Abstract
Background:
Human papillomavirus (HPV) and Epstein-Barr virus (EBV) are important etiological factors for several cancers. This study aimed to determine the prevalence of HPV and EBV infection in penile cancer.
Methods:
Forty-three formalin-fixed paraffin-embedded penile cancer tissue samples were analyzed for the HPV-induced p16INK4A protein by immunohistochemistry and Epstein-Barr encoding region in situ hybridization. Demographic data and overall survival were analyzed.
Results:
The median age of patients was 59 years, ranging from 23 to 91 years old. Most of the tumors (86%) were located at the tip of the penis. HPV infection was positive in 12/43 (27.9%) patients. EBV infection was observed in 2/43 (4.6%) of cases and there was no co-infection detected in this cohort. Patients who had p16INK4A overexpression had a trend toward longer survival compared to those without; the median survival time of 104.4 vs 89 months, the hazard ratio of 0.48 (95% CI: 0.16-1.42, p = 0.173).
Conclusions:
One-third of penile cancer patients were positive for HPV-induced p16INK4A expression and there was a trend toward better survival in HPV-positive patients. EBV infection was infrequent in penile cancer in Thailand.
Key Words: Human papillomavirus, Epstein-Barr virus, penile cancer, prevalence, p16
Introduction
Squamous cell carcinoma of the penile is a rare cancer with a prevalence of 0.4-0.6% worldwide. The incidence varies according to geographic region, in Asia and Africa the incidence reaches approximately 10% [1, 2]. Several factors increase the risk of developing penile cancer including phimosis, balanitis, poor hygiene, smoking, and sexually transmitted infections, specifically human papillomavirus (HPV) infection [3, 4].
HPV infection was reported in 20-80% of invasive penile cancers, the variation mostly due to different geographic backgrounds and various methods of HPV detection [5-7]. Evidence suggests that HPV infection is likely a carcinogenesis of penile cancer [8], even though exact pathways have not been fully elucidated. The high-risk oncogenic strains, subtypes 16 and 18 [9], encoded the E6 and E7 oncogenes, which leads to the degradation of p53 and pRb resulting in the increased expression of p16INK4a. Immunohistochemical staining of p16INK4a is a surrogate marker for the high-oncogenic risk HPV infection, especially subtype 16 [7, 10, 11].
The Epstein-Barr virus (EBV) is related to several cancers including Burkitt’s lymphoma, Hodgkin’s disease, nasopharyngeal carcinoma, and gastric cancer. The co-infection of EBV and HPV has been linked to cervical cancer, with an increased likelihood of aggressiveness [12]. However, the presence and role of EBV in penile cancer remain unclear [11, 13], and more studies are needed.
Thus, this study aimed to determine the prevalence of HPV and EBV infection and the co-infection rate in a series of Thai penile cancer cases.
Materials and Methods
Patients and clinico-pathological data
Fifty-two penile SCC patients who underwent surgical resection in Srinagarind Hospital between January 2009 and December 2017, were included. Nine patients were excluded due to unavailable formalin-fixed paraffin-embedded (FFPE) tissue. A total of 43 patients were included in the final analysis. All patients underwent partial or total penectomy and none received neoadjuvant chemotherapy or radiotherapy.
Age, Eastern Cooperative Oncology Group (ECOG) performance status [14], and survival time were retrieved. The histological subtype, grading, and pathological staging according to the 8th edition American Joint Committee on Cancer (AJCC) staging system [15] were recorded.
Immunohistochemistry for p 16INK4a
FFPE tissue specimens were sectioned at 3.5 µm and were prepared using a microtome and mounted on adhesive glass slides. Immunohistochemistry (IHC) assay was performed using an antibody to p16INK4a (clone G175-405, catalog number Z2117, company name ZETA corporation). Antibody was diluted at 1: 100, and the slides were stained and incubated at 37 °C for 32 minutes.
