and enhancing their ability to cope with crude oil pollution52. Conversely, genes linked to metabolism, genetic information processing, cellular processes, human diseases aging, and carbohydrate metabolism pathways displayed a decrease in abundance. This decline might reflect the inhibitory effect of adding crude oil on microbial community metabolic function and potential ecological pressure53. Redundancy analysis revealed a significant positive correlation between soil acid phosphatase (ACP) activity and multiple KEGG pathways, highlighting the crucial role of ACP in regulating microbial metabolism. ACP plays a vital role in the synthesis and degradation of microbial cell walls, which is essential for their survival and adaptation to environmental changes54. Furthermore, pathways such as Signaling molecules and interaction, Cancer: specific types exhibited a positive correlation with the activities of polyphenol oxidase (PPO), ACP, N-acetylglucosaminidase (NAG), and catalase (CAT) enzymes. This suggests that these enzymes play an important role in microbial response to environmental stress and signal transduction55. In conclusion, KEGG pathway analysis reveals the impact of crude oil addition on soil microbial community functionality while emphasizing the critical role of soil enzyme activity. These findings provide significant scientific evidence for comprehending and addressing issues related to crude oil pollution while offering novel insights into the protection and restoration of soil microbial functionality. Future research can further explore the response mechanisms of microbial communities to crude oil addition and promote the recovery of soil microbial functionality through regulation of key enzyme activities for biodegradation.
Conclusion
This study enhances our understanding of the mechanisms underlying variations in soil bacterial, fungal, archaeal, and viral communities under different levels of crude oil addition. The dominant phyla for bacteria, fungi, archaea, and viruses were Proteobacteria, Actinobacteria, Ascomycota, Basidiomycota, Mucoromycota, Thaumarchaeota, and Uroviricota, respectively. With an increase in crude oil addition levels, the diversity of soil bacterial, fungal, archaeal, and viral communities exhibited an upward trend with significantly higher microbial community diversity observed in the F40 treatment compared to other treatments. Ecological network analysis revealed a transition from cooperative interactions to competitive relationships among soil bacterial, fungal, archaeal, and viral communities as crude oil addition levels increased. Furthermore, the fungal and viral communities as well as soil enzyme activity demonstrated a significant positive correlation with plant growth while the bacterial and archaeal communities displayed a significant negative correlation. This experiment provides a theoretical foundation for remediation strategies against crude oil pollution using Calamagrostis epigejos by investigating changes in bacterial, fungal, archaeal, and viral communities under varying levels of crude oil exposure and their driving factors.
Materials and methods
Experimental materials
The seeds of Calamagrostis epigejos utilized in this experiment were collected from Sihemu Nature Reserve in Wuhai City, Inner Mongolia, in June 2020. The crude oil was obtained from Ansai Oilfield located in northern Shaanxi Province. Soil samples for testing purposes were extracted from the newly established area of Xi’an Botanical Garden in Shaanxi Province. Topsoil (0–20 cm) was gathered, dried, sieved to eliminate impurities, and mixed with river sand at a volume ratio of 3:1. After thorough mixing, the crude oil dissolved in petroleum ether at a volume ratio of 1:1 was added and thoroughly blended before being poured into the soil samples. After vigorous stirring and kneading to ensure uniform distribution of pollutants within the soil as an indicator of stress on Calamagrostis epigejos’s biochemical properties, the contaminated soil was further combined with the remaining soil. To simulate recently polluted surface soil conditions, the contaminated soil sample underwent natural homogenization treatment for 30 days under shade.
Experimental design
Based on the actual soil oil pollution situation in the production area of Yan’an Oilfield in northern Shaanxi and incorporating previous research findings, this experiment established three levels of crude oil contamination at 0 g/kg, 10 g/kg, and 40 g/kg with three replicates for each treatment level. Feather grass used in this study was initially cultivated in a seedling tray and subsequently transplanted into plastic flower pots measuring 10 cm × 10 cm upon reaching four leaves. Three plants exhibiting consistent growth were selected for each pot. Once they reached approximately 10 cm height, the plants were rinsed with running water before being transplanted into plastic flower pots measuring 20 cm × 15 cm to undergo crude oil stress treatment. Each pot contained 1.6 kg of treated soil, and there were three biological replicates for each treatment level. The cultivation took place in a greenhouse under regular moisture management without fertilization or weed removal over a period of five months from June12th, 2023 to November 2nd, 2023.
