Abstract
Preeclampsia (PE) is a common hypertensive disease in women with pregnancy. With the development of bioinformatics, WGCNA was used to explore specific biomarkers to provide therapy targets efficiently. All samples were obtained from gene expression omnibus (GEO), then we used a package named “WGCNA” to construct a scale-free co-expression network and modules related to PE. Next, the search tool for the retrieval of interacting genes database (STRING) was adopted to structure the protein-protein interaction (PPI) of genes in the hub module. Furthermore, the MCODE plug-in was applied to discern hub clusters of the PPI network. We also utilized clusterprofiler to execute the functional analysis. Finally, hub genes were selected via Venn Plot and confirmed by quantitative real-time polymerase chain reaction. Through the co-expression networks and modules, we ensured the turquoise module was the most significant one related to PE. Functional analysis implied these genes were mainly enriched in the organic hydroxy compound metabolic process and Phosphatidylinositol signal system. Due to connectivity, the PPI network showed that GAPDH and VEGFA were the most conspicuous. Lastly, the Venn Plot screened eight hub genes (LDHA, ENG, OCRL, PIK3CB, FLT1, HK2, PKM, and LEP). LDHA was confirmed to be downregulated in PE tissues (P<0.001). This study revealed vital module and hub genes associated with preeclampsia and indicated that LDHA might be a therapeutic target in the future.
Keywords: Preeclampsia, WGCNA, Module, Hub genes
Subject terms: Computational biology and bioinformatics, Cellular signalling networks, Functional clustering, Gene regulatory networks
Introduction
Preeclampsia (PE) is a hypertensive disease that exists in pregnant women, which usually contributes to maternal mortality1. The global incidence of PE is approximately 4.6%, with 76,000 maternal deaths per year, accounting for 16% of global maternal deaths, most of which occur in developing countries2. However, the cause of PE has not been determined yet. Existing evidence indicates that PE might be a genetic disease related to the immune system. in addition, placental hypoxia and oxidative stress are involved in the occurrence of this disease3,4. Therefore, it is important to explore the key regulators and mechanism of PE progression.
Genomics and transcriptomics have developed rapidly since the application of high-throughput sequencing. Weighted gene co-expression network analysis (WGCNA) is an algorithm that illustrates the co-expression relationship and presents highly correlated modules so as to identify ultimately hub genes5. Plenty of complex diseases, especially cancer, need to explore mechanisms to enhance the effectiveness of treatment, provide new therapy targets, or improve the prognosis. WGCNA is now widely used to study tumor-related biomarkers and pathogenesis, such as up-regulated LMNB1 could contribute to predicting the prognosis of prostate cancer, while down-regulation of PCAT18 and LINC01133 may have a role in tumor suppression in the development of gastric cancer6. WGCNA application improved the data analysis capacity and enhanced the efficiency of searching critical factors in exploring tumors, therefore providing potential possibilities for novel treatment strategies and prognosis of cancer.
In this study, we used WGCNA to construct a scale-free co-expression network and PE related modules, and obtained 8 key hub genes (LDHA, ENG, OCRL, PIK3CB, FLT1, HK2, PKM, LEP) related to the PE development. Subsequently, it was found by qPCR that the expression of LDHA mRNA in PE placenta tissue was significantly lower than that in normal placenta tissue, suggesting that LDHA may play a certain role in the pathogenesis of PE. This study might provide a novel insight into PE progression and treatment.
Materials and methods
Data selection
The RNAseq of placentas was downloaded from the GSE75010 dataset (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE75010), including 80 samples from the 80 PE patients and 77 samples from the 77 health puerperae, and 21,045 genes were obtained. The R package “limma” was utilized to compared the differential genes in two tissue groups (|LogFC|>1 & p.adj < 0.05), and 5261 genes (the top-quality quartile of differential genes) were selected for further analysis.