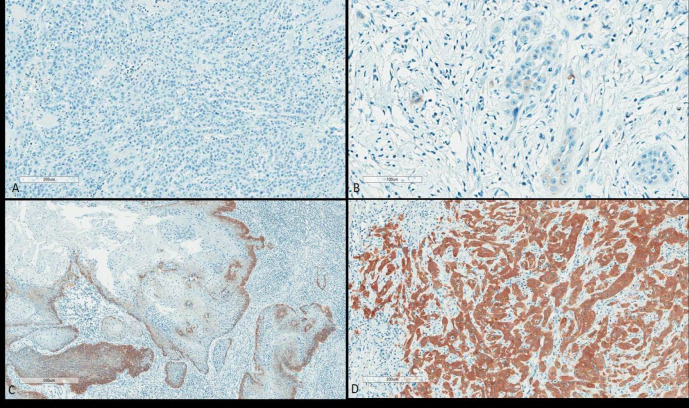
Four p16INK4a expression patterns were classified as follows (Figure 1):
Figure 1.
Four p16INK4a Expression Pattern (A) Pattern 0; Complete absence. (B) Pattern 1; Positive scattered tumor cells, weak intensity. (C) Pattern 2; positive focal, discontinuous, moderate intensity. (D) Pattern 3; Diffuse positive, strong intensity.
Pattern 0: A complete absence of staining in all neoplastic cells.
Pattern 1: Irregular and discontinuous individual staining in some of the neoplastic cells.
Pattern 2: A more extensive, although discontinuous, staining pattern with small clusters of positive neoplastic cells.
Pattern 3: Continuous and complete cytoplasmic and nuclear staining in all neoplastic cells.
Pattern 3 was considered positive for p16INK4a expression, while patterns 0, 1, and 2 were all negative.
Epstein-Barr encoding region in situ hybridization
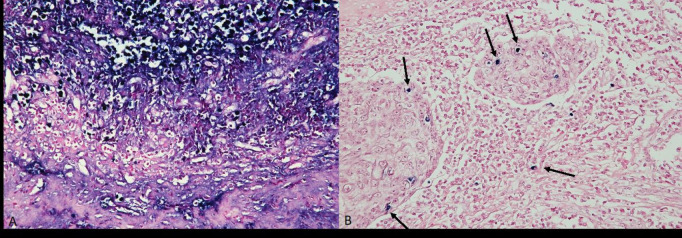
In situ hybridization for Epstein-Barr encoding RNA was performed using EBV-ISH detection kit (INFORM EBER Virus Early RNA probe, catalog number 800-2842, lot number J12038), under protocol 236 EBER of BenchMark XT Automated Slide Staining system. A known EBV-positive sample from the local laboratory was used as a control specimen (Figure 2).
Figure 2.
EBER in Situ Hybridization. (A) EBER positive control. (B) positive EBER in scattered inflammatory cells.
Statistical analysis
SPSS software, KKU license version 27, was performed to analyze the association between p16 expression, and clinico-pathological parameters (including tumor size, histological grading, histologic subtype, staging, and survival time) through Chi-square or Fisher’s exact test as appropriate. The differences of continuous data between the two dependent groups were analyzed by either independent t-test (parametric test) or Mann–Whitney test (non-parametric test). Values were presented as the mean ± SD. The survival analysis was conducted and analyzed using Kaplan-Meier estimation with Log-rank and Cox regression tests. Statistical significance was determined at p-value < 0.05.
Results
The median age was 59 years old (range, 23-91 years) as shown in Table 1. Most of the patients were diagnosed with squamous cell carcinoma, and the tumors were mostly well-differentiated. The most common site of the tumor was the tip of the penis. All patients had ECOG performance scores of 0-1; 86% were ECOG 0 and 14% were ECOG 1. According to the 8th AJCC staging system, 27/43 cases (62.8%) were T3-T4, 12/43 cases (27.9%) had nodal metastasis and 20/42 cases (47.6%) were stage III-IV.
Table 1.
