Abstract
Brainstem hemorrhage is a severe neurological condition with high mortality and poor prognosis. This study aims to develop and validate a prognostic model for brainstem hemorrhage to facilitate early prediction of patient outcomes, thereby supporting clinical decision-making. Clinical data from 140 patients with brainstem hemorrhage were collected. A prognostic model was constructed through multivariate logistic regression analysis, and a nomogram was developed for clinical use. The model’s performance was evaluated using ROC curves, PR curves, and calibration curves, and was validated through cross-validation and an independent validation cohort. Additionally, decision curve analysis was conducted to assess the model’s clinical benefit. The study identified hematoma expansion (adjusted OR = 12.92, 95% CI: 2.39–69.79, P = 0.003), GCS score (adjusted OR = 0.77, 95% CI: 0.63–0.93, P = 0.008), hematoma type (OR = 8.01, 95% CI: 2.02–31.78, P = 0.003), and hematoma volume (OR = 1.75, 95% CI: 1.26–2.43, P = 0.001) as independent risk factors for poor prognosis in patients with brainstem hemorrhage. The nomogram prognostic model demonstrated excellent performance in predicting clinical outcomes, with an AUC of 0.95, outperforming individual predictors (volume: 0.94, type: 0.8, GCS: 0.78, expansion: 0.59). Calibration curves showed a high degree of agreement between the model and the ideal curve. Moreover, decision curve analysis indicated that the model provided significant net clinical benefit. This nomogram can effectively provide a basis for prognostic judgment in brainstem hemorrhage, helping clinicians optimize treatment decisions and improve patient outcomes.
Keywords: Brainstem hemorrhage, Prognostic model, Nomogram, Risk factors, Clinical decision-making
Subject terms: Nomograms, Risk factors, Stroke, Stroke
Introduction
Stroke has become the leading cause of death among the Chinese population, with a mortality rate of 149 per 100,000 individuals1. Primary intracerebral hemorrhage (ICH) accounts for 19.6% of all strokes2,3. Intracerebral hemorrhage, a type of non-traumatic bleeding within the brain parenchyma, is the second most common type of stroke, affecting 15%-30% of patients and associated with high mortality and disability rates4. Primary brainstem hemorrhage (PBH) is the most severe subtype, constituting 6%-10% of all primary ICH cases5. Although the incidence of ICH generally increases with age, PBH tends to occur in a relatively younger population, predominantly between the ages of 40 and 606,7. PBH presents acutely and severely, with reported mortality rates ranging from 47 to 80%8,9. Huang et al. found that patients with primary brainstem hemorrhage who had a hematoma volume greater than 10 ml and a GCS score below 5 at admission had a 100% mortality rate within 30 days10.
Both conservative medical treatment and surgical intervention are employed for PBH patients. Conservative treatment is typically used for patients with milder conditions, while surgical intervention may be considered for those with severe consciousness impairment. Studies have shown that surgical intervention can significantly improve the prognosis and reduce the mortality rate of PBH patients11. Regardless of the treatment approach, the prognosis for PBH remains poor, with high mortality and disability rates. Therefore, early prevention and intervention are crucial. Identifying and avoiding risk factors in daily life can help prevent PBH and improve patient outcomes.
Despite extensive research on the etiology and risk factors of PBH, the exact causes and mechanisms remain unclear. Several studies suggest that the clinical outcomes of PBH patients may be associated with factors such as blood pressure at admission, volume of hemorrhage, location of hemorrhage, GCS score, and clinical interventions. Therefore, this study aims to develop a clinical prediction model to identify factors that may influence the mortality and prognosis of PBH patients, thereby enhancing our understanding of the disease and providing a reference for clinical prognosis assessment.
Materials and methods
This study was approved by the Ethics Committee of The Affiliated Hospital of Xuzhou Medical University (Approval No.: XYFY2024-KL197-01). Given the retrospective nature of the data analysis and the fact that no personally identifiable information of patients was disclosed, the Ethics Committee granted a waiver of informed consent. The study strictly adhered to the principles of the Declaration of Helsinki and other relevant ethical guidelines to ensure the privacy and confidentiality of patient data. All collected clinical and imaging data were used solely for the purposes of this study.The authors were unable to obtain information that could identify individual participants during or after data collection.
Study subjects
The study included 140 patients with brainstem hemorrhage who were treated at The Affiliated Hospital of Xuzhou Medical University and Changshu Hospital Affiliated to Soochow University from January 2021 to January 2024. All patients met the following inclusion criteria: diagnosed with brainstem hemorrhage, aged 18 years or older, and having complete clinical and imaging data records during hospitalization. Exclusion criteria were: a history of severe neurological disorders, patients with severe comorbidities, and patients with missing data for unknown reasons.
Admission characteristics
Upon admission, the following data were recorded for each patient: Glasgow Coma Scale (GCS) score, presence of hydrocephalus, intubation status, presence of hypertension (Hp) or Diabetes Mellitus (DM), and Body Mass Index (BMI).
