Abstract
Gastric cancer is a common malignant tumor with high incidence and mortality. The overexpression of Human epidermal growth factor receptor 2 (HER2) is associated with increased metastatic potential and poor clinical outcome in gastric cancer. Despite the proven clinical response rates of approved HER2-targeted therapies, including Trastuzumab combined with chemotherapy, their limited long-term clinical benefits and inevitable disease progression still pose significant challenges to the clinical treatment of gastric cancer. Hence, exploring novel strategies to enhance therapeutic outcomes for HER2-positive patients is extremely crucial and urgent. Here, we reported that DX126-262, a novel HER2-targeted antibody-drug conjugate, generated by conjugating a potent Tubulysin B analogue (Tub-114) to humanized anti-HER2 monoclonal antibody, exhibited a significant synergistic inhibitory effect with both Cisplatin and 5-FU in HER2-positive gastric cancer NCI-N87 cells. Moreover, the triple-drug combination strategy of DX126-262 combined with Cisplatin and 5-FU showed much better in vitro and in vivo therapeutic efficacy than monotherapy or double-drug combination (Cisplatin plus 5-FU) or first-line standard-of-care (SOC, Herceptin plus Cisplatin and 5-FU), and comparable or even superior in vivo efficacy than third-line SOC (DS-8201a) in NCI-N87 cells and xenograft models. Meanwhile, the triple-drug combination therapy did not exhibit superimposed toxicity. Taken together, our findings provide compelling evidence that DX126-262 in combination with Cisplatin and 5-FU exerts synergistic antitumor activity and is a promising strategy to improve the clinical efficacy of HER2-positive advanced or metastatic gastric cancer.
Keywords: Antibody-drug conjugate, target therapy, Cisplatin, 5-Fluorouracil, combination therapy, tumor, metastasis
Introduction
Gastric cancer is one of the most prevalent malignancies, ranking fifth in global cancer diagnosis and fourth leading cause of cancer-related death worldwide [1,2]. Notably, China bears the heaviest burden, with the incidence of gastric cancer ranking first globally, accounting for an astounding 44% of all new cases [2,3]. The current clinical therapeutic for gastric cancer in China is primarily dominated by chemotherapy and molecular targeted therapies. However, the arsenal of targeted drugs for gastric cancer is constrained, primarily comprising anti-HER2 agents as first-line therapy and anti-angiogenic therapies as second/third-line treatment, and there remains a pressing need for the development of alternative, efficacious targeted treatment strategies to address the ongoing challenge posed by this malignancy.
HER2 plays an important role in cell proliferation, differentiation, apoptosis, migration and tumorigenesis [4]. The overexpression or amplification of HER2 has been consistently associated with increased metastatic potential and poor clinical outcome and has been verified in many malignant tumors, including breast cancer, lung cancer, gastric cancer [4-6]. Globally, the latest reported incidence of HER2 overexpression in gastric cancer ranging from 7.3% to 20.2% [4,7], while the positive rate of HER2 among Chinese gastric cancer patients is 13%-18% [8-10]. In 2010, ToGA Trial firstly confirmed that integration of anti-HER2 targeted therapy with chemotherapy markedly enhanced response rates and prolonged the survival of patient, making it highly recommended as the first-line standard-of-care (SOC) for individuals with HER2-positive advanced gastric cancer, and opening the era of anti-HER2 targeted therapy for gastric cancer [11,12]. Unfortunately, after the ToGA study, progress in the development of treatments for gastric cancer stalled for nearly a decade [13]. The emergence of Disitamab vedotin (RC48) and Trastuzumab deruxtecan (DS-8201a), two novel HER2 targeting antibody-drug conjugates (ADC), brought a new breakthrough in the therapeutic landscape for HER2-positive gastric cancer [4,14]. However, due to the complexity inherent in the disease, patients diagnosed with HER2-positive advanced gastric cancer inevitably face disease progression over time. Even with the first-line regimen of Trastuzumab in combination with chemotherapy, the long-term benefits are still limited, as evidenced by a median overall survival (OS) of 13.8 months and an objective response rate (ORR) of merely 47%, only 12% increase of ORR for the group receiving Trastuzumab in combination with chemotherapy compared to chemotherapy alone [11]. Although RC48 and DS-8201a have demonstrated significant clinical efficacy over chemotherapy regimens, their advantage in terms of prolonging long-term benefits is not pronounced. For example, RC48-C008 trial indicated that RC48 achieved an ORR of 24.8% in the later-line treatment (≥3rd line) of HER2-overexpressed advanced gastric cancer, yet the median progression-free survival (PFS) and median OS were only 4.1 months and 7.9 months, respectively [15]. Additionally, while the DESTINY-Gastric01 study reported even more promising clinical data for DS-8201a in the later-line treatment of HER2-positive gastric cancer, with an ORR of 42%, the median PFS was still only 5.6 months, and the median OS was 12.5 months, failing to provide long-term benefits for patients [16]. Therefore, it is particularly important to identify potential strategies to overcome anti-HER2 resistance and develop novel anti-HER2 approaches so as to improve the overall therapeutic outcomes and benefits for patients with HER2-positive gastric cancer.
