Abstract
Maize (Zea mays L.) faces significant challenges to its growth and productivity from heavy metal stress, particularly Chromium (Cr) stress, which induces reactive oxygen species (ROS) generation and damages photosynthetic tissues. This study aimed to investigate the effects of fulvic acid (FA) application, via foliar spray or root irrigation, on mitigating chromium stress in maize by evaluating its impact on antioxidant activity and growth parameters. Two maize varieties, P3939 and 30Y87, were subjected to chromium stress (CrCl3·6H2O) at concentrations of 300 µM and 100 µM for a duration of 5 weeks. The experiment was conducted in a wire house under natural environmental conditions at the Seed Centre, Institute of Botany, University of the Punjab, Lahore, Pakistan. Physiological assessments included electrolyte leakage, chlorophyll pigment content, malondialdehyde (MDA) levels, and activities of antioxidant enzymes such as catalase (CAT), ascorbate peroxidase (APX), and guaiacol peroxidase (GPX) in maize leaves. Growth parameters were also monitored. The results revealed that chromium stress significantly reduced chlorophyll content and increased oxidative stress, as evidenced by elevated MDA levels and electrolyte leakage. However, FA application notably mitigated these effects: chlorophyll content improved by 15%, and MDA levels decreased significantly. Irrigation with FA was particularly effective, reducing MDA levels by 40% compared to the 300 µM chromium treatment. Furthermore, while chromium stress enhanced antioxidant enzyme activities, FA application further boosted total soluble protein levels and antioxidant enzyme activities under stress conditions. In conclusion, FA application demonstrates potential in improving maize tolerance to heavy metal stress by enhancing the antioxidant defense system and preserving photosynthetic pigments. These findings highlight FA’s promise as a practical strategy for mitigating the negative impacts of chromium stress on maize, promoting sustainable agricultural practices in contaminated environments.
Keywords: Antioxidant enzymes, Chromium toxicity, Maize growth, Oxidative damage, Reactive oxygen species, Stress mitigation
Subject terms: Plant physiology, Plant stress responses
Introduction
Heavy metal contamination is a major problem across the world owing to its negative impacts on plant development, production, and food quality1. Heavy metals are introduced into soil by both natural (volcanism and weathering) and human activities, i.e., wastes and chemicals from industries2. In Pakistan, heavy metal contamination is primarily driven by industrialization, urbanization, wastewater irrigation, and the use of pesticides. Notably, approximately 600 leather tanneries are operating in Karachi, Kasur, and Sialkot3. The excessive release of wastewater and effluents from these tanneries has been identified as a major source of soil and water contamination with heavy metals4. Chromium is a widespread heavy metal released by tanning and plating industries5. Chromium (VI), in particular, is highly toxic due to its high oxidation potential and solubility compared to chromium (III)6. Chromium toxicity in plants leads to various harmful effects, including inhibited growth, pigment degradation, suppression of antioxidant enzyme activity (SOD, POD, APX, and CAT), and the generation of reactive oxygen species (ROS), which cause cellular damage. In response, antioxidants like ascorbic acid, glutathione, α-tocopherol, flavonoids, and carotenoids (CAR) become essential, as they play a pivotal role in neutralizing ROS and mitigating oxidative stress7,8. Chromium-induced stress disrupts electron transport in the thylakoidal membrane during photosynthesis, leading to oxidative stress and a decrease in photosynthetic capacity9. One potential short-term solution to mitigate chromium’s adverse effects is the application of soil amendments or foliar sprays containing antioxidant compounds10.
Humic substances, such as fulvic acid, play an essential role in improving plant growth, enzyme activity, and soil chemistry. Fulvic acid is the acid-soluble fraction of humic substances. These organic compounds have a tendency to form bonds with heavy metals, thereby reducing the detrimental impacts of metal stress on plant development11. Fulvic acid is used as natural soil amendment which is formed by biological and chemical decomposition of plants12. The major functional groups of fulvic acid include aromatic rings, phenolic hydroxyls and carboxyl groups13. Fulvic acid promotes maize growth and photosynthesis while reducing toxic element uptake by forming chemical bonds with heavy metals. Fulvic acid can enhance seed germination and stimulate plant metabolism14. It enhances antioxidant enzyme activity, including catalase (CAT) and peroxidase (POD), as well as other enzymes like alkaline phosphatase (e.g., improving phosphate mobilization in plants under stress conditions)15.Maize (Zea mays L.) is one of the most widely cultivated cereal crops globally, playing a crucial role in food security, animal feed, and industrial applications16. It is rich in starch, proteins, and fiber, making it crucial for both human and animal nutrition17. Due to its economic and nutritional importance, maize is often grown in regions that face environmental challenges, including soil contamination from heavy metals. Chromium, a persistent and toxic heavy metal, can severely affect maize growth and development, leading to reduced yields and compromised nutritional quality18. As a staple crop, maize is particularly vulnerable to heavy metal stress, which inhibits its physiological functions, including photosynthesis and antioxidant enzyme activity19. Although maize is known to be vulnerable to heavy metal stress, the specific mechanisms by which chromium affects its physiological functions, including photosynthesis and antioxidant enzyme activity, are not fully understood. The objectives of this study were to evaluate the detrimental effects of chromium on maize growth and antioxidant enzyme activity and to assess the potential mitigating role of fulvic acid in counteracting chromium toxicity. While various mitigation strategies have been explored, the role of fulvic acid in enhancing antioxidant defense under chromium stress in maize remains underexplored. The working hypothesis proposes that fulvic acid application enhances maize’s antioxidant defense system under chromium stress, offering an innovative approach to improve tolerance and alleviate oxidative damage caused by chromium toxicity.
Materials and methods
Experimental setup and chromium stress with fulvic acid application in maize cultivars P3939 and 30Y87
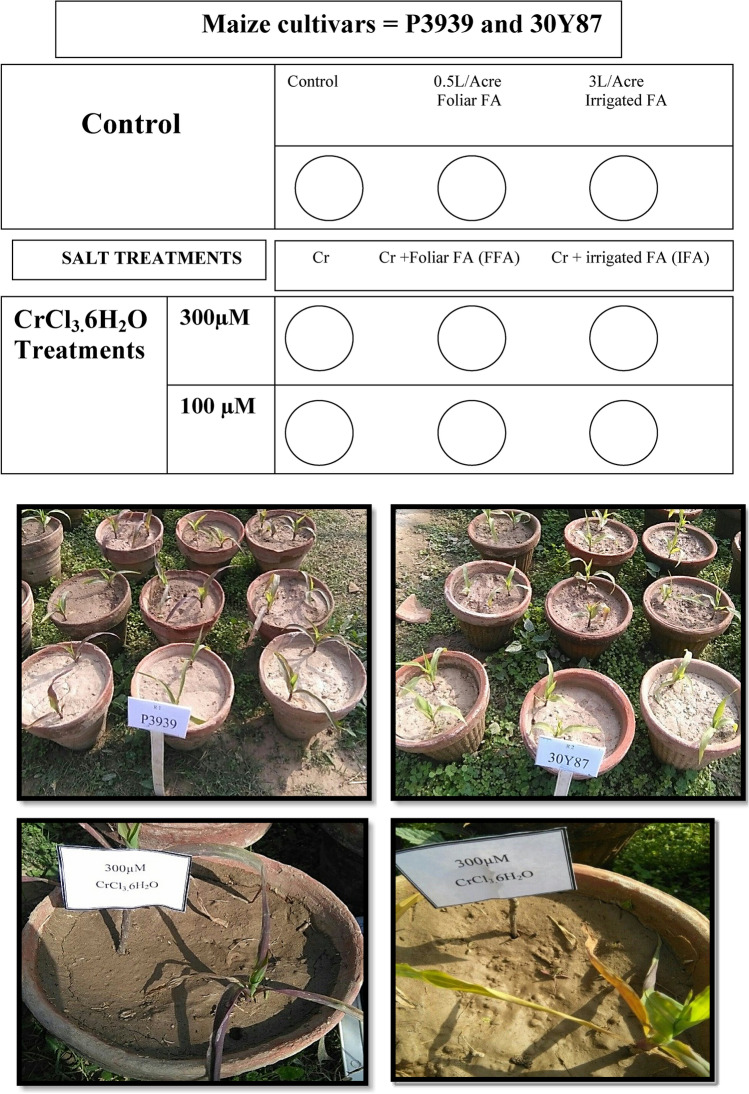
To evaluate the impact of fulvic acid on two maize cultivars, a pot experiment was conducted at the Seed Centre, Institute of Botany, University of the Punjab, Lahore, Pakistan, in November 2018 utilizing a complete randomized design (CRD) as shown in Fig. 1. Seeds of maize cultivars P3939 and 30Y87 were sourced from Pioneer Seed Company in Sahiwal. The fulvic acid used in the experiment was purchased from Sigma-Aldrich. Pots were grown in a wire house under natural environmental conditions, with regular irrigation twice a week and a foliar spray of N, P, K applied to meet nutritional requirements. The experiment included three replicates for each treatment. Chromium stress was induced using chromium chloride (100 µM20 and 300 µM21) by dissolving the appropriate amounts in distilled water at the 4th leaf stage. After one week of exposure to heavy metal stress, fulvic acid applications via foliar spray (0.5 L/A)22 and root application (300 L/A) with irrigated water, were randomly allocated to pots to minimize potential bias and environmental variability.
Fig. 1.
The experimental layout includes two maize cultivars, P3939 and 30Y87, subjected to different chromium (Cr) stress levels and treatments with fulvic acid. The figure depicts the arrangement of the pots and highlights the visual symptoms in maize plants exposed to Cr, such as leaf discoloration.
Evaluation of growth parameters
To investigate the effects of fulvic acid on the development of maize cultivars under to chromium stress, several growth parameters (leaf length, leaf breadth, leaf area, plant height, and number of leaves) were evaluated.