Construction of weighed genes co-expression network
We analyzed the 5261 differential genes using the “WGCNA” R package to build a scale-free co-expression network. First, Pearson’s correlation matrices were constructed for all pairwise genes. Then, a weighted adjacency matrix was established using the power function aij = |cor(xi,xj)|β (cor(xi,xj) = Pearson’s correlation between gene xi and gene xj; aij = adjacency between gene xi and gene x), where β was the soft-thresholding power, which can decrease the weight of weak correlations and increase the weight of strong correlations. After choosing an appropriate β value, we transformed the adjacency matrix into a topological overlap matrix (TOM), which could measure the network degree of a gene. It not only contained information on direct correlations between coterminous genes but also included information on the indirect correlations if genes were nonadjacent; that is a sum-based matrix. Finally, we created average linkage hierarchical clustering due to the TOM-based dissimilarity (1-TOM) measure with a minimum size (gene group) of 50 for the gene dendrogram; therefore, genes with similar expression profiles could be classified into the same module.
Identification of crucial module in PE
Gene significance (GS) represents the association between the individual genes and the interest trait. Module significance (MS) represented modules’ connection to clinical traits, which could be regarded as the weighed mean of GS. Then, the module was selected for the following research according to absolute MS. In addition, through the principal component analysis (PCA), the module eigengene (ME) was calculated for each module.
Gene ontology (GO) and Kyoto encyclopedia of genes and genomes (KEGG) analysis
The function analysis proceeded so that we could understand the molecular mechanisms of these genes selected from the optimal module. GO and KEGG analyses illustrated the pathway that might be involved in PE7–9. The package “clusterProfiler” was utilized to perform the GO and KEGG analysis, and the p.adj < 0.05 was considered as statistical significance.
Protein-protein interaction (PPI) network and sub-network clusters
We applied STRING to acquire interactions between genes in the hub module. Moreover, we utilized Cytoscape software(version 3.9.1) to construct a PPI network for visualization. The Molecular Complex Detection (MCODE) plug-in was employed to discover clustered sub-networks and we set the default parameters: node score cutoff = 0.2, k-core = 2, degree cutoff = 2, and max depth = 100. According to the above results, “Clusterprofiler” was performed to explore the function of hub clusters10.
Hub genes selection
We determined the hub genes through the Venny 2.1 platform (https://bioinfogp.cnb.csic.es/tools/venny/). One part consisted of the top 30 genes in the PPI network according to connectivity, and the other comprised 68 genes in the PE-tightly correlated module (MM > 0.8 and GS > 0.2). Then, the intersection of the two parts was identified as hub genes. This combination of the PPI network and clinical trait-associated module may improve the accuracy and significance of the results.
Tissue samples
27 PE and 27 normal tissues were collected from the Second Affiliated Hospital of Nantong University from 22nd April 2019 to 8th July 2020. All donors signed consent forms, and this research was approved by the Human Ethics Committees of the Nantong First People’s Hospital (2021KT016).
Quantitative real-time polymerase chain reaction (qRT-PCR)
Total RNA was extracted from tissues by Trizol regent (#R0016, Beyotime, China) and then transcribed into cDNA via First Strand cDNA Synthesis Kit (#K1612, ThermoFisher Scientific, USA). Fluorescence quantitative amplification was performed using the BeyoFast SYBR Green qPCR Mix (#D7260, Beyotime) in a StepOne Real-Time PCR System (#4376357, ThermoFisher Scientific). Each sample contained 50 ng cDNA, and PCR reaction conditions were as follow: 95℃ for 30 s; 95℃ for 5 s and then 60℃ for 30 s, 40 cycles; 95℃ for 15 s, 60℃ for 1 min and 95℃ for 15 s .The primers were shown in (Table S1). The β-actin was utilized as the internal reference gene. The relative quantitative value of the target gene was calculated as  .
.
Statistical analysis
All statistical analyses were performed in the R software (version 4.3.1). Statistical analysis was processed by SPSS 16.0. P < 0.05 was considered statistically significant. To decrease the error, all analyses were done three times.