ADCs represent a highly selective and efficient delivery system for anticancer agents, precisely targeting tumor tissue. ADCs exhibit more pronounced anti-tumor advantages over monoclonal antibody drugs due to the combination of tumor-targeting properties of antibody drugs and the potent killing effects of small molecule chemotherapeutic drugs, while maintaining a broad therapeutic window, and offering significant advantages in cancer treatment [17,18]. We have developed DX126-262, a HER2-targeted ADC that conjugates a novel Tubulysin B analogue to a recombinant humanized anti-HER2 monoclonal antibody. Innovations of DX126-262 have been made in the selection of small molecular payloads and linkers. As the next-generation tubulin inhibitor, Tubulysin B has been proven to exhibit stronger anti-tumor activity and more potent killing characteristics against drug-resistant tumor cells than conventional highly active molecules, such as DM1 and MMAE [19,20]. We embarked on with a potent Tubulysin B analogue Tub-114, conducted meticulous screening and structural modification to further enhance its biological activity. Incorporation of a lysine residue and branched sustained-releasing moieties into the linker markedly decreases the rate of payload release and degradation. This modification enables a more controlled and sustained delivery of the therapeutic payload, thereby enhancing the overall efficacy and safety profile of the ADC. The preclinical evaluation of DX126-262, encompassing efficacy studies, pharmacokinetics analysis, toxicology assessments, and safety evaluations, demonstrates exceptional drug-ability, efficacy and toxicology profile significantly outperforming its counterpart Kadcyla (T-DM1) (data not shown). The pharmacological effect of DX126-262 is similar to that of DS-8201a [21]. After being exposed to the HER2 antigen on the surface of the tumor, DX126-262 binds to and accumulates on the surface, and then is internalized into the tumor cells. The antibody and linker are degraded by proteinases, releasing Tub-114-cys which binds to tubulin and inhibits the polymerization process necessary for cell survival and proliferation, thereby killing tumor cells.
Combination therapy stands as one of the potent strategies aiming at enhancing clinical efficacy. Preclinical and clinical evidence have so far demonstrated varying success in combination therapy of ADCs and chemotherapeutic agents [22]. For example, the combinations of ravtansine-based ADCs with carboplatin or doxorubicin have shown initial efficacy in platinum-sensitive and resistant ovarian cancer [23,24]. Consistently, notable response rates have been observed in early phase trials of testing the combinations of deruxtecan-based ADCs with Capecitabine or Cisplatin in gastric cancer [25,26]. This accumulating knowledge has yielded invaluable insights, serving as a cornerstone for guiding the subsequent development of novel therapeutic modalities. In consideration of the notable clinical benefits and favorable tolerability profile demonstrated by Herceptin in combination with Cisplatin and 5-Fluorouracil (5-FU) as a first-line treatment for HER2-positive gastric cancer, the aim of present study is to evaluate the antitumor effect of the combination of DX126-262 with Cisplatin and 5-FU, so as to provide support for the clinical use of HER2 targeted therapeutic strategies for gastric cancer. Indeed, we demonstrate here that triple-drug combination therapy of DX126-262 plus Cisplatin and 5-FU exhibits superior in vitro and in vivo therapeutic efficacy compared to monotherapy (DX126-262, Cisplatin, 5-FU or Herceptin), dual-drug combinations (Cisplatin plus 5-FU) and firstline SOC (Herceptin plus Cisplatin and 5-FU). Notably, this triple-drug combination also demonstrated comparable or even superior in vivo efficacy against human gastric cancer NCI-N87 cells in comparison to DS-8201a. Importantly, the triple-drug combination therapy does not elicit superimposed toxicity, as assessed by monitoring mouse body weights. Collectively, these findings provide compelling evidence that DX126-262 in combination with Cisplatin and 5-FU is a promising therapeutic strategy to enhance clinical efficacy and outcome for patients with HER2-positive gastric cancer.
Materials and methods
Antitumor agents
Trastuzumab (Herceptin) was obtained from Roche Pharmaceuticals (Basel, Switzerland). Cisplatin was purchased from QILU Pharmaceutical (Shandong, China). 5-Fluorouracil (5-FU) was purchased from Shanghai Aladdin Biochemical Technology Co., Ltd. (Shanghai, China). DS-8201a was purchased from Daiichi Sankyo (Tokyo, Japan).
Synthesis of DX126-262
DX126-262 was independently developed and prepared by Hangzhou DAC Biotechnology Co., Ltd. A Tubulysin B analogue (Tub-114) was conjugated to the recombinant humanized anti-HER2 monoclonal antibody (DX-CHO9, IgG1) to produce DX126-262. In Tub-114, the original 1-methylpiperidine group of the natural Tubulysin B was substituted with N,N-dimethylpropan-2-amine to reduce hepatic toxicity, and a branched hydrophilic ethylene glycol side chain was also incorporated. The antibody was harvested from CHO cell line culture and purified in three chromatographic steps (protein A, anion exchange and cation exchange). The ADC conjugation procedure was performed at pH 6.5-6.8 PBS buffer, wherein DX-CHO9 was subjected to reduction with 3.5 equivalents of TCEP for a duration of 1 hour, followed by a 2-hour reaction with Tub-114. The yielded DX126-262 was then isolated and purified using either chromatography or ultrafiltration/diafiltration (UF/DF). The major metabolite of DX126-262 found in animal plasma was Tub-114-cys, an adduct of Tub-114 and a cysteine residue.
Cell culture
The human gastric carcinoma cell line NCI-N87 was purchased from the National Collection of Authenticated Cell Cultures and SNU-16 was purchased from American Type Culture Collection (ATCC). Both cell lines were maintained in RPMI-1640 supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin in a humidified 5% CO2 incubator at 37°C.
Flow cytometry
For the detection of HER2 expression in NCI-N87 and SNU-16 cell lines, 1×105 cells were incubated on ice for 1 hour with anti-human HER2 mAb from in-house or Human IgG1 Kappa Isotype Control from Sino Biological, Inc. (Beijing, China). Subsequently, cells were stained with Goat anti-Human IgG Fc Secondary Antibody-PE from Thermo Fisher Scientific (Massachusetts, USA) for 30 min. After washing, the labeled cells were analyzed by CytoFlex S flow cytometer (Beckman Coulter, Brea, USA). Relative mean fluorescence intensity (rMFI) was calculated by the following equation: rMFI = MFI of anti-HER2 Ab-PE/MFI of isotype control-PE.