Harvesting
After four weeks of applying fulvic acid treatment, maize plants were harvested and carefully divided into shoots and roots. Prior to harvesting, samples were collected from both young and fully matured leaves of each plant. These samples were then subjected to detailed analysis to assess various growth parameters and biochemical indicators. This analysis included measurements of electrolyte leakage, malondialdehyde (MDA) content (a marker of lipid peroxidation), chlorophyll pigment content, total soluble proteins (TSP), and activities of antioxidant enzymes. These assessments provided insights into the physiological responses and biochemical changes induced by the fulvic acid treatment in maize plants.
Determination of chlorophyll content
Chlorophyll content was determined according to the method of Arnon,23 with slight modifications. For determination of chlorophyll content, leaf 0.1 g of each plant tissue was collected in labelled Eppendorf tubes 1.5 mL and DMSO (Dimethyl sulfoxide.) 1 mL was added. After the Eppendorf were placed in the dark for 48 h, the absorbance of each sample was then measured at 663 nm and 645 nm.
Total Chlorophyll Content = [(0.00802) (D – 663) + (0.0202) (D – 645) (mL of solvent)] / gram fresh weight of the plant.
Electrolyte-leakage determination
Leaf samples of uniform size were made using a cork borer and put in 20 mL distilled water. These tubes were then shaken for 24 h on an orbital shaker to break the cell membranes. The electrical conductivity (EC1) was measured after 24 h then autoclaved at 121 °C for 1 h before being put on an orbital shaker for 24 h (120 rpm) to measure electrical conductivity (EC2)24. Electrolyte leakage was calculated by using following formula.
Electrolyte leakage = EC1/EC2 × 100
Evaluation of MDA content
MDA content and antioxidant enzyme activity (CAT, APX, and GPX) were measured using the technique of (He et al., 2001). 0.25 g of leaves were crushed in 150 mM phosphate buffer (4 mL) having pH 7.0 and centrifuged at 14,000 rpm for 20 min (4 °C). For MDA content determination, 1 ml of enzyme extract was mixed with 2 ml of a reaction solution containing 20% TCA and 0.5% TBA. After that, the solution was heated in a water bath (95 °C) for 30 min before being centrifuged at 12,000 rpm for 15 min. The absorbance then recorded at 532 and 600 nm 25
Determination of antioxidant enzymes (CAT, APX, and GPX)
Catalase activity was assessed using a modified version of the technique described by Chance and Maehly26. Reaction solution containing pH 7.0 (3 mL), hydrogen peroxide (H2O2) 45 mM, and enzyme extract 100 µL were collected. A spectrophotometer was used to measure changes in absorbance of each sample at 240 nm.
The approach of27 with certain changes was used to determine APX activity. 3 (mL) of the reaction solution 100 mM sodium acetate (pH 5.8), H2O2 (5 mM), Ethylenediaminetetraacetic acid (EDTA) 0.003 mM, 10 mM ascorbic acid, and 100 µL extracted solution) was collected and the reaction was started by adding enzyme extract. A spectrophotometer was used to measure changes in absorbance at 290 nm every 10 s for 60 s.
With minor adjustments, GPX activity was assessed using the approach of26. 3 mL reaction solution comprising 0.1 mol/L sodium acetate buffer, 0.25% guaiacol, 0.75% H2O2, and 50 µL enzyme extract were collected. Spectrophotometer was used to measure changes in absorbance at 460 nm every 10 s for 60 s.
Determination of total soluble protein
The total soluble protein content of the leaf was calculated by adding supernatant 100 µL from extract of plant material then mixed with 3 mL of colour reagent. After 5 min, the absorbance of each sample was measured at 595 nm28.
Statistical analysis
Using the SAS (Statistical Analysis System) statistical software programme, the data was statistically analyzed using procedure mixed (PROC MIXED) and procedure generalized linear model (PROC GLM) (SAS Institute). The Duncan Multiple range test was used to detect mean differences. Correlation and principal component analyses were performed using RStudio.’
Results
Effects of fulvic acid on growth parameters of maize cultivars
The study investigated the effects of different treatments on the growth parameters of two maize cultivars, P3939 and 30Y87. Significant variations were observed in leaf length, leaf width, leaf area, plant height, and number of leaves across treatments (Table 1). For both cultivars, the control group exhibited intermediate values for most parameters. The application of 100 µM Cr notably reduced leaf length, leaf width, leaf area, and plant height compared to the control, indicating a negative impact on growth. Conversely, treatments combining 100 µM Cr with fulvic acid applied as a foliar spray (FFA, 0.5 L/A) or through the rooting medium (IFA, 300 L/A) showed varying effects. The application of 100 µM Cr + FFA generally mitigated some of the adverse effects associated with chromium exposure, whereas 100 µM Cr + IFA yielded mixed outcomes, occasionally intensifying the harmful impacts of chromium. Overall, these findings highlight the complex interactions between chromium exposure and fulvic acid application methods on the growth metrics of these maize cultivars.
Table 1.
Effects of fulvic acid applied as a foliar spray (FFA, 0.5 L/A) and through the rooting medium irrigation (IFA, 300 L/A) on growth parameters of maize cultivars (P3939 and 30Y87).
| Treatments | Cultivar P3939 | Cultivar 30Y87 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Leaf length (cm) | Leaf width (cm) | Leaf area (cm2) | Plant height (cm) | No. of leaves | Leaf length (cm) | Leaf width (cm) | Leaf area (cm2) | Plant height (cm) | No. of leaves | |
| Control (0 µM) | 23.92 ± 1.12 b | 1.55 ± 0.08 b | 28.91 ± 2.47 b | 33.73 ± 1.86 a-b | 6.52 ± 0.33 ab | 22.82 ± 1.12 b | 1.41 ± 0.08 b | 26.91 ± 2.47 b | 32.62 ± 1.86 b | 6.58 ± 0.33 ab |
| FFA | 28.62 ± 1.12 a | 1.77 ± 0.08 a | 38.78 ± 2.47 a | 35.92 ± 1.86 a | 7.12 ± 0.33 a | 28.62 ± 1.12 a | 1.77 ± 0.08 a | 37.78 ± 2.47 a | 35.82 ± 1.86 a | 7.12 ± 0.33 a |
| IFA | 24.00 ± 1.12 b | 1.67 ± 0.08 ab | 29.80 ± 2.47 b | 34.73 ± 1.86 a | 6.70 ± 0.33 ab | 23.00 ± 1.12 a-b | 1.47 ± 0.08 a-b | 28.80 ± 2.47 a-b | 33.66 ± 1.86 a-b | 6.50 ± 0.33ab |
| 100 µM Cr | 17.15 ± 1.46 e | 1.15 ± 0.05 d | 15.91 ± 1.73 e | 23.80 ± 0.64 d | 5.90 ± 0.50 b-c-d | 16.90 ± 1.56 c-d | 1.19 ± 0.09 c-d | 15.81 ± 2.67 c | 24.35 ± 1.85 d | 5.55 ± 0.25 d |
| 100 µM Cr + FFA | 21.30 ± 0.92 c | 1.30 ± 0.05 b-c | 21.82 ± 1.57 c | 26.30 ± 1.36 b | 6.40 ± 0.50 b | 19.90 ± 0.77 b-c | 1.30 ± 0.05 b-c | 19.52 ± 1.55 b-c | 29.50 ± 2.70 c | 6.25 ± 0.47 b |
| 100 µM Cr + IFA | 19.22 ± 1.78 c-d | 1.25 ± 0.08 c | 19.66 ± 2.64 c-d | 25.02 ± 3.04 b-c | 6.25 ± 0.47 b | 19.00 ± 1.08 b-c | 1.27 ± 0.05 b-c | 18.96 ± 1.57 b-c | 28.20 ± 5.06 a-c | 6.00 ± 0.40 b-c |
| 300 µM Cr | 15.45 ± 1.15 e | 1.05 ± 0.05 d-e | 14.09 ± 1.36 e | 20.50 ± 0.98 b-e | 5.50 ± 0.70 b-c | 15.55 ± 0.87 d | 1.10 ± 0.05 d | 14.10 ± 1.53 d-e | 23.15 ± 1.65 d-e | 5.15 ± 0.62 d-e |
| 300 µM Cr + FFA | 17.60 ± 2.19 d | 1.27 ± 0.05 c-d | 19.09 ± 1.81 c-d | 24.85 ± 3.63 b-c | 5.75 ± 0.25 b-c | 16.50 ± 0.54 c-d | 1.20 ± 0.08 cd | 15.94 ± 1.50 c | 25.00 ± 0.99 c-d | 5.05 ± 0.47 c |
| 300 µM Cr + IFA | 16.65 ± 2.64 d-e | 1.23 ± 0.09 d | 17.93 ± 3.78 d | 22.57 ± 0.73 d | 5.61 ± 0.28 d | 17.15 ± 1.21 c | 1.20 ± 0.08 cd | 15.43 ± 2.23 cd | 24.65 ± 3.05 c-d | 5.00 ± 0.40 c-d |
Values represent mean ± standard error (SE), with lowercase letters indicating significant differences among treatments.