Results
Weighed gene co-expression network and key module
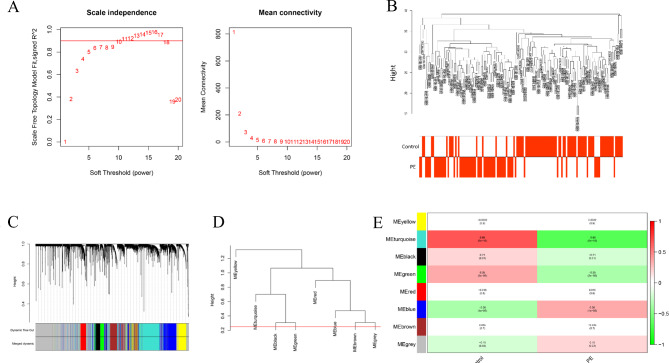
All samples’ data profiles from GEO containing 80 PE patients and 77 normal specimens were used to construct a scale-free network using the “WGCNA” package. First, we set the power β = 10 (scale-free R2 = 0.88) as the soft-thresholding parameter (Fig. 1A) to emphasize the strong correlations. Based on the above, Samples with similar and different clinical traits were drawn to a dendrogram and a heatmap (Fig. 1B), which we can overview. Next, we classified the given genes with similar patterns into a module via average linkage clustering (Fig. 1C). Furthermore, we selected a height cut of 0.25 to merge similar modules (Fig. 1D). As a result, eight modules were identified, including black, blue, brown, green, grey, red, turquoise, and yellow modules, containing 181, 753, 546, 196, 1975, 193, 994, and 423 genes, respectively.
Fig. 1.
Construction of WGCNA. (A) The soft-thresholding parameter. (B) Sample dendrogram and trait heatmap. (C) Cluster dendrogram. (D) Clustering of module eigengenes. (E) Module-trait relationships.
As shown in Fig. 1E, among all modules correlated to trait, the turquoise module was the most relevant to PE (correlation coefficient=−0.65, P = 9e− 18). Moreover, it could be inferred that genes in the turquoise module had a strong negative correlation with PE. Therefore, the turquoise module will be deemed a key module for subsequent research.
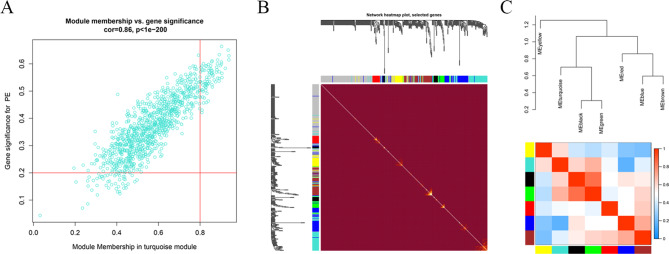
Intriguingly, by intramodular analysis, we know that GS and MM are highly correlated (cor = 0.86, p < 1e-200), and genes highly significantly associated with PE are often the most central elements of the selected module (Fig. 2A). The genes in the turquoise module were also exhibited in the heatmap (Fig. 2B). Similarly, from the module dendrogram, the intermodular relevance was presented in Fig. 2C; either of the dendrogram or heatmap illustrated seven modules could be divided into 3 clusters: one is the yellow module, one is the turquoise, black, and green module, another is left. Modules in the same cluster had a similar expression profile; in other words, they might synergistically affect biological functions.
Fig. 2.
The key module identification. (A) Relationship between module membership in turquoise module and gene significance for PE. The red line represent gene significance (GS) module membership (MM). (B) Network heatmap plot. (C) The intermodular relevance.
Functional enrichment analysis
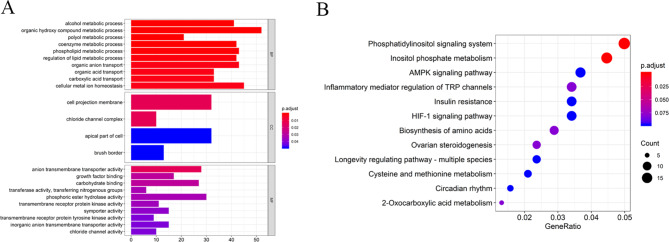
Regarding genes in the turquoise, we conducted a functional enrichment analysis to uncover the biological process and signal pathway that might regulate the PE progression. The GO analysis is shown in Fig. 3A; the GO-biological process (BP) displayed genes mainly enriched in the organic hydroxy compound metabolic process, and the GO-cellular component (CC) implied cell projection membrane was the most meaningful; for GO-cellular component (MF), these genes remarkably enriched in anion transmembrane transporter activity. The KEGG enrichment analysis revealed these genes were primarily enriched in the phosphatidylinositol signal system and inositol phosphate metabolism (Fig. 3B).
Fig. 3.