Baseline Characteristics in Positive and Negative HPV Infection in Penile SCC Patients.
| Features | Total n=43 |
p
16INK4A
Positive (n=12, 27.9%) |
p
16INK4A Negative (n=31, 72.1%) |
P-value |
|---|---|---|---|---|
| Age | ||||
| Median 59 (range 23-91) | ||||
| <59 years | 20 (46.5) | 6 (50) | 14 (45.2) | 0.775 |
| >59 years | 23 (53.5) | 6 (50) | 17 (54.8) | |
| ECOG score | ||||
| 0 | 37 (86) | 9 (75) | 28 (90.3) | 0.325 |
| 1 | 6 (14) | 3 (25) | 3 (9.7) | |
| Location | ||||
| Tip | 37 (86) | 10 (83.3) | 27 (87.1) | 1 |
| Shaft | 6 (14) | 2 (16.7) | 4 (12.9) | |
| Histological Grade | ||||
| 1 | 35 (83.7) | 9 (75) | 26 (83.9) | 0.665 |
| 2-3 | 8 (16.3) | 3 (25) | 5 (16.1) | |
| T stage | ||||
| T1-2 | 16 (37.2) | 4 (38.7) | 12 (33.3) | 0.744 |
| T3-4 | 27 (62.8) | 8 (61.3) | 19 (66.7) | |
| Lymph node metastasis (N stage) | ||||
| Negative | 31 (72.1) | 11 (91.7) | 20 (64.5) | 0.13 |
| Positive | 12 (27.9) | 1 (8.3) | 11 (35.5) | |
| TNM Stage | ||||
| I-II | 22 (52.4) | 8 (66.7) | 14 (46.7) | 0.315 |
| III-IV | 20 (47.6) | 4 (33.3) | 16 (53.3) | |
| HIV status | ||||
| Negative | 42 (97.7) | 12 (100) | 30 (96.8) | 1 |
| Positive | 1 (2.3) | 0 (0) | 1 (3.2) | |
| EBER | ||||
| Negative | 41 (95.3) | 12 (100) | 29 (93.5) | |
| Positive | 2 (4.6) | 0 (0) | 2 (6.5) | 1 |
P16 expression was positive in twelve cases (28%). No significant differences between the HPV-positive and negative patients regarding age, ECOG score, tumor location, histological grading, T stage, N status, and TNM staging at presentation (Table 1).
The prevalence of EBV in this penile cancer cohort was 2/43 cases (4.6%). There was no HPV and EBV co-infection in this cohort. Table 2 shows the characteristics of the two patients who harbored positive EBER.
Table 2.
Patient Characteristics in Positive Epstein-Barr Virus (EBV) Infection in Penile SCC Patients.
| Case | Age | Location | Grade | T | N | Staging | HPV | HIV | Survival (months) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 67 | Tip | 1 | 1 | Positive | III | Neg. | Neg. | 146 |
| 2 | 56 | Tip | 2 | 3 | Positive | IV | Neg. | Neg. | 6 |
Abbreviations; T, tumor; N, node; M, metastasis
Survival outcome of penile SCC patients
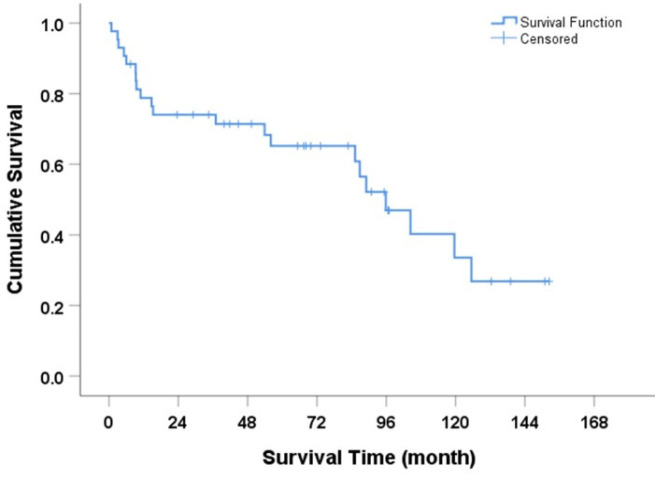