Cell viability assay
NCI-N87 and SNU-16 cells were seeded in a 96-well plate at 6×103 cells/well. After overnight incubation, a serially diluted solution of ADC, anti-HER2 monoclonal antibody, Tub-114-cys or chemotherapeutic drugs were added according to the single drug or combination design. Cell viability was evaluated after 5 days using Cell Counting Kit-8 (CCK-8, Life-iLab, AC11L054) according to the manufacturer’s instructions. The absorbance at 450 nm was measured by SpectraMax 190 microplate reader with reference wavelength of 630 nm.
Analysis of synergistic effects
Drug combination studies were set up using a 4×4 matrix design around IC50 of each drug in NCI-N87 cell line. SynergyFinder plus (https://synergyfinder.org/) and DrugComb (https://drugcomb.org/) online tools were used to analyze the synergistic effects of combinations of DX126-262 and Cisplatin or 5-FU. The dose-response data was imported into the software according to the instructions. The software then performed a comprehensive analysis of the potential synergistic interactions between the two drugs, taking into account their individual mechanisms of action and potential side effects. SynergyFinder plus was used to collect the dose-response matrix (inhibition) for the two drugs of interest from in vitro experiments. The inhibition ratio is positively correlated to the degree of red. DrugComb software was used to conduct quantitative analysis of the synergistic effects of two drugs in combination. Combination sensitivity score (CSS) was used to determine the sensitivity of a drug pair, and S score was applied to detect true synergistic drug combination at an accuracy level comparable to that using the full matrix design. Moreover, ZIP, Bliss, Loewe and HSA models were further used to quantify their synergistic interactions as previously described [27], a score significantly greater than 1 indicates synergy, less than 1 indicates antagonism, and close to 1 suggests additivity. The data obtained from these analyses were then visualized using isobolograms, providing a comprehensive and informative representation of the interactions between drug combinations.
Combination index (CI) score was calculated using Compusyn software to further quantify and analyze the synergistic effect as previously described [28]. The CI score quantitatively defines synergism (CI<1), additive effect (CI=1) and antagonism (CI>1) in drug combinations.
In vivo tumor growth inhibition studies
Specific pathogen-free (SPF) female BALB/c nude mice aged 5-7 weeks were purchased from Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China). NCI-N87 Xenograft model was established by subcutaneous injecting 5×106 cells in the flanks of the mice. After 8-10 days, the tumor-bearing mice were randomized into treatment and control groups based on the tumor volume, and dosing initiated (day 1). NCI-N87 xenografts were treated with saline (solvent control), DX126-262 (1.5, 3.0, 6.4 mg/kg, Intravenous injection (iv), Q3W×2), Herceptin (1.5, 3.0, 6.4 mg/kg, iv, Q3W×2), DS-8201a (1.5, 3.0, 6.4 mg/kg, iv, Q3W×2), Cisplatin (1 mg/kg, iv, Q3W×2) plus 5-FU (15 mg/kg, iv, QW×5), or the combination of DX126-262 or Herceptin and Cisplatin plus 5-FU according to their grouping. Tumor volumes and body weights were measured twice a week, and the volume was calculated as follows: “V = (a×b2)/2, where a refers to tumor length and b refers to tumor width”. When tumor volume reached to about 1,500 mm3, mice were euthanized by cervical dislocation and tumors were weighted and collected. Tumor growth inhibition (TGI, %) was calculated according to the following equation: TGI = (1 - TmRTV/CmRTV) ×100%; TmRTV and CmRTV are the mean relative tumor volumes of the treatment and control groups, respectively, on a given day. RTV (Relative Tumor Volume) = Vt/V0, V0 is the individual’s tumor volume on the day of randomization (day 1), Vt is its tumor volume after the treatment on a given day (day t). All mice were maintained in SPF facilities at the laboratory animal public service platform of MSLT BIOTECH (Hangzhou) Co., Ltd. All animal procedures were approved by the Institutional Animal Care and Use Committee of MSLT BIOTECH (Hangzhou) Co., Ltd. in accordance with the ethical regulations regarding animal research.
Statistical analysis
The data are presented as Mean ± SD (SD: Standard Deviation). Statistical analyses and graphical representations were conducted employing the SPSS 18.0 and GraphPad Prism 6.0 software. For the purpose of comparisons, a one-way ANOVA or two-way ANOVA were performed, followed by either Tukey’s or Dunnett’s post hoc tests, depending on the suitability of the data. A value of P<0.05 was considered statistically significantly different.
Results
DX126-262 significantly inhibited the growth of HER2-positive gastric cancer NCI-N87 cells
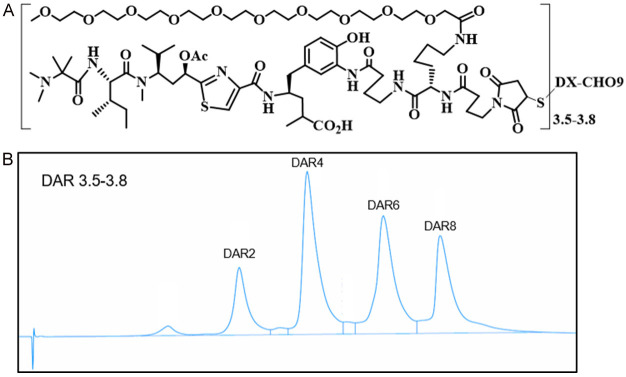
As shown in Figure 1, DX126-262 is a Tubulysin B analogue (Tub-114) conjugate containing branched hydrophilic polyethylene glycol linker. This linker is attached to the HER2 antibody using thiol-maleimide chemistry, resulting in a drug-to-antibody ratio (DAR) of 3.5-3.8 (Figure 1A, 1B).
Figure 1.
Structure and characterization of DX126-262. A. The chemical structure of DX126-262. B. The conjugated drug distribution by HIC-HPLC analysis.