Collection of soil samples
The plant samples are manually divided into aboveground parts and underground parts (root system). The soil samples are divided into three portions: one portion is poured into a sterilized centrifuge tube and placed in a foam box with ice packs, transported to the laboratory, and stored at -80 °C freezer for subsequent extraction of total DNA from the soil; another portion is brought back to the laboratory and kept cool in a 4 °C refrigerator for determination of soil enzyme activity; the remaining portion is air-dried and used for measuring the physicochemical properties of the soil.
Soil enzyme determination method
The determination of soil enzyme activity was conducted using the microplate fluorescence method56. 4-methylumbelliferone (MUB) was employed as a standard control for β-glucosidase (BG), N-acetylglucosaminidase (NAG), cellulose hydrolyzing enzymes (CBH), and acid phosphatase (ACP) activities. Frozen soil samples at -20 °C were thawed in a refrigerator at 4 °C for 5 days. Subsequently, fresh soil weighing 1.5 g was added to a sodium acetate solution with a volume of 125 mL and mixed thoroughly on a magnetic stirrer for approximately one minute to obtain a homogeneous soil slurry. Using an eight-channel pipette, 200µL of the soil slurry was transferred onto each well of a 96-well microplate in the sample group, followed by adding corresponding enzyme substrates with a volume of 50µL. For the control groups, acetic acid, standard control substances, and specific substrates were used respectively. The microplate containing the samples was incubated at 25 °C for three hours before promptly measuring the readings under excitation light at both wavelengths of 365 nm and 450 nm using an enzyme analyzer (Synergy H1). Soil polyphenol oxidase activity was determined using the pyrogallol colorimetric method, while catalase activity was measured through potassium permanganate titration57,58.
Metagenomic sequencing of soil microorganisms
The total genomic DNA was extracted from the samples using the E.Z.N.A.® Soil DNA Kit (Omega Bio-tek, Norcross, GA, USA). The concentration and purity of the extracted DNA were determined using TBS-380 and NanoDrop2000 spectrophotometers, respectively. The quality of the DNA extraction solution was assessed by 1% agarose gel electrophoresis. Subsequently, the extracted DNA fragments were fragmented to an average size of approximately 400 bp using Covaris M220 software (Gene Company Limited, China) and constructed into paired-end libraries. Paired-end libraries were prepared utilizing the NEXTflexTM Rapid DNA-Seq kit (Bioo Scientific, Austin, TX, USA), where adapters containing complete sequencing primer hybridization sites were ligated to blunt ends of the fragments. Paired-end sequencing was performed on Illumina NovaSeq/Hiseq Xten instruments (Illumina Inc., San Diego, CA, USA) with NovaSeq Reagent kit/Hiseq X Reagent kit at Aijibaike Biotechnology Co., Ltd. in Wuhan, China.
Sequence quality control and genome assembly: The Majorbio Cloud platform (cloud.majorbio.com) is utilized for accessing the fastp tool59 (https://github.com/OpenGene/fastp, version 0.20.0) online, which enables accessing the fastp tool59 online to remove adapter sequences, trim and filter low-quality reads containing N bases below a minimum length threshold of 50 bp or falling below a minimum quality threshold of 20 in order to generate clean reads. Subsequently, MEGAHIT60 (with parameters kmer min = 47, kmer_max = 97, step = 10) (https://github.com/voutcn/megahit, version 1.1.2) is employed for efficient de Bruijn graph-based assembly of these high-quality reads into contigs using a de Bruijn graph-based approach. The final assembly results consist of contigs with lengths equal to or exceeding 300 bp.