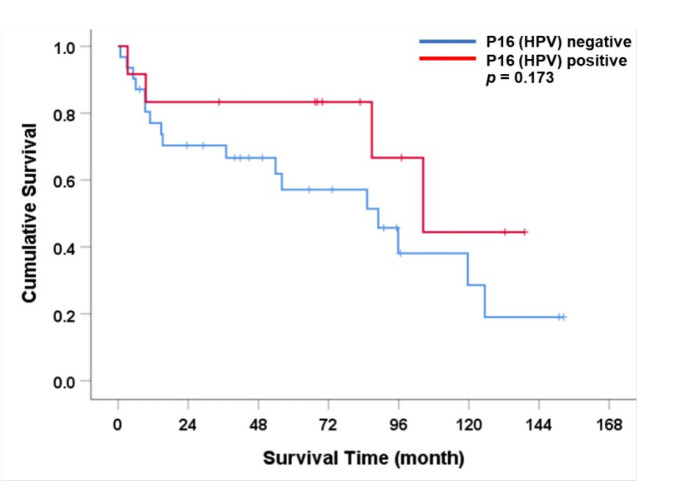
The median overall survival for the entire cohort was 95.9 months (95% CI: 75.6-116.1) as shown in Figure 3. Patients with positive p16 expression had a longer overall survival compared to those without of 104.4 vs 89 months (HR = 0.48, p=0.173) as shown in Figure 4. Histological grade 2-3, tumor location at shaft, positive lymph node, and higher TNM stage were factors associated with shorter survival time (Table 3). In multivariable analysis, histological grade 2-3 was the only independent factor to predict survival outcomes of penile SCC patients in this cohort (HR = 4.34, p=0.041)
Figure 3.
Overall Survival Rate for Participants with Penile Cancer (Kaplan-Meier method)
Figure 4.
Overall Survival Rate for Participants with Penile Cancer According to HPV Infection.
Table 3.
Univariate and Multivariate Analysis for Overall Survival
| Characteristic | Median survival (months) | Univariate | Multivariate | ||
|---|---|---|---|---|---|
| HR (95%CI) | p-value | HR (95%CI) | p-value | ||
| Age | |||||
| < 59 | NR | 1 | - | ||
| > 59 | 86.82 | 1.38 (0.55-3.48) | 0.495 | - | - |
| ECOG | |||||
| 0 | 89.04 | 1 | - | ||
| 1 | NR | 0.36 (0.08-1.62) | 0.166 | - | - |
| Histological Grade | |||||
| 1 | 104.4 | 1 | 1 | ||
| 2-3 | 9.29 | 4.34 (1.53-12.29) | 0.003* | 3.59 (1.05-12.45) | 0.041* |
| Location | |||||
| Tip | 104.39 | 1 | 1 | ||
| Shaft | 10.91 | 3.43 (1.04-11.29) | 0.031* | 4.01 (0.86-18.81) | 0.078 |
| T stage | |||||
| T1-2 | 125.46 | 1 | - | ||
| T3-4 | 86.82 | 2.00 (0.73-5.47) | 0.167 | - | - |
| Lymph node metastasis (N) | |||||
| N0 | 104.39 | 1 | 1 | ||
| N1 | 36.96 | 2.44 (1.02-5.81) | 0.038* | 1.15 (0.29-4.60) | 0.842 |
| TNM stage | |||||
| Early (I-II) | 125.46 | 1 | 1 | ||
| Late (III-IV) | 53.89 | 3.65 (1.39-9.60) | 0.005* | 2.47 (0.66-9.29) | 0.181 |
| p16INK4A status | |||||
| Negative | 89.04 | 1 | - | ||
| Positive | 104.39 | 0.48 (0.16-1.42) | 0.173 | - | - |
*, Statical significance, p<0.05; NR (not reached), the overall survival did not decrease to the median while analyzing the data; Abbreviations; ECOG, Eastern Cooperative Oncology Group; T, tumor; N, node; M, metastasis
Figure 4 Overall survival rate for participants with penile cancer according to HPV infection.
