Abstract
Neurotrophic tyrosine kinase receptor (NTRK)-rearranged uterine sarcoma is a rare type of uterine sarcoma. This paper presents a case of a 49-year-old female who was admitted to the hospital due to lower abdominal pain and subsequently diagnosed with tropomyosin 3 (TPM3)::NTRK1-rearranged uterine sarcoma. To our knowledge, TPM3::NTRK1-rearranged sarcomas almost always occur in the cervix, and this is a novel case of uterine corpus occurrence. The patient received chemotherapy and radiotherapy after surgery. No recurrence or metastasis was observed after 9 months of treatment. Moreover, all reported clinicopathological features, treatment methods, and prognoses of NTRK-rearranged uterine sarcoma patients are reviewed herein.
Keywords: NTRK rearrangement, uterine tumors, pan-tyrosine kinase (TRK)
Introduction
NTRK-rearranged sarcoma is a rare type of sarcoma that originates mainly from the cervix and rarely occurs in the uterine corpus. This malignancy predominantly affects females of reproductive age, but it can be found in children and elderly women (13-72 years, with an average age of 38 years). Chiang [1] reported this type of sarcoma for the first time in 2011. In the fifth edition of the World Health Organization (WHO) Classification of Tumors of the Female Reproductive System in 2020, NTRK-rearranged uterine sarcoma was classified under fibroblastic and myofibroblastic tumors of mesenchymal origin in the female reproductive system, without an International Classification of Diseases for Oncology (ICD-O) code [2]. The pathological features and prognosis of NTRK-rearranged uterine sarcoma vary significantly from case to case, making diagnosis difficult.
This paper reports a case of NTRK-rearranged sarcoma that occurred in the uterine corpus. Herein, the morphological, immunohistochemical, and molecular features are discussed; a literature review of the reported cases of NTRK-rearranged uterine tumors is described; and the clinicopathological features, treatment methods, and prognosis of this disease are summarized.
Case report
A 49-year-old postmenopausal patient was admitted to a local hospital due to lower abdominal pain and space occupation in the uterine cavity for 2+ months. Gynecological ultrasound revealed that the echo of the uterine myometrium was not uniform, and a hypoechoic area of approximately 3.1*1.0 cm located from the lower segment of the uterus to the cervix appeared to be closely related to the posterior myometrium. A possible submucosal fibroid originating from the endometrium was considered. The patient subsequently underwent intrauterine curettage at a local hospital. During the operation, a smooth, sheet-like tumor 3*2*0.5 cm in size was observed in the lower segment of the uterus, with an indistinct boundary with the posterior uterine wall. The local hospital considered this to be an endometrial stromal tumor, so the patient was sent to our hospital for consultation. Microscopic examination revealed that the tumor tissue was composed of diffuse spindle cells, with mild atypia, rare mitotic figures, staghorn blood vessels, and no necrosis. The tumor cells exhibit strongly and diffusely positive for pan-TRK and diffusely positive for CD34 and S-100. According to the morphological features and immunohistochemistry (IHC) results, the tumor was preliminarily diagnosed as a spindle cell sarcoma with NTRK translocation. The patient was admitted to our hospital for further surgical treatment.
Preoperative computed tomography (CT) revealed that the uterus was enlarged, with an intrauterine mass that was not clearly demarcated from the myometrium; the parametrial space was blurred, and the right part of the bladder trigone of the cervix was partially blurred (Figure 1). The patient underwent multi-incision laparoscopic subtotal hysterectomy, bilateral salpingo-oophorectomy, and dissection of the lymph nodes adjacent to the right external iliac artery. Gross observation revealed a mass measuring 2.5*1.5*1.5 cm on the lower segment of the myometrium of the uterus, protruding into the uterine cavity; the cut surface of the mass was pale yellow, solid, fine, and homogeneous, with an indistinct boundary with the myometrium, invading less than half the thickness of the myometrium (Figure 2). No obvious mass was found in the adnexa on either side.
Figure 1.

CT image showing a mass occupying the uterine cavity with an unclear boundary between the mass and the myometrium.
Figure 2.

Gross image of a NTRK1-rearranged uterine sarcoma (the red box indicates the location of the tumor).