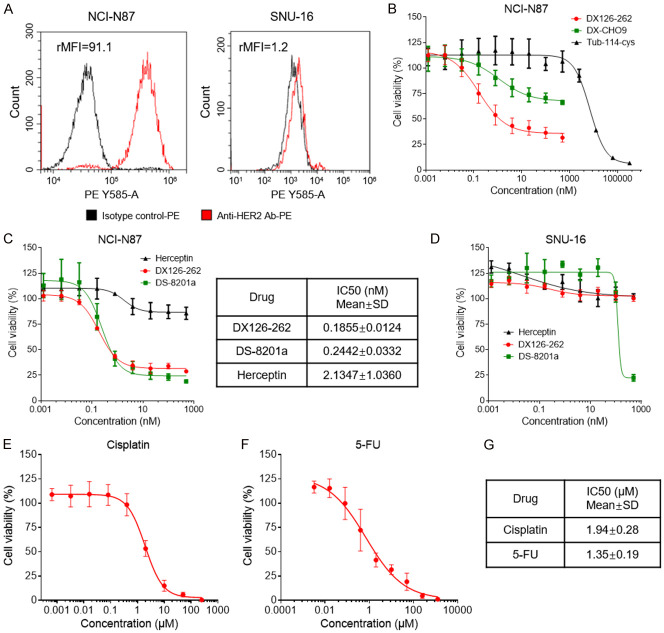
HER2 expression levels on the surface of NCI-N87 and SNU-16 cells were quantified utilizing flow cytometric analysis (Figure 2A). The relative MFI (rMFI) of NCI-N87 was 91.1, which is similar to the previous findings reported by Yusuke Ogitani et al. [21], indicating that HER2 is clearly expressed on the cell surface. The rMFI of SNU-16 was 1.2, suggesting lack of HER2 expression in the SNU-16 cell. To determine the inhibitory effects of DX126-262 on the proliferation of gastric cancer cells that overexpress HER2, we conducted an in vitro assessment of DX126-262’s antiproliferative activity against the NCI-N87 cell line. Additionally, Herceptin (Trastuzumab, a well-established anti-HER2 monoclonal antibody) and DS-8201a (a HER2-targeted ADC) were employed as positive controls. As shown in Figure 2B, 2C, DX126-262 exhibited a remarkable dose-dependent inhibitory activity of cell growth, with IC50 of 0.1855±0.0123 nM, and its antitumor activity was significantly enhanced when compared to its monoclonal antibody (DX-CHO9) and payload (Tub-114-cys) (Figure 2B). Although Herceptin also demonstrated growth-inhibitory activity against NCI-N87 cells, its potency was substantially weaker, as evidenced by IC50 of 2.1347±1.0360 nM (Figure 2C). Furthermore, the positive control DS-8201a also displayed excellent inhibition of NCI-N87 cell growth, with IC50 of 0.2442±0.0332 nM, slightly inferior to DX126-262 (Figure 2C). These results indicated that a substantial enhancement in the cell growth-inhibitory activity of anti-HER2 mAb when conjugated to payload Tub-114-cys. Moreover, we also elucidated the target specificity of DX126-262. As shown in Figure 2D, DX126-262 did not show cell growth-inhibitory activity in HER2-negative SNU-16 cells, similar to the results observed with Herceptin. In contrast, DS-8201a demonstrated a certain degree of antitumor activity at high concentrations. Taken together, these data demonstrates that DX126-262 exhibits a target-specific and potent growth inhibition against the HER2-positive gastric cancer cell line, highlighting its potential as a HER2-targeted therapeutic agent.
Figure 2.
HER2 expression and in vitro cell growth inhibitory activity in NCI-N87 and SNU-16 cell lines. (A) Flow cytometric analysis of HER2 expression at the cell surface of NCI-N87 and SNU-16 cells. Relative mean fluorescence intensity (rMFI) values for HER2 are shown above. (B) In vitro cell growth inhibitory activity in HER2-positive NCI-N87 cell line. The cells were treated with DX126-262, DX-CHO9, and Tub-114-cys for 5 days. Each point represents the Mean and SD (n=3). (C) In vitro cell growth inhibitory activity in the NCI-N87 cell line. The cells were treated with DX126-262, DS-8201a, and Herceptin for 5 days. The IC50 values were calculated by logistic regression using the GraphPad Prism software and shown in the table on the right. (D) In vitro cell growth inhibitory activity in HER2-negative SNU-16 cell line. The cells were treated with DX126-262, DS-8201a, and Herceptin for 5 days. (E, F) Cell viability determined using a Cell Counting Kit-8 (CCK-8) in NCI-N87 cells after exposure to Cisplatin (E) or 5-FU (F) at different doses for 5 days. (G) The IC50 value of the cell growth inhibitory activity of Cisplatin or 5-FU in NCI-N87 cells are shown as Mean ± SD. Each experiment was repeated at least 3 times.