Discussion
In this study, we explored the prevalence of HPV and EBV in penile cancer cases in Thailand. The prevalence of HPV and EBV in penile cancer in Thailand was 27.9% and 4.6%, and there was no co-infection of HPV and EBV in this cohort.
The HPV prevalence was low (28%) compared to the reported global prevalence of 30-75% [5, 6, 9, 16]. Martinez-Ballon et al described the HPV rate in penile cancer in Mexico and found that the HPV DNA detection rate was as high as 59% [17]. However, the p16INK4a expression in the study was 30.9% which is comparable to our study. Moreover, recent reports in the high endemic area also revealed a positive p16 expression of 22-26% [16, 18]. Different molecular techniques, types of samples, and populations result in different sensitivity and specificity for HPV detection. The report from Brazil, a highly endemic area of penile cancer, found that p16INK4a expression was observed in 40% while HPV DNA was detected in 89% of cases [6]. The lower rate of HPV prevalence is mainly due to the detection method of only p16INK4a expression which correlates with only the high-risk HPV genotypes. The most common genotype reported in penile cancer cases in Thailand was the high-risk HPV-18 either single or multiple infection [19].
HPV-induced p16INK4a positive patients had a trend toward longer overall survival compared to HPV-negative patients but did not reach statistical significance. The results are in line with the earlier larger report on penile and oropharyngeal cancer [11, 16, 20-21]. The p16 expression is a surrogate marker of HPV infection and is an important prognostic factor for oropharyngeal cancer leading to the classification in the TNM staging and determining the treatment modality [22]. Treatment response is improved in patients receiving radiotherapy with or without chemotherapy. In penile cancer, however, the evidence is not yet strong enough and the multi-modality treatment is still the standard of care for locally advanced disease [23].
EBV infection is an emerging importance for viral carcinogenesis. In this study, the prevalence of EBV in penile malignancy was 4.6% and there was no co-infection with HPV. The number is much lower than reports from Brazil where the prevalence of EBV was 30-46% and the co-infection rate was 26-29% [11]. We used the EBER as the detection method rather than the EBV PCR which explains the lower positive rate. Whether EBV is the cofactor for the development of penile cancer is still controversial.
There is growing evidence that HPV vaccination prevents cervical, anal, and potentially oropharyngeal cancer [20, 24, 25]. HPV vaccination has been implemented in the immunization program for young girls in Thailand recently. Nevertheless, no data for penile cancer prevention is available. Since the vaccination led to herd protection against oral HPV in unvaccinated males [26], future trends in HPV-related cancer, including penile cancer, might change.
Our study has several shortcomings. First, the number of patients is limited. Second, the staging data was largely pathological data which did not reflect the clinical staging for nodal or distant metastasis. Sentinel lymph node biopsy and nodal dissection were not routinely done. Lastly, no data regarding HPV and EBV DNA.
In conclusion, HPV is positive in one-third of penile cancer patients in Thailand. Penile cancer patients who had HPV-positive have a trend toward better survival time than HPV-negative cases. EBV infection in penile cancer patients in Thailand is rare, and there was no co-infection.
Author Contribution Statement
Conceptualization: SS, JC, and WS. Data curation: SS, TT, JC, UR, PK, SL, PT and WS. Investigation: SS, TT, WS, and JC. Methodology: WS, UR, PK, SL, PT and JC. Writing—review & editing: SS, JC and WS. Approval of final manuscript: SS, TT, JC, UR, PK, SL, PT and WS.
Acknowledgements
None.
Ethical consideration
Ethical approval was provided by the Khon Kaen University Ethics Committee (KKUEC) as instituted by the Declaration of Helsinki (HE611509). For this type of study, informed consent was not required in accordance with the ethics committee and institutional guidelines. All the data was fully anonymized and maintained with confidentiality.
Funding
This study was granted by the Faculty of Medicine, Khon Kaen University, Thailand (Grant Number IN61149).
Availability of data and materials
The data are available from corresponding author per requested.
Conflict of interest
All authors declare no conflict of interest.