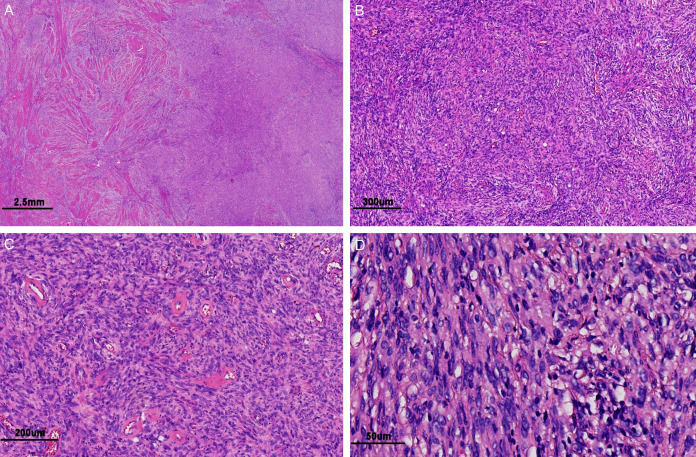
Microscopically, the tumor showed infiltrative growth, invaded the full myometrium of the uterus, and involved the cervical mucosa and stroma as well as the right parametrium (Figure 3A). The tumor was composed mainly of densely arranged spindle cells with abundant collagen in the stroma, and scattered, infiltrating inflammatory cells could be observed in some areas; however, there was no necrosis (Figure 3B). Many thick-walled vessels with hyalinization were found in the tumor stroma (Figure 3C). Under high magnification, the cytoplasm of the tumor cells was eosinophilic, mostly showing mild nuclear atypia, with moderate nuclear atypia in some areas; mitotic figures were rare, seen in approximately 1-2/10 high-power fields (HPFs) (Figure 3D). No obvious intravascular infiltration was found, the tumor did not involve the right external iliac lymph node tissue, and the incision margins of both the lateral pelvic walls, the vaginal cuff, and the left and right adnexa.
Figure 3.
Microscopic appearance of NTRK1-rearranged uterine sarcoma. A: The tumor tissue infiltrated the myometrium (HE magnification of 10×). B: The tumor was composed mainly of relatively uniform spindle cells, and abundant collagen was visible in the stroma (HE magnification of 60×). C: Numerous thick-walled vessels with hyalinization were observed in the tumor stroma (HE magnification of 100×). D: The tumor cells showed mild to moderate nuclear atypia (HE magnification was 400×).
IHC was performed using the EnVision method. IHC staining revealed that the tumor cells diffusely expressed pan-TRK (Figure 4A), CD34 (Figure 4B), S-100 (Figure 4C) and cyclin D1; the focal area expressed CD10 (Figure 4D), DOG, and SMA; there was no expression of Desmin (Figure 4E), ALK (Figure 4F), SOX-10 (Figure 4G), CD117, pan-CK, ER, PR, HMB45, or STAT6; p53 showed a wild-type expression pattern; and the Ki67 proliferation index was approximately 5% (Figure 4H). Fluorescence in situ hybridization (FISH) revealed that the NTRK1 gene separation probe exhibited red and green separation signals in more than 15% of the tumor cells (Figure 5A), whereas separation signals were not detected with the NTRK2 or NTRK3 separation probes. Next-generation sequencing (NGS) revealed that the tumor was of the TPM3::NTRK1 fusion subtype, with the 5’-end breakpoint located at chr.1:154142876 and the 3’-end breakpoint located at chr.1:156844363. The fusion mode of the two genes is shown in Figure 5B.
Figure 4.
IHC performed using the EnVision method revealed features of NTRK1-rearranged uterine sarcoma. A: Diffusely and strongly positive staining for pan-TRK (magnification of 100×). B: Diffusely and strongly positive staining for CD34 (magnification of 100×). C: Positive for S-100 (magnification of 100×). D: Focally positive staining for CD10 (magnification of 100×). E: The tumor cells were negative for Desmin, and the normal myometrium was stained positively (magnification of 40×). F: Negative for ALK (magnification of 100×). G: Negative for SOX-10 (magnification of 40×). H: The percentage of Ki67-positive cells was approximately 5% (magnification of 40×).
Figure 5.
Molecular characteristics of NTRK1-rearranged uterine sarcoma. A: NTRK1 FISH detection revealed separation of red and green signals, confirming NTRK1 gene translocation (magnification of 1000×). B: The TPM3::NTRK1 fusion gene was detected via RNA sequencing, with the breakpoint marked in red.
In summary, the patient was diagnosed with TPM3::NTRK1-rearranged uterine sarcoma. The tumor was staged as IIB according to the 2018 International Federation of Gynecology and Obstetrics (FIGO) staging system for uterine tumors.