Imaging data
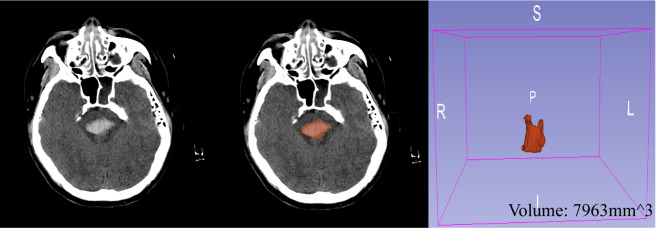
All patients underwent a non-contrast head CT scan to confirm the hemorrhage location in the brainstem. The hemorrhage area was delineated layer by layer using 3D-slicer software (Version5.2.2)12, and the hemorrhage volume was calculated. An illustration of this process is shown in Fig. 1. Hematomas were classified using the “Zhejiang University Second Affiliated Hospital, Z2 classification”13 into four types: Type 0 (fourth ventricle casting), Type 1 (unilateral, not crossing the midline), Type 2 (bilateral, but not crossing the 3/4 boundary of both sides of the brainstem), and Type 3 (crossing the 3/4 boundary of both sides of the brainstem). For statistical purposes, Types 0–1 were grouped as low-grade, and Types 2–3 as high-grade.
Fig. 1.
Hemorrhage Volume Delineation Diagram.
Treatment methods
Treatment methods were categorized into conservative and surgical treatments. Surgical treatments included ventricular drilling, hematoma cavity drilling, craniotomy hematoma evacuation, hematoma cavity + ventricular drilling, and craniotomy hematoma evacuation + ventricular drilling. Indications for surgery included: 1) hematoma volume ≥ 5 mL with GCS ≤ 7; 2) hematoma volume 3–5 mL with GCS ≤ 5 after 72 h of conservative treatment or with acute hydrocephalus; 3) strong family request for surgery; 4) age < 65 years.
Prognosis assessment
Some brainstem hemorrhage patients were admitted to the ICU, where they might still have a heartbeat at discharge but require ventilator support and have unstable vital signs. Prognosis was assessed using the GOS score at discharge (or at 30 days for those hospitalized longer). The GOS score includes: 5 (good recovery, normal life despite minor deficits), 4 (moderate disability, independent living, able to work under supervision), 3 (severe disability, dependent on daily care), 2 (persistent vegetative state with eye movements and sleep–wake cycles), and 1 (death). Patients scoring below 3 (excluding 3) were defined as having a poor prognosis, while those scoring 3 and above were defined as having a good prognosis.
Model development and evaluation
A prognostic model was constructed using multivariate logistic regression analysis. First, univariate analysis was performed on all variables to identify potential factors associated with prognosis. Significant variables were then included in the multivariate analysis to construct the preliminary model. The predictive performance of the model was evaluated using ROC curves, PR curves, and confusion matrices. The area under the ROC curve (AUC) was used to measure the overall performance, while the PR curve assessed precision and recall at different thresholds. All models were validated through cross-validation and an independent validation cohort to ensure robustness and generalizability.
Statistical analysis
Data analysis was performed using statistical software packages such as Python and R. Continuous variables were presented as mean ± standard deviation, and categorical variables as frequencies and percentages. Group comparisons were conducted using t-tests or Mann–Whitney U tests for continuous variables and χ2 tests for categorical variables. All statistical tests were two-sided, with P < 0.05 considered statistically significant.
Results
A total of 140 patients with brainstem hemorrhage were included in this study, comprising 105 males (75%) and 35 females (25%), with a mean age of 53 years (SD ± 11.89 years) and a mean BMI of 27.3 (SD ± 4.95). Among all patients, 80% had a history of hypertension, and 14.29% had a history of diabetes. Upon admission, the median GCS score was 7 (IQR 5.0–13.0); 28 patients (20%) underwent surgery, while 112 patients (80%) received conservative treatment. During hospitalization, 32 patients (22.86%) developed hydrocephalus, while 108 patients (77.14%) did not. Tracheal intubation and mechanical ventilation were required in 51 patients (36.43%), whereas 89 patients (63.57%) did not undergo intubation. Imaging data collected during hospitalization revealed an average hemorrhage volume of approximately 3.6 ml (IQR 1.7 ml–7.1 ml). Hematoma types were evenly distributed, with 70 patients (50%) classified as Type 1 and 70 patients (50%) as Type 2. Hematoma expansion was observed in 34 patients (24.29%), while 106 patients (75.71%) did not experience hematoma expansion. The baseline characteristics of the patients are shown in Table 1.
Table 1.