References
- 1.Christodoulidou M, Sahdev V, Houssein S, Muneer A. Epidemiology of penile cancer. Curr Probl Cancer. 2015;39(3):126–36. doi: 10.1016/j.currproblcancer.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 2.Montes Cardona CE, García-Perdomo HA. Incidence of penile cancer worldwide: Systematic review and meta-analysis. Rev Panam Salud Publica. 2017;41:e117. doi: 10.26633/RPSP.2017.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pow-Sang MR, Ferreira U, Pow-Sang JM, Nardi AC, Destefano V. Epidemiology and natural history of penile cancer. Urology. 2010;76(2 Suppl 1):S2–6. doi: 10.1016/j.urology.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 4.Thomas A, Necchi A, Muneer A, Tobias-Machado M, Tran ATH, Van Rompuy AS, et al. Penile cancer. Nat Rev Dis Primers. 2021;7(1):11 . doi: 10.1038/s41572-021-00246-5. [DOI] [PubMed] [Google Scholar]
- 5.Miralles-Guri C, Bruni L, Cubilla AL, Castellsagué X, Bosch FX, de Sanjosé S. Human papillomavirus prevalence and type distribution in penile carcinoma. J Clin Pathol. 2009;62(10):870–8. doi: 10.1136/jcp.2008.063149. [DOI] [PubMed] [Google Scholar]
- 6.Martins VA, Pinho JD, Teixeira Júnior AAL, Nogueira LR, Silva FF, Maulen VE, et al. P16ink4a expression in patients with penile cancer. PLoS One. 2018;13(10):e0205350. doi: 10.1371/journal.pone.0205350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Olesen TB, Sand FL, Rasmussen CL, Albieri V, Toft BG, Norrild B, et al. Prevalence of human papillomavirus DNA and p16(ink4a) in penile cancer and penile intraepithelial neoplasia: A systematic review and meta-analysis. Lancet Oncol. 2019;20(1):145–58. doi: 10.1016/S1470-2045(18)30682-X. [DOI] [PubMed] [Google Scholar]
- 8.Diorio GJ, Giuliano AR. The role of human papilloma virus in penile carcinogenesis and preneoplastic lesions: A potential target for vaccination and treatment strategies. Urol Clin North Am. 2016;43 4:419–25. doi: 10.1016/j.ucl.2016.06.003. [DOI] [PubMed] [Google Scholar]
- 9.Backes DM, Kurman RJ, Pimenta JM, Smith JS. Systematic review of human papillomavirus prevalence in invasive penile cancer. Cancer Causes Control. 2009;20(4):449–57. doi: 10.1007/s10552-008-9276-9. [DOI] [PubMed] [Google Scholar]
- 10.Mannweiler S, Sygulla S, Winter E, Regauer S. Two major pathways of penile carcinogenesis: Hpv-induced penile cancers overexpress p16ink4a, hpv-negative cancers associated with dermatoses express p53, but lack p16ink4a overexpression. J Am Acad Dermatol. 2013;69(1):73–81. doi: 10.1016/j.jaad.2012.12.973. [DOI] [PubMed] [Google Scholar]
- 11.Martins V, Cunha IW, Figliuolo G, Rondon H, de Souza PM, Torres Silva FL, et al. Presence of hpv with overexpression of p16ink4a protein and ebv infection in penile cancer-a series of cases from brazil amazon. PLoS One. 2020;15(5):e0232474. doi: 10.1371/journal.pone.0232474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aromseree S, Pientong C, Swangphon P, Chaiwongkot A, Patarapadungkit N, Kleebkaow P, et al. Possible contributing role of epstein-barr virus (ebv) as a cofactor in human papillomavirus (hpv)-associated cervical carcinogenesis. J Clin Virol. 2015;73:70–6. doi: 10.1016/j.jcv.2015.10.015. [DOI] [PubMed] [Google Scholar]
- 13.Afonso LA, Carestiato FN, Ornellas AA, Ornellas P, Rocha WM, Cordeiro TI, et al. Human papillomavirus, epstein-barr virus, and methylation status of p16(ink4a) in penile cancer. J Med Virol. 2017;89(10):1837–43. doi: 10.1002/jmv.24833. [DOI] [PubMed] [Google Scholar]
