ABSTRACT
Objectives
To evaluate the quality and types of care individuals with mild‐to‐moderate knee osteoarthritis receive in the Canadian Maritime provinces, and determine associations with demographic, social, and patient‐reported factors.
Methods
Individuals with knee osteoarthritis were invited to complete a healthcare quality survey based on the British Columbia Osteoarthritis (BC OA) survey. The cross‐sectional descriptive observational survey assessed four healthcare quality indicators: advice to exercise, advice to lose weight, assessment of ambulatory function, and assessment of non‐ambulatory function. Pass‐rates were calculated overall and for each quality indicator. Binary logistic regressions determined associations between quality indicators and demographic, social, and patient‐reported outcomes. Patient‐reported use of exercise and diet as arthritis treatments were added to the quality indicator eligibility criteria as a sensitivity analysis.
Results
Participants (n = 241) had a mean age of 67 (7) years, body mass index of 30.7 (7.5) kg/m2 and were 77% female. The overall pass rate was 42.9% using the BC OA criteria, and 49.3% in the sensitivity analysis. Individual quality indicator pass‐rates ranged from 4.3% for non‐ambulatory function to 85.7% for ambulatory function assessments. The sensitivity analysis increased pass‐rates for advice to exercise (61.9%–69.3%) and advice to lose weight (27.9%–35.1%). Pass‐rates were not driven by demographic, social, or patient‐reported factors.
Conclusions
Over half of individuals with mild‐to‐moderate knee osteoarthritis did not receive recommended core treatments in the Maritimes, highlighting a need to improve care for this patient group. Quality indicators should be routinely evaluated to determine whether clinical care aligns with best practice guidelines.
Keywords: community survey, exercise, health care quality assessment, healthcare quality indicator, knee osteoarthritis, quality indicator survey
1. Introduction
Knee osteoarthritis is a common chronic and disabling joint condition that affects one in every eight Canadian adults aged 55 years or older (Global Burden of Disease Collaborative Network 2020), with the highest prevalence in the Maritime provinces (Nova Scotia, New Brunswick, and Prince Edward Island) (Statistics Canada 2022). Knee osteoarthritis substantially contributes to joint pain, mobility impairments, and decreased quality of life (Vina and Kwoh 2018). Evidence‐based clinical practice guidelines consistently recommend non‐surgical and non‐pharmacological treatments to manage knee osteoarthritis. Core management strategies include education and self‐management, physical activity, therapeutic exercise, and weight management (Bannuru et al. 2019; Kolasinski et al. 2020). Evidence suggests that exercise improves pain and physical function (Zampogna et al. 2020), and these benefits are consistent across supervised and unsupervised exercise (Hinman et al. 2023). Additionally, weight loss, when medically indicated, reduces joint loads and inflammation and improves clinical outcomes (Messier et al. 2020). Despite international consensus on core management, whether and when individuals with knee osteoarthritis receive evidence‐based care in practice remains unclear.
No cure for knee osteoarthritis exists, and traditional clinical management strategies focus on symptom relief using medication on a pathway to end‐stage joint replacement surgery (Crawford, Miller, and Block 2013). Recent data show that only 21%–47% of individuals waiting for a knee joint replacement in the Maritime provinces received the surgery within national benchmark timeframes in 2023 (Canadian Institute for Health Information 2024), indicating that surgical demand greatly exceeds healthcare system capacity. Osteoarthritis treatments are traditionally coordinated by general practitioners (Finley et al. 2018), although patients may attempt treatment independently before consulting a physician (Horn et al. 2021). Further, recent recommendations suggest that multidisciplinary healthcare teams should be involved to provide core management (Jayakumar, Moore, and Bozic 2019), as this model may further improve patient outcomes (Hunter et al. 2022). A critical shift in knee osteoarthritis management is required to improve healthcare quality and patient outcomes (Eyles et al. 2019).
Osteoarthritis quality indicator sets have been developed to assess healthcare quality, represent the minimum acceptable standard of care, and focus on care processes provided to patients. For example, the Arthritis Foundation Quality Indicator Project developed 14 quality indicators related to osteoarthritis assessment, treatment, and follow‐up (MacLean et al. 2004). A sub‐set of these quality indicators align with treatments related to exercise, weight loss, and assistive devices, and can therefore be used to examine the quality of these core treatments. Furthermore, healthcare quality can be assessed from the clinician or patient perspective; however, evidence suggests that medical records can be discordant with patient perceptions of care received (Jordan, Jinks, and Croft 2006). Therefore, patient self‐reported quality indicator metrics are the preferred method to monitor and evaluate osteoarthritis healthcare quality because they reflect the quality of care as perceived by the patient (Østerås et al. 2013).
Studies provide a wide range of results when examining the quality and types of knee osteoarthritis care consistent with clinical practice guidelines in Canada (Glazier et al. 2003; King et al. 2020; Li et al. 2011) and globally (Ganz et al. 2006; Grønhaug, Østerås, and Hagen 2014 2015; Ingelsrud et al. 2020; Larmer et al. 2019; Oomen et al. 2022; Østerås et al. 2013 2015). Previous research suggests that the quality and types of care received were dependent on demographic and social factors including age (Glazier et al. 2003; King et al. 2020; Li et al. 2011), sex (King et al. 2020; Li et al. 2011), and education level (King et al. 2020; Li et al. 2011), as well as country (Østerås et al. 2015), or province within Canada (Glazier et al. 2003; King et al. 2020; Li et al. 2011). Additionally, much of the previous quality indicator work has been completed with a focus on late‐stage knee osteoarthritis management, defined as severe symptoms (e.g., pain) or awaiting knee joint replacement surgery (Glazier et al. 2003; Ingelsrud et al. 2020; King et al. 2020; Oomen et al. 2022). While later‐stage interventions prior to joint replacement may help optimise post‐surgical outcomes (Gränicher et al. 2022) or delay surgery (Skou et al. 2018), they have less potential to slow or potentially halt knee osteoarthritis progression. Alternatively, implementing non‐surgical and non‐pharmacological care at earlier stages of knee osteoarthritis may contribute to slowing or preventing symptoms or structural disease progression and minimising the burden of knee osteoarthritis (Mahmoudian et al. 2021). Thus, individuals with earlier stage knee osteoarthritis (i.e., mild‐to‐moderate, as opposed to severe, symptoms and functional impairments) represent an important target for knee osteoarthritis management to optimise patient outcomes and healthcare pathways.
The purpose of this study was to evaluate the quality and types of care individuals with mild‐to‐moderate knee osteoarthritis receive in the Maritime provinces (Nova Scotia, New Brunswick, Prince Edward Island). This research aimed to determine the quality of non‐surgical and non‐pharmacological care strategies prescribed, and the association between the quality and types of care received with demographic and social factors, and patient‐reported outcomes.
2. Methods
This cross‐sectional descriptive observational study adheres to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines (von Elm et al. 2007). This study was approved by the Atlantic Partnership for Tomorrow's Health (PATH) Data Access Committee and the Nova Scotia Health Research Ethics Board (file # 1025913). All participants provided written informed consent. The funders played no role in the design, conduct, or reporting of this study.