Effects of fulvic acid on chlorophyll content maize cultivars
In both maize cultivar chlorophyll concentration was measured across varying Cr concentrations and foliar application treatments. In cultivar P3939, under normal conditions (0 µM Cr), chlorophyll levels were relatively consistent across treatments: 1.0096 µg/mg FW without FFA, 1.3013 µg/mg FW with FFA, and 1.2113 µg/mg FW with IFA. However, exposure to 100 µM Cr resulted in a decrease in chlorophyll concentration across all treatments: 0.9713 µg/mg FW without FFA, 1.3022 µg/mg FW with FFA, and 0.9992 µg/mg FW with IFA. At 300 µM Cr, chlorophyll concentrations varied further: 1.177 µg/mg FW without FFA, 1.1362 µg/mg FW with FFA, and 1.1399 µg/mg FW with IFA (Fig. 2A). Under control conditions (0 µM Cr), In cultivar 30Y87 chlorophyll levels varied with foliar treatments: 1.0096 µg/mg FW without FFA, 1.3013 µg/mg FW with FFA, and 1.2113 µg/mg FW with IFA. Exposure to 100 µM Cr resulted in a decline in chlorophyll concentration across all treatments: 0.9324 µg/mg FW without FFA, 0.9342 µg/mg FW with FFA, and 0.8633 µg/mg FW with IFA. At 300 µM Cr, chlorophyll concentrations continued to decrease: 0.9747 µg/mg FW without FFA, 1.1332 µg/mg FW with FFA, and 0.8657 µg/mg FW with IFA. These findings indicate that while foliar application of fulvic acid (FFA) generally maintained or slightly elevated chlorophyll levels compared to controls, chromium exposure consistently diminished chlorophyll production (Fig. 2B).
Fig. 2.
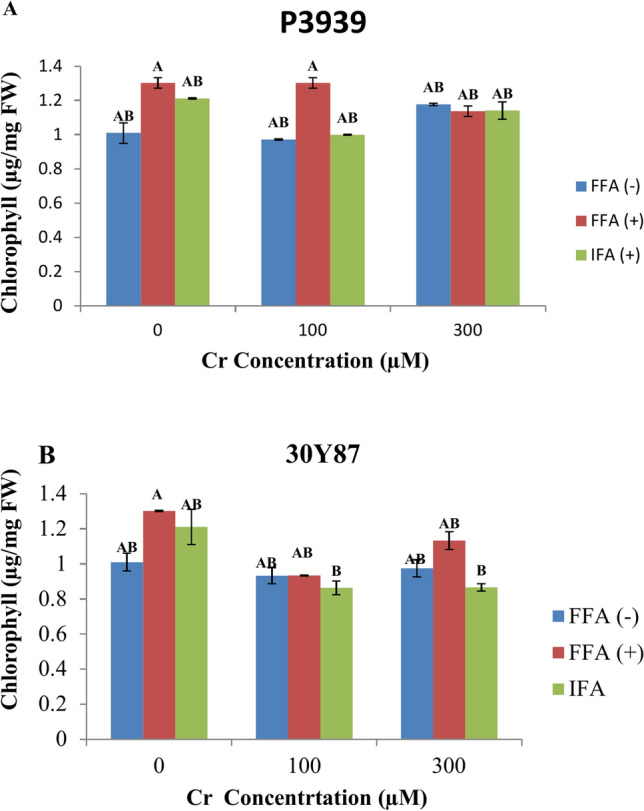
Effects of fulvic acid applied as a foliar spray (FFA, 0.5 L/A) and through the rooting medium irrigation (IFA, 300 L/A) on Chlorophyll content of maize cultivars P3939 (A) and 30Y87 (B) under chromium stress. Values represent mean ± standard error (SE), with lowercase letters indicating significant differences among treatments. -FFA indicates no fulvic acid application (control). + FFA represents foliar application of fulvic acid, sprayed on leaves. IFA refers to fulvic acid application through irrigation, delivered via the rooting medium.
Effects of fulvic acid on oxidative stress (electrolyte leakage and MDA) content maize cultivars
Electrolyte leakage (%)
In maize cultivar P3939, the impact of Cr concentrations on electrolyte leakage percentage was examined across different foliar application treatments. Under normal conditions (0 µM Cr), electrolyte leakage percentages were 84.96% without FFA (fulvic acid), 57.34% with FFA, and 70.67% with IFA (fulvic acid through the rooting medium). As chromium concentration increased to 100 µM, electrolyte leakage decreased to 64.03% without FFA, 56.71% with FFA, and 58.5% with IFA. At the highest Cr level tested (300 µM), electrolyte leakage showed mixed trends: 68.08% without FFA, 55.3% with FFA, and 49.38% with IFA (Fig. 3A).
Fig. 3.
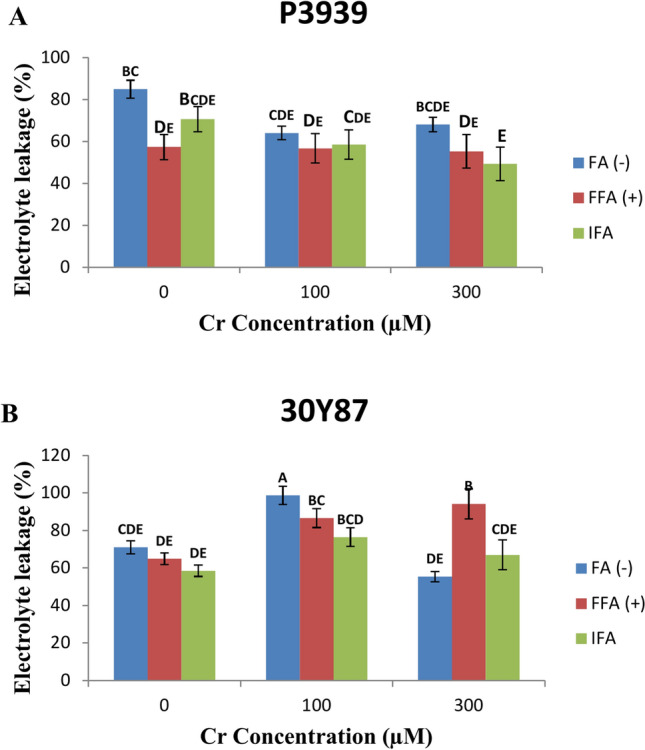
Effects of fulvic acid applied as a foliar spray (FFA, 0.5 L/A) and through the rooting medium irrigation (IFA, 300 L/A) on Electrolyte leakage (EL) of maize cultivars P3939 (A) and 30Y87 (B) under chromium stress. Values represent mean ± standard error (SE), with lowercase letters indicating significant differences among treatments. -FFA indicates no fulvic acid application (control). + FFA represents foliar application of fulvic acid, sprayed on leaves. IFA refers to fulvic acid application through irrigation, delivered via the rooting medium.
In maize cultivar 30Y87, electrolyte leakage percentages were examined across varying Cr concentrations and foliar application treatments. At 100 µM Cr, electrolyte leakage increased compared to the control (0 µM Cr) across all treatments: FFA (-) increased by 38.97%, FFA ( +) by 33.30%, and IFA ( +) by 30.68%. However, at 300 µM Cr, the responses varied: FFA (-) showed a decrease in leakage by 21.92%, FFA ( +) exhibited an increase by 45.12%, and IFA ( +) showed a modest increase by 14.52% (Fig. 3B).
MDA content (nm/g FW)
Maize cultivar P3939 demonstrates distinct changes in MDA (Malondialdehyde) content (nM/g FW) in response to varying concentrations of Cr and fulvic acid (FA). At 0 µM Cr, the MDA content for the cultivar starts at 7.4516 nM/g FW under FA(-) conditions. As the Cr concentration increases to 100 µM, MDA levels show a slight rise to 7.8194 nM/g FW. However, a more notable increase occurs at 300 µM Cr, where MDA content significantly jumps to 14.2065 nM/g FW. These observations indicate that maize cultivar P3939 exhibits a concentration-dependent response to Cr (Fig. 4A). Maize cultivar 30Y87 displays distinct changes in MDA (Malondialdehyde) content (nM/g FW) in response to varying concentrations of chromium (Cr) and different foliar treatments with fulvic acid (FA). Under control conditions (0 µM Cr), the MDA content is recorded at 7.4516 nM/g FW. When exposed to 100 µM Cr without any foliar treatment (FA-), the MDA content increases to 8.3613 nM/g FW. Interestingly, the addition of foliar fulvic acid (FFA +) at the same Cr concentration (100 µM) results in a slight decrease in MDA to 6.4258 nM/g FW, suggesting a potential mitigating effect of FFA against Cr-induced oxidative stress. Further analysis at 300 µM Cr with irrigated fulvic acid (IFA +) shows a rise in MDA content to 6.8129 nM/g FW, indicating a moderate increase compared to the control (Fig. 4B).
Fig. 4.
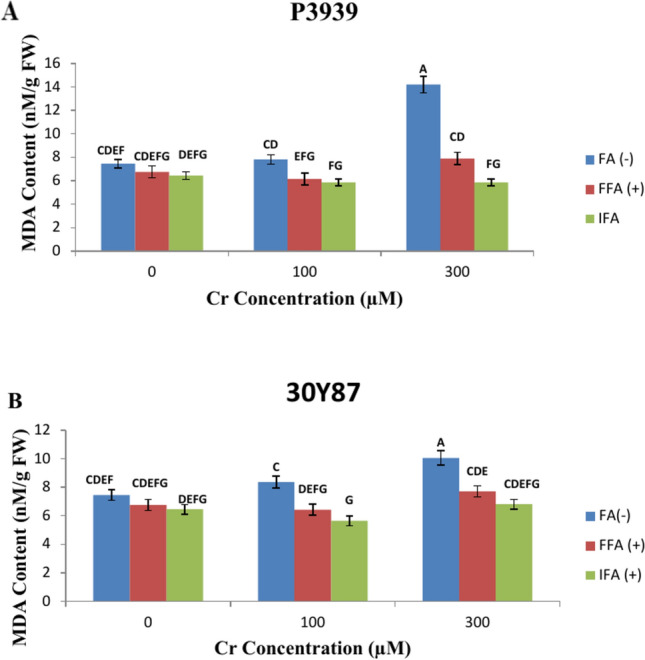
Effects of fulvic acid applied as a foliar spray (FFA, 0.5 L/A) and through the rooting medium irrigation (IFA, 300 L/A) on MDA content of maize cultivars P3939 (A) and 30Y87 (B) under chromium stress. Values represent mean ± standard error (SE), with lowercase letters indicating significant differences among treatments. -FFA indicates no fulvic acid application (control). + FFA represents foliar application of fulvic acid, sprayed on leaves. IFA refers to fulvic acid application through irrigation, delivered via the rooting medium.