- 14.Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, et al. Toxicity and response criteria of the eastern cooperative oncology group. Am J Clin Oncol. 1982;5(6):649–55. [PubMed] [Google Scholar]
- 15.Motaparthi K, Kapil JP, Velazquez EF. Cutaneous squamous cell carcinoma: Review of the eighth edition of the american joint committee on cancer staging guidelines, prognostic factors, and histopathologic variants. Adv Anat Pathol. 2017;24(4):171–94. doi: 10.1097/PAP.0000000000000157. [DOI] [PubMed] [Google Scholar]
- 16.Teixeira Júnior AAL, da Costa Melo SP, Pinho JD, Sobrinho TBM, Rocha TMS, Duarte DRD, et al. A comprehensive analysis of penile cancer in the region with the highest worldwide incidence reveals new insights into the disease. BMC Cancer. 2022;22(1):1063 . doi: 10.1186/s12885-022-10127-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martínez-Bailón C, Mantilla-Morales A, Méndez-Matías G, Alvarado-Cabrero I, Maldonado-Rodríguez R, Quintero-Becerra J, et al. Human papillomavirus genotypes and p16ink4a expression in squamous penile carcinoma in mexican patients. BMC Infect Dis. 2019;19(1):1068 . doi: 10.1186/s12879-019-4696-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Santos RDS, Hirth CG, Pinheiro DP, Bezerra MJB, Silva-Fernandes IJL, Paula DS, et al. Hpv infection and 5mc/5hmc epigenetic markers in penile squamous cell carcinoma: New insights into prognostics. Clin Epigenetics. 2022;14(1):133 . doi: 10.1186/s13148-022-01360-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Senba M, Kumatori A, Fujita S, Jutavijittum P, Yousukh A, Moriuchi T, et al. The prevalence of human papillomavirus genotypes in penile cancers from northern thailand. J Med Virol. 2006;78(10):1341–6. doi: 10.1002/jmv.20703. [DOI] [PubMed] [Google Scholar]
- 20.Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tân PF, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363(1):24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bezerra SM, Chaux A, Ball MW, Faraj SF, Munari E, Gonzalez-Roibon N, et al. Human papillomavirus infection and immunohistochemical p16(ink4a) expression as predictors of outcome in penile squamous cell carcinomas. Hum Pathol. 2015;46(4):532–40. doi: 10.1016/j.humpath.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 22.National comprehensive cancer network. Head and neck cancers (version 1.2024) Available from: https://www.Nccn.Org/professionals/physician_gls/pdf/head-and-neck.Pdf. [DOI] [PMC free article] [PubMed]
- 23.Sirithanaphol W, Sookprasert A, Rompsaithong U, Kiatsopit P, Wirasorn K, Chindaprasirt J. Prognostic factors for penile cancer and survival in response to multimodality therapy. Res Rep Urol. 2020;12:29–34. doi: 10.2147/RRU.S238147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beachler DC, Kreimer AR, Schiffman M, Herrero R, Wacholder S, Rodriguez AC, et al. Multisite hpv16/18 vaccine efficacy against cervical, anal, and oral hpv infection. J Natl Cancer Inst. 2016;108:1. doi: 10.1093/jnci/djv302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lei J, Ploner A, Elfström KM, Wang J, Roth A, Fang F, et al. Hpv vaccination and the risk of invasive cervical cancer. N Engl J Med. 2020;383(14):1340–8. doi: 10.1056/NEJMoa1917338. [DOI] [PubMed] [Google Scholar]
- 26.Chaturvedi AK, Graubard BI, Broutian T, Xiao W, Pickard RKL, Kahle L, et al. Prevalence of oral hpv infection in unvaccinated men and women in the united states, 2009-2016. Jama. 2019;322(10):977–9. doi: 10.1001/jama.2019.10508. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data are available from corresponding author per requested.