The patient refused the use of NTRK1 inhibitors. The patient started chemotherapy with ifosfamide (IFO) + epirubicin 1 month after surgery and radiation therapy 2 months after surgery. The patient underwent 5 cycles of chemotherapy and 28 cycles of external beam radiation therapy in the 9 months after surgery. No metastasis or recurrence was detected via imaging.
Discussion
NTRK-rearranged uterine tumors are rare and were first reported in 2011. To date, approximately 60 cases have been reported in the literature. Table 1 shows the clinical characteristics of the reported patients in the literature and the patients included in this study. This malignancy predominantly affects females of reproductive age, but it can be found in children and elderly women (13-72 years, with an average age of 38 years). The majority of NTRK-rearranged uterine tumors are located primarily in the cervix (53/61 patients) and rarely in the uterine corpus (8/61 patients). At the initial diagnosis, tumors are confined mostly to the primary site and rarely metastasize to distant sites. Most patients have vaginal bleeding as the first symptom, while some patients have no first symptoms but are diagnosed through examination.
Table 1.
The reported cases of NTRK-rearranged uterine sarcomas
| Case | Author | Age | Site | Stage | pan-TRK | S-100 | CD34 | NTRK fusion | Follow up |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Chiang [1] | 46 | Cervix | IB | +++ | + | - | TPM3::NTRK1 | AWD |
| 2 | 27 | Corpus | IB | + | + | - | LMNA::NTRK1 | NED | |
| 3 | 47 | Cervix | IB | ++ | + | - | RBPMS::NTRK3 | DOD | |
| 4 | 42 | Cervix | IB | + | + | - | TPR::NTRK1 | NED | |
| 5 | Goulding [9] | 13 | Cervix | IB | +++ | +++ | + | TPM3::NTRK1 | NED |
| 6 | Boyle [10] | 42 | Cervix | IB | +++ | - | + | TPM3::NTRK1 | NED |
| 7 | Croce [11] | 39 | Cervix | NA | +++ | +++ | +++ | TPM3::NTRK1 | NA |
| 8 | 44 | Cervix | IA | +++ | +++ | +++ | TPM3::NTRK1 | NED | |
| 9 | 26 | Cervix | IB | +++ | +++ | +++ | EML4::NTRK3 | AWD | |
| 10 | 23 | Cervix | IA | +++ | +++ | +++ | TPM3::NTRK1 | NED | |
| 11 | 30 | Cervix | IA | +++ | +++ | + | TPM3::NTRK1 | NED | |
| 12 | 60 | Cervix | IIIB | +++ | NA | +++ | TPM3::NTRK1 | DOD | |
| 13 | 33 | Cervix | IA | +++ | +++ | +++ | TPM3::NTRK1 | NED | |
| 14 | 23 | Cervix | IIA | +++ | + | +++ | TPM3::NTRK1 | AWD | |
| 15 | Devereaux [12] | 24 | Cervix | IB | +++ | + | ++ | SPECC1L::NTRK3 | NED |
| 16 | 39 | Cervix | IB | - | +++ | +++ | TPM3::NTRK1 | NED | |
| 17 | 66 | Cervix | IA | +++ | +++ | +++ | TPM3::NTRK1 | NED | |
| 18 | 40 | Cervix | IA | + | + | +++ | TPR::NTRK1 | NED | |
| 19 | 37 | Cervix | ≥IB | +++ | +++ | +++ | IRF2BP2::NTRK1 | LTFU | |
| 20 | 35 | Corpus | IB | + | +++ | +++ | C16orf72::NTRK1 | NED | |
| 21 | Rabban [13] | 49 | Cervix | NA | ++ | POS | - | TPR::NTRK1 | NED |
| 22 | Hodgson [14] | 50 | Cervix | IA | NA | +++ | +++ | SPECC1L::NTRK3 | NED |
| 23 | Wong [15] | 31 | Cervix | NA | POS | + | +++ | NTRK3 | LTFU |
| 24 | Michal [16] | 26 | Uterine | ≥IB | +++ | +++ | +++ | STRN::NTRK3 | NED |
| 25 | Wells [17] | 30 | Cervix | IB | NA | POS | POS | TPM3::NTRK1 | NED |