Effects of fulvic acid on total soluble protein (TSP) content (mg/g FW)
Maize cultivar P3939 displays varying levels of total soluble protein (TSP) (mg/g) in response to different concentrations of Cr and foliar treatments with fulvic acid (FA). Under normal conditions without Cr (0 µM), TSP levels are 0.95275 mg/g for FA (-), 0.934 mg/g for FFA ( +), and 0.963 mg/g for IFA ( +). As the Cr concentration increases to 100 µM, there is a noticeable decrease in TSP across all foliar treatments: 0.8555 mg/g for FA (-), 0.877 mg/g for FFA ( +), and 0.849 mg/g for IFA ( +). This reduction suggests that chromium stress may negatively impact protein synthesis or stability in maize cultivar P3939. At the highest Cr concentration tested (300 µM), TSP levels continue to decrease: 0.848 mg/g for FA (-), 0.86 mg/g for FFA ( +), and 0.9125 mg/g for IFA ( +) (Fig. 5A). Under normal conditions without Cr (0 µM), the TSP levels are relatively stable across all foliar treatments: 0.95275 mg/g for FA (-), 0.934 mg/g for FFA ( +), and 0.963 mg/g for IFA ( +). However, as the Cr concentration increases to 100 µM and 300 µM, TSP levels show slight fluctuations among the treatments. At 100 µM Cr, TSP levels increase to 0.9585 mg/g for FA (-), 1.0005 mg/g for FFA ( +), and 0.954 mg/g for IFA ( +). This suggests a potential response of the cultivar to moderate stress induced by chromium, possibly influencing protein metabolism or synthesis. At 300 µM Cr, TSP levels decrease slightly to 0.933 mg/g for FA (-), 1.005 mg/g for FFA ( +), and 0.9465 mg/g for IFA ( +), indicating a more pronounced effect of higher chromium concentrations on protein levels in the maize plants (Fig. 5B).
Fig. 5.
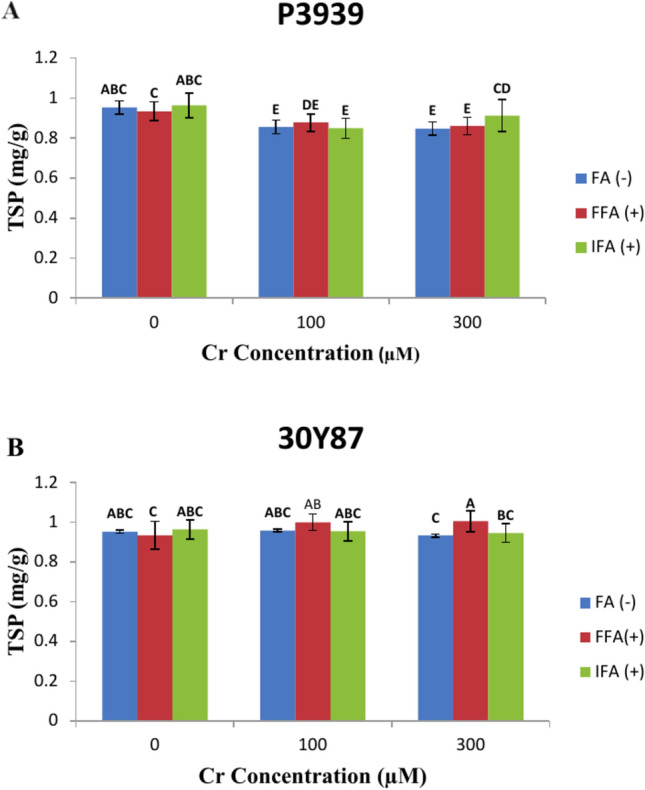
Effects of fulvic acid applied as a foliar spray (FFA, 0.5 L/A) and through the rooting medium irrigation (IFA, 300 L/A) on Total Soluble protein (TSP) of maize cultivars P3939 (A) and 30Y87 (B) under chromium stress. Values represent mean ± standard error (SE), with lowercase letters indicating significant differences among treatments. -FFA indicates no fulvic acid application (control). + FFA represents foliar application of fulvic acid, sprayed on leaves. IFA refers to fulvic acid application through irrigation, delivered via the rooting medium.
Effects of fulvic acid on antioxidant enzymatic activity under chromium stress
In the results, it was found that fulvic acid played a significant role in modulating antioxidant enzymatic activity in plants under chromium stress conditions. Specifically, enzymes such as catalase (CAT), ascorbate peroxidase (APX), and glutathione peroxidase (GPX) were observed to be crucial components of the antioxidant defense system that helped mitigate oxidative damage caused by chromium toxicity.
Catalase activity (units mg-1 protein)
Maize cultivar P3939 exhibits varying catalase (CAT) activity (U mg-1 protein) in response to different concentrations of Cr and foliar treatments with fulvic acid (FA). Under control conditions without Cr (0 µM), CAT activity levels are measured at 0.006649 U mg-1 protein for FA (-), 0.009183 mg-1 protein for FFA ( +), and 0.009027 mg-1 protein for IFA ( +). As the Cr concentration increases to 100 µM and 300 µM, changes in CAT activity are observed across all foliar treatments. At 100 µM Cr, CAT activity decreases to 0.003534 mg-1 protein for FA (-), 0.006842 U mg-1 protein for FFA ( +), and 0.007068 U mg-1 protein for IFA ( +). This reduction suggests a potential suppression of CAT enzyme function under moderate chromium stress conditions. At 300 µM Cr, CAT activity shows further variations: 0.007076 U mg-1 protein for FA (-), 0.005585 U mg-1 protein for FFA ( +), and 0.006243 U mg-1 protein for IFA ( +) (Fig. 6A). Maize cultivar 30Y87 exhibits varying levels of catalase (CAT) activity (mg-1 protein) in response to different concentrations of chromium (Cr) and foliar treatments with fulvic acid (FA). Under normal conditions without Cr (0 µM), CAT activity levels are recorded at 0.008452 U mg-1 protein for FA (-), 0.009154 U mg-1 protein for FFA ( +), and 0.008106 U mg-1 protein for IFA ( +). As the Cr concentration increases to 100 µM, changes in CAT activity are observed across the foliar treatments. CAT activity slightly increases to 0.010042 U mg-1 protein for FA (-), remains stable at 0.010112 U mg-1 protein for FFA ( +), and decreases to 0.005225 U mg-1 protein for IFA ( +). At 300 µM Cr, further variations in CAT activity are noted: 0.013391 U mg-1 protein for FA (-), 0.008314 U mg-1 protein for FFA ( +), and 0.007919 U mg-1 protein for IFA ( +) (Fig. 6B).
Fig. 6.
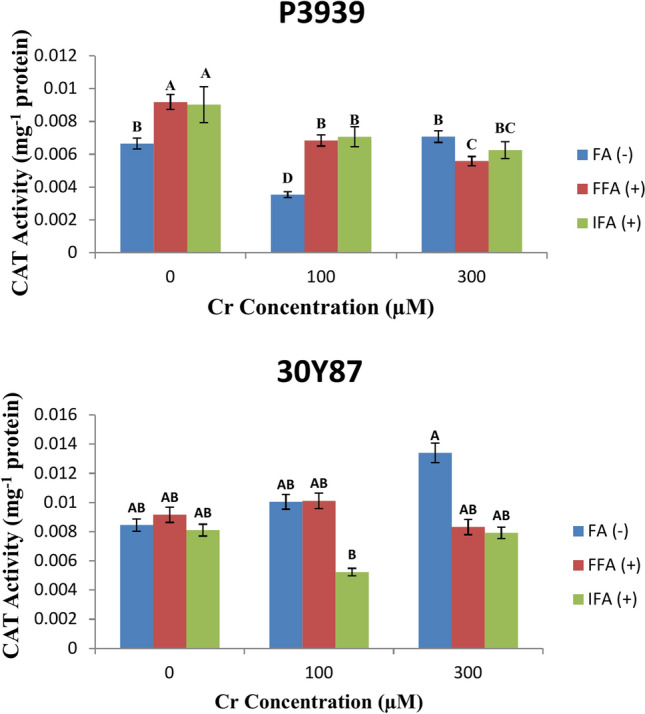
Effects of fulvic acid applied as a foliar spray (FFA, 0.5 L/A) and through the rooting medium irrigation (IFA, 300 L/A) on Catalase enzyme (CAT) of maize cultivars P3939 (A) and 30Y87 (B) under chromium stress. Values represent mean ± standard error (SE), with lowercase letters indicating significant differences among treatments. -FFA indicates no fulvic acid application (control). + FFA represents foliar application of fulvic acid, sprayed on leaves. IFA refers to fulvic acid application through irrigation, delivered via the rooting medium.