Baseline Characteristics of Patients.
| Variables | Total (n = 140) | GOS 3–5 (n = 61) | GOS 0–1 (n = 79) | P value |
|---|---|---|---|---|
| Age, Mean ± SD (years) | 53.33 ± 11.89 | 54.61 ± 12.92 | 52.34 ± 11.01 | 0.265 |
| Sex, n (%) | 0.922 | |||
| Male | 105 (75.00) | 45 (73.77) | 60 (75.95) | |
| Female | 35 (25.00) | 16 (26.23) | 19 (24.05) | |
| BMI, Mean ± SD | 27.30 ± 4.95 | 26.59 ± 5.06 | 27.84 ± 4.83 | 0.140 |
| GCS, median (Q1, Q3) | 7.00 (5.00, 13.00) | 13.00 (8.00, 15.00) | 6.00 (5.00, 7.00) | < 0.001 |
| Volume, median (Q1, Q3)(mL) | 3.60 (1.70, 7.10) | 1.600 (0.800, 2.800) | 6.90 (4.20, 8.95) | < 0.001 |
| Surgery, n (%) | < 0.001 | |||
| No | 112 (80.00) | 58 (95.08) | 54 (68.35) | |
| Yes | 28 (20.00) | 3 (4.92) | 25 (31.65) | |
| Hydrocephalus, n (%) | 0.071 | |||
| No | 108 (77.14) | 52 (85.25) | 56 (70.89) | |
| Yes | 32 (22.86) | 9 (14.75) | 23 (29.11) | |
| Intubation, n (%) | < 0.001 | |||
| No | 89 (63.57) | 51 (83.61) | 38 (48.10) | |
| Yes | 51 (36.43) | 10 (16.39) | 41 (51.90) | |
| Hp, n (%) | 0.250 | |||
| No | 28 (20.00) | 9 (14.75) | 19 (24.05) | |
| Yes | 112 (80.00) | 52 (85.25) | 60 (75.95) | |
| DM, n (%) | 0.384 | |||
| No | 120 (85.71) | 50 (81.97) | 70 (88.61) | |
| Yes | 20 (14.29) | 11 (18.03) | 9 (11.39) | |
| Type, n (%) | < 0.001 | |||
| No | 70 (50.00) | 52 (85.25) | 18 (22.78) | |
| Yes | 70 (50.00) | 9 (14.75) | 61 (77.22) | |
| Expand, n (%) | < 0.001 | |||
| No | 106 (75.71) | 56 (91.80) | 50 (63.29) | |
| Yes | 34 (24.29) | 5 (8.20) | 29 (36.71) |
In the univariate logistic regression analysis, several clinical variables were evaluated to predict the prognosis of patients with brainstem hemorrhage. The results indicated that intubation during hospitalization (OR = 5.5, 95% CI: 2.45–12.36, P < 0.001), hematoma expansion (OR = 6.5, 95% CI: 2.34–18.06, P < 0.001), GCS score (OR = 0.65, 95% CI: 0.59–0.76, P < 0.001), hydrocephalus (OR = 2.37, 95% CI: 1.01–5.6, P = 0.048), surgical treatment (OR = 8.95, 95% CI: 2.56–31.35, P = 0.001), hematoma type (OR = 19.58, 95% CI: 8.11–47.28, P < 0.001), and hematoma volume (OR = 2.32, 95% CI: 1.75–3.08, P < 0.001) were statistically significant predictors of prognosis. Other evaluated variables did not show significant predictive ability (P > 0.05).
In the multivariate logistic regression analysis, all variables that were significant in the univariate analysis were included to assess their independent predictive ability. The results demonstrated that hematoma expansion (adjusted OR = 12.92, 95% CI: 2.39–69.79, P = 0.003), GCS score (adjusted OR = 0.77, 95% CI: 0.63–0.93, P = 0.008), hematoma type (adjusted OR = 8.01, 95% CI: 2.02–31.78, P = 0.003), and hematoma volume (adjusted OR = 1.75, 95% CI: 1.26–2.43, P = 0.001) remained significant predictors after adjusting for other factors. Other variables did not retain significance in the multivariate model. These results indicate that hematoma expansion, GCS score, hematoma type, and hematoma volume are important independent predictors of prognosis in patients with brainstem hemorrhage, providing valuable reference for clinicians. The detailed results of univariate and multivariate logistic regression are presented in Table 2.
Table 2.
Univariate and Multivariate Logistic Regression Analysis Results.