| 26 | Gatalica [18] | NA | Cervix | NA | POS | NA | NA | TPM3::NTRK1 | NA |
| 27 | NA | Uterine | NA | POS | NA | NA | SPECC1L::NTRK3 | NA | |
| 28 | Nilforoushan [19] | 54 | Cervix | IA | NA | POS | POS | SPECC1L::NTRK3 | NED |
| 29 | 52 | Cervix | IB | NA | +++ | +++ | TPM3::NTRK1 | NED | |
| 30 | Moh [20] | 69 | Corpus | IB | WWOX::NTRK2 | NED | |||
| 31 | Tsai [21] | 47 | Cervix | NA | POS | POS | POS | TPM3::NTRK1 | AWD |
| 32 | 53 | Cervix | NA | POS | POS | POS | TPM3::NTRK1 | AWD | |
| 33 | Costigan [4] | 35 | Corpus | IB | NA | NA | NA | C16orf72::NTRK1 | LTFU |
| 34 | 35 | Cervix | IA | +++ | + | POS | TPM3::NTRK1 | LTFU | |
| 35 | 47 | Cervix | IB | +++ | NA | NA | TPR::NTRK1 | DOD | |
| 36 | 30 | Cervix | IIB | ++ | - | NA | TPR::NTRK1 | NED | |
| 37 | 39 | Cervix | NA | NA | POS | POS | TPM3::NTRK1 | LTFU | |
| 38 | 16 | Cervix | IA | +++ | ++ | ++ | TPR::NTRK1 | NED | |
| 39 | 26 | Cervix | IIB | + | ++ | ++ | EML4::NTRK3 | NED | |
| 40 | 26 | Cervix | ≥IB | ++ | - | - | TFG::NTRK3 | DOD | |
| 41 | 26 | Cervix | NA | + | ++ | - | Failed QC | DOD | |
| 42 | 61 | Cervix | IB | ++ | +++ | ++ | SPECC1L:NTRK3 | NED | |
| 43 | 24 | Cervix | IA | +++ | + | +++ | TPM3::NTRK1 | NED | |
| 44 | 42 | Cervix | NA | ++ | +++ | - | Insufficient tissue | LTFU | |
| 45 | 42 | Cervix | IB | ++ | +++ | - | TPR::NTRK1 | NED | |
| 46 | 46 | Cervix | IB | +++ | ++ | NA | IRF2BP2::NTRK1 | LTFU | |
| 47 | 26 | Cervix | NA | +++ | ++ | NA | TPM3::NTRK1 | LTFU | |
| 48 | Moura [5] | NA | Cervix | Subcutaneous metastasis | +++ | NA | NA | LMNA::NTRK1 | NA |
| 49 | NA | Corpus | NA | +++ | NA | NA | EML4::NTRK3 | NA | |
| 50 | NA | Cervix | NA | +++ | NA | NA | SPECCL1::NTRK3 | NA | |
| 51 | NA | Cervix | NA | +++ | NA | NA | TPM3::NTRK1 | NA | |
| 52 | NA | Cervix | NA | - | NA | NA | SPECCL1::NTRK3 | NA | |
| 53 | Dang [22] | 33 | Cervix | IIA | +++ | +++ | +++ | EML4::NTRK3 | DOD |
| 54 | Munkhdelger [23] | 72 | Cervix | Lung metastasis | NA | - | NA | DLG2::NTRK2 | AWD |
| 55 | Huang [24] | NA | Cervix | NA | POS | POS | POS | TPR::NTRK1 | NA |
| 56 | NA | Cervix | NA | POS | POS | POS | TPR::NTRK1 | NA | |
| 57 | NA | Cervix | NA | POS | POS | POS | TPM3::NTRK1 | NA | |
| 58 | NA | Cervix | NA | POS | POS | POS | TRIM67::NTRK1 | NA | |
| 59 | NA | Cervix | NA | POS | POS | POS | NTRK1 | NA | |
| 60 | NA | Cervix | NA | POS | POS | POS | NTRK1 | NA | |
| 61 | NA | Cervix | NA | POS | POS | POS | NTRK1 | NA | |
| 62 | The present study | 49 | Corpus | IIB | +++ | + | +++ | TPM3::NTRK1 | NED |
AWD = alive with disease; DOD = died of disease; HPF = high power fields; POS = positive; LTFU = lost to follow up; NED = no evidence with disease; NA = not applicable; NP = not performed; LMNA = Lamin A/C; RBPMS = RNA Binding Protein, MRNA Processing Factor; EML4 = EMAP Like 4; IRF2BP2 = Interferon Regulatory Factor 2 Binding Protein 2; C16orf72 = Previous HGNC Symbols for HAPSTR1 Gene; STRN = Striatin; WWOX = WW Domain Containing Oxidoreductase; TFG = Trafficking From ER To Golgi Regulator; DLG2 = Discs Large MAGUK Scaffold Protein 2; TRIM67 = Tripartite Motif Containing 67.