APX activity (units mg-1 protein)
Maize cultivar P3939 demonstrates varying ascorbate peroxidase (APX) activity (U mg-1 protein) in response to different concentrations of Cr and foliar treatments with fulvic acid (FA). Under normal conditions without Cr (0 µM), APX activity levels are measured at 0.010163 U mg-1 protein for FA (-), 0.011059 U mg-1 protein for FFA ( +), and 0.010007 mg-1 protein for IFA ( +). As the Cr concentration increases to 100 µM, APX activity shows fluctuations across the foliar treatments. APX activity increases to 0.015154 mg-1 protein for FA (-), remains relatively stable at 0.011348 U mg-1 protein for FFA ( +), and slightly increases to 0.011529 U mg-1 protein for IFA ( +). At 300 µM Cr, further variations in APX activity are observed: 0.01151 U mg-1 protein for FA (-), 0.011373 U mg-1 protein for FFA ( +), and 0.015043 U mg-1 protein for IFA ( +) (Fig. 7A). Maize cultivar 30Y87 displays varying levels of ascorbate peroxidase (APX) activity (U mg-1 protein) in response to different concentrations of chromium (Cr) and foliar treatments with fulvic acid (FA). Under control conditions without Cr (0 µM), APX activity levels are relatively consistent: 0.010163 U mg-1 protein for FA (-), 0.011059 U mg-1 protein for FFA ( +), and 0.010007 U mg-1 protein for IFA ( +). As the Cr concentration increases to 100 µM, APX activity shows minor fluctuations across the foliar treatments. APX activity decreases slightly to 0.010361 U mg-1 protein for FA(-), decreases to 0.009479 U mg-1 protein for FFA ( +), and increases marginally to 0.010184 U mg-1 protein for IFA( +).At 300 µM Cr, further variations in APX activity are observed: 0.010944 U mg-1 protein for FA(-), 0.011579 U mg-1 protein for FFA ( +), and 0.010261 U mg-1 protein for IFA ( +) (Fig. 7B).
Fig. 7.
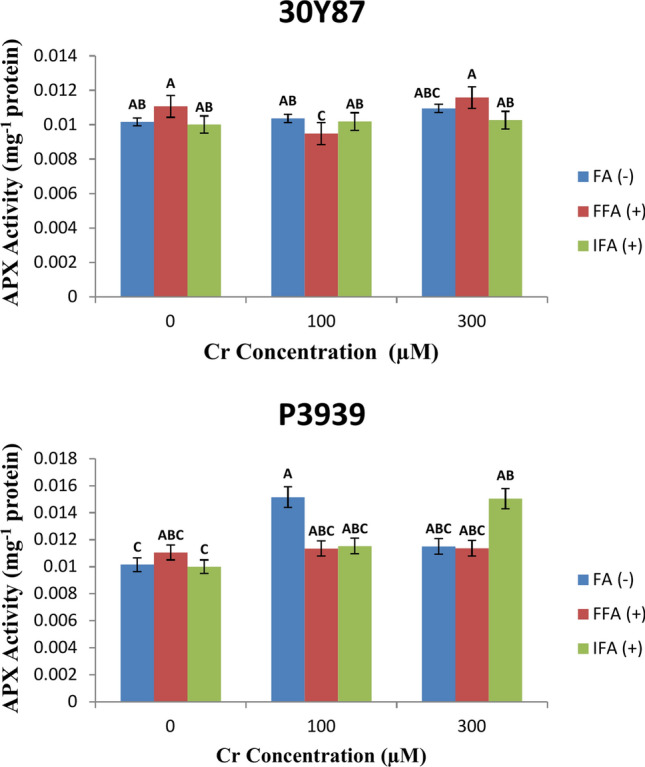
Effects of fulvic acid applied as a foliar spray (FFA, 0.5 L/A) and through the rooting medium irrigation (IFA, 300 L/A) on Ascorbate enzyme (APX) of maize cultivars P3939 (A) and 30Y87 (B) under chromium stress. Values represent mean ± standard error (SE), with lowercase letters indicating significant differences among treatments. -FFA indicates no fulvic acid application (control). + FFA represents foliar application of fulvic acid, sprayed on leaves. IFA refers to fulvic acid application through irrigation, delivered via the rooting medium.
GPX activity (units mg-1 protein)
Maize cultivar P3939 is evaluated for its response to varying concentrations of Cr, focusing on the enzymatic activity of glutathione peroxidase (GPX). The study examines GPX activity in terms of micromoles (µM) of Cr at concentrations of 0, 100, and 300 µM. Results indicate that at increasing Cr concentrations, GPX activity in P3939 maize cultivar remains within a consistent range, suggesting a robust antioxidative response. The levels of FA (-), FFA ( +), and IFA ( +) in the cultivar show differential responses to Cr exposure, with fluctuations observed across the treatments. Specifically, FA (-) exhibits a slight increase from 0.005377 to 0.006086 U mg-1 protein as Cr levels rise from 0 to 300 µM, while FFA( +) and IFA( +) show more varied responses (Fig. 8A). Maize cultivar 30Y87 was investigated for its response to increasing concentrations of chromium (Cr), focusing on the activity of glutathione peroxidase (GPX) and the levels of FA (-), FFA ( +), and IFA ( +) in its tissues. The study measured GPX activity in units per milligram of protein across Cr concentrations of 0, 100, and 300 µM. Results indicate varying responses in GPX activity as Cr levels increase, suggesting a nuanced antioxidative capacity in cultivar 30Y87. Specifically, GPX activity fluctuates, with a noticeable decrease from 0.007192 to 0.009094 U mg-1 protein as Cr concentration rises from 0 to 300 µM. This variability underscores the cultivar’s adaptability to oxidative stress induced by chromium. In terms of FA (-), FFA ( +), and IFA ( +) levels, cultivar 30Y87 displays differential responses across the Cr treatments. FA (-) levels notably increase from 0.005377 U mg-1 protein at 0 µM Cr to 0.009157 U mg-1 protein at 300 µM Cr, while FFA ( +) and IFA ( +) show more complex patterns of change (Fig. 8B).
Fig. 8.
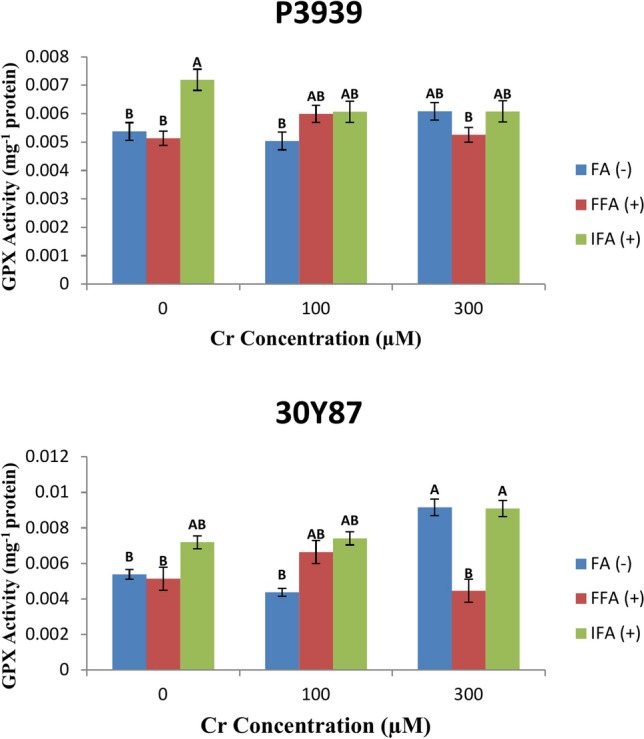
Effects of fulvic acid applied as a foliar spray (FFA, 0.5 L/A) and through the rooting medium irrigation (IFA, 300 L/A) on glutathione peroxidase (GPX) of maize cultivars P3939 (A) and 30Y87 (B) under chromium stress. Values represent mean ± standard error (SE), with lowercase letters indicating significant differences among treatments. -FFA indicates no fulvic acid application (control). + FFA represents foliar application of fulvic acid, sprayed on leaves. IFA refers to fulvic acid application through irrigation, delivered via the rooting medium.
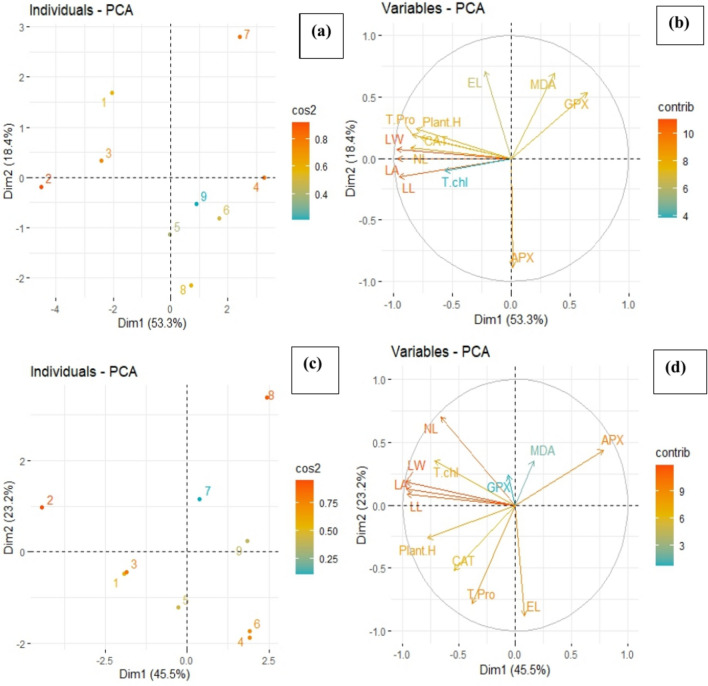
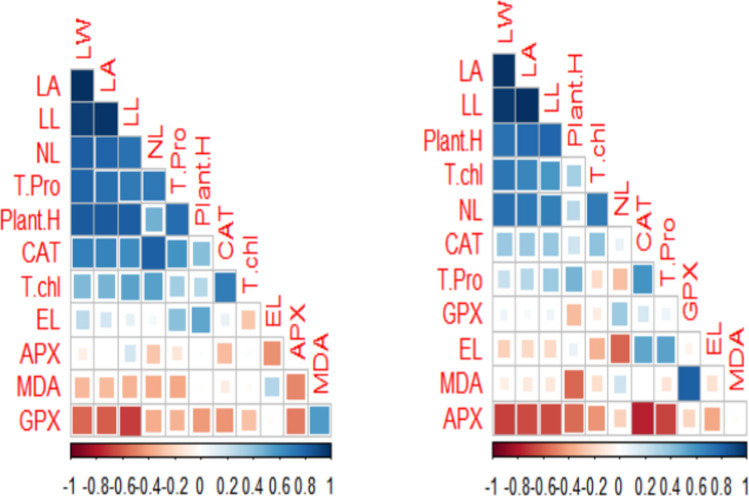
Principal component analysis (PCA) and correlation analysis
This study employed Principal Component Analysis (PCA) and correlation analysis to assess the biochemical and morphological parameters of maize cultivars under varying levels of Cr stress and application of fulvic acid (FFA) and/or inorganic fulvic acid (IFA) through foliar and irrigated methods (Fig. 9). All parameters were standardized to ensure variables were comparable by scaling to zero mean and unit variance. PC scores were analyzed to identify patterns and relationships among parameters across different experimental treatments. This helped in understanding how Cr stress and fulvic acid applications influenced the biochemical and morphological characteristics of maize cultivars. Pearson correlation coefficients were calculated to determine the strength and direction of linear relationships between pairs of parameters within each experimental group (Fig. 10).