| Characteristics | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| OR | 95%CI | P value | OR | 95%CI | P value | |
| Age | 0.98 | 0.96–1.01 | 0.264 | |||
| BMI | 1.05 | 0.98–1.13 | 0.141 | |||
| Intubation | 5.5 | 2.45–12.36 | < 0.001 | 2.48 | 0.63–9.85 | 0.195 |
| DM | 0.58 | 0.23–1.52 | 0.269 | |||
| Expand | 6.5 | 2.34–18.06 | < 0.001 | 12.92 | 2.39–69.79 | 0.003 |
| GCS | 0.67 | 0.59–0.76 | < 0.001 | 0.77 | 0.63–0.93 | 0.008 |
| Gender | 0.89 | 0.41–1.92 | 0.768 | |||
| Hp | 0.55 | 0.23–1.31 | 0.176 | |||
| Hydrocephalus | 2.37 | 1.01–5.6 | 0.048 | 0.34 | 0.06–2.07 | 0.242 |
| Surgery | 8.95 | 2.56–31.35 | 0.001 | 2.78 | 0.39–19.78 | 0.307 |
| Type | 19.58 | 8.11–47.28 | < 0.001 | 8.01 | 2.02–31.78 | 0.003 |
| Volume | 2.32 | 1.75–3.08 | < 0.001 | 1.75 | 1.26–2.43 | 0.001 |
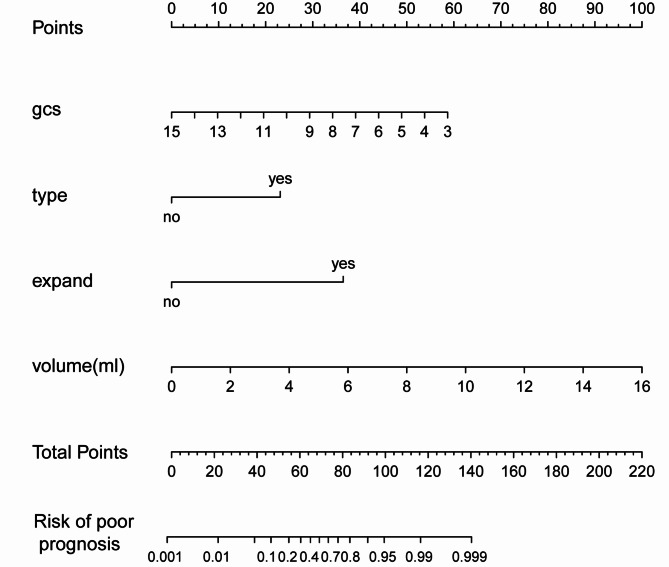
The nomogram prognostic model was constructed using four variables identified from multivariate logistic regression analysis (Fig. 2). We compared the performance of the Nomogram model with other univariate models (gcs, type, expand, and volume) (Table 3). The results demonstrated that the Nomogram model excelled in all metrics. It had a ROC value of 0.95, significantly higher than the other models; an AIC value of 52.39, indicating greater simplicity and a better balance between goodness of fit and model complexity; and a Brier score of 0.062, reflecting superior predictive accuracy. These results underscore the potential clinical utility of the Nomogram model in predicting brainstem hemorrhage outcomes.
Fig. 2.
Nomogram for Predicting Prognosis in Brainstem Hemorrhage Patients.
Table 3.
Model Performance Comparison.
| Models | ROC | AIC | Brier |
|---|---|---|---|
| Nomogram | 0.97 | 52.39 | 0.062 |
| gcs | 0.89 | 85.90 | 0.123 |
| type | 0.80 | 103.5 | 0.152 |
| expand | 0.66 | 126.2 | 0.203 |
| volume | 0.91 | 79.95 | 0.118 |
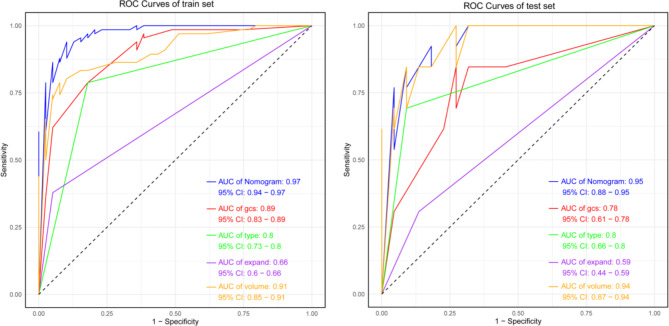
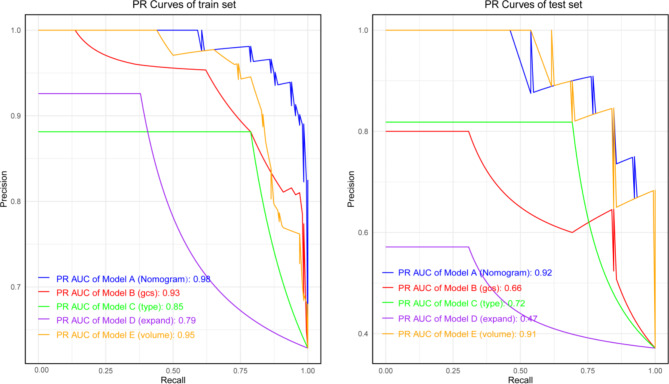
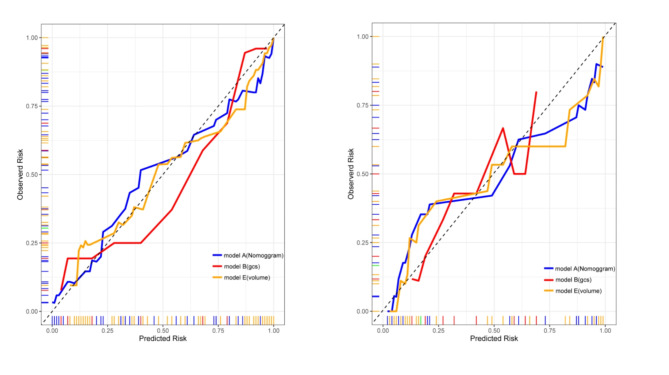
The model’s area under the ROC curve (AUC) was 0.95 (95% CI: 0.88–0.95), indicating high discriminative ability. The Nomogram model outperformed other univariate models (Fig. 3). The precision-recall (PR) curve showed that the model maintained high precision and recall rates across different thresholds (Fig. 4). The calibration curve for the Nomogram model demonstrated good agreement between predicted probabilities and observed incidence rates (Fig. 5), indicating accurate predictive capability across different risk levels. This further validates the Nomogram model’s reliability and practical applicability in clinical settings.