2.1. Participant Recruitment
Individuals from the three Maritime provinces with self‐reported osteoarthritis were recruited for this study from the Atlantic PATH cohort (Sweeney et al. 2017). The Atlantic PATH is a regional cohort of the Canadian Partnership for Tomorrow's Health study, which consists of over 330,000 participants within seven regional cohorts across 10 provinces, and represents Canada's largest population health study (Dummer et al. 2018). Individuals from the Atlantic PATH cohort were recruited for this study if they (a) self‐reported osteoarthritis at baseline (i.e., responded ‘yes’ to the question ‘has a doctor ever told you that you had osteoarthritis?’), (b) currently resided in Nova Scotia, New Brunswick, or Prince Edward Island, and (c) provided an email address as a method of contact. Individuals self‐reporting osteoarthritis from the Atlantic PATH cohort have been shown to represent a healthier and less clinically severe group compared with the wider Canadian population with osteoarthritis (Kozey et al. 2023).
2.2. Data Collection
Participants were invited to complete an electronic healthcare quality survey. This 62‐question survey replicated the British Columbia Osteoarthritis (BC OA) Survey (Li et al. 2011), which is based on recommendations provided by the Arthritis Foundation Quality Indicator Project (MacLean et al. 2004). The questionnaire collected information on (a) general health and arthritis, including health services used to manage arthritis, (b) comorbidities including diabetes, high blood pressure, heart conditions, liver conditions, kidney and/or bladder conditions, lung conditions, intestinal or stomach ulcers, bowel disorder, fibromyalgia, osteoporosis, cancer, and depression, and (c) osteoarthritis outcomes using the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) (Bellamy et al. 1988). Supplementary osteoarthritis‐specific instruments included the Knee injury and Osteoarthritis Outcome Score (function during sport and recreational activities sub‐scale; KOOS‐Sport) (Roos et al. 1998), Intermittent and Constant Osteoarthritis Pain (ICOAP) questionnaire (Hawker et al. 2008), the 5‐level Euro‐QOL 5‐dimension (EQ‐5D‐5L) questionnaire (Herdman et al. 2011), and the Oxford Knee Score (Dawson et al. 1998). All survey responses were collected through the secure Research Electronic Data Capture (REDCap) online portal hosted within Nova Scotia Health.
2.3. Data Processing
Four healthcare quality indicators were derived from BC OA survey responses: (1) advice to exercise, (2) advice to lose weight, (3) assessment of ambulatory function (e.g., mobility), and (4) assessment of non‐ambulatory function (e.g., dressing). Each quality indicator included two components: the ‘IF’ statement that determined a participant's eligibility to receive the specified care, and the ‘THEN’ statement that determined the care process that should be performed. A pass‐rate for each quality indicator was calculated by dividing the number of individuals receiving care (i.e., achieved the ‘THEN’ statement) by the number of individuals eligible to receive the care (i.e., achieved the ‘IF’ statement), signifying the proportion of eligible individuals who received recommended care.
Given recent evidence supporting the benefits of both supervised and unsupervised exercise (Hinman et al. 2023) and recommendations for various healthcare practitioners to provide knee osteoarthritis care (Jayakumar, Moore, and Bozic 2019), the quality indicator criteria were updated for advice to exercise and advice to lose weight as a sensitivity analysis. These updated criteria reflect dichotomous (yes/no) patient‐reported use of exercise and diet as arthritis treatments, consistent with recent quality indicator surveys (King et al. 2020; Østerås et al. 2013). The adapted BC OA criteria for achieving each of the eligibility statements for each quality indicator, along with the sensitivity analysis criteria, are portrayed in Supporting Information S1: Appendix A.
2.4. Statistical Analysis
Participant demographics were summarised using descriptive statistics. Survey data were summarised using frequencies to represent the pass rate for each quality indicator. The overall pass rate across all four quality indicators was calculated using Equation (1).
| (1) |
Binary logistic regression models were used to determine associations between each quality indicator and five independent demographic and social variables: (1) age, (2) sex (female, male), (3) education level (university degree, trade certificate, high school diploma, less than high school), (4) employment (employed, retired due to medical reasons, unemployed/retired), and (5) number of comorbidities (maximum 12). Additionally, binary logistic regression models were used to determine associations between each quality indicator and patient‐reported outcome (WOMAC, KOOS‐Sport, ICOAP, EQ‐5D‐5L, Oxford Knee Score). Summary scores were calculated for each patient‐reported outcome. The WOMAC score was calculated as the sum of all items ranging from 0 (no difficulty) to 96 (extreme difficulty) (Bellamy et al. 1988). The KOOS‐Sport score was calculated as the transformed average of the 5‐item KOOS sport and recreation function sub‐scale ranging from 0 (extreme problems) to 100 (no problems) (Roos et al. 1998). The ICOAP score was calculated as the percentage score of all items ranging from 0 (no pain) to 100 (maximal pain) (Hawker et al. 2008). The EQ‐5D‐5L score was calculated as the sum of the five health state items ranging from 5 (no symptoms) to 25 (worst symptoms) (Herdman et al. 2011). The Oxford Knee Score was calculated as the sum of all items ranging from 0 (worst outcomes) to 48 (best outcomes) (Dawson et al. 1998).
Outcomes were calculated as unadjusted and adjusted odds ratios (OR) and 95% confidence intervals (CI) for all regression models. All regression models were run first using the pass rates calculated using the BC OA criteria and then with pass rates calculated using the updated quality indicator criteria as a sensitivity analysis (patient‐reported use of exercise and diet as arthritis treatments). Statistical testing was performed using SPSS (IBM SPSS Statistics for Windows, Version 28.0.1.1 Armonk, NY), with an alpha value of 0.05 to represent statistical significance.
3. Results
A total of 421 individuals from the Atlantic PATH cohort who had a self‐reported history of osteoarthritis consented to participate (Figure 1). Of those who consented, 85% (n = 359/421) completed the survey, and 57% (n = 241/421) indicated they had osteoarthritis in one or both knees and were included in final analyses. Almost all participants with self‐reported knee osteoarthritis indicated that they had had an x‐ray to confirm their arthritis (n = 228/241, 94.6%), and would therefore satisfy the American College of Rheumatology classification criteria of clinical and radiographic knee osteoarthritis (Altman et al. 1986). Among those with knee osteoarthritis, participants reported having osteoarthritis at the knee only (n = 50/241, 20.7%), both knee and hip (n = 17/241, 7.1%), knee and other (e.g., shoulder, back) joint (n = 90/241, 37.3%), or knee, hip, and other joint (n = 84/241, 34.9%). A total of 118 participants indicated they had osteoarthritis at a joint other than the knee, including hip only (n = 11), hip and other joint (n = 41), other joint only (n = 61), or no osteoarthritis (n = 5), and were therefore excluded from the analyses.
FIGURE 1.
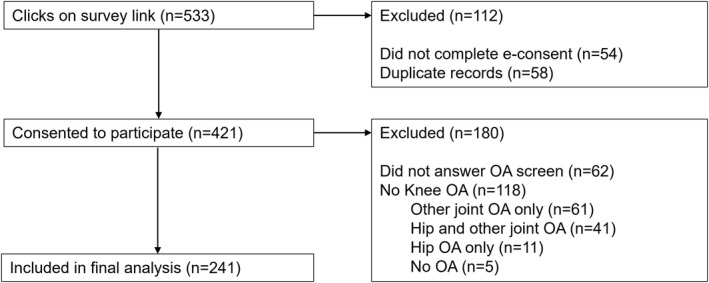
Flow chart of included participants (n = 241). OA = osteoarthritis.