The combination of DX126-262 and chemotherapy showed a significant synergistic inhibitory effect on NCI-N87 cell proliferation in vitro
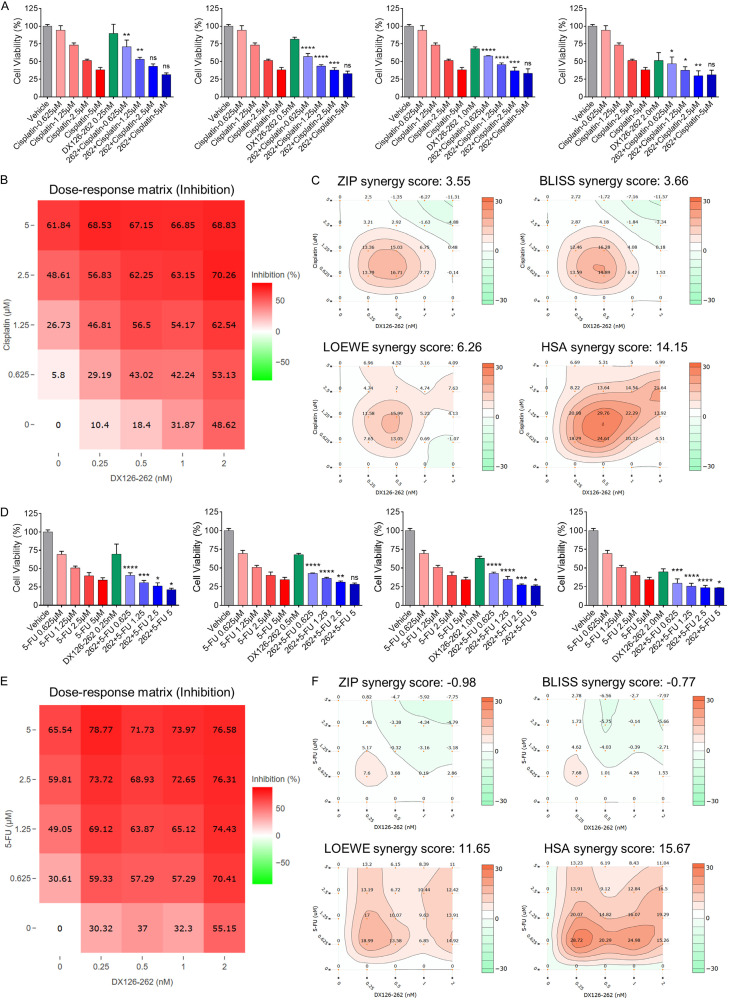
Trastuzumab (Herceptin) combined with Cisplatin and 5-FU is currently the first-line SOC for patients with HER2-positive advanced metastatic gastric cancer. In light of the remarkable superiority of DX126-262 over Herceptin in terms of in vitro growth inhibition, it was expected that the combination strategy of DX126-262 with Cisplatin and 5-FU could potentially elicit a more pronounced and synergistic inhibitory effect, thereby enhancing therapeutic efficacy. The results of cellular viability assessment confirmed that the dose-dependent cell growth-inhibitory activity of Cisplatin and 5-FU on NCI-N87 cell proliferation, with IC50 of 1.94±0.28 μM, 1.35±0.19 μM, respectively (Figure 2E-G). Subsequently, NCI-N87 cells were treated with DX126-262 or Cisplatin, either monotherapy or in combination using a dose-matrix approach to evaluate the proliferation inhibition of different drug combinations. The combination group exhibited different degrees of enhanced proliferative inhibitory activities as compared to the monotherapy treatments (Figure 3A). A dose-response matrix of proliferation inhibition for 4 concentrations of DX126-262 and 4 concentrations of Cisplatin in a 2-fold dilution scheme was summarized in Figure 3B. ZIP, Bliss, Loewe Additivity and Highest Single Agent (HAS) models were used to quantify their synergistic interactions by using DrugComb online tool, respectively. The resulting synergy heatmaps vividly illustrate that DX126-262 and Cisplatin exhibit pronounced synergistic efficacy (depicted as red areas within the model graphs) in inhibiting cell proliferation across a broad spectrum of drug combination ratios (Figure 3C; Table 1). Moreover, the results of the synergy analysis showed that the Combination Synergy Score (CSS) and S score of DX126-262 and Cisplatin were 60.40 and 28.95, respectively, confirming the existence of synergy between DX126-262 and Cisplatin (Table 1). Similar findings were also observed in the analysis of synergistic effect between DX126-262 and 5-FU (Figure 3D-F; Table 1). The drug combination index (CI) score, as calculated by Compusyn software, provided further corroboration of the significant synergistic interaction between DX126-262 and both Cisplatin and 5-FU (Table 2). Collectively, these findings suggested that a significant synergistic effect between DX126-262 and Cisplatin or 5-FU, providing a crucial basis for the potential application of a triple combination therapy.
Figure 3.
The combination of DX126-262 and Cisplatin or 5-FU showed a significant synergistic effect in Her2-positive gastric cancer cells in vitro. (A, D) In vitro cell growth inhibitory activities for combination treatment of DX126-262 and Cisplatin (A) or 5-FU (D) against NCI-N87 cells were evaluated after 5 days using CCK-8. ns: no significance, *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001, combination versus single drug. (B, E) Dose-response matrix (inhibition) for DX126-262 and Cisplatin (B) or 5-FU (E) was analyzed by SynergyFinder plus. The inhibition ratio is positively correlated to the degree of redness. (C, F) The synergy landscape over the dose-response matrix of DX126-262 and Cisplatin (C) or 5-FU (F) in NCI-N87 cells were analyzed by DrugComb online tool.
Table 1.
The synergy scores of DX126-262 combined with Cisplatin or 5-FU were calculated by DrugComb
| Drug A | Drug B | Cell line | CSS | S score | ZIP | BLISS | LOEWE | HSA |
|---|---|---|---|---|---|---|---|---|
| DX126-262 | Cisplatin | NCI-N87 | 60.40 | 28.95 | 3.55 | 3.66 | 6.26 | 14.15 |
| DX126-262 | 5-FU | NCI-N87 | 65.63 | 22.11 | -0.98 | -0.77 | 11.65 | 15.67 |
Table 2.