Species and functional annotation: The representative sequences of the non-redundant gene catalog were annotated using blastp with DIAMOND v0.9.19, based on the NCBI NR database. Classification annotation was performed using DIAMOND61 (http://www.diamondsearch.org/index.php, version 0.8.35) with an e-value cutoff of 1e-5. KEGG annotation was conducted by employing Diamond61 (http://www.diamondsearch.org/index.php, version 0.8.35) to annotate against the Kyoto Encyclopedia of Genes and Genomes database (http://www.genome.jp/keeg/, version 94.2), with an e-value cutoff of 1e− 5.
Data analysis
The data on soil physicochemical properties, bacterial, fungal, archaeal, and viral community composition were processed using SPSS 26.0 and Excel 2010. Significance analysis of differences was conducted through one-way analysis of variance (ANOVA) followed by multiple comparisons (LSD method, P = 0.05). Bacterial, fungal, archaeal, and viral community composition and diversity analyses were performed utilizing the Aigibike-Sanger cloud platform. Molecular ecological networks were calculated using R language and visualized with Gephi software. Redundancy analysis (RDA) was employed to explore the relationship between microbial communities and soil environmental factors for plotting purposes using CANOCO 5.0 software. Graphs were further refined using Adobe Illustrator CS6.
Author contributions
Ying Wei: Data curation, Investigation, Methodology, Software, Writing – original draft. Ming Yue: Conceptualization, Funding acquisition, Methodology, Writing – review & editing. Zhuxin Mao: Conceptualization, Data curation, Methodology, Writing – review & editing. Yukun Zhu, Chen Chen and Liqun Yang: Conceptualization, Investigation, Methodology, Writing – review & editing. Yuchao Wang and Qian Li: Investigation, Methodology, Software, Data curation, Writing – review & editing. Yang Li: Investigation, Project administration, Software, Supervision, Writing – review & editing. Jinlin Lv: Investigation, Methodology, Software, Writing – review & editing. Wenyan Xue: Data curation, Formal analysis, Investigation, Software, Supervision, Writing – original draft.
Funding
This work was supported by “Xi’an Agricultural Technology R&D Projects(22NYYF029)”, “Natural Science Foundation of Science and Technology Department of Shaanxi Province (S2024-JC-YB-2574)”, “Xi’an Science Technology Bureau Fund (2023JH-NJGG-0167)”, “Natural Science Foundation of Science and Technology Department of Shaanxi Province ( 2020ZDLSF06-01)” and “the Project of the First Investigation of Wild Plants Resources in Xi’an (KRDL K6-2207039)” ,“Strategic Reserve Talent Training Program of Shaanxi Academy of Sciences (2021K-25) ”.
Data availability
The sequence data associated with this project have been deposited in NCBI database under accession number PRJNA1158518.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ming Yue, Email: yueming196710@163.com.
Zhuxin Mao, Email: zhuxinmao@gmail.com.
References
- 1.Liang, W. & Yang, M. Urbanization, economic growth and environmental pollution: Evidence from China. Sustain. Comput. Inf. Syst.21, 1–9 (2019). [Google Scholar]
- 2.Zhang, B., Matchinski, E. J., Chen, B., Ye, X., Jing, L. & Lee, K. Marine oil spills—oil pollution, sources and effects. In World seas: an environmental evaluation. 391–406 (Elsevier, 2019).
- 3.Schlatter, D. C., Kahl, K., Carlson, B., Huggins, D. R. & Paulitz, T. Soil acidification modifies soil depth-microbiome relationships in a no-till wheat cropping system. Soil Biol. Biochem.149, 107939 (2020). [Google Scholar]
- 4.O’Brien, S. L. et al. Spatial scale drives patterns in soil bacterial diversity. Environ. Microbiol.18(6), 2039–2051 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koshila Ravi R, Anusuya S, Balachandar M, Muthukumar T. Microbial interactions in soil formation and nutrient cycling. Mycorrhizosphere and pedogenesis. 363–382 (2019).