Knee osteoarthritis participants (n = 241) were 77% female and had a mean age of 67.2 (6.9) years (Table 1). Participants had a mean body mass index (BMI) of 30.7 (7.5) kg/m2, and 63.9% (n = 154/241) had a BMI > 27 kg/m2. Participants had a median of two comorbidities (IQR 1.0‐3.0; range 0–8), and the most common comorbidities included high blood pressure (n = 103/241, 42.7%), depression (n = 49/241, 20.3%), and cancer (n = 40/241, 16.6%). Most participants (n = 200/241, 83.0%) rated their general health as good, very good, or excellent, and many (n = 140/241, 58.1%) reported that they were first told by a health professional that they had arthritis more than 11 years ago, followed by 6–10 years ago (n = 75/241, 31.1%), 1–5 years ago (n = 23/241, 9.5%), and less than one year ago (n = 1/241, 0.4%). The vast majority were eligible for at least one quality indicator (n = 231/241, 95.9%).
TABLE 1.
Participant sociodemographic and patient‐reported outcomes (n = 241).
| Characteristic | |
|---|---|
| Age, years: Mean (SD) | 67 (7) |
| Body mass index, kg/m2: Mean (SD) | 30.7 (7.5) |
| Sex, n (%) | |
| Female | 185 (76.8) |
| Male | 56 (23.2) |
| Education, n (%) | |
| University degree | 112 (46.5) |
| Trade certificate | 91 (37.8) |
| High school diploma | 36 (14.9) |
| Less than high school | 1 (0.4) |
| Missing data | 1 (0.4) |
| Employment status, n (%) | |
| Employed | 94 (39.0) |
| Retired due to medical reasons | 32 (13.3) |
| Unemployed/retired | 113 (46.9) |
| Missing data | 2 (0.8) |
| Number of comorbidities, 0‐12: Median (IQR); range | 2 (1.0, 3.0); 0‐8 |
| Eligibility for quality indicators, n (%) | |
| 0 quality indicators | 10 (4.1) |
| 1 quality indicator | 83 (34.4) |
| 2 quality indicators | 120 (49.8) |
| 3 quality indicators | 25 (10.4) |
| 4 quality indicators | 3 (1.2) |
| General health, n (%) | |
| Excellent | 12 (5.0) |
| Very good | 93 (38.6) |
| Good | 95 (39.4) |
| Fair | 37 (15.4) |
| Poor | 3 (1.2) |
| WOMAC score a , 0–96: Mean (SD); range | 27.4 (15.2); 2‐86 |
| KOOS‐Sport score b , 0–100: Mean (SD); range | 43.1 (26.2); 0‐100 |
| ICOAP score a , 0–100: Mean (SD); range | 23.5 (16.4); 0‐75 |
| EQ‐5D‐5L score a , 5–25: Mean (SD); range | 9.4 (2.6); 5‐19 |
| Oxford Knee Score b , 0–48: Mean (SD); range | 34.6 (7.8); 14‐48 |
Abbreviations: EQ‐5D‐5L = 5‐level Euro‐QOL 5‐dimension questionnaire, ICOAP = Intermittent and Constant Osteoarthritis Pain questionnaire, IQR = Inter‐quartile range, KOOS‐Sport = Knee injury and Osteoarthritis Outcome Score, sport and recreation function sub‐scale, SD = standard deviation, WOMAC = Western Ontario and McMaster Universities Osteoarthritis Index.
Higher scores indicate worse patient‐reported outcomes.
Higher scores indicate better patient‐reported outcomes.
The overall pass rate for all quality indicators using the BC OA criteria was 42.9% and increased to 49.3% when patient‐reported use of exercise and diet as arthritis treatments was included in the sensitivity analysis. Individual quality indicator pass‐rates ranged from 4.3% to 85.7% (Table 2). Few participants were eligible for or received care for assessment of ambulatory and non‐ambulatory functions; therefore, statistical testing was exclusively performed for advice to exercise and advice to lose weight.
TABLE 2.
Pass rates of each quality indicator for individuals with knee osteoarthritis (n = 241).
| BC OA criteria | Sensitivity analysis a | ||||
|---|---|---|---|---|---|
| Quality indicator | People eligible for care, n (% of survey participants) | People who received care, n | Pass rate (%) | People who received care, n | Pass rate (%) |
| Advice to exercise | 202 (83.8) | 125 | 61.9 | 140 | 69.3 |
| Advice to lose weight | 154 (63.9) | 43 | 27.9 | 54 | 35.1 |
| Assessment of ambulatory function | 7 (2.9) | 6 | 85.7 | — | — |
| Assessment of non‐ambulatory function | 47 (19.5) | 2 | 4.3 | — | — |
| Overall | 410 | 176 | 42.9 | 202 | 49.3 |
Abbreviation: BC OA = British Columbia Osteoarthritis.
The sensitivity analysis includes patient‐reported use of exercise and diet as arthritis treatments for advice to exercise and advice to lose weight, respectively. Criteria were not updated for assessment of ambulatory function or assessment of non‐ambulatory function (i.e., left blank in the table) because there were no relevant patient‐reported treatments for these quality indicators. The overall pass rate for the updated criteria was calculated using the updated pass rates for advice to exercise and advice to lose weight and the BC OA pass rates for ambulatory and non‐ambulatory functions.
There were no significant differences between age, sex, education level, employment, or comorbidities for individuals who did, or did not, receive advice to exercise or advice to lose weight using the BC OA criteria or within the sensitivity analysis (Table 3). Additionally, univariate analysis using the BC OA criteria indicated that the odds of receiving advice to exercise were significantly higher for those who reported better function during sport and recreational activities using the KOOS‐Sport sub‐scale (OR 1.02, 95% CI 1.00, 1.03; p = 0.02). There were no other significant associations between patient‐reported outcomes and advice to exercise or advice to lose weight in unadjusted or adjusted analyses using the BC OA criteria or within the sensitivity analysis (Table 4).
TABLE 3.