The Combination index (CI) scores of DX126-262 combined with Cisplatin or 5-FU were calculated by Compusyn software
| CI: NCI-N87 | DX126-262 (nM) | ||||
|
| |||||
| 0.25 | 0.5 | 1 | 2 | ||
|
| |||||
| Cisplatin (μM) | 0.625 | 0.6458 | 0.4018 | 0.4122 | 0.2944 |
| 1.25 | 0.7133 | 0.5294 | 0.5691 | 0.4380 | |
| 2.5 | 1.0473 | 0.8829 | 0.8575 | 0.6744 | |
| 5 | 1.4324 | 1.5020 | 1.5180 | 1.4180 | |
|
| |||||
| CI: NCI-N87 | DX126-262 (nM) | ||||
|
| |||||
| 0.25 | 0.5 | 1 | 2 | ||
|
| |||||
| 5-FU (μM) | 0.625 | 0.2659 | 0.3044 | 0.3047 | 0.1234 |
| 1.25 | 0.2705 | 0.3917 | 0.3595 | 0.1806 | |
| 2.5 | 0.3817 | 0.5486 | 0.4152 | 0.3092 | |
| 5 | 0.4985 | 0.8910 | 0.7485 | 0.6041 | |
The combination of DX126-262 with Cisplatin and 5-FU exhibited promising therapeutic efficacy both in vitro and in vivo
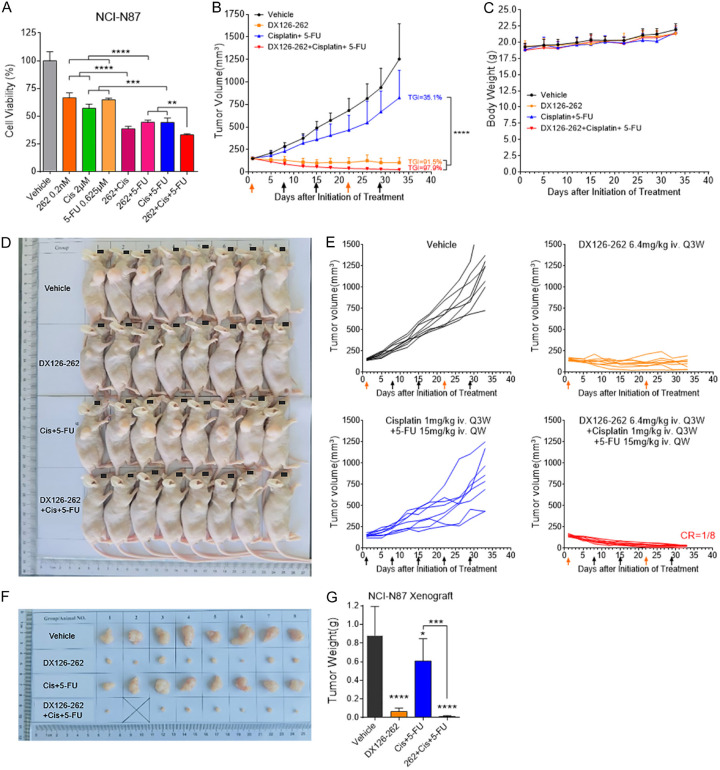
An in vitro proliferation inhibition study was first initiated to evaluate the potential of DX126-262 when combined with Cisplatin and 5-FU. As shown in Figure 4A, triple-drug combination therapy (DX126-262+Cisplatin+5-FU) exhibited superior efficacy in proliferation inhibition in NCI-N87 cells when compared to monotherapy of any individual drug (DX126-262, Cisplatin or 5-FU) or the dual chemotherapeutic combination (Cisplatin+5-FU). Subsequently, we evaluated the efficacy of this triple combination in an NCI-N87 mouse xenograft model. The antitumor effect was evident when compared to vehicle control, as monotherapy of DX126-262 (6.4 mg/kg, day 1, 22) demonstrated significance at day 8 (P<0.05), and the combinations of Cisplatin (1 mg/kg, day 1, 22) and 5-FU (15 mg/kg, day 1, 8, 15, 22, 29) reached statistical significance at day 18 (P<0.01) (Figure 4B). Notably, the combined administration of DX126-262 plus Cisplatin and 5-FU elicited a superior antitumor effect, outperforming the combination of Cisplatin and 5-FU as early as day 12 (P<0.0001) (Figure 4B). The TGI rates for vehicle, DX126-262, Cisplatin+5-FU, and combination of DX126-262+Cisplatin+5-FU at day 33 were 0%, 35.1%, 91.5%, and 97.9%, respectively (Figure 4B, 4D-G). Furthermore, a remarkable complete response (CR) in one of the eight mice was observed within the triple-drug combination therapy group (CR=1/8, Figure 4D-F). All treatment groups were well-tolerated, with no mortality or severe body weight loss observed in any of the mice, indicating the feasibility and safety of this therapeutic approach (Figure 4C). These results clearly demonstrated the benefit of DX126-262 in combination with Cisplatin and 5-FU, without significant superimposed toxicity.
Figure 4.
DX126-262 combined with Cisplatin and 5-FU showed better efficacy than single drug (DX126-262) or Cisplatin plus 5-FU in vitro and in vivo. (A) In vitro cell growth inhibitory activities of combination treatment of DX126-262 plus Cisplatin and 5-FU against NCI-N87 cells were evaluated after 5 days using CCK-8. **P<0.01, ***P<0.001, ****P<0.0001. (B) Antitumor efficacy of DX126-262 combined with chemotherapy (Cisplatin and 5-FU) in NCI-N87 xenograft model. The tumor-bearing mice were intravenously administered with DX126-262 (6.4 mg/kg, day 1, 22), Cisplatin (1 mg/kg, day 1, 22) plus 5-FU (15 mg/kg, day 1, 8, 15, 22, 29) or the combination of DX126-262 plus Cisplatin and 5-FU. The arrow indicates the date of each intravenous administration. Each point represents the Mean tumor volume and SD (n=8). ****P<0.0001. (C) Body weight-time curve of BALB/c nude mice bearing NCI-N87 xenograft. Each point represents the Mean body weight and SD. (D) Summary of the growth of NCI-N87 xenografts under the treatment of control, DX126-262, Cisplatin plus 5-FU or DX126-262 plus Cisplatin and 5-FU. (E) Individual tumor growth curve of the indicated treatments in (B), CR represents complete remission of the tumor. (F) Sizes of exfoliated NCI-N87 xenograft tissues. (G) Tumor weights of NCI-N87 xenografts were represented as Mean ± SD. ***P<0.001, ****P<0.0001.