NTRK-rearranged uterine sarcomas exhibit significant morphological variations [3]. Most tumors show infiltrative growth patterns, and a few tumors demonstrate a pushing growth pattern. Microscopic observation revealed that these tumors have features of fibrosarcomas and are composed mainly of relatively uniform spindle-shaped cells. Small blood vessels, staghorn blood vessels, and thick-walled blood vessels with perivascular hyalinization can be observed in the tumor stroma. There is considerable variation in tumor cell atypia, with most cells showing mild to moderate nuclear atypia, and the degree of cell atypia can also vary within the same tumor. In these types of tumors, the nuclei are mostly oval or short spindle shaped with clumped chromatin and small nucleoli in the nuclei. The number of mitotic figures varies widely in previously reported cases, from 0-50 per 10 high-power fields of view (with an average of 13/10 HPFs). Some authors [4] have argued that the prognostic significance of tumor cell nuclear atypia is not clear, but a large number of mitotic figures is correlated with a poor prognosis. In some cases, focal mucoid degeneration can be observed in the tumor, and some tumor stroma can have inflammatory cell infiltration.
The IHC staining characteristics of NTRK-rearranged uterine sarcoma are as follows: Pan-TRK is an IHC marker with high sensitivity and specificity and is commonly used for screening NTRK-rearranged tumors. Positive staining is defined as staining in ≥1% of tumor cells (cytoplasm, membrane, nuclear or perinuclear) [5]. Among the reported cases, 86.9% (53/61) were positive for pan-TRK. Among the remaining 8 patients, 6 did not have available data for IHC staining for pan-TRK; therefore, the percentage of patients who were positive for pan-TRK may be greater. Among the 53 positive cases, only 8 had pan-TRK staining that was weakly positive or focally positive, and the staining intensity of the remaining 45 cases was moderately to strongly positive. The pan-TRK antibody binds to the extracellular domain of the NTRK family; it is unable to differentiate between NTRK fusion proteins and wild-type proteins and is unable to identify the specific isoforms of the NTRK family. Hondelink [6] reported that the false-negative rate of pan-TRK IHC staining in NTRK rearranged tumors was approximately 18%, and false negatives were more common during NTRK3 rearrangement. Momeni-Boroujeni [6] reported that the positive rate of pan-TRK can reach 91.4% in high-grade uterine stromal sarcomas, which may be related to the upregulation of NTRK3 mRNA content in such tumors, although the specific mechanism involved remains unclear. Makino [7] reported that the expression of NTRK2 is upregulated in uterine leiomyosarcoma cells and that pan-TRK staining is positive. Positive staining for S-100 (92%, 46/50) and CD34 (81.3%, 39/48) may indicate NTRK-rearranged uterine sarcoma. Therefore, some scholars believe that at microscopic level, these tumors are mainly composed of spindle cells. When IHC results are positive for pan-TRK, S-100, and CD34 but negative for myogenic markers such as desmin and caldesmon, fluorescence in situ hybridization (FISH) or RNA sequencing should be performed to confirm the presence of NTRK rearrangement. Notably, the myogenic marker SMA can be weakly positively expressed in some NTRK-rearranged tumors.
The NTRK-rearranged uterine sarcoma is genetically characterized by NTRK rearrangement. NTRK1 rearrangement is most common (43/61), with TPM3::NTRK1 rearrangement as the main pattern (25/61), followed by translocated promoter region (TPR)::NTRK1 rearrangement (9/61). Among the existing cases of TPM3::NTRK1 rearrangement, all tumors occurred in the cervix. The case in the present study is a novel case in which TPM3::NTRK1 rearrangement was found in the uterine corpus. NTRK3 rearrangement is less common (13/61), and the main fusion gene is sperm antigen with calponin homology and coiled-coil domains 1 like (SPECC1L)::NTRK3. NTRK2 rearrangement is rare and is often accompanied by multiple other genetic changes. Research has shown that NTRK3 rearrangement may be associated with a larger tumor volume and a poorer prognosis [1].