Logistic regression models for advice to exercise and advice to lose weight, including demographic and social factors.
| Independent variable | British Columbia Osteoarthritis criteria | Sensitivity analysis | ||||
|---|---|---|---|---|---|---|
| n (received care)/n (needed care) | Unadjusted OR (95% CI) | Adjusted OR (95% CI) | n (received care)/n (needed care) | Unadjusted OR (95% CI) | Adjusted OR (95% CI) | |
| Advice to exercise (n = 201 included in adjusted analysis) | ||||||
| Age, years | — | 0.98 (0.94, 1.02) | 0.98 (0.93, 1.03) | — | 0.99 (0.95, 1.03) | 0.99 (0.94, 1.04) |
| Sex (reference: Male) | ||||||
| Female | 97/154 | 1.22 (0.63, 2.35) | 1.16 (0.56, 2.43) | 107/154 | 1.04 (0.51, 2.08) | 0.98 (0.45, 2.12) |
| Male | 28/47 | 1 | 1 | 33/47 | 1 | 1 |
| Education (reference: High school diploma) | ||||||
| University degree | 72/100 | 2.25 (0.97, 5.21) | 2.37 (0.99, 5.66) | 77/100 | 1.94 (0.81, 4.66) | 2.04 (0.83, 5.04) |
| Trade certificate | 37/71 | 0.93 (0.39, 2.17) | 0.87 (0.36, 2.12) | 44/71 | 0.91 (0.38, 2.20) | 0.93 (0.37, 2.32) |
| High school diploma | 16/30 | 1 | 1 | 19/30 | 1 | 1 |
| Employment (reference: Unemployed/retired) | ||||||
| Employed | 51/80 | 1.16 (0.63, 2.14) | 0.99 (0.48, 2.04) | 57/80 | 1.15 (0.60, 2.19) | 1.06 (0.50, 2.25) |
| Retired for medical reasons | 15/23 | 1.24 (0.48, 3.20) | 1.73 (0.56, 5.31) | 16/23 | 1.06 (0.40, 2.83) | 1.51 (0.48, 4.81) |
| Unemployed/retired | 59/98 | 1 | 1 | 67/98 | 1 | 1 |
| Number of comorbidities | — | 0.91 (0.76, 1.08) | 0.87 (0.71, 1.07) | — | 0.90 (0.74, 1.07) | 0.87 (0.70, 1.06) |
| Advice to lose weight (n = 153 included in adjusted analysis) | ||||||
| Age, years | — | 0.96 (0.91, 1.01) | 0.96 (0.90, 1.02) | — | 0.96 (0.91, 1.00) | 0.96 (0.91, 1.01) |
| Sex (reference: Male) | ||||||
| Female | 34/114 | 1.40 (0.60, 3.26) | 1.29 (0.53, 3.14) | 41/114 | 1.11 (0.51, 2.39) | 0.99 (0.44, 2.24) |
| Male | 9/39 | 1 | 1 | 13/39 | 1 | 1 |
| Education (reference: High school diploma) | ||||||
| University degree | 18/61 | 1.20 (0.43, 3.32) | 1.17 (0.40, 3.44) | 21/61 | 1.05 (0.40, 2.74) | 1.07 (0.39, 2.94) |
| Trade certificate | 18/64 | 1.12 (0.40, 3.10) | 0.94 (0.32, 2.73) | 24/64 | 1.20 (0.47, 3.09) | 1.08 (0.40, 2.91) |
| High school diploma | 7/27 | 1 | 1 | 9/27 | 1 | 1 |
| Less than high school | 0/1 | — | — | 0/1 | — | — |
| Employment (reference: Unemployed/retired) | ||||||
| Employed | 20/64 | 1.49 (0.68, 3.25) | 1.07 (0.43, 2.68) | 25/64 | 1.52 (0.73, 3.17) | 1.10 (0.47, 2.58) |
| Retired for medical reasons | 8/25 | 1.54 (0.55, 4.26) | 1.09 (0.34, 3.47) | 10/25 | 1.58 (0.60, 4.14) | 1.05 (0.36, 3.12) |
| Unemployed/retired | 15/64 | 1 | 1 | 19/64 | 1 | 1 |
| Number of comorbidities | — | 1.09 (0.89, 1.34) | 1.10 (0.88, 1.37) | — | 1.10 (0.90, 1.34) | 1.11 (0.90, 1.38) |
Abbreviations: CI = confidence interval, OR = Odds ratio.
TABLE 4.
Logistic regression models for advice to exercise and advice to lose weight, including patient‐reported factors.
| British Columbia Osteoarthritis criteria | Sensitivity analysis | |||
|---|---|---|---|---|
| Independent variable | Unadjusted OR (95% CI) | Adjusted OR (95% CI) | Unadjusted OR (95% CI) | Adjusted OR (95% CI) |
| Advice to exercise (n = 171 included in adjusted analysis) | ||||
| WOMAC | 0.98 (0.96, 1.00) | 0.97 (0.94, 1.01) | 0.99 (0.97, 1.01) | 1.00 (0.96, 1.04) |
| KOOS‐Sport | 1.02 (1.00, 1.03) | 1.02 (1.00, 1.03) | 1.01 (0.99, 1.03) | 1.01 (1.00, 1.03) |
| ICOAP | 1.01 (0.97, 1.05) | 1.07 (0.99, 1.15) | 1.01, 0.97, 1.06) | 1.06 (0.98, 1.14) |
| EQ‐5D‐5L | 0.96 (0.84, 1.09) | 1.04 (0.87, 1.25) | 0.95 (0.83, 1.09) | 0.96 (0.80, 1.16) |
| Oxford Knee Score | 1.02 (0.98, 1.06) | 1.01 (0.93, 1.10) | 1.02 (0.97, 1.06) | 1.02 (0.94, 1.11) |
| Advice to lose weight (n = 119 included in adjusted analysis) | ||||
| WOMAC | 1.00 (0.98, 1.03) | 0.98 (0.94, 1.03) | 1.00 (0.98, 1.02) | 0.99 (0.95, 1.03) |
| KOOS‐Sport | 1.00 (0.98, 1.01) | 1.01 (0.99, 1.02) | 1.00 (0.98, 1.01) | 1.00 (0.99, 1.02) |
| ICOAP | 1.04 (0.99, 1.09) | 1.04 (0.96, 1.13) | 1.05 (1.00, 1.10) | 1.07 (0.99, 1.15) |
| EQ‐5D‐5L | 0.99 (0.86, 1.14) | 0.91 (0.72, 1.14) | 0.99 (0.87, 1.13) | 0.90 (0.73, 1.11) |
| Oxford Knee Score | 0.97 (0.93, 1.02) | 0.95 (0.86, 1.05) | 0.98 (0.93, 1.02) | 0.97 (0.89, 1.06) |
Note: Bolded values indicate statistical significance at p < 0.05.
Abbreviations: CI = confidence interval, EQ‐5D‐5L = 5‐level Euro‐QOL 5‐dimension questionnaire, ICOAP = Intermittent and Constant Osteoarthritis Pain questionnaire, KOOS‐Sport = Knee injury and Osteoarthritis Outcome Score, sport and recreation function sub‐scale, OR = Odds ratio, WOMAC = Western Ontario and McMaster Universities Osteoarthritis Index.
4. Discussion
This study quantifies the quality of care for individuals with mild‐to‐moderate knee osteoarthritis in the Maritime provinces and explores associations between the quality and types of care received with participant demographic, social, and patient‐reported factors. Findings suggest that the quality of osteoarthritis care is low across four non‐surgical and non‐pharmacological quality indicators, with overall pass rates of 42.9% when using the original BC OA criteria, and 49.3% when incorporating patient‐reported use of exercise and diet as arthritis treatments (Table 2). Compared to the original BC OA survey (Li et al. 2011), the overall pass rate increased by 20.5%, driven by an almost 37% increase in advice to exercise, with very little change (3% increase) in advice to lose weight; the current eligibility for assessment of ambulatory and non‐ambulatory function remains too low for robust comparison. Overall, less than half of the individuals with knee osteoarthritis in the Maritimes received recommended core treatment (Bannuru et al. 2019; Kolasinski et al. 2020), and these pass rates were not driven by participant demographic, social, or patient‐reported factors.