Functional enrichment analysis of the genes in turquoise module. (A) GO analysis. (B) KEGG analysis. BP biological process, CC cellular component, MF molecular function.
PPI network and analysis of hub clusters
A PPI network synthesized genes’ interaction in a turquoise module was constructed using the STRING (Fig. 4A); the network consisted of 937 nodes and 3046 edges. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was the most outstanding among all proteins due to its high connectivity (connecting 100 nodes), while vascular endothelial growth factor A (VEGFA), connecting 84 nodes, came second (Fig. 4B).
Fig. 4.
PPI network of the genes in turquoise module. (A) PPI network. (B) Key proteins in the PPI.
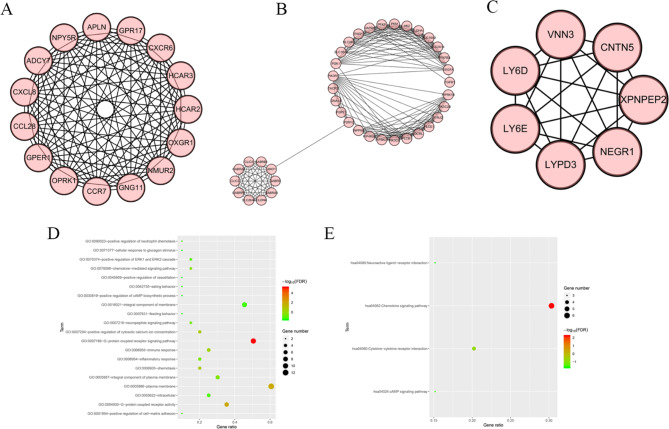
To further detect the hub clustering sub-network, we applied the MCODE plug-in for scoring to select the eligible results by setting some default parameters. Consequently, 26 clusters were appeared as the k-core = 2. Cluster 1 acquired the highest score (score = 15) with 15 nodes and 105 edges (Fig. 5A). Following is the cluster 2 (score = 10.105, node = 39, edge = 192) (Fig. 5B). The third is cluster 3, which got seven scores and owned 7 nodes and 21 edges (Fig. 5C). To summarize, the genes in these three significant clusters might be more relative to PE. We also used cluster profiler to carry out the function analysis of cluster 1. GO analysis demonstrated the genes in cluster 1 were mainly enriched in the G-protein coupled receptor signaling pathway, G-protein coupled receptor activity, and plasma membrane (Fig. 5D). KEGG analysis verified the enrichment of these genes in the chemokine signaling pathway (Fig. 5E).
Fig. 5.
Hub clusters in PPI network. (A) Cluster (1) (B) Cluster (2) (C) Cluster (3) (D) GO analysis of genes in the Cluster 1. (E) KEGG analysis of genes in the Cluster 1.
Hub genes selection
To screen out the hub genes, we combined the PPI network and turquoise module to take advantage of these closely related data and obtain more optimal results. As a result, the top 30 genes in the PPI network complex and 68 genes in the turquoise module were identified for painting the Venn Plot (Fig. 6A); it was apparent that the intersection of two parts was the hub genes, which contained eight genes. The hub genes were LDHA, ENG, OCRL, PIK3CB, FLT1, HK2, PKM, and LEP specifically. Subsequently, the mRNA expressions of these eight hub genes were detected in 27 PE tissues and 27 normal tissues. The results confirmed that only LDHA was downregulated in PE tissues, while ENG, PIK3CB, FLT1, PKM, and LEP were upregulated in PE tissues (P<0.001, Fig. 6B–I).
Fig. 6.
Hub genes identified in PE. (A) The hub genes shared by PPI network and turquoise module. The mRNA expressions of LDHA (B), ENG (C), OCRL (D), PIK3CB (E), FLT1 (F), HK2 (G), PKM (H) and LEP (I) in PR tissues and normal tissues.