Fig. 3.
ROC Curves for Training and Testing Sets.
Fig. 4.
PR Curves for Training and Testing Sets.
Fig. 5.
Calibration Curves for Training and Testing Sets.
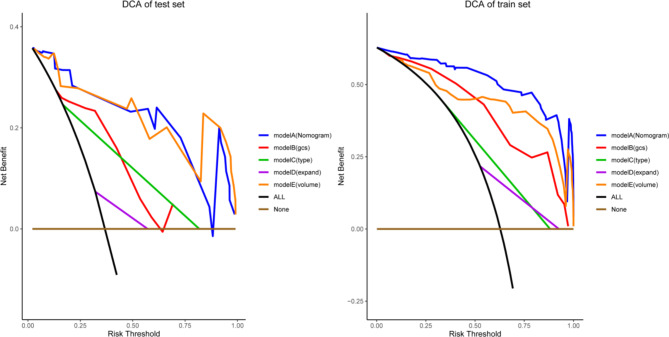
Decision curve analysis (Fig. 6) indicated that the constructed prognostic model exhibited high net benefits across different threshold ranges, suggesting significant clinical value.
Fig. 6.
Decision Curve Analysis (DCA) for Training and Testing Sets.
Discussion
Hypertensive brainstem hemorrhage accounts for 6–10% of all brainstem hemorrhages, with pontine hemorrhage being the most common type14,15. The hemorrhage primarily originates from the paramedian perforating arteries supplying the brainstem16. Similar to intracerebral hemorrhage (ICH), the most common cause of primary brainstem hemorrhage (PBH) is hypertension. Hypertensive hemorrhages occur in areas supplied by the penetrating branches of the major cerebral arteries. These smaller arteries are directly exposed to the relatively high pressure of the larger parent arteries, lacking the protective gradual tapering of vessel diameter17.
Several mechanisms of brain injury in brainstem hemorrhage are generally recognized. Initially, hematoma expansion and cytotoxic edema cause primary mechanical disruption and compression of the surrounding brain tissue. Both the hematoma and the resultant perilesional edema can create a mass effect and increase intracranial pressure (ICP), leading to reduced cerebral perfusion and ischemic injury. In severe cases, this can further cause brain herniation18. Research indicates that necrosis of the brain tissue surrounding the hematoma begins within six hours after onset, worsens after 12 h, and peaks within 24 hours19. If the hematoma extends downward, it may compress the medulla, affecting the brainstem’s vital centers and causing respiratory and circulatory dysfunction. Upward extension can compress the midbrain’s reticular activating system, quickly leading the patient into a coma.
Given the high mortality and disability rates and generally poor prognosis of primary brainstem hemorrhage, promptly identifying key factors affecting the prognosis and survival of PBH patients is crucial for clinical decision-making and treatment. Through comparative analysis of surviving and deceased patients, this study preliminarily identified several potential key risk factors, including the Glasgow Coma Scale (GCS) score at admission, specific types of brainstem hematomas, initial hematoma volume, and subsequent hematoma expansion. These findings provide essential references for the clinical management of PBH patients.
Previous studies have shown that the GCS score is an independent risk factor for predicting mortality in PBH. Geng et al. reported that a GCS score of < 9 at admission significantly increased the 30-day mortality rate20. In our study, multivariate analysis further revealed that a GCS score ≤ 6 was significantly associated with poor prognosis within 30 days for brainstem hemorrhage patients (P < 0.001). The GCS score at admission is a reliable parameter for assessing the prognosis of traumatic brain injury; the lower the score, the worse the prognosis, and the higher the potential mortality risk. Even if patients survive, they may suffer severe sequelae21. Therefore, dynamically monitoring the GCS score at admission is crucial. It helps healthcare providers promptly understand changes in the patient’s condition and quickly take effective measures.