This study focuses on individuals with mild‐to‐moderate knee osteoarthritis from the Maritime provinces. Individuals with self‐reported osteoarthritis were recruited from the Atlantic PATH cohort to participate; however, nearly all participants reported that they had also received a radiograph to confirm their diagnosis of osteoarthritis. Thus, only 5% of this sample did not satisfy the traditional radiographic‐focused definition of knee osteoarthritis (Altman et al. 1986). Moreover, findings from the patient‐reported questionnaires (e.g., WOMAC, ICOAP) suggest that this sample group had mild‐to‐moderate pain, symptoms, and functional limitations (Bellamy et al. 1988; Dawson et al. 1998; Hawker et al. 2008; Herdman et al. 2011; Roos et al. 1998). The current sample displayed similar demographics and magnitudes of patient‐reported outcomes compared to previous cohorts with radiographically and clinically confirmed mild‐to‐moderate knee osteoarthritis (Astephen et al. 2008; Naili et al. 2019). Therefore, the current sample group represents a knee osteoarthritis cohort that may be an optimal target for early healthcare intervention to slow disease progression (Mahmoudian et al. 2021).
Notwithstanding strong recommendations regarding core treatment (Bannuru et al. 2019; Kolasinski et al. 2020), the overall quality of knee osteoarthritis care remains low. Our low‐to‐moderate overall pass rates (43%–49%) are comparable to previous quality indicator surveys focused on similarly aged community‐dwelling individuals (Glazier et al. 2003; Grønhaug, Østerås, and Hagen 2014 2015; Larmer et al. 2019; Oomen et al. 2021; Østerås et al. 2015). Observed pass rates are higher than those reported over a decade ago (Li et al. 2011; Østerås et al. 2013), which may imply a general improvement over time; however, evidence suggests that the quality of osteoarthritis care has not clearly or meaningfully improved over time (Bennell et al. 2021). Interestingly, the current overall pass rates are notably lower than those reported with more severe knee osteoarthritis populations, including individuals awaiting total knee arthroplasty (King et al. 2020) and physically frail samples (Ganz et al. 2006). These findings highlight a gap in the healthcare pathway in which secondary prevention strategies may be poorly implemented for individuals with milder symptoms or disease. Low adherence to non‐surgical and non‐pharmacological quality indicators may reflect low healthcare provider knowledge of the benefits of, or confidence in prescribing, core treatments (Selten et al. 2017), or patients' inadequate access to healthcare and community services (Dobson et al. 2016). Further exploration of patient access to healthcare resources in the Maritimes may be required to better understand and address potential gaps in healthcare utilization and implementation. This evidence indicates that osteoarthritis healthcare requires targeted improvement to ensure individuals with mild‐to‐moderate knee osteoarthritis receive optimal care.
Despite physical activity and therapeutic exercise being recognized as core treatments for knee osteoarthritis (Bannuru et al. 2019; Kolasinski et al. 2020), over 30% of patients reported that they did not receive advice to exercise. Observed pass rates for advice to exercise (62%–69%) are generally higher than those reported in earlier quality indicator surveys (Ganz et al. 2006; Glazier et al. 2003; Østerås et al. 2013), including the original BC OA survey (Li et al. 2011), but lower than more recent surveys (Grønhaug, Østerås, and Hagen 2014 2015; Ingelsrud et al. 2020; King et al. 2020; Larmer et al. 2019; Oomen et al. 2021; Østerås et al. 2015). Although the current findings for advice to exercise are encouraging, these results emphasise that more individuals with knee osteoarthritis in the Maritimes should receive advice to exercise, which has been achieved in other countries (Grønhaug, Østerås, and Hagen 2014 2015; Larmer et al. 2019; Oomen et al. 2021; Østerås et al. 2015) and with more severe knee osteoarthritis populations (King et al. 2020). Pass‐rates for advice to exercise may be attenuated by individual‐level barriers including lack of motivation or beliefs that exercise will worsen symptoms, social barriers including underprovided advice from healthcare practitioners or lacking social support, or environmental barriers including exercise programs that are unavailable (e.g., within rural communities) or inaccessible (e.g., transportation or cost) (Dobson et al. 2016; Gay et al. 2018). Wider dissemination of reported exercise facilitators, including education about exercise benefits, increased social support, and improved access to exercise programs and facilities (Dobson et al. 2016; Gay et al. 2018), is therefore needed to further increase the quality of exercise‐based osteoarthritis care.
Increased weight is a well‐established risk factor for knee osteoarthritis incidence and progression (Solanki et al. 2023); however, provision of advice to lose weight remains low. Our low pass rates for advice to lose weight (28%–35%) are lower than previous reports (Ganz et al. 2006; Glazier et al. 2003; Grønhaug, Østerås, and Hagen 2014 2015; Ingelsrud et al. 2020; King et al. 2020; Larmer et al. 2019; Li et al. 2011; Oomen et al. 2022; Østerås et al. 2013 2015). Specifically, current pass‐rates are lower than weight loss advice previously given to Canadians awaiting total knee arthroplasty (King et al. 2020) and community‐dwelling knee osteoarthritis populations in other countries (Grønhaug, Østerås, and Hagen 2014 2015; Ingelsrud et al. 2020; Larmer et al. 2019; Oomen et al. 2022; Østerås et al. 2015). Further, advice to lose weight has shown no distinct improvement from the original BC OA survey (Li et al. 2011). Evidence suggests that physicians report limited knowledge on weight management, mistrust of dieticians, and difficulties discussing weight loss with patients (Selten et al. 2017), likely contributing to low pass rates for weight management. Patients have expressed complex challenges associated with weight loss (Allison et al. 2019), and have stated that their healthcare providers may not supply adequate resources to support weight loss (Horn et al. 2021); thus, advice to lose weight requires improvements to enhance the quality of osteoarthritis care for overweight and obese individuals.
The regression analyses suggest that healthcare quality was not driven by participant demographic, social, or patient‐reported factors. Unadjusted models indicated that individuals reporting a higher KOOS‐Sport score (signifying fewer functional limitations) may be more likely to receive advice to exercise, although this finding did not persist in adjusted analyses. These results are in contrast to previous literature that determined that older age, male sex, and lower education levels were associated with lower odds of receiving recommended osteoarthritis care (Glazier et al. 2003; King et al. 2020; Li et al. 2011). Additionally, previous quality indicator surveys have reported that worse patient‐reported outcomes (e.g., pain and function) were associated with higher odds of receiving recommended non‐surgical and non‐pharmacological care (King et al. 2020; Li et al. 2011). The current findings should be confirmed with a larger and more diverse sample to determine whether there are potential inequities in osteoarthritis care in the Maritimes.