Both NTRK mutation and posttranscriptional processing may affect the occurrence and development of tumors, but NTRK rearrangement is the most important cause of pathogenicity for TRK genes. NTRK fusion proteins often retain the kinase domain at the 3’ end, while the 5’ end is replaced, causing the fusion protein to lose the autoinhibitory part of the kinase, become a ligand-independent kinase, or change its subcellular localization, leading to oncogenic effects [8], making this domain the most common target of TRK inhibitors. TPM3 encodes a member of the actin-binding protein tropomyosin family, and chromosomal rearrangement of this gene and other genes often leads to tumor development. The specific oncogenic mechanism of TPM3::NTRK1 rearrangement in uterine sarcoma remains to be studied.
The differential diagnosis is as follows. (1) Leiomyosarcoma: The tumor cell nuclei are “cigar-like” or “rod-shaped”; cells often have moderate to severe atypia; mitotic figures are readily visible (≥10 cells/10 HPF); tumor necrosis can be observed; IHC myogenic markers are positive; and genetic testing does not reveal NTRK rearrangement. (2) Endometrial stromal sarcoma: The tumor shows infiltrative growth. In low-grade endometrial stromal sarcoma, tumor cells can be short-spindle shaped, with little nuclear atypia and few mitotic figures; in high-grade endometrial stromal sarcoma, significant cellular atypia and frequent mitotic figures are evident. IHC staining reveals CD10 expression, and pan-TRK positivity can be observed in high-grade endometrial stromal sarcoma in the absence of NTRK rearrangement. Definitive diagnosis can be achieved through FISH testing or RNA sequencing. (3) Inflammatory myofibroblastoma: Inflammatory myofibroblastoma with anaplastic lymphoma kinase (ALK) rearrangement occurs mostly in the uterine corpus and has a spindle-shaped cell morphology and infiltration by lymphocytes and plasma cells. The infiltrating inflammatory cells in NTRK-rearranged uterine sarcoma are often lymphocytes and lack plasma cells. ALK rearrangement can be detected in inflammatory myofibroblastoma, which can be confirmed by genetic testing. Inflammatory myofibroblastoma can also be associated with NTRK rearrangement, but some scholars currently believe that tumors with inflammatory myofibroblastoma morphology accompanied by NTRK rearrangement should be classified as NTRK rearrangement sarcoma [5]. (4) Collagen type I alpha 1 chain (COL1A1)::platelet-derived growth factor subunit B (PDGFB)-rearranged uterine sarcoma: Spindle cell tumors have mild to moderate atypia, and focal myxoid areas with inflammatory infiltration consisting of lymphocytes and eosinophils are observed. Tumors of this type are positive for CD34 and CD10 and negative for S-100. RNA sequencing indicates COL1A1::PDGFB translocation.
In the present study, the patient was 49 years old with a tumor that presented a fibrosarcoma-like morphology and expressed pan-TRK, CD34, and S-100 on IHC. FISH revealed NTRK1 rearrangement, which was verified as TPM3::NTRK1 rearrangement by next-generation sequencing (NGS), leading to a precise diagnosis in this case.
The treatment of NTRK-rearranged uterine sarcomas includes surgical resection, chemotherapy, radiotherapy, and targeted therapy with TRK inhibitors. Currently, the commonly used clinical methods are surgical resection and chemotherapy + radiotherapy. Among TRK inhibitors, larotrectinib is currently the most commonly used and has the best therapeutic effect, and it can selectively inhibit TRK receptor kinase. Entrectinib, an orally administered pan-TRK inhibitor, also has inhibitory effects on ROS proto-oncogene 1 (ROS1, a receptor tyrosine kinase) and ALK proteins [8].
Among the 61 patients with NTRK-rearranged uterine sarcoma, there was a disease-free survival rate of 42.6% (26/61), a disease-with survival rate of 13.1% (8/61), and a mortality rate of 11.5% (7/61) within the study period, and follow-up data for the remaining 20 patients were not collected. Costigan [4] proposed that individuals with one of the following features are at high risk: mitotic figures ≥8/10 HPF, lymphovascular invasion, necrosis, and NTRK3 fusion. The patient in the present study received chemotherapy and radiotherapy after surgical resection. During the 9-month follow-up, no metastasis or recurrence was found.
Conclusion
In summary, NTRK-rearranged uterine sarcoma is a rare form of uterine sarcoma with a histological morphology similar to that of various common uterine mesenchymal tumors, making its diagnosis challenging and requiring molecular testing for confirmation.
Disclosure of conflict of interest
None.