- 6.Ma, S. et al. Inconsistent responses of soil microbial community structure and enzyme activity to nitrogen and phosphorus additions in two tropical forests. Plant Soil.460, 453–468 (2021). [Google Scholar]
- 7.Khatoon, H. et al. Role of microbes in organic carbon decomposition and maintenance of soil ecosystem. Int. J. Chem. Stud.5(6), 1648–1656 (2017). [Google Scholar]
- 8.Evans, P. N. et al. An evolving view of methane metabolism in the Archaea. Nat. Rev. Microbiol.17(4), 219–232 (2019). [DOI] [PubMed] [Google Scholar]
- 9.Kuzyakov, Y. & Mason-Jones, K. Viruses in soil: Nano-scale undead drivers of microbial life, biogeochemical turnover and ecosystem functions. Soil Biol. Biochem.127, 305–317 (2018). [Google Scholar]
- 10.Lumactud, R., Shen, S. Y., Lau, M. & Fulthorpe, R. Bacterial endophytes isolated from plants in natural oil seep soils with chronic hydrocarbon contamination. Front. Microbiol.7, 755 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang, G., Shen, Z. & Fu, G. Geo-distribution patterns of soil fungal community of Pennisetum flaccidum in Tibet. J. Fungi8(11), 1230 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zubairi, T., Jabeen, K., Khalid, S. & Iqbal, S. Isolation and molecular characterization of causal agent of blue mold on Allium cepa L. and its control by Pennisetum flaccidum Griseb. Saudi J. Biol. Sci.28(12), 6774–6781 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spitzer, C. M. et al. Root traits and soil micro-organisms as drivers of plant–soil feedbacks within the sub-arctic tundra meadow. J. Ecol.110(2), 466–478 (2022). [Google Scholar]
- 14.Asemoloye, M. D., Jonathan, S. G. & Ahmad, R. Synergistic plant-microbes interactions in the rhizosphere: A potential headway for the remediation of hydrocarbon polluted soils. Int. J. Phytoremediation21(2), 71–83 (2019). [DOI] [PubMed] [Google Scholar]
- 15.Maddhesiya, P. K., Singh, K. & Singh, R. P. Effects of perennial aromatic grass species richness and microbial consortium on soil properties of marginal lands and on biomass production. Land Degrad. Dev.32(2), 1008–1021 (2021). [Google Scholar]
- 16.Bai, B. et al. The root microbiome: Community assembly and its contributions to plant fitness. J. Integrative Plant Biol.64(2), 230–243 (2022). [DOI] [PubMed] [Google Scholar]
- 17.Zhang, J. et al. NRT1. 1B is associated with root microbiota composition and nitrogen use in field-grown rice. Nat. biotechnol.37(6), 676–684 (2019). [DOI] [PubMed] [Google Scholar]
- 18.Koprivova, A. & Kopriva, S. Plant secondary metabolites altering root microbiome composition and function. Curr. Opin. Plant Biol.67, 102227 (2022). [DOI] [PubMed] [Google Scholar]
- 19.Jog, R., Pandya, M., Nareshkumar, G. & Rajkumar, S. Mechanism of phosphate solubilization and antifungal activity of Streptomyces spp. isolated from wheat roots and rhizosphere and their application in improving plant growth. Microbiology160(4), 778–788 (2014). [DOI] [PubMed] [Google Scholar]
- 20.Galloway, J. N. et al. Nitrogen cycles: Past, present, and future. Biogeochemistry70, 153–226 (2004). [Google Scholar]
- 21.Sun, L. et al. The volatile organic compounds of Floccularia luteovirens modulate plant growth and metabolism in Arabidopsis thaliana. Plant Soil456, 207–221 (2020). [Google Scholar]
- 22.Kanehisa, M., Goto, S., Kawashima, S. & Nakaya, A. The KEGG databases at GenomeNet. Nucleic Acids Res.30(1), 42–46 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Calvo, C. et al. Biostimulation of crude oil-polluted soils: Influence of initial physicochemical and biological characteristics of soil. Int. J. Environ. Sci. Technol.16, 4925–4934 (2019). [Google Scholar]