In recent years, increasing research has highlighted the strong correlation between hematoma expansion and the prognosis of patients with intracerebral hemorrhage (ICH)22,23. Due to the small volume of the brainstem, hematoma expansion in this region can have a more severe impact on patient survival and prognosis. Hematoma morphology-related signs, such as the spot sign, have been recognized as key imaging markers indicative of increased risk for hematoma expansion. A retrospective analysis by Delgado et al. of 367 acute ICH patients found that approximately 19% had the spot sign, which was independently associated with hematoma expansion24. Moreover, the number of spot signs is considered an important predictor of hematoma expansion25. Therefore, taking appropriate measures to limit hematoma expansion is crucial for improving the prognosis of PBH patients. Hemostatic agents such as tranexamic acid, which stabilize intracerebral hematomas through their antifibrinolytic effects, can help prevent further hematoma expansion. Additionally, it is necessary to avoid the excessive use of dehydration agents, as studies have shown that the use of mannitol in the ultra-early phase of hemorrhage may lead to hematoma expansion26. Treating hemorrhage-associated fever is also important, as persistent fever is associated with poor prognosis and hematoma expansion27,28.
Hematoma volume is also considered an independent predictor of poor prognosis in primary brainstem hemorrhage patients. A 2023 study by Yu et al. found that when hematoma volume exceeded 4 ml, the 30-day mortality rate significantly increased, reaching saturation at 10 ml. This aligns with our findings, which show that when the hemorrhage volume exceeds 6 ml, the 30-day prognosis significantly worsens21.
Given the highly dangerous course of primary brainstem hemorrhage, rapid identification and diagnosis are critical. With the widespread use of CT technology, precise classification of brainstem hematomas has become a research focus. Russell et al. first proposed classifying brainstem hematomas into central, dorsolateral tegmental, and basal tegmental types in 198629. Later, Chung et al. proposed a more detailed classification: massive, bilateral tegmental, basal-tegmental, and unilateral small hematoma types, with survival rates of 7.1%, 14.3%, 26.1%, and 94.1%, respectively30. Wessels et al. found that dorsal brainstem hematomas generally had better prognoses compared to ventral hematomas31. In our study, we found that the severity of the hematoma (high-grade type) was significantly associated with poor prognosis. We speculate that this difference may stem from damage to the reticular activating system (RAS) caused by the hematoma. The RAS is a crucial part of the brain responsible for arousal and sleep–wake cycles, closely related to the level of consciousness and wakefulness32. Therefore, understanding the detailed classification of brainstem hematomas and their impact on the RAS is essential for predicting and improving clinical outcomes.
Currently, the treatment of primary brainstem hemorrhage is primarily conservative, centered on neurocritical care33 Common surgical approaches for primary brainstem hemorrhage include traditional craniotomy for hematoma evacuation, stereotactic hematoma aspiration, lateral ventricular drainage in cases of acute hydrocephalus, and decompressive craniectomy when signs of intracranial hypertension appear. In theory, hematoma removal can alleviate the mass effect of the hemorrhage and reduce intracranial pressure, while also eliminating the inflammatory and cytotoxic effects caused by the hematoma on brain tissue34.Selecting an appropriate surgical approach through a safe entry zone to evacuate the hematoma requires adherence to the principles of "no traction on the brainstem, gentle aspiration of the hematoma, and mild electrocoagulation of responsible vessels." However, such procedures demand highly skilled surgical expertise35. A long-standing point of debate regarding surgical intervention for primary brainstem hemorrhage is the potential for the surgery itself to cause unavoidable damage to the brainstem. Brainstem hemorrhages often occur in deep regions of the brainstem, and traditional hematoma evacuation surgery necessitates cutting through surface brainstem tissue, which increases the risk of damage to the brainstem.
Stereotactic aspiration, guided by precise technology, appears to hold great promise for the treatment of primary brainstem hemorrhage. A Japanese team36,37 was among the first to apply stereotactic aspiration in brainstem hemorrhage and found that the surgical group had significantly better clinical outcomes compared to the conservative group. With advancements in technology, stereotactic minimally invasive puncture for hematoma aspiration has been developed. This technique uses preoperative computer software for 3D reconstruction and evaluation of the hematoma and utilizes the natural channels formed by the hematoma to avoid important intracranial conduction pathways and dense neural nuclei. During the procedure, mechanical traction, electrocoagulation, and tissue dissection on the brainstem can be minimized, thereby reducing potential damage to the brainstem to the greatest extent.Zhou and colleagues13, based on their classification study, found that for critically ill patients upon admission (GCS 10 ml) with type 3 hemorrhages, proactive stereotactic aspiration is recommended. This approach can simultaneously improve short-term survival and long-term neurological recovery outcomes, reducing the mortality rate of this patient group to just 20%, whereas previous studies reported a 30-day mortality rate as high as 100% for such cases10. For patients with hematoma volumes > 5 ml and GCS < 8 upon admission with type 2 or 3 hemorrhages, stereotactic aspiration may also be considered.
However, most current guidelines do not recommend surgical treatment for primary brainstem hemorrhage, as infratentorial hemorrhages are generally excluded from large-scale randomized clinical trials on intracerebral hemorrhage. Thus, there remains a lack of high-quality evidence to support the efficacy and safety of surgical intervention. Future large-scale clinical trials are needed to provide more detailed clinical data and evidence. Therefore, the establishment of multicenter collaborative groups to further assess prognostic factors and re-evaluate the indications and timing for surgical intervention is necessary.