Fig. 9.
Score (a, c) and loading plots (b, d) of principal component analysis (PCA) on various studied parameters of maize cultivars grown in Cr stressed soil. Score plot represents separation of treatments as (1) Control (without Cr contamination); (2) FFA (3) IFA (4) 100 µM Cr level, 5) 100 µM Cr + FFA 6) 100 µM Cr + IFA, 7) 300 µM Cr, 8) 300 µM Cr + FFA 9) 300 µM Cr + IFA respectively. The abbreviations of parameters are as follows: Plant H: plant height; NL: number of leaves; LW: leaf width; T chl: total chlorophyll; EL: electrolyte leakage; MDA: Lipid peroxidation; T pro: total protein; CAT: catalase; APX: Ascorbate peroxidase GPX: Guaiacol peroxidases.
Fig. 10.
Correlation between growth and physiological parameters of maize cultivars grown in Cr stressed soil.
Discussion
In this study, we investigated the role of different modes of fulvic acid application on the antioxidant activity of maize (Zea mays L.) subjected to artificial chromium stress. Chromium contamination in agricultural soils is a significant environmental concern, posing detrimental effects on plant growth and productivity7. Fulvic acid, known for its chelating properties and ability to enhance plant stress tolerance, was applied through various methods to assess its efficacy in mitigating chromium-induced oxidative stress in maize plants29. The antioxidant activity of maize plants serves as a critical indicator of their ability to counteract reactive oxygen species (ROS) accumulation under chromium stress conditions30. By examining the effects of different fulvic acid application modes, this study aims to provide insights into optimizing agricultural practices for sustainable crop production in chromium-contaminated soils. A non-significant increase in growth parameters was observed in metal treated plants and Cr treated plants with FA application. However, these non-significant findings may be attributed to the inherent variability among treatments or the stage of plant development at the time of assessment. Our results indicated a reduction in growth parameters in chromium treated plants. These findings are in consistent with work of Arshed et al.7 who observed the reduction in growth parameters in Brassica juncea under chromium stress. Alshegaihi et al.30 also reported reduction in growth parameters in cooper metal treated plants of wheat. These reductions in growth parameters can be attributed to ultra-structural and physio-chemical alterations in plants exposed to Cr stress31. This decrease in growth metrics, such as shoot and root length and biomass, is linked to diminished nutrient uptake32–34. Our results indicated a reduction in leaf area under chromium stress. This reduction in leaf area could be linked to restricted water availability impacting leaf expansion, similar to observations under potassium deficiency35. Fluoride toxicity in wheat has similarly been shown to reduce leaf area36,37.
In our experiment, foliar fulvic acid application showed an increase in various growth parameters such as leaf length, width, area, and number of leaves. Our study is consistent with previous studies that demonstrated the effects of fulvic acid application on plant growth and nutrient content. For instance, one study focused on the effect of foliar application of fulvic acid on plant growth and fruit quality of tomato (Lycopersicon esculentum L.). Another study investigated the effects of soil application of fulvic acid on the growth and nutrient content of cucumber (Cucumis sativus) plants38,39. The capacity of FA to penetrate plant roots and stimulate the expression of genes involved in cell division supports its role in promoting vegetative growth40.
Chlorophyll content was reduced in plants of both maize cultivars due to chromium stress. This reduction aligns with findings in Triticum aestivum under antibiotic stress41. Chromium stress results in the production of reactive oxygen species that reacts with pigment-protein complexes and damages the thylakoid membranes of chlorophyll due to the substitution of magnesium with H+ ions42. This biochemical disruption underscores the vulnerability of photosynthetic machinery under heavy metal stress. On the other hand, foliar application of fulvic acid showed an increase in chlorophyll content. For example, 15% increase in chlorophyll content due to foliar application of fulvic acid was observed at 100 µM Cr (1.3022 µg/mg FW) in plants of maize cultivar P3939. Moreover, foliar application of fulvic acid caused a 12% increase in chlorophyll content in maize plants grown under normal conditions. These results are similar to some earlier studies, in which an increase in chlorophyll content by the fulvic acid application has observed in maize plants43. This increase suggests that FA mitigates oxidative damage by reducing free Cr ion concentration through adsorption44.
Electrolyte leakage is an indicator of cellular damage induced by heavy metal stress and other abiotic stresses. Addition of chromium (100 &300 µM) to the growth medium increased the electrolyte leakage in the leaves of both maize cultivars. The rise in leakage is consistent with overproduction of ROS under metal stress, contributing to oxidative damage45. However, fulvic acid application (foliar + irrigated) showed reduced electrolyte leakage in chromium treated plants. About 16% decrease in electrolyte leakage was observed with IFA application at 300 µM Cr as compared with 300 µM Cr treatment alone. Malondialdehyde content is a reliable indicator of oxidative stress induced by abiotic stresses, heavy metal stress and pathogen attack46. A significant increase in MDA content (P < 0.05) was observed at 300 µM Cr concentrations in both varieties. This aligns with findings in sunflower and turnip, where Cr-induced oxidative damage elevated MDA levels47,48 where Cr-induced ROS reacted with lipids, causing membrane disruption and cellular damage49. Fulvic acid application showed reduced MDA content in chromium treated plants of both cultivars. However, the application of FA through the soil was found more effective in alleviating oxidative stress by free radical detoxification.
Our results indicate relatively reduced total soluble protein content at 300 μM Cr and 100 µM Cr treatments as compared to control. Previous study also reported a decrease in total soluble protein content under Cr stress50. This decline in TSP may also stem from increased Cr uptake interfering with protein stability51. On the other hand, application of fulvic acid through rooting medium enhanced the total soluble protein content in metal treated plants. These results are in consistent with work of Askari et al.52 who also recorded an increase in TSP content in Cr treated wheat plants. Increase in total soluble protein might be due to positive effects of fulvic acid on plant growth and photosynthetic pigments. This improvement underscores the role of FA in supporting protein synthesis and stability under stress. In our study investigating the role of different modes of fulvic acid application on the antioxidant activity of maize under artificial chromium stress, a significant increase in key antioxidant enzymes and compounds was observed. Specifically, levels of catalase (CAT), ascorbate peroxidase (APX), peroxidase (POD), and glutathione reductase (GPX) showed marked enhancement. These enzymes play critical roles in mitigating oxidative stress by decomposing ROS, preventing cellular damage, and maintaining cellular homeostasis53. Catalase is an important component of antioxidant defense system that is involved in breakdown of hydrogen peroxide generated during photorespiration and fatty acids oxidation54,55. Our results indicated no significant increase in CAT activity in chromium treated plants of both cultivars. An increase in catalase activity was recorded at a higher concentration of Cr i.e. 300 µM as compared with control and 100 µM Cr. Jabeen et al.56 also recorded increase in CAT activity in bean plants under Cr stress. The decrease in CAT activity at 100 µM Cr might be due to reason that catalase is iron porphyrin molecule and Cr may react with iron affecting the availability of the active form of iron57. Fulvic acid (foliar) application showed an increase in catalase activity at 100 µM Cr concentration. About 33% increase in CAT activity was observed with foliar FA at100µM Cr as compared with 100 µM Cr alone treatment. Peroxidase that breakdown H2O2 by using ascorbate as an electron donor is known as ascorbate peroxidase. APX is an essential antioxidant enzyme present in peroxisomes, cytosol and chloroplasts58. Our results showed reduced APX activity at a higher concentration of Cr (300 µM). The decrease in APX was also reported in Indian mustard59, oil seed rape60 and Zea mays61. The reason for the decline in APX activity might be that higher Cr toxicity can inhibit the antioxidant defence system responsible for detoxification of ROS. However, foliar application of fulvic acid increased APX activity in chromium stressed plants of cultivar 30Y87. These findings are in consistent with work of Wang et al.22 also recorded an increase in APX activity with fulvic acid application in metal treated plants. About 30% increase in APX activity was observed with foliar FA application at 300 µM Cr treatment. GPX is an antioxidant enzyme that breaks down H2O2 by using guaiacol as an electron donor62. GPX plays vital role in lignifications; defense and wound healing63. The activity of GPX did not change due to chromium stress or exogenous application of fulvic acid. An increase in antioxidant enzyme activities (CAT and GPX) under Cr stress is might in response to generation of superoxide radicals by Cr induced blockage of electron transport chain in mitochondria. The decrease in antioxidant enzyme activity (APX) might be due to the inhibitory effects of Cr ions on the enzyme64. In our experiment, FA application improved the activity of antioxidant enzymes (CAT and APX) under chromium stress. It might be due to reason that FA acts as a free radical scavenger and antioxidant. FA has the ability to react with both positive and negatively charges unpaired electrons and hence detoxify the free radicals might be due to enhanced uptake of nutrients65.