References
- 1.Chiang S, Cotzia P, Hyman DM, Drilon A, Tap WD, Zhang L, Hechtman JF, Frosina D, Jungbluth AA, Murali R, Park KJ, Soslow RA, Oliva E, Iafrate AJ, Benayed R, Ladanyi M, Antonescu CR. NTRK fusions define a novel uterine sarcoma subtype with features of fibrosarcoma. Am J Surg Pathol. 2018;42:791–798. doi: 10.1097/PAS.0000000000001055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO Classification of Tumours Editorial board. WHO classification of tumours. Female genital tumours. 5th edition. Lyon: IARC Press; 2020. [Google Scholar]
- 3.Croce S, Hostein I, McCluggage WG. NTRK and other recently described kinase fusion positive uterine sarcomas: a review of a group of rare neoplasms. Genes Chromosomes Cancer. 2021;60:147–159. doi: 10.1002/gcc.22910. [DOI] [PubMed] [Google Scholar]
- 4.Costigan DC, Nucci MR, Dickson BC, Chang MC, Song S, Sholl LM, Hornick JL, Fletcher CDM, Kolin DL. NTRK-rearranged uterine sarcomas: clinicopathologic features of 15 cases, literature review, and risk stratification. Am J Surg Pathol. 2022;46:1415–1429. doi: 10.1097/PAS.0000000000001929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moura MS, Costa J, Velasco V, Kommoss F, Oliva E, Le Loarer F, McCluggage WG, Razack R, Treilleux I, Mills A, Longacre T, Devouassoux-Shisheboran M, Hostein I, Azmani R, Blanchard L, Hartog C, Soubeyran I, Khalifa E, Croce S. Pan-TRK immunohistochemistry in gynaecological mesenchymal tumours: diagnostic implications and pitfalls. Histopathology. 2024;84:451–462. doi: 10.1111/his.15082. [DOI] [PubMed] [Google Scholar]
- 6.Momeni-Boroujeni A, Mohammad N, Wolber R, Yip S, Köbel M, Dickson BC, Hensley ML, Leitao MM Jr, Antonescu CR, Benayed R, Ladanyi M, Lee CH, Chiang S. Targeted RNA expression profiling identifies high-grade endometrial stromal sarcoma as a clinically relevant molecular subtype of uterine sarcoma. Mod Pathol. 2021;34:1008–1016. doi: 10.1038/s41379-020-00705-6. [DOI] [PubMed] [Google Scholar]
- 7.Makino K, Kawamura K, Sato W, Kawamura N, Fujimoto T, Terada Y. Inhibition of uterine sarcoma cell growth through suppression of endogenous tyrosine kinase B signaling. PLoS One. 2012;7:e41049. doi: 10.1371/journal.pone.0041049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cocco E, Scaltriti M, Drilon A. NTRK fusion-positive cancers and TRK inhibitor therapy. Nat Rev Clin Oncol. 2018;15:731–747. doi: 10.1038/s41571-018-0113-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goulding EA, Morreau P, De Silva M, Watson M, van Vliet C, Leung B, Eva LJ. Case report: NTRK1-rearranged cervical sarcoma with fibrosarcoma like morphology presenting in a 13-year-old managed with a neo-adjuvant TRK-inhibitor and surgical excision. Gynecol Oncol Rep. 2021;37:100845. doi: 10.1016/j.gore.2021.100845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boyle W, Williams A, Sundar S, Yap J, Taniere P, Rehal P, Ganesan R. TMP3-NTRK1 rearranged uterine sarcoma: a case report. Case Rep Womens Health. 2020;28:e00246. doi: 10.1016/j.crwh.2020.e00246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Croce S, Hostein I, Longacre TA, Mills AM, Pérot G, Devouassoux-Shisheboran M, Velasco V, Floquet A, Guyon F, Chakiba C, Querleu D, Khalifa E, Mayeur L, Rebier F, Leguellec S, Soubeyran I, McCluggage WG. Uterine and vaginal sarcomas resembling fibrosarcoma: a clinicopathological and molecular analysis of 13 cases showing common NTRK-rearrangements and the description of a COL1A1-PDGFB fusion novel to uterine neoplasms. Mod Pathol. 2019;32:1008–1022. doi: 10.1038/s41379-018-0184-6. [DOI] [PubMed] [Google Scholar]