Discussion
Preeclampsia is an important cause of morbidity and mortality in the gestation period11. The mechanisms for the occurrence and progression of PE are complex and undetermined. In recent studies, a variety of molecules have been verified to be associated with PE, but this is far from enough. WGCNA was applied widely to identify biomarkers in various diseases. Previous studies that have performed WGCNA on PE focused on the peripheral blood gene expression or small sample size in placenta tissues12,13, but the gene levels in placenta tissues might be different from these in peripheral blood, and small sample size might induce systemic error. Hence, we employed this efficient method to identify PE-related biomolecules in placenta tissues to reveal more comprehensive mechanisms of PE and offer potential therapy targets. In our study, a total of 5261 differential genes between normal placentas and PE tissues were selected for WGCNA method. WGCNA identified 8 modules in which genes had similar expression patterns, and the turquoise module had the most significance, with genes showing an inhibition function on PE progression.
GO analysis showed genes were primarily enriched in the organic hydroxy compound metabolic process. KEGG analysis showed that these genes were enriched mainly in the phosphatidylinositol signal system. Negre-Salvayre et al. elaborated that lipid peroxidation (LPO) product accumulation, like 4-hydroxynonenal (HNE), in fetal vascular results in PE via targeting tissue and cellular dysfunction14. Xu et al. testified overexpression of lncRNA-H19 accelerated the pathogenesis of PE by activating the PI3K/AKT/mTOR pathways15. In addition, accumulating evidence verified the Phosphatidylinositol signal pathway facilitated the pathogenesis and pathophysiology of PE or other vascular diseases and tumors16–18.
Based on the turquoise module, we constructed a PPI network to present the complex interaction preliminarily. Next, we obtained hub clusters related to PE according to k-core = 2. Above all, we selected some crucial genes in the PPI network based on its degree of connectivity. GAPDH and VEGFA were the two most remarkable genes. GAPDH is a housekeeping gene that participates in multiple biological processes. Recent studies demonstrated that GAPDH was associated with cell death via causing mitochondrial dysfunction19, and elevated GAPDH was significantly related to cancer proliferation and invasion20, while GAPDH suppression resulted in a decrease in cell proliferation and metastasis in colon cancer21. However, the relationship between GAPDH and PE hasn’t been researched. Concerning this pity, the mechanisms of GAPDH in PE deserve to be studied in order to elucidate them in the future. Biwei et al. manifested that VEGFA regulated by TLR9 participated in the PE development as the TLR9 could suppress angiogenesis22. Similarly, Zhixiong et al. illuminated that microRNA-199a-5p could give rise to PE occurrence via targeting VEGFA23. Thus, VEGFA could be a potential therapeutic target.
Finally, we used Venn Plot to combine the top 30 genes in the PPI network with the 68 genes in the turquoise module to ensure the hub genes. The results showed eight genes (LDHA, ENG, OCRL, PIK3CB, FLT1, HK2, PKM, and LEP) were identified as hub genes for further study. To further identify the critical genes in PE progression, we detected the mRNA of those eight genes in 27 PE tissues and 27 normal tissues. Compared with normal tissues, lactate dehydrogenase A (LDHA) was the only hub gene downregulated in PE tissues.
LDHA participates in glycolysis metabolism, and its primary function is to convert pyruvate to lactic acid, which significantly enhances cell invasion and the triggers of immune escape24. More and more research has demonstrated that LDHA is overexpressed in multiple tumors, affecting the biological activities of cancer cells25–27. Shi et al. confirmed that the knockdown of LDHA decreased migration to 57.4% in hepatoma cells28. Fumartenase-deficient cells with strong invasion potential overexpressed LDHA and inhibition of LDHA activity could inhibit cell mobility29. Considering the heterogeneity of tumors, the biological effects of LDHA in different tissues and diseases need to be further evaluated. Similarly to our research, Shouling et al. suggested that LDHA might be a novel target for PE treatment and contribute to PE progression30. Uterine spiral artery remodeling disorder (USARD) is currently recognized as the primary PE pathogenesis, and the weakened invasion of trophoblast cells is the leading cause of USARD31–33. If decreased LDHA could impair the migration and invasion of trophoblast cells, just like in the previous studies, it would lead to PE development through inducing USARD. Still, more studies are necessary to confirm this hypothesis finding.
There were some limitations in this study. First, our data analysis was based on the RNAseq of 80 PE tissues and 77 normal tissues at a single center, and more samples from multiple centers were needed to verify our results. Second, we found that LDHA was the key differential gene and downregulated in PE tissues, but the potential role of LDHA in PE progression needs to be further confirmed in vivo and in vitro.