Conclusion
This study developed a prognostic model for brainstem hemorrhage based on multivariate logistic regression, demonstrating high predictive accuracy and reliability. Key predictive factors include hematoma expansion, GCS score, hematoma type, and hematoma volume. The model shows significant value in clinical applications, effectively assisting clinicians in risk assessment and treatment decision-making, ultimately improving patient prognosis.
Author contributions
S.W and L.G. conceptualized the study, outlined the study design,collected data, analyzed and interpreted results, and wrote the manuscript. Y.F. and P.J. preprocessed input data, built machine learning models, analyzed data, and wrote the manuscript. L.Y. collected data and preprocessed input data. F.L. and S.M. helped to adjust the ideas of the manuscript, suggested changes, and revised the manuscript. All authors agreed to take responsibility for their contributions and read and approved the final manuscript.
Funding
The paper is sponsored by Jiangsu Provincial Key Medical Discipline (ZDXK202228) and High-level hospital construction (2023601001).
Data availability
The datasets used and analyzed during our study are available from the corresponding author upon reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Consent statement
Informed consent has been obtained from all participants and/or their legal guardians for this study. Before the study began, the participants and/or their legal guardians were fully informed of the purpose, procedures, potential risks and benefits of the study and had the opportunity to ask any questions. All participants and/or their legal guardians signed the informed consent form and agreed to voluntarily participate in this study. This statement ensures that the study meets ethical requirements and proves that the participants or their legal guardians have been fully informed and are willing to participate in the study.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Shuo Wei and Longyuan Gu.
Contributor Information
Fengda Li, Email: fdcivilization@163.com.
Shuhong Mei, Email: mshsjwk@163.com.
References
- 1.Zhou, M. et al. Mortality, morbidity, and risk factors in China and its provinces, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet394(10204), 1145–1158 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sacco, S., Marini, C., Toni, D., Olivieri, L. & Carolei, A. Incidence and 10-year survival of intracerebral hemorrhage in a population-based registry. Stroke40(2), 394–399 (2009). [DOI] [PubMed] [Google Scholar]
- 3.Sarti, C., Rastenyte, D., Cepaitis, Z. & Tuomilehto, J. International trends in mortality from stroke, 1968 to 1994. Stroke31(7), 1588–1601 (2000). [DOI] [PubMed] [Google Scholar]
- 4.Seo, B. B. et al. Post-traumatic intradiploic lep tomeningal cyst of the posterior fossa in an adult. Clin. Neuro Sci.16, 1367–1369 (2009). [DOI] [PubMed] [Google Scholar]
- 5.Delcourt, C. et al. Intracerebral hemorrhage location and outcome among INTERACT2 participants. Neurology88, 1408 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dinsdale, H. B. Spontaneous hemorrhage in the posterior fossa. A study of primary cerebellar and pontine hemorrhages with observations on their pathogenesis. Arch. Neurol.10(2), 200–217 (1964). [DOI] [PubMed] [Google Scholar]
- 7.van Asch, C. J. et al. Incidence, case fatality, and functional outcome of intracerebral haemor- rhage over time, according to age, sex, and ethnic origin: a systematic review and meta-analysis. Lancet Neurol.9(2), 167–176 (2010). [DOI] [PubMed] [Google Scholar]
- 8.Nilsson, O. G., Lindgren, A., Brandt, L. & Säveland, H. Prediction of death in patients with primary intracerebral hemorrhage: A prospective study of a defined population. J. Neurosurg.97(3), 531–536 (2002). [DOI] [PubMed] [Google Scholar]
- 9.Murata, Y. et al. Relationship between the clinical manifestations, computed tomographic findings and the outcome in 80 patients with primary pontine hemorrhage. J. Neurol. Sci.167(2), 107–111 (1999). [DOI] [PubMed] [Google Scholar]
- 10.Huang, K. et al. Development and validation of a grading scale for primary pontine hemorrhage. Stroke48(1), 63–69 (2017). [DOI] [PubMed] [Google Scholar]
- 11.Murata, Y. et al. Relationship between the clinical manifestations, computed tomographic findings and the outcome in 80 patients with primary pontine hemorrhage. Neurol. Sci.167, 107–108 (1999). [DOI] [PubMed] [Google Scholar]
- 12.Zhou, J. et al. ZJUSAH classification: A new classification for primary brainstem hemorrhage. Life (Basel)13(3), 846 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fedorov, A. et al. 3D Slicer as an image computing platform for the Quantitative Imaging Network. Magn. Reson. Imaging30(9), 1323–1341 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang, S. S. et al. Management of brainstem haemorrhages. Swiss Med. Wkly.5(149), w20062 (2019). [DOI] [PubMed] [Google Scholar]