Importantly, these findings should be interpreted with an understanding of the quality indicator criteria that may over‐ or under‐report pass rates. For instance, receiving advice to exercise included a criterion of visiting a physiotherapist within the past year, alluding to participation in supervised exercise. However, other quality indicator surveys have analysed exercise and physiotherapy separately (King et al. 2020). Therefore, our criteria may over‐report pass rates for advice to exercise because it encompasses both exercise and physiotherapy. Similarly, criteria descriptors identify receiving care as seeing a specialist related to each indicator (e.g., physiotherapist, dietician, occupational therapist). Therefore, these criteria may be interpreted as receiving a specialist referral, while other quality indicator surveys separate advice (i.e., receiving information) from referral (i.e., access to specialists) care processes (Østerås et al. 2013). Adding patient‐reported use of exercise or diet as a sensitivity analysis may have captured more participants who underwent exercise or weight loss, yet our criteria may still underestimate the number of participants who received information related to these quality indicators but did not pursue seeing a specialist or begin an exercise or weight‐loss programme. Finally, our conservative eligibility criteria included participants who said ‘yes’, and excluded those who said ‘maybe’, to having knee osteoarthritis. Consequently, it is possible that we excluded participants who had not yet received a formal diagnosis but who were experiencing symptoms and could potentially fulfil a clinical diagnosis of knee osteoarthritis (National Institute for Health and Care Excellence 2022), resulting in a lower proportion of individuals who were eligible to receive care.
4.1. Limitations
This study may be limited by the smaller sample size compared with previous Canadian quality indicator analyses (Glazier et al. 2003; King et al. 2020; Li et al. 2011). The relatively small sample size may have limited the power for understanding the factors associated with receiving care. However, our high survey completion rate (85%) is similar to other recent quality indicator surveys (Ingelsrud et al. 2020; Larmer et al. 2019; Oomen et al. 2021), and is a strength of this study. Compared to the wider Atlantic PATH population with self‐reported osteoarthritis (Kozey et al. 2023), the current sample was older but had a comparable BMI, sex distribution, and comorbidity profile. Additionally, the sample group included in this study provided limited information on assessment of ambulatory and non‐ambulatory function, precluding detailed statistical analysis or interpretation for these two quality indicators. Furthermore, this study did not collect information from healthcare providers; therefore, results and interpretation are limited to the patient perspective of care received. However, patient‐reported quality indicator tools are the preferred approach for quality indicator evaluations due to the discordance between patient and provider perspectives (Jordan, Jinks, and Croft 2006), and the knowledge gleaned from patient perceptions on care quality (Østerås et al. 2013). Finally, the survey was completed online, which may disproportionately exclude individuals who are older, less educated, or report a lower health status (Kelfve et al. 2020).
5. Conclusions
In conclusion, these results suggest that the quality of osteoarthritis care in the Maritimes is sub‐optimal, and over half of individuals with mild‐to‐moderate knee osteoarthritis did not receive recommended core treatments. Although healthcare quality was not driven by patient demographic, social, or patient‐reported factors, an inclusive shift in management strategies is needed to improve overall care for individuals with mild‐to‐moderate knee osteoarthritis, and earlier healthcare intervention is needed for this patient group. Quality indicators should be routinely evaluated to determine whether clinical care aligns with current best practice guidelines and identify areas for intervention in the care pathway.
Author Contributions
Aleksandra R. Budarick: formal analysis, investigation, data curation, writing–original draft, writing–review & editing, project administration. Cheryl L. Hubley‐Kozey: conceptualisation, methodology, writing–review & editing, supervision, funding acquisition. Linda C. Li: conceptualisation, methodology, writing–review & editing. Olga Theou: methodology, writing–review & editing. William D. Stanish: methodology, writing–review & editing. Rebecca F. Moyer: conceptualisation, methodology, writing–review & editing, supervision, funding acquisition.
Conflicts of Interest
The authors declare no conflicts of interest.
Supporting information
Supporting Information S1
Acknowledgements
The authors gratefully acknowledge the contributions of the Atlantic Partnership for Tomorrow's Health (PATH) participants and research personnel, and the in‐kind support provided by the Maritime SPOR SUPPORT Unit. Additionally, we acknowledge Dr. Judith Godin, PhD, who provided statistical guidance.
Funding: This research was supported by a Nova Scotia Health Research Foundation Development and Innovation Grant. Aleksandra Budarick was supported by an MSSU Student Award (Maritime SPOR SUPPORT Unit), a Nova Scotia Research and Innovation Graduate Scholarship, a Scotia Scholar Doctoral Award (Research Nova Scotia), and a Killam Level II Predoctoral Scholarship. The funders played no role in the design, conduct, or reporting of this study.
Data Availability Statement
Data supporting the findings of this study are available from the corresponding author upon request.
References
- Allison, K. , Delany C., Setchell J., et al. 2019. “A Qualitative Study Exploring the Views of Individuals With Knee Osteoarthritis on the Role of Physiotherapists in Weight Management: A Complex Issue Requiring a Sophisticated Skill Set.” Musculoskeletal Care 17, no. 2: 206–214. 10.1002/msc.1391. [DOI] [PubMed] [Google Scholar]
- Altman, R. , Asch E., Bloch D., et al. 1986. “Development of Criteria for the Classification and Reporting of Osteoarthritis: Classification of Osteoarthritis of the Knee.” Arthritis & Rheumatism 29, no. 8: 1039–1049. 10.1002/art.1780290816. [DOI] [PubMed] [Google Scholar]
- Astephen, J. L. , Deluzio K. J., Caldwell G. E., Dunbar M. J., and Hubley‐Kozey C. L.. 2008. “Gait and Neuromuscular Pattern Changes Are Associated With Differences in Knee Osteoarthritis Severity Levels.” Journal of Biomechanics 41, no. 4: 868–876. 10.1016/j.jbiomech.2007.10.016. [DOI] [PubMed] [Google Scholar]
- Bannuru, R. R. , Osani M. C., Vaysbrot E. E., et al. 2019. “OARSI Guidelines for the Non‐surgical Management of Knee, Hip, and Polyarticular Osteoarthritis.” Osteoarthritis and Cartilage 27, no. 11: 1578–1589. 10.1016/j.joca.2019.06.011. [DOI] [PubMed] [Google Scholar]