In summary, we elaborated on PE-related modules and hub genes using WGCNA and other bioinformatics methods. LDHA might a vital role in PE development. Our study promotes the understanding of the molecular process of PE and provides a possible therapeutic target. However, more trials should be conducted to ensure the exact mechanisms.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Author contributions
Conceptualization, J.L. and W.T.; bioinformatic analysis, writing—review and editing, J.L. and L.J.; data curation, H.K., Y.Z. and J.C.; Supervision, Project administration, Funding acquisition, W.T. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Jiangsu Provincial Health Commission Maternal and Child Health Research Project (Grant No: F202110), Clinical Medicine Project of Nantong University Scientific Research Fund (Grant No: 2022JY006), Research Fund Project of Nantong Health Commission (Grant No: MS2023040), Research on population development in Nantong City (Grant No: 20231114).
Data availability
The data in this study are available from the corresponding author.
Declarations
Competing interests
The authors declare no competing interests.
Ethical approval
This research was approved by the Human Ethics Committees of the Nantong First People’s Hospital (2021KT016) and performed in accordance with the Declaration of Helsinki. The informed consent was obtained from all participants and/or their legal guardians.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-025-85599-7.
References
- 1.Irminger-Finger, I., Jastrow, N., Irion, O. & Preeclampsia A danger growing in disguise. Int. J. Biochem. Cell Biol.40, 1979–1983 (2008). [DOI] [PubMed] [Google Scholar]
- 2.Abalos, E., Cuesta, C., Grosso, A. L., Chou, D. & Say, L. Global and regional estimates of preeclampsia and eclampsia: a systematic review. Eur. J. Obstet. Gynecol. Reprod. Biol.170, 1–7 (2013). [DOI] [PubMed] [Google Scholar]
- 3.Lu, H. Q. & Hu, R. The role of immunity in the pathogenesis and development of pre-eclampsia. Scand. J. Immunol.90, e12756 (2019). [DOI] [PubMed] [Google Scholar]
- 4.Miller, E. Preeclampsia and cerebrovascular disease. Hypertension74, 5–13 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peter & LangfelderSteve Horvath. WGCNA: an R package for weighted correlation network analysis. BMC Bioinform.9, 559 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Foroughi, K. et al. Tissue-specific down-regulation of the long non-coding RNAs PCAT18 and LINC01133 in gastric cancer development. Int. J. Mol. Sci.19, 3881 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kanehisa, M. & Goto, S. KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res.28, 27–30 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kanehisa, M. Toward understanding the origin and evolution of cellular organisms. Protein Sci.28, 1947–1951 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kanehisa, M., Furumichi, M., Sato, Y., Kawashima, M. & Ishiguro-Watanabe, M. KEGG for taxonomy-based analysis of pathways and genomes. Nucleic Acids Res.51, D587–D92 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu, G., Wang, L. G., Han, Y. & He, Q. Y. clusterProfiler: an R package for comparing biological themes among gene clusters. Omic16, 284–287 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Espinoza, J., Vidaeff, A., Pettker, C. M. & Simhan, H. Gestational hypertension and preeclampsia. MCN Am. J. Maternal Child. Nurs.44, 170 (2019). [DOI] [PubMed] [Google Scholar]
- 12.Li, Q. et al. Development and validation of preeclampsia predictive models using key genes from bioinformatics and machine learning approaches. Front. Immunol.15, 1416297 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin, J., Meng, Y., Song, M. F. & Gu, W. Network-based analysis reveals novel biomarkers in peripheral blood of patients with preeclampsia. Front. Mol. Biosci.9, 757203 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Negre-Salvayre, A. et al. Pathological aspects of lipid peroxidation. Free Radic. Res.44, 1125 (2010). [DOI] [PubMed] [Google Scholar]
- 15.Xu, J. et al. Overexpression of long non-coding RNA H19 promotes invasion and autophagy via the PI3K/AKT/mTOR pathways in trophoblast cells. Biomed. Pharmacother. Biomed. Pharmacother.101, 691–697 (2018). [DOI] [PubMed] [Google Scholar]