- 24.Egobueze, F. E., Ayotamuno, J. M., Iwegbue, C. M., Eze, C. & Okparanma, R. N. Effects of organic amendment on some soil physicochemical characteristics and vegetative properties of Zea mays in wetland soils of the Niger Delta impacted with crude oil. Int. J. Recycling Org. Waste Agric.8, 423–435 (2019). [Google Scholar]
- 25.Akhanova, T. R. et al. Complex restoration of oil-contaminated soils with new organomineral reagents. Water Air Soil Pollut.234(11), 686 (2023). [Google Scholar]
- 26.Zhang, B., Zhang, L. & Zhang, X. Bioremediation of petroleum hydrocarbon-contaminated soil by petroleum-degrading bacteria immobilized on biochar. RSC Adv.9(60), 35304–35311 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vasilyeva, G., Kondrashina, V., Strijakova, E. & Ortega-Calvo, J.-J. Adsorptive bioremediation of soil highly contaminated with crude oil. Sci. Total Environ.706, 135739 (2020). [DOI] [PubMed] [Google Scholar]
- 28.Fatima, K., Imran, A., Amin, I., Khan, Q. & Afzal, M. Plant species affect colonization patterns and metabolic activity of associated endophytes during phytoremediation of crude oil-contaminated soil. Environ. Sci. Pollut. Res.23, 6188–6196 (2016). [DOI] [PubMed] [Google Scholar]
- 29.Bahram, M. et al. Structure and function of the global topsoil microbiome. Nature560(7717), 233–237 (2018). [DOI] [PubMed] [Google Scholar]
- 30.Li, L. et al. Long-term phosphorus fertilization reveals the phosphorus limitation shaping the soil micro-food web stability in the Loess Plateau. Front. Microbiol.14, 1256269 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun, W. et al. Microbial communities inhabiting oil-contaminated soils from two major oilfields in Northern China: Implications for active petroleum-degrading capacity. J. Microbiol.53, 371–378 (2015). [DOI] [PubMed] [Google Scholar]
- 32.Shahi, A., Aydin, S., Ince, B. & Ince, O. Reconstruction of bacterial community structure and variation for enhanced petroleum hydrocarbons degradation through biostimulation of oil contaminated soil. Chem. Eng. J.306, 60–66 (2016). [Google Scholar]
- 33.Aranda, E. Promising approaches towards biotransformation of polycyclic aromatic hydrocarbons with Ascomycota fungi. Curr. Opin. Biotechnol.38, 1–8 (2016). [DOI] [PubMed] [Google Scholar]
- 34.Daccò, C. Selection of new fungal strains and development of a microbial consortium for the bioremediation of complex hydrocarbon mixtures (2021).
- 35.Horak, R. E. et al. Relative impacts of light, temperature, and reactive oxygen on thaumarchaeal ammonia oxidation in the North Pacific Ocean. Limnol. Oceanograph.63(2), 741–757 (2018). [Google Scholar]
- 36.Lian, J., Zou, D., Trebuch, L. M., Duan, C. & Li, M. Exploring the interactions between algae and archaea. Marine Life Sci. Technol., 1–16 (2024).
- 37.Hsieh, S.-Y. Investigating the Human Intestinal Virome, (University of East Anglia; 2020).
- 38.Khakbazan, M. et al. Effects of crop rotation on energy use efficiency of irrigated potato with cereals, canola, and alfalfa over a 14-year period in Manitoba, Canada. Soil Tillage Res.195, 104357 (2019). [Google Scholar]
- 39.Kayikcioglu, H. H., Duman, İ, Asciogul, T. K., Bozokalfa, M. K. & Elmacı, Ö. L. Effects of tomato-based rotations with diversified pre-planting on soil health in the Mediterranean soils of Western Turkey. Agric. Ecosyst. Environ.299, 106986 (2020). [Google Scholar]
- 40.Redfern, L. K. Microbial Communities and Polycyclic Aromatic Hydrocarbons: Exposure Related Adaptations in Environmental Microbiomes and Their Potential for Bioremediation, (Duke University; 2017).