DX126-262+Cisplatin+5-FU triple therapy showed better in vivo efficacy when compared with first-line or third-line clinical treatment options
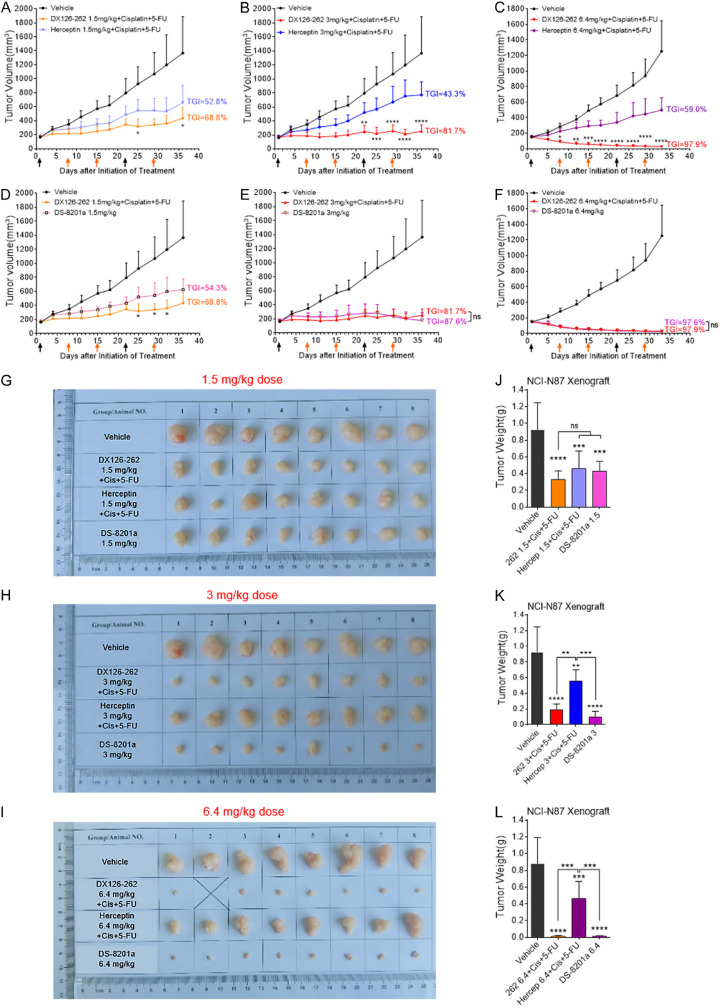
To assess whether the therapeutic efficacy of DX126-262 in combination with Cisplatin and 5-FU is better than the current clinical treatment options, we conducted a comparative analysis of its in vivo efficacy against the standard first-line regimen of Herceptin combined with Cisplatin and 5-FU, as well as the third-line treatment of DS-8201a. The results revealed that varying doses of DX126-262 (1.5, 3, 6.4 mg/kg, iv, day 1, 22), when combined with a fixed dose of Cisplatin (1 mg/kg, iv, day 1, 22) and 5-FU (15 mg/kg, iv, day 1, 8, 15, 22, 29), exhibited a markedly superior in vivo efficacy compared to the Herceptin+Cisplatin+5-FU triplet regimen at equivalent dosing. More importantly, with the increase of the dosage of DX126-262, the overall therapeutic advantage became increasingly pronounced. Specifically, at day 36 post-treatment, the TGI rate for 1.5 mg/kg DX126-262 triple-drug combination group was 68.8%, while only 52.8% TGI was observed in Herceptin triple-drug combination group (Figure 5A). Furthermore, in the 3 mg/kg and 6.4 mg/kg dose cohorts, the TGI rates for the DX126-262 triple-drug group surpassed those of the Herceptin triple-drug group (81.7% vs 43.3%, and 97.9% vs 59.0%) (Figure 5B, 5C). In comparison with DS-8201a, DX126-262 group demonstrated a significantly superior in vivo anti-tumor effect even at a low dosage of 1.5 mg/kg. On day 36, the TGI rate for DX126-262 triple-drug combination group was 68.8%, whereas the DS-8201a group exhibited a TGI of 54.3% (Figure 5D). Further increase in the dose of DX126-262 showed enhanced in vivo efficacy, similar to DS-8201a (Figure 5E, 5F). Comparable results were observed in terms of both the size of exfoliated tissues and the tumor weights in NCI-N87 xenograft model (Figure 5G-L). All together, the above results indicated that DX126-262+Cisplatin+5-FU triple therapy demonstrated a clearly superior in vivo efficacy compared to the first-line Herceptin+Cisplatin+5-FU triple therapy. Furthermore, this therapeutic approach also exhibited either superior or comparable in vivo anti-tumor efficacy against DS-8201a therapy.
Figure 5.
DX126-262+Cisplatin+5-FU triple therapy showed relatively better in vivo efficacy when compared with Herceptin+Cisplatin+5-FU triple therapy or DS-8201a. (A-C) Antitumor efficacy of DX126-262 combined with Cisplatin and 5-FU were compared with the first-line standard of care of Herceptin triple therapy at the same dose in NCI-N87 xenograft model. The tumor-bearing mice were intravenously administered with three different doses of DX126-262 or Herceptin (1.5 mg/kg dose in A, 3 mg/kg dose in B, 6.4 mg/kg dose in C, day 1, 22), at the same time combined with the same dose of Cisplatin (1 mg/kg, day 1, 22) plus 5-FU (15 mg/kg, day 1, 8, 15, 22, 29). The arrow indicates the date of each intravenous administration. Each point represents the Mean tumor volume and SD (n=8). *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001, DX126-262+Cisplatin+5-FU versus Herceptin+Cisplatin+5-FU. (D-F) Antitumor efficacy of DX126-262 combined with Cisplatin and 5-FU was compared with the same dose group of DS-8201a in NCI-N87 xenograft model. *P<0.05, DX126-262+Cisplatin+5-FU versus DS-8201a. (G-I) Sizes of exfoliated NCI-N87 xenograft tissues. (J-L) Tumor weights of NCI-N87 xenografts were represented as Mean ± SD. ns: no significance, **P<0.01, ***P<0.001, ****P<0.0001.