- 16.Chaudhary, P., Babu, G. S., Sobti, R. C. & Gupta, S. K. HGF regulate HTR-8/SVneo trophoblastic cells migration/invasion under hypoxic conditions through increased HIF-1α expression via MAPK and PI3K pathways. J. Cell. Commun. Signal.13, 503–521 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marialuisa, P., Giuseppe, L. & Daniela, C. The multifaceted roles of PI3Kγ in hypertension, vascular biology, and inflammation. Int. J. Mol. Sci.17, 1858 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thorpe, L. M., Yuzugullu, H. & Zhao, J. J. PI3K in cancer: divergent roles of isoforms, modes of activation and therapeutic targeting. Nat. Rev. Cancer15, 7–24 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakajima, H. et al. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) aggregation causes mitochondrial dysfunction during oxidative stress-induced cell death. J. Biol. Chem.292, 4727–4742 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hao, L. et al. Elevated GAPDH expression is associated with the proliferation and invasion of lung and esophageal squamous cell carcinomas %J proteomics. Proteomics15, 3087–3100 (2015). [DOI] [PubMed] [Google Scholar]
- 21.Kaiyan et al. Glyceraldehyde-3-phosphate dehydrogenase promotes cancer growth and metastasis through upregulation of SNAIL expression. Int. J. Oncol.50, 252–262 (2017). [DOI] [PubMed] [Google Scholar]
- 22.He, B. et al. TLR9 (toll-Like receptor 9) agonist suppresses angiogenesis by differentially regulating VEGFA (vascular endothelial growth factor A) and sFLT1 (soluble vascular endothelial growth factor receptor 1) in preeclampsia. Hypertension71, 671–680 (2018). [DOI] [PubMed] [Google Scholar]
- 23.Mei, Z., Huang, B., Zhang, Y., Qian, X. & Mo, Y. Histone deacetylase 6 negatively regulated microRNA-199a-5p induces the occurrence of preeclampsia by targeting VEGFA in vitro. Biomed. Pharmacother. 114, 108805 (2019). [DOI] [PubMed] [Google Scholar]
- 24.Seth, P. et al. Deletion of lactate dehydrogenase—A in myeloid cells triggers antitumor immunity. Cancer Res.77, 3632–3643 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cai, H., Li, J., Zhang, Y., Liao, Y. & Hou, J. LDHA promotes oral squamous cell carcinoma progression through facilitating glycolysis and epithelial–mesenchymal transition. Front. Oncol.9, 1446 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang, Y., Lin, S., Yan, C., Fei, Y. & Liu, S. LDH-apromotes epithelial-mesenchymal transition by upregulating ZEB2 in intestinal-type gastric cancer. Oncotarg. Ther.11, 2363–2373 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hou, X. M., Yuan, S. Q., Zhao, D., Liu, X. J. & Wu, X. A. LDH-A promotes malignant behavior via activation of epithelial-to-mesenchymal transition in lung adenocarcinoma. Biosci. Rep. (2018). [DOI] [PMC free article] [PubMed]
- 28.Sheng, S. L. et al. Knockdown of lactate dehydrogenase a suppresses tumor growth and metastasis of human hepatocellular carcinoma. FEBS J.279, 3898–3910 (2012). [DOI] [PubMed] [Google Scholar]
- 29.Xie, H. et al. LDH-A inhibition, a therapeutic strategy for treatment of hereditary leiomyomatosis and renal cell cancer. Mol. Cancer Ther.8, 626–635 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Luo, S., Cao, N., Tang, Y. & Gu, W. Identification of key microRNAs and genes in preeclampsia by bioinformatics analysis. PLoS One12, e0178549 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen, S. N. et al. Maternal pregnancy-induced hypertension increases the subsequent risk of neonatal candidiasis: a nationwide population-based cohort study. Taiwan. J. Obstet. Gynecol.58, 261–265 (2019). [DOI] [PubMed] [Google Scholar]
- 32.Fs, A., Fb, B., Fb, A. & Ma, C. Association between quality and quantity of dietary carbohydrate and pregnancy-induced hypertension: a case–control study-ScienceDirect. Clin. Nutr. ESPEN33, 158–163 (2019). [DOI] [PubMed] [Google Scholar]
- 33.Geusens, N. et al. Changes in endovascular trophoblast invasion and spiral artery remodelling at term in a transgenic preeclamptic rat model. Placenta31, 320–326 (2010). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data in this study are available from the corresponding author.