Conclusion
Chromium toxicity adversely affects antioxidant enzyme activities, photosynthetic pigments, and overall plant growth. Fulvic acid application (irrigated) led to an increase in chlorophyll content and total soluble proteins, although these results were statistically non-significant. Additionally, fulvic acid reduced malondialdehyde (MDA) content and electrolyte leakage in chromium-treated plants. Foliar fulvic acid application significantly enhanced the activities of antioxidant enzymes, particularly catalase (CAT) and ascorbate peroxidase (APX), while it did not affect the activity of guaiacol peroxidase in metal-treated plants. The increased activities of CAT and APX suggest that foliar fulvic acid application may improve plant resilience against chromium stress by effectively scavenging reactive oxygen species (ROS). The potential mechanism of fulvic acid’s action in this context involves enhancing antioxidant enzyme activities, which helps mitigate oxidative stress caused by chromium exposure. Although chromium stress resulted in reduced growth parameters, foliar and root irrigated fulvic acid application had a positive impact on various growth measures. We recommend fulvic acid application as a more effective approach for mitigating chromium toxicity, given its significant impact on the antioxidant defense system. Future research should explore the long-term effects of foliar versus irrigated fulvic acid, as well as its potential to alleviate other types of abiotic stress.
Acknowledgements
The study was supported by the Ministry of Science, Technological Development and Innovation of the Republic of Serbia (grants no. 451−03−66/2024−03/200032). The authors extend their appreciation to Researchers Supporting Project number (RSP2025R390), King Saud University, Riyadh, Saudi Arabia.
Author contributions
Conceptualization, F.I., A.Z., and A.K.; methodology, F.I.; software, A.S., and M.Z.A.; validation and formal analysis, A.S., and F.I.; resources, I.D., and P.V.P.; data curation, U.Z.; writing—original draft preparation, F.I., A.Z., A.S., U.Z., and M.Z.A,; writing—review and editing, I.D., P.V.P., and W.S.; supervision, A.Z. All authors have read and agreed to the published version of the manuscript.
Funding
The study was supported by the Ministry of Science, Technological Development and Innovation of the Republic of Serbia (grants no. 451 − 03 − 66/2024 − 03/200032). The authors extend their appreciation to Researchers Supporting Project number (RSP2025R390), King Saud University, Riyadh, Saudi Arabia.
Data availability
All data generated or analyzed during this study are included in this published article.
Declarations
Ethics approval and consent to participate
All methods were performed in accordance with the relevant guidelines and regulations. We have obtained permission to collect plant material and seedlings.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Usman Zulfiqar, Email: usman.zulfiqar@iub.edu.pk.
Ivica Djalovic, Email: ivica.djalovic@ifvcns.ns.ac.rs.
References
- 1.Fatima, A., Shabaan, M., Ali, Q., Malik, M., Asghar, H.N., Aslam, M., Zulfiqar, U., Hameed, A., Nazim, M., Mustafa, A.E.Z.M. and Elshikh, M.S. Integrated application of metal tolerant P. fluorescens and press mud for conferring heavy metal tolerance to aloe vera (Aloe barbadensis). Plant Stress, 11, 100333 (2024).
- 2.Sarwar, M.J., Shabaan, M., Asghar, H.N., Ayyub, M., Ali, Q., Zulfiqar, U., Nazim, M., Alarjani, K.M. and Elshikh, M.S. Interaction of chromium (Cr) resistant plant growth promoting rhizobacteria with compost to phytostabilize Cr in spinach rhizosphere. Plant Stress, 10, 100261 (2023).
- 3.Ali, H. Q. et al. Tanneries impact on groundwater quality: A case study of Kasur city in Pakistan. Environ. Monit. Assess.194(11), 823 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mehdiniya Afra, J. et al. Influence of chemical fertilizers and animal manure on morphological traits of medicinal plants in Northern Iran. Res. Crop Ecophysiol.17(2), 86–101 (2022). [Google Scholar]
- 5.Laxmi, V. & Kaushik, G. Toxicity of hexavalent chromium in environment, health threats, and its bioremediation and detoxification from tannery wastewater for environmental safety. In Bioremediation of industrial waste for environmental safety: volume I: industrial waste and its management (eds Saxena, G. & Bharagava, R. N.) 223–243 (Springer Singapore, 2020). [Google Scholar]
- 6.Zulfiqar, U., Haider, F.U., Ahmad, M., Hussain, S., Maqsood, M.F., Ishfaq, M., Shahzad, B., Waqas, M.M., Ali, B., Tayyab, M.N. and Ahmad, S.A., 2023. Chromium toxicity, speciation, and remediation strategies in soil-plant interface: A critical review. Front Plant Sci. 13, 1081624 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arshed, M. Z., Zulfiqar, A. & Saleem, A. Alleviation of chromium stress in Brassica juncea L. and soil remediation by plant growth promoting (PGP) bacteria. Pak. J. Sci. Ind. Res. Ser. Biol. Sci.67(1), 42–48 (2024). [Google Scholar]
- 8.Sheteiwy, M. S. et al. Elevated CO2 differentially attenuates beryllium-induced oxidative stress in oat and alfalfa. Physiol. Plant.175(5), e14036 (2023). [DOI] [PubMed] [Google Scholar]
- 9.Hussnain, M., Shabaan, M., Ali, Q., Ashraf, S., Ahmad, M., Ghafoor, U., Akhtar, M.J., Zulfiqar, U., Hussain, S., Al-Ashkar, I. and Elshikh, M.S. Microbial phytoremediation of chromium-contaminated soil with biogas slurry for enhancing the performance of Vigna radiata L. Plant Stress, 10, 100206 (2023). [Google Scholar]
- 10.Younis, U., Danish, S., Datta, R., Alahmadi, T. A. & Ansari, M. J. Sustainable remediation of chromium-contaminated soils: Boosting radish growth with deashed biochar and strigolactone. BMC Plant Biol.24(1), 115 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nardi, S., Schiavon, M. & Francioso, O. Chemical structure and biological activity of humic substances define their role as plant growth promoters. Molecules26(8), 2256 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goenadi, D. H. Fulvic acid–a small but powerful natural substance for agricultural and medical applications. Menara Perkeb.10.22302/iribb.jur.mp.v89i1.424 (2021). [Google Scholar]
- 13.Gong, G. et al. Extraction of fulvic acid from lignite and characterization of its functional groups. ACS Omega5(43), 27953–27961 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shahrajabian, M. H. & Sun, W. The importance of salicylic acid, humic acid and fulvic acid on crop production. Lett. Drug Des. Discov.21(9), 1465–1480 (2024). [Google Scholar]
- 15.Mohamed, M. H. et al. Impacts of effective microorganisms, compost tea, fulvic acid, yeast extract, and foliar spray with seaweed extract on sweet pepper plants under greenhouse conditions. Plants10(9), 1927 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.García-Lara, S., Serna-Saldivar, S. O. Corn history and culture. In Corn 1-18 (2019).