- Bellamy, N. , Buchanan W. W., Goldsmith C. H., Campbell J., and Stitt L. W.. 1988. “Validation Study of WOMAC: A Health Status Instrument for Measuring Clinically Important Patient Relevant Outcomes to Antirheumatic Drug Therapy in Patients With Osteoarthritis of the Hip or Knee.” Journal of Rheumatology 15, no. 12: 1833–1840. [PubMed] [Google Scholar]
- Bennell, K. L. , Bayram C., Harrison C., et al. 2021. “Trends in Management of Hip and Knee Osteoarthritis in General Practice in Australia over an 11‐year Window: A Nationwide Cross‐Sectional Survey.” Lancet Regional Health ‐ Western Pacific 12: 100187. 10.1016/j.lanwpc.2021.100187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canadian Institute for Health Information . 2024. “Explore Wait Times for Priority Procedures across Canada.” Ottawa, ON: Canadian Institute for Health Information. https://www.cihi.ca/en/explore‐wait‐times‐for‐priority‐procedures‐across‐canada. [Google Scholar]
- Crawford, D. C. , Miller L. E., and Block J. E.. 2013. “Conservative Management of Symptomatic Knee Osteoarthritis: A Flawed Strategy?.” Orthopedic Reviews 5, no. 1: 2. 10.4081/or.2013.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson, J. , Fitzpatrick R., Murray D., and Carr A.. 1998. “Questionnaire on the Perceptions of Patients About Total Knee Replacement.” Journal of Bone and Joint Surgery. British Volume 80‐B, no. 1: 63–69. 10.1302/0301-620X.80B1.0800063. [DOI] [PubMed] [Google Scholar]
- Dobson, F. , Bennell K. L., French S. D., et al. 2016. “Barriers and Facilitators to Exercise Participation in People With Hip And/or Knee Osteoarthritis: Synthesis of the Literature Using Behavior Change Theory.” American Journal of Physical Medicine & Rehabilitation 1, no. 5: 372–389. 10.1097/PHM.0000000000000448. [DOI] [PubMed] [Google Scholar]
- Dummer, T. J. B. , Awadalla, P. , Boileau, C. , et al., … with the CPTP Regional Cohort Consortium . 2018. “The Canadian Partnership for Tomorrow Project: A Pan‐Canadian Platform for Research on Chronic Disease Prevention.” Canadian Medical Association Journal, 190(23): E710–E717. 10.1503/cmaj.170292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyles, J. P. , Hunter D. J., Bennell K. L., et al. 2019. “Priorities for the Effective Implementation of Osteoarthritis Management Programs: An OARSI International Consensus Exercise.” Osteoarthritis and Cartilage 27, no. 9: 1270–1279. 10.1016/j.joca.2019.05.015. [DOI] [PubMed] [Google Scholar]
- Finley, C. R. , Chan D. S., Garrison S., et al. 2018. “What Are the Most Common Conditions in Primary Care? Systematic Review.” Canadian Family Physician Medecin De Famille Canadien 64, no. 11: 832–840. [PMC free article] [PubMed] [Google Scholar]
- Ganz, D. A. , Chang J. T., Roth C. P., et al. 2006. “Quality of Osteoarthritis Care for Community‐Dwelling Older Adults.” Arthritis & Rheumatism 55, no. 2: 241–247. 10.1002/art.21844. [DOI] [PubMed] [Google Scholar]
- Gay, C. , Eschalier B., Levyckyj C., Bonnin A., and Coudeyre E.. 2018. “Motivators for and Barriers to Physical Activity in People With Knee Osteoarthritis: A Qualitative Study.” Joint Bone Spine 85, no. 4: 481–486. 10.1016/j.jbspin.2017.07.007. [DOI] [PubMed] [Google Scholar]
- Glazier, R. H. , Badley E. M., Wright J. G., et al. 2003. “Patient and Provider Factors Related to Comprehensive Arthritis Care in a Community Setting in Ontario, Canada.” Journal of Rheumatology 30, no. 8: 1846–1850. [PubMed] [Google Scholar]
- Global Burden of Disease Collaborative Network . 2020. “Global Burden of Disease Study 2019 (GBD 2019) Results.” Seattle, WA: Institute for Health Metrics and Evaluation. http://ghdx.healthdata.org/gbd‐results‐tool. [Google Scholar]
- Gränicher, P. , Mulder L., Lenssen T., Scherr J., Swanenburg J., and De Bie R.. 2022. “Prehabilitation Improves Knee Functioning Before and Within the First Year After Total Knee Arthroplasty: A Systematic Review With Meta‐Analysis.” Journal of Orthopaedic & Sports Physical Therapy 52, no. 11: 709–725. 10.2519/jospt.2022.11160. [DOI] [PubMed] [Google Scholar]
- Grønhaug, G. , Hagfors J., Borch I., Østerås N., and Hagen K. B.. 2015. “Perceived Quality of Health Care Services Among People With Osteoarthritis – Results From a Nationwide Survey.” Patient Preference and Adherence: 1255. 10.2147/PPA.S82441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grønhaug, G. , Østerås N., and Hagen K. B.. 2014. “Quality of Hip and Knee Osteoarthritis Management in Primary Health Care in a Norwegian County: A Cross‐Sectional Survey.” BMC Health Services Research 14, no. 1: 598. 10.1186/s12913-014-0598-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawker, G. A. , Davis A. M., French M. R., et al. 2008. “Development and Preliminary Psychometric Testing of a New OA Pain Measure – an OARSI/OMERACT Initiative.” Osteoarthritis and Cartilage 16, no. 4: 409–414. 10.1016/j.joca.2007.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herdman, M. , Gudex C., Lloyd A., et al. 2011. “Development and Preliminary Testing of the New Five‐Level Version of EQ‐5D (EQ‐5D‐5L).” Quality of Life Research 20, no. 10: 1727–1736. 10.1007/s11136-011-9903-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinman, R. S. , Hall M., Comensoli S., and Bennell K. L.. 2023. “Exercise & Sports Science Australia (ESSA) Updated Position Statement on Exercise and Physical Activity for People With Hip/knee Osteoarthritis.” Journal of Science and Medicine in Sport 26, no. 1: 37–45. 10.1016/j.jsams.2022.11.003. [DOI] [PubMed] [Google Scholar]
- Horn, D. B. , Damsgaard C., Earles K., Mathew S., and Nelson A. E.. 2021. “Engagement Between Patients With Obesity and Osteoarthritis and Primary Care Physicians: A Cross‐Sectional Survey.” Postgraduate Medicine 133, no. 8: 979–987. 10.1080/00325481.2021.1982588. [DOI] [PubMed] [Google Scholar]
- Hunter, D. J. , Bowden, J. L. , Hinman, R. S. , et al., and the PARTNER Study Team . 2022. “Effectiveness of a New Service Delivery Model for Management of Knee Osteoarthritis in Primary Care: A Cluster Randomized Controlled Trial.” Arthritis Care & Research, 75, (6), acr: 1320–1332, 10.1002/acr.25037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingelsrud, L. H. , Roos E. M., Gromov K., Jensen S. S., and Troelsen A.. 2020. “Patients Report Inferior Quality of Care for Knee Osteoarthritis Prior to Assessment for Knee Replacement Surgery—A Cross‐Sectional Study of 517 Patients in Denmark.” Acta Orthopaedica 91, no. 1: 82–87. 10.1080/17453674.2019.1680180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayakumar, P. , Moore M. L. G., and Bozic K. J.. 2019. “Team Approach: A Multidisciplinary Approach to the Management of Hip and Knee Osteoarthritis.” JBJS Reviews 7, no. 6: e10. 10.2106/JBJS.RVW.18.00133. [DOI] [PubMed] [Google Scholar]