- 12.Devereaux KA, Weiel JJ, Mills AM, Kunder CA, Longacre TA. Neurofibrosarcoma revisited: an institutional case series of uterine sarcomas harboring kinase-related fusions with report of a novel FGFR1-TACC1 fusion. Am J Surg Pathol. 2021;45:638–652. doi: 10.1097/PAS.0000000000001644. [DOI] [PubMed] [Google Scholar]
- 13.Rabban JT, Devine WP, Sangoi AR, Poder L, Alvarez E, Davis JL, Rudzinski E, Garg K, Bean GR. NTRK fusion cervical sarcoma: a report of three cases, emphasising morphological and immunohistochemical distinction from other uterine sarcomas, including adenosarcoma. Histopathology. 2020;77:100–111. doi: 10.1111/his.14069. [DOI] [PubMed] [Google Scholar]
- 14.Hodgson A, Pun C, Djordjevic B, Turashvili G. NTRK-rearranged cervical sarcoma: expanding the clinicopathologic spectrum. Int J Gynecol Pathol. 2021;40:73–77. doi: 10.1097/PGP.0000000000000669. [DOI] [PubMed] [Google Scholar]
- 15.Wong DD, Vargas AC, Bonar F, Maclean F, Kattampallil J, Stewart C, Sulaiman B, Santos L, Gill AJ. NTRK-rearranged mesenchymal tumours: diagnostic challenges, morphological patterns and proposed testing algorithm. Pathology. 2020;52:401–409. doi: 10.1016/j.pathol.2020.02.004. [DOI] [PubMed] [Google Scholar]
- 16.Michal M, Hájková V, Skálová A, Michal M. STRN-NTRK3-rearranged mesenchymal tumor of the uterus: expanding the morphologic spectrum of tumors with NTRK fusions. Am J Surg Pathol. 2019;43:1152–1154. doi: 10.1097/PAS.0000000000001292. [DOI] [PubMed] [Google Scholar]
- 17.Wells AE, Mallen AM, Bui MM, Reed DR, Apte SM. NTRK-1 fusion in endocervical fibroblastic malignant peripheral nerve sheath tumor marking eligibility for larotrectinib therapy: a case report. Gynecol Oncol Rep. 2019;28:141–144. doi: 10.1016/j.gore.2019.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gatalica Z, Xiu J, Swensen J, Vranic S. Molecular characterization of cancers with NTRK gene fusions. Mod Pathol. 2019;32:147–153. doi: 10.1038/s41379-018-0118-3. [DOI] [PubMed] [Google Scholar]
- 19.Nilforoushan N, Wethington SL, Nonogaki H, Gross J, Vang R, Xing D. NTRK-fusion sarcoma of the uterine cervix: report of 2 cases with comparative clinicopathologic features. Int J Gynecol Pathol. 2022;41:642–648. doi: 10.1097/PGP.0000000000000834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moh M, Johnson CM, Geurts J, Bishop E. Uterine sarcoma with a novel WWOX-NTRK2 fusion in a postmenopausal woman with Li-Fraumeni-Like syndrome: a case that expands the spectrum of NTRK-rearranged uterine tumors. AJSP Rev Rep. 2021;26:304–306. [Google Scholar]
- 21.Tsai JW, Lee JC, Hsieh TH, Huang SC, Lee PH, Liu TT, Kao YC, Chang CD, Weng TF, Li CF, Lin JC, Liang CW, Su YL, Chang IY, Wang YT, Chang NY, Yu SC, Wang JC, Huang HY. Adult NTRK-rearranged spindle cell neoplasms of the viscera: with an emphasis on rare locations and heterologous elements. Mod Pathol. 2022;35:911–921. doi: 10.1038/s41379-021-01005-3. [DOI] [PubMed] [Google Scholar]
- 22.Dang X, Xiang T, Zhao C, Tang H, Cui P. EML4-NTRK3 fusion cervical sarcoma: a case report and literature review. Front Med (Lausanne) 2022;9:832376. doi: 10.3389/fmed.2022.832376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Munkhdelger J, Shimooka T, Koyama Y, Ikeda S, Mikami Y, Fukuoka J, Hori T, Bychkov A. Basaloid squamous cell carcinoma of the uterine cervix: report of a case with molecular analysis. Int J Surg Pathol. 2021;29:770–774. doi: 10.1177/1066896921997132. [DOI] [PubMed] [Google Scholar]
- 24.Huang HJ, Wang C, Fan DG, He YH, Chen X, Zheng SL. NTRK-rearranged uterine sarcoma: a clinicopathological analysis of seven cases. Zhonghua Bing Li Xue Za Zhi. 2024;53:189–191. doi: 10.3760/cma.j.cn112151-20230728-00036. [DOI] [PubMed] [Google Scholar]