- 15.Behrouz, R. Prognostic factors in pontine haemorrhage: A systematic review. Eur. Stroke J.3(2), 101–109 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen, L. H. et al. The microsurgical treatment for primary hypertensive brainstem hemorrhage: Experience with 52 patients. Asian J. Surg.44(1), 123–130. 10.1016/j.asjsur.2020.04.016 (2021). [DOI] [PubMed] [Google Scholar]
- 17.Garcia, J. H. & Ho, K. L. Pathology of hypertensive arteriopathy. Neurosurg. Clin. N. Am.3, 497 (1992). [PubMed] [Google Scholar]
- 18.Xu, B. N. et al. Pathophysiology of brain swelling after acute experimental brain compression and decompression. Neurosurgery32, 289 (1993). [DOI] [PubMed] [Google Scholar]
- 19.Zhang, X. Q. et al. Exploring the optimal operation time for patients with hypertensive intracerebral hemorrhage: tracking the expression and progress of cell apoptosis of prehematomal brain tissues. Chin. Med. J. (Engl).123(10), 1246–1250 (2010). [PubMed] [Google Scholar]
- 20.Geng, Y. et al. How to predict the outcome of primary brainstem hemorrhage: Six-year results of a single-center retrospective analysis. Medicine (Baltimore)102(37), e35131 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu, Z. et al. Effect of hematoma volume on the 30-day mortality rate of patients with primary hypertensive brainstem hemorrhage: A retrospective cohort study. Front Surg.5(10), 1136296 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li, Z. et al. Hematoma expansion in intracerebral hemorrhage: an update on prediction and treatment. Front. Neurol.11, 702 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davis, S. M. et al. Hematoma growth is a determinant of mortality and poor outcome after intracerebral hemorrhage. Neurology66, 1175–1181 (2006). [DOI] [PubMed] [Google Scholar]
- 24.Delgado Almandoz, J. E. et al. The spot sign score in primary intracerebral hemorrhage identifies patients at highest risk of in-hospital mortality and poor outcome among survivors. Stroke41, 54 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huynh, T. J. et al. Spot sign number is the most important spot sign characteristic for predicting hematoma expansion using first-pass computed tomography angiography: Analysis from the PREDICT study. Stroke44, 972 (2013). [DOI] [PubMed] [Google Scholar]
- 26.Ding, W. L., Xiang, Y. S., Liao, J. C., Wang, S. Y. & Wang, X. Y. Early tracheostomy is associated with better prognosis in patients with brainstem hemorrhage. J. Integr. Neurosci.19, 437–442 (2020). [DOI] [PubMed] [Google Scholar]
- 27.Schwarz, S., Häfner, K., Aschoff, A. & Schwab, S. Incidence and prognostic significance of fever following intracerebral hemorrhage. Neurology54(2), 354–361 (2000). [DOI] [PubMed] [Google Scholar]
- 28.Rincon, F., Lyden, P. & Mayer, S. A. Relationship between temperature, hematoma growth, and functional outcome after intracerebral hemorrhage. Neurocrit Care18(1), 45–53 (2013). [DOI] [PubMed] [Google Scholar]
- 29.Russell, B., Rengachary, S. S. & McGregor, D. Primary pontine hematoma presenting as a cerebellopontine angle mass. Neurosurgery19(1), 129–133 (1986). [DOI] [PubMed] [Google Scholar]
- 30.Chung, C. S. & Park, C. H. Primary pontine hemorrhage: A new CT classification. Neurology42(4), 830–834 (1992). [DOI] [PubMed] [Google Scholar]
- 31.Wessels, T., Möller-Hartmann, W., Noth, J. & Klötzsch, C. CT findings and clinical features as markers for patient outcome in primary pontine hem- orrhage. AJNR Am. J. Neuroradiol25(2), 257–260 (2004). [PMC free article] [PubMed] [Google Scholar]
- 32.Wang, S. S. et al. Management of brainstem haemorrhages. Swiss Med. Wkly149, w20062 (2019). [DOI] [PubMed] [Google Scholar]
- 33.Gujjar, A. R. et al. Mechanical ventilation for ischemic stroke and intracerebral hemorrhage: indications, timing, and outcome. Neurology51(2), 447–451(1998). [DOI] [PubMed]
- 34.Broderick, J. P. The STICH trial: what does it tell us and where do we go from here? Stroke36(7), 1619–1620 (2005). [DOI] [PubMed]
- 35.Tao, C. et al. Predictors of surgical results in patients with primary pontine hemorrhage. Turk Neurosurg26(1), 77–83 (2016). [DOI] [PubMed]
- 36.Takahama, H., Morii, K., Sato, M., Sekiguchi, K. & Sato, S. Stereotactic aspiration in hypertensive pontine hemorrhage: comparative study with con- servative therapy. No Shinkei Geka17(8), 733–739 (1989). [PubMed]
- 37.Hara, T. et al. Functional outcome of primary pontine hemorrhage: conservative treatment or stereotaxic surgery. No Shinkei Geka29(9), 823–829 (2001). [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analyzed during our study are available from the corresponding author upon reasonable request.