- 41.Rivas, M. N. et al. A microbiota signature associated with experimental food allergy promotes allergic sensitization and anaphylaxis. J. Allergy Clin. Immunol.131(1), 201–212 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhong, Y. et al. Intercropping tea plantations with soybean and rapeseed enhances nitrogen fixation through shifts in soil microbial communities. Front. Agric. Sci. Eng.9(3), 344–355 (2022). [Google Scholar]
- 43.Gui, H. et al. Continental-scale insights into the soil microbial co-occurrence networks of Australia and their environmental drivers. Soil Biol. Biochem.186, 109177 (2023). [Google Scholar]
- 44.Zhou, Y. et al. Driving mechanisms for the adaptation and degradation of petroleum hydrocarbons by native microbiota from seas prone to oil spills. J. Hazard. Mater.476, 135060 (2024). [DOI] [PubMed] [Google Scholar]
- 45.Chrobak, G. et al. Graph enhanced co-occurrence: Deep dive into urban park soundscape. Ecol. Indicators165, 112172 (2024). [Google Scholar]
- 46.Cheng, W. et al. Nutrient availability contributes to structural and functional diversity of microbiome in Xinjiang oilfield. Front. Microbiol.15, 1450226 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chunyan, X., Qaria, M. A., Qi, X. & Daochen, Z. The role of microorganisms in petroleum degradation: Current development and prospects. Sci. Total Environ.865, 161112 (2023). [DOI] [PubMed] [Google Scholar]
- 48.Bidja Abena, M. T. et al. Microbial diversity changes and enrichment of potential petroleum hydrocarbon degraders in crude oil-, diesel-, and gasoline-contaminated soil. 3 Biotech10, 1–15 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang, Y., Wang, J., Leng, F. & Chen, J. Effects of oil pollution on indigenous bacterial diversity and community structure of soil in Fushun, Liaoning Province, China. Geomicrobiol. J.38(2), 115–126 (2021). [Google Scholar]
- 50.Gao, Y. et al. Metagenomics analysis identifies nitrogen metabolic pathway in bioremediation of diesel contaminated soil. Chemosphere271, 129566 (2021). [DOI] [PubMed] [Google Scholar]
- 51.Gkorezis, P. et al. The interaction between plants and bacteria in the remediation of petroleum hydrocarbons: An environmental perspective. Front. Microbiol.7, 1836 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xu, X. et al. Petroleum hydrocarbon-degrading bacteria for the remediation of oil pollution under aerobic conditions: a perspective analysis. Front. Microbiol.9, 2885 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Huang, L., Ye, J., Jiang, K., Wang, Y. & Li, Y. Oil contamination drives the transformation of soil microbial communities: Co-occurrence pattern, metabolic enzymes and culturable hydrocarbon-degrading bacteria. Ecotoxicol. Environmental Saf.225, 112740 (2021). [DOI] [PubMed] [Google Scholar]
- 54.Kondakova, T. et al. Glycerophospholipid synthesis and functions in Pseudomonas. Chem. Phys. Lipids190, 27–42 (2015). [DOI] [PubMed] [Google Scholar]
- 55.Pu S, Liu S. Extracellular Enzymes in Environments: Responses to Collaborative Remediation of Contaminated Soil and Groundwater. Elsevier; 2023.
- 56.Frankenberger, W. & Johanson, J. Factors affecting invertase activity in soils. Plant Soil74, 313–323 (1983). [Google Scholar]
- 57.Moore, C. E. et al. The effect of increasing temperature on crop photosynthesis: from enzymes to ecosystems. J. Exp. Botany72(8), 2822–2844 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kumarathunge, D. P. et al. Acclimation and adaptation components of the temperature dependence of plant photosynthesis at the global scale. New Phytologist.222(2), 768–784 (2019). [DOI] [PubMed] [Google Scholar]
- 59.Chen, S., Zhou, Y., Chen, Y. & Gu, J. fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics34(17), i884–i890 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li, D., Liu, C.-M., Luo, R., Sadakane, K. & Lam, T.-W. MEGAHIT: An ultra-fast single-node solution for large and complex metagenomics assembly via succinct de Bruijn graph. Bioinformatics31(10), 1674–1676 (2015). [DOI] [PubMed] [Google Scholar]
- 61.Buchfink, B., Xie, C. & Huson, D. H. Fast and sensitive protein alignment using DIAMOND. Nat. Methods12(1), 59–60 (2015). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The sequence data associated with this project have been deposited in NCBI database under accession number PRJNA1158518.