- Jordan, K. , Jinks C., and Croft P.. 2006. “Health Care Utilization: Measurement Using Primary Care Records and Patient Recall Both Showed Bias.” Journal of Clinical Epidemiology 59, no. 8: 791–797.e2. 10.1016/j.jclinepi.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Kelfve, S. , Kivi M., Johansson B., and Lindwall M.. 2020. “Going Web or Staying Paper? the Use of Web‐Surveys Among Older People.” BMC Medical Research Methodology 20, no. 1: 252. 10.1186/s12874-020-01138-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King, L. K. , Marshall D. A., Faris P., et al. 2020. “Use of Recommended Non‐surgical Knee Osteoarthritis Management in Patients Prior to Total Knee Arthroplasty: A Cross‐Sectional Study.” Journal of Rheumatology 47, no. 8: 1253–1260. 10.3899/jrheum.190467. [DOI] [PubMed] [Google Scholar]
- Kolasinski, S. L. , Neogi T., Hochberg M. C., et al. 2020. “2019 American College of Rheumatology/Arthritis Foundation Guideline for the Management of Osteoarthritis of the Hand, Hip, and Knee.” Arthritis & Rheumatology 72, no. 2: 220–233. 10.1002/art.41142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozey, C. , Moyer R., Grant A., et al. 2023. Current Management and Health Care Use for People with Osteoarthritis [Summary Report]. Halifax, NS: Maritime SPOR Support Unit. https://mssu.ca/wp‐content/uploads/2023/02/OA‐Maritimes‐Summary‐Report_2023‐02‐10.pdf. [Google Scholar]
- Larmer, P. J. , Bennett K., Baldwin J. N., Bassett S., and O’Brien D. W.. 2019. “Quality Indicators for Hip and Knee Osteoarthritis Management in New Zealand: A Patient Survey.” New Zealand Journal of Physiotherapy 47, no. 3: 183–192: c8h. 10.15619/NZJP/47.3.06. [DOI] [Google Scholar]
- Li, L. C. , Sayre E. C., Kopec J. A., Esdaile J. M., Bar S., and Cibere J.. 2011. “Quality of Nonpharmacological Care in the Community for People With Knee and Hip Osteoarthritis.” Journal of Rheumatology 38, no. 10: 2230–2237. 10.3899/jrheum.110264. [DOI] [PubMed] [Google Scholar]
- MacLean, C. H. , Saag K. G., Solomon D. H., Morton S. C., Sampsel S., and Klippel J. H.. 2004. “Measuring Quality in Arthritis Care: Methods for Developing the Arthritis Foundation’s Quality Indicator Set: Measuring Quality in Arthritis Care.” Arthritis Care & Research 51, no. 2: 193–202. 10.1002/art.20248. [DOI] [PubMed] [Google Scholar]
- Mahmoudian, A. , Lohmander L. S., Mobasheri A., Englund M., and Luyten F. P.. 2021. “Early‐stage Symptomatic Osteoarthritis of the Knee—Time for Action.” Nature Reviews Rheumatology 17, no. 10: 621–632. 10.1038/s41584-021-00673-4. [DOI] [PubMed] [Google Scholar]
- Messier, S. P. , Beavers D. P., Mihalko S. L., et al. 2020. “The Effects of Intensive Dietary Weight Loss and Exercise on Gait in Overweight and Obese Adults With Knee Osteoarthritis. The Intensive Diet and Exercise for Arthritis (IDEA) Trial.” Journal of Biomechanics 98: 109477. 10.1016/j.jbiomech.2019.109477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naili, J. E. , Broström E. W., Clausen B., and Holsgaard‐Larsen A.. 2019. “Measures of Knee and Gait Function and Radiographic Severity of Knee Osteoarthritis—A Cross‐Sectional Study.” Gait & Posture 74: 20–26. 10.1016/j.gaitpost.2019.08.003. [DOI] [PubMed] [Google Scholar]
- National Institute for Health and Care Excellence . 2022. Osteoarthritis in Over 16s: Diagnosis and Management. NICE Guideline [NG226]. Manchester, UK: National Institute for Health and Care Excellence. https://www.nice.org.uk/guidance/ng226. [PubMed] [Google Scholar]
- Oomen, J. M. H. , Peters Y. A. S., Ende C. H. van den, et al. 2022. “Quality of Knee Osteoarthritis Care in the Netherlands: A Survey on the Perspective of People with Osteoarthritis.” BMC Health Services Research 22: 631. 10.1186/s12913-022-08014-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Østerås, N. , Garratt A., Grotle M., et al. 2013. “Patient‐Reported Quality of Care for Osteoarthritis: Development and Testing of the OsteoArthritis Quality Indicator Questionnaire: OA Care and Quality Indicators.” Arthritis Care & Research 65, no. 7: 1043–1051. 10.1002/acr.21976. [DOI] [PubMed] [Google Scholar]
- Østerås, N. , Jordan K. P., Clausen B., et al. 2015. “Self‐reported Quality Care for Knee Osteoarthritis: Comparisons Across Denmark, Norway, Portugal and the UK.” RMD Open 1, no. 1: e000136. 10.1136/rmdopen-2015-000136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos, E. M. , Roos H. P., Lohmander L. S., Ekdahl C., and Beynnon B. D.. 1998. “Knee Injury and Osteoarthritis Outcome Score (KOOS)—Development of a Self‐Administered Outcome Measure.” Journal of Orthopaedic & Sports Physical Therapy 28, no. 2: 88–96. 10.2519/jospt.1998.28.2.88. [DOI] [PubMed] [Google Scholar]
- Selten, E. M. H. , Vriezekolk J. E., Nijhof M. W., et al. 2017. “Barriers Impeding the Use of Non‐pharmacological, Non‐surgical Care in Hip and Knee Osteoarthritis: The Views of General Practitioners, Physical Therapists, and Medical Specialists.” Journal of Clinical Rheumatology 23, no. 8: 405–410. 10.1097/RHU.0000000000000562. [DOI] [PubMed] [Google Scholar]
- Skou, S. T. , Roos E. M., Laursen M. B., et al. 2018. “Total Knee Replacement and Non‐surgical Treatment of Knee Osteoarthritis: 2‐year Outcome From Two Parallel Randomized Controlled Trials.” Osteoarthritis and Cartilage 26, no. 9: 1170–1180: Embase. 10.1016/j.joca.2018.04.014. [DOI] [PubMed] [Google Scholar]
- Solanki, P. , Hussain S. M., Abidi J., et al. 2023. “Association Between Weight Gain and Knee Osteoarthritis: A Systematic Review.” Osteoarthritis and Cartilage 31, no. 3: 300–316. 10.1016/j.joca.2022.10.023. [DOI] [PubMed] [Google Scholar]
- Statistics Canada . 2022, August 26. “Health Characteristics, Annual Estimates: Table 13‐10‐0096‐01.” Ottawa, ON: Government of Canada. 10.25318/1310009601-eng. [DOI] [Google Scholar]
- Sweeney, E. , Cui Y., DeClercq V., et al. 2017. “Cohort Profile: The Atlantic Partnership for Tomorrow’s Health (Atlantic PATH) Study.” International Journal of Epidemiology 46, no. 6: 1762–1763i. 10.1093/ije/dyx124. [DOI] [PubMed] [Google Scholar]
- Vina, E. R. , and Kwoh C. K.. 2018. “Epidemiology of Osteoarthritis: Literature Update.” Current Opinion in Rheumatology 30, no. 2: 160–167. 10.1097/BOR.0000000000000479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Elm, E. , Altman D. G., Egger M., Pocock S. J., Gøtzsche P. C., and Vandenbroucke J. P., and STROBE Initiative . 2007. “The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies.” Epidemiology 18, no. 6: 800–804. 10.1097/EDE.0b013e3181577654. [DOI] [PubMed] [Google Scholar]
- Zampogna, B. , Papalia R., Papalia G. F., et al. 2020. “The Role of Physical Activity as Conservative Treatment for Hip and Knee Osteoarthritis in Older People: A Systematic Review and Meta‐Analysis.” Journal of Clinical Medicine 9, no. 4: 1167. 10.3390/jcm9041167. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information S1
Data Availability Statement
Data supporting the findings of this study are available from the corresponding author upon request.