- 17.Huma, B., Hussain, M., Ning, C. & Yuesuo, Y. Human benefits from maize. Sch. J. Appl. Sci. Res.2(2), 4–7 (2019). [Google Scholar]
- 18.Vasilachi, I. C., Stoleru, V. & Gavrilescu, M. Analysis of heavy metal impacts on cereal crop growth and development in contaminated soils. Agriculture13(10), 1983 (2023). [Google Scholar]
- 19.AbdElgawad, H. et al. Maize roots and shoots show distinct profiles of oxidative stress and antioxidant defense under heavy metal toxicity. Environ. Pollut.258, 113705 (2020). [DOI] [PubMed] [Google Scholar]
- 20.Huang, Q. et al. Silicon dioxide nanoparticles enhance plant growth, photosynthetic performance, and antioxidants defence machinery through suppressing chromium uptake in Brassica napus L.. Environ. Pollut.342, 123013 (2024). [DOI] [PubMed] [Google Scholar]
- 21.Kamran, M. et al. Jasmonic acid-mediated enhanced regulation of oxidative, glyoxalase defense system and reduced chromium uptake contributes to alleviation of chromium (VI) toxicity in choysum (Brassica parachinensis L.). Ecotoxicol. Environ. Saf.208, 111758 (2021). [DOI] [PubMed] [Google Scholar]
- 22.Wang, Y., Yang, R., Zheng, J., Shen, Z. & Xu, X. Exogenous foliar application of fulvic acid alleviate cadmium toxicity in lettuce (Lactuca sativa L.). Ecotoxicol. Environ. Saf.167, 10–19 (2019). [DOI] [PubMed] [Google Scholar]
- 23.Arnon, D. I. Copper enzymes in isolated chloroplasts Polyphenoloxidase in Beta vulgaris. Plant physiol.24(1), 1 (1949). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bajji, M., Kinet, J.-M. & Lutts, S. The use of the electrolyte leakage method for assessing cell membrane stability as a water stress tolerance test in durum wheat. Plant Growth Regul.36, 61–70 (2002). [Google Scholar]
- 25.Heath, R. L. & Packer, L. Photoperoxidation in isolated chloroplasts: II. Role of electron transfer. Arch. Biochem. Biophys.125(3), 850–857 (1968). [DOI] [PubMed] [Google Scholar]
- 26.Chance, B., Maehly A. [136] Assay of catalases and peroxidases (1955). [DOI] [PubMed]
- 27.Nakano, Y. & Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol.22(5), 867–880 (1981). [Google Scholar]
- 28.Kruger, N. J. The Bradford method for protein quantitation. In The protein protocols handbook (ed. Walker, J. M.) 17–24 (Humana Press, 2009) Totowa, NJ. [Google Scholar]
- 29.Ali, S. et al. Fulvic acid mediates chromium (Cr) tolerance in wheat (Triticum aestivum L.) through lowering of Cr uptake and improved antioxidant defense system. Environ. Sci. Pollut. Res.22, 10601–10609 (2015). [DOI] [PubMed] [Google Scholar]
- 30.Alshegaihi, R. M. et al. Silicon and titanium dioxide mitigate copper stress in wheat (Triticum aestivum L.) through regulating antioxidant defense mechanisms. J. Plant Growth Regul.43, 1519–1535 (2023). [Google Scholar]
- 31.Saud, S. et al. The impact of chromium ion stress on plant growth, developmental physiology, and molecular regulation. Front. Plant Sci.13, 994785 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Komal, Shabaan, M., Ali, Q., Asghar, H.N., Zahir, Z.A., Yousaf, K., Aslam, N., Zulfiqar, U., Ejaz, M., Alwahibi, M.S. and Ali, M.A. Exploring the synergistic effect of chromium (Cr) tolerant Pseudomonas aeruginosa and nano zero valent iron (nZVI) for suppressing Cr uptake in Aloe Vera. Int J Phytoremed.26, 1474-1485 (2024). [DOI] [PubMed] [Google Scholar]
- 33.Saleem, A. et al. Alkaline and acidic soil constraints on iron accumulation by Rice cultivars in relation to several physio-biochemical parameters. BMC Plant Biol.23(1), 397 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saleem, A., Zulfiqar, A., Saleem, M. Z., Saleem, M. H. & Fahad, S. Elevated expression of YSL genes and enhanced physiological responses in rice subjected to iron application. J. Plant Growth Regul.43, 1–19 (2024). [Google Scholar]
- 35.Hu, W. et al. The reduction in leaf area precedes that in photosynthesis under potassium deficiency: The importance of leaf anatomy. New Phytol.227(6), 1749–1763 (2020). [DOI] [PubMed] [Google Scholar]
- 36.Zulfiqar, A. et al. Fluoride resistant bacteria alleviate fluoride stress in Triticum aestivum l. through modulating gas exchange characteristics and enhanced plant growth. Fluoride55(3), 280–294 (2022). [Google Scholar]
- 37.Zulfiqar, A., Fatima, R., Ahmed, S., Saleem, A., Sardar, R., Ahmad, M. N., Yasin N. A. Mechanistic insights into the interaction of fluoride resistant bacteria with wheat roots toward enhancing plant productivity by alleviating fluoride stress. Fluoride56(3) (2023)
- 38.Suh, H. Y., Yoo, K. S. & Suh, S. G. Effect of foliar application of fulvic acid on plant growth and fruit quality of tomato (Lycopersicon esculentum L.). Hortic. Environ. Biotechnol.55, 455–461 (2014). [Google Scholar]
- 39.Rauthan, B. & Schnitzer, M. Effects of a soil fulvic acid on the growth and nutrient content of cucumber (Cucumis sativus) plants. Plant Soil63, 491–495 (1981). [Google Scholar]
- 40.Li, Z. et al. Controlled-release urea combined with fulvic acid enhanced carbon/nitrogen metabolic processes and maize growth. J. Sci. Food Agric.102(9), 3644–3654 (2022). [DOI] [PubMed] [Google Scholar]
- 41.Saleem, A., Zulfiqar, A., Ahmed, Z. Effect of 2-(phenylsulfonyl) hydrazine carbothioamide on photosynthetic pigments in wheat (Triticum aestivum L.) varieties. J. Plantarum.2. 1-9 (2020).
- 42.Wakeel, A., Xu, M. & Gan, Y. Chromium-induced reactive oxygen species accumulation by altering the enzymatic antioxidant system and associated cytotoxic, genotoxic, ultrastructural, and photosynthetic changes in plants. Int. J. Mol. Sci.21(3), 728 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gao, F. et al. The combined application of urea and fulvic acid solution improved maize carbon and nitrogen metabolism. Agronomy12(6), 1400 (2022). [Google Scholar]
- 44.Yang, R. et al. Effects of Fe (III)-fulvic acid on Cu removal via adsorption versus coprecipitation. Chemosphere197, 291–298 (2018). [DOI] [PubMed] [Google Scholar]
- 45.Sachdev, S., Ansari, S. A., Ansari, M. I., Fujita, M. & Hasanuzzaman, M. Abiotic stress and reactive oxygen species: Generation, signaling, and defense mechanisms. Antioxidants10(2), 277 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Haider, F.U., Zulfiqar, U., ul Ain, N., Hussain, S., Maqsood, M.F., Ejaz, M., Yong, J.W.H. Li, Y. Harnessing plant extracts for eco-friendly synthesis of iron nanoparticle (Fe-NPs): Characterization and their potential applications for ameliorating environmental pollutants. Ecotoxicol. Environ. Saf. 281, 116620 (2024). [DOI] [PubMed] [Google Scholar]
- 47.Farid, M. et al. Glutamic acid-assisted phytomanagement of chromium contaminated soil by sunflower (Helianthus annuus L.): Morphophysiological and biochemical alterations. Front. Plant Sci.11, 1297 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gill, R. A. et al. Chromium-induced physio-chemical and ultrastructural changes in four cultivars of Brassica napus L.. Chemosphere120, 154–164 (2015). [DOI] [PubMed] [Google Scholar]
- 49.Feng, M. et al. Hexavalent chromium induced oxidative stress and apoptosis in Pycnoporus sanguineus. Environ. Pollut.228, 128–139 (2017). [DOI] [PubMed] [Google Scholar]
- 50.Seleiman, M. F. et al. Chromium resistant microbes and melatonin reduced Cr uptake and toxicity, improved physio-biochemical traits and yield of wheat in contaminated soil. Chemosphere250, 126239 (2020). [DOI] [PubMed] [Google Scholar]
- 51.Anjum, S. A. et al. Phyto-toxicity of chromium in maize: Oxidative damage, osmolyte accumulation, anti-oxidative defense and chromium uptake. Pedosphere27(2), 262–273 (2017). [Google Scholar]
- 52.Askari, S. H., Ashraf, M. A., Ali, S., Rizwan, M. & Rasheed, R. Menadione sodium bisulfite alleviated chromium effects on wheat by regulating oxidative defense, chromium speciation, and ion homeostasis. Environ. Sci. Pollut. Res.28, 36205–36225 (2021). [DOI] [PubMed] [Google Scholar]
- 53.Sharma, M., Dey, S. R. & Kumar, P. Antioxidant Defence: a key mechanism of chromium tolerance. In Chromium in Plants and Environment (eds Kumar, N. et al.) 91–116 (Springer, 2023). [Google Scholar]
- 54.Ayub, A., Shabaan, M., Malik, M., Asghar, H.N., Zulfiqar, U., Ejaz, M., Alarjani, K.M. and Al Farraj, D.A. Synergistic application of Pseudomonas strains and compost mitigates lead (Pb) stress in sunflower (Helianthus annuus L.) via improved nutrient uptake, antioxidant defense and physiology. Ecotoxicol. Environ. Saf. 274, 116194 (2024). [DOI] [PubMed] [Google Scholar]
- 55.Dawood, M. F., Tahjib-Ul-Arif, M., Sohag, A. A. M. & Abdel Latef, A. A. H. Role of acetic acid and nitric oxide against salinity and lithium stress in Canola (Brassica napus L.). Plants13(1), 51 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jabeen, N. et al. Glycinebetaine mediates chromium tolerance in mung bean through lowering of Cr uptake and improved antioxidant system. Arch. Agron. Soil Sci.62(5), 648–662 (2016). [Google Scholar]
- 57.Abu-Omar, M. M. High-valent iron and manganese complexes of corrole and porphyrin in atom transfer and dioxygen evolving catalysis. Dalton Trans.40(14), 3435–3444 (2011). [DOI] [PubMed] [Google Scholar]
- 58.Sousa, R. H. et al. Impairment of peroxisomal APX and CAT activities increases protection of photosynthesis under oxidative stress. J. Exp. Bot.70(2), 627–639 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nouairi, I. et al. Antioxidant defense system in leaves of Indian mustard (Brassica juncea) and rape (Brassica napus) under cadmium stress. Acta Physiol. Plant.31, 237–247 (2009). [Google Scholar]
- 60.Wu, Z. et al. Antioxidant enzyme systems and the ascorbate–glutathione cycle as contributing factors to cadmium accumulation and tolerance in two oilseed rape cultivars (Brassica napus L.) under moderate cadmium stress. Chemosphere138, 526–536 (2015). [DOI] [PubMed] [Google Scholar]
- 61.Chugh, V., Kaur, N., Gupta, A. K. Evaluation of oxidative stress tolerance in maize (Zea mays L.) seedlings in response to drought (2011). [PubMed]
- 62.Dawood, M. F., Tahjib-Ul-Arif, M., Sohag, A. A. M. & Abdel Latef, A. A. H. Fluoride mitigates aluminum-toxicity in barley: Morpho-physiological responses and biochemical mechanisms. BMC Plant Biol.22(1), 287 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bala, S., Asthir, B. & Bains, N. Activities of cell wall peroxidases in relation to lignification in six wheat (Triticum aestivum L.) genotypes under heat and drought stress. J. Environ. Biol.37(3), 437 (2016). [Google Scholar]
- 64.Yilmaz, S. H., Kaplan, M., Temizgul, R. & Yilmaz, S. Antioxidant enzyme response of sorghum plant upon exposure to aluminum, chromium and lead heavy metals. Turk. J. Biochem.42(4), 503–512 (2017). [Google Scholar]
- 65.Wang, W., Chen, M., Wang, D., Yan, M. & Liu, Z. Different activation methods in sulfate radical-based oxidation for organic pollutants degradation: Catalytic mechanism and toxicity assessment of degradation intermediates. Sci. Total Environ.772, 145522 (2021). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.