The combination of DX126-262 with Cisplatin and 5-FU did not result in significant overlapping toxicities in NCI-N87 xenograft model
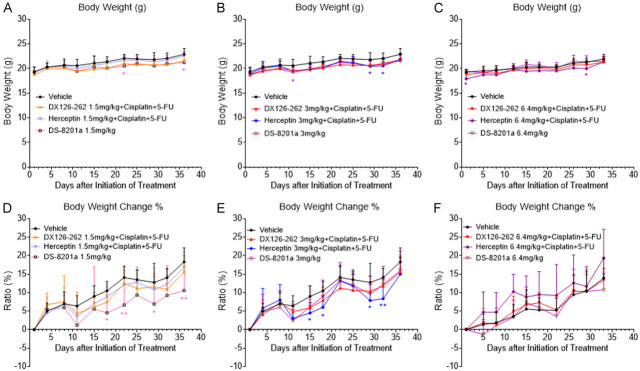
Finally, we further investigated whether the combination of DX126-262 with Cisplatin and 5-FU would result in overlapping toxicities. As shown in Figure 6A-C, varying doses of DX126-262 (1.5, 3, 6.4 mg/kg, iv, day 1, 22) were administered in combination with a fixed dose of Cisplatin (1 mg/kg, iv, day 1, 22) and 5-FU (15 mg/kg, iv, day 1, 8, 15, 22, 29), and no significant mortality or severe body weight loss were observed in any of the DX126-262 triple-drug group mice when compared to the control group (Vehicle). Consistent findings were also observed in the percentage of body weight change of the mice (Figure 6D-F), indicating good tolerability of mice to this triple combination therapy. In contrast, varying degrees of weight loss were observed in the groups treated with the combination of Herceptin with Cisplatin and 5-FU, as well as in the groups treated with DS-8201a monotherapy (Figure 6A-F). Moreover, no significant adverse reactions or symptoms were observed in all groups of mice throughout the entire treatment. These findings suggested that within the current dosage combination range, the triple combination of DX126-262+Cisplatin+5-FU does not exhibit significant overlapping toxicity and demonstrates better safety compared to standard first-line and third-line treatments.
Figure 6.
The combination of DX126-262 with Cisplatin and 5-FU did not result in significant overlapping toxicities in NCI-N87 xenograft models. (A-C) Body weight-time curves of BALB/c nude mice under treatment with Control, different doses of DX126-262 or Herceptin (1.5, 3, 6.4 mg/kg) combined with a fixed dose of Cisplatin (1 mg/kg) and 5-FU (15 mg/kg), or DS-8201a (1.5, 3, 6.4 mg/kg) in NCI-N87 xenograft models. Each point represents the mean body weight and SD (n=8). *P<0.05; * indicates significant differences between the group corresponding to the color and the Vehicle group. (D-F) The percentages of body weight changes of BALB/c nude mice under different treatments (1.5 mg/kg dose in D, 3 mg/kg dose in E, 6.4 mg/kg dose in F) were calculated and shown as Mean ± SD. *P<0.05, **P<0.01; * indicates significant differences between the group corresponding to the color and the Vehicle group.
Discussion
The combination therapy strategy has emerged as an important approach in cancer therapy, holding the potential to significantly enhance clinical efficacy and advance ADC drugs from later-line treatments to first-line/second-line therapies [22]. For example, despite the remarkable efficacy demonstrated by DS-8201a monotherapy in the clinical treatment of advanced gastric cancer, researchers are still continuously exploring the combination therapies involving DS-8201a, hoping to further improve the clinical outcomes. Recently, a phase Ib/II clinical trial has demonstrated that the combination of DS-8201a with Capecitabine or Cisplatin exhibited initial efficacy in the treatment of HER2 positive gastric cancer [25,26].
In our study, we have confirmed that DX126-262 exhibits significant synergistic effects when combined with both Cisplatin and 5-FU in various synergy analysis models. These results provide an important foundation for the design of the DX126-262 triple-drug combination.
Both in vitro cell proliferation inhibition assays and the in vivo NCI-N87 xenograft model have demonstrated that the triple therapy of DX126-262 in combination with Cisplatin and 5-FU is significantly more effective than monotherapy or the dual chemotherapeutic combination of Cisplatin and 5-FU. Its efficacy was also significantly superior to that of first-line SOC (Herceptin in combination with chemotherapy at the same dosage) in NCI-N87 xenograft model. This indicates that DX126-262 exhibits better synergistic effects when combined with Cisplatin and 5-FU compared to Herceptin.
The reasons for this difference can be attributed to the following two aspects: Firstly, the antibody component of DX126-262 has similar molecular mechanism as Herceptin, which is closely related to the inhibition of HER2-PI3K-AKT signal transduction, downregulation of nucleotide excision repair cross-complementation 1 (ERCC1), and interference with cell cycle distribution in HER2-positive gastric cancer cells [29]. Secondly, the payload conjugated to DX126-262 is a tubulin inhibitor Tub-114. After DX126-262 is internalized into the tumor cells, the antibody and linker are degraded by proteinases, releasing its metabolite Tub-114-cys, which can effectively inhibit cell cycle progression and promote tumor cell apoptosis. Furthermore, numerous studies have confirmed that tubulin inhibitors exhibited significant synergistic effects with Cisplatin and 5-FU [30-32]. Additionally, a phase II clinical trial results have found that the combination of Docetaxel (a Tubulin inhibitor) with Trastuzumab, Cisplatin and 5-FU has also been proved to be effective and safe in patients with metastatic HER2-positive gastric or gastro-esophageal junction adenocarcinoma [33]. Moreover, the results of NCI-N87 xenograft model have shown that DX126-262 exhibited superior in vivo antitumor activity compared to DX-CHO9 and Tub-114-cys, alone and in combination (Supplementary Figure 1). This result validates that the format of ADC can further enhance the cytotoxicity of payload towards tumor cells via tumor-specific delivery, thereby improving the therapeutic efficacy [34]. Taken together, these previous literature data and the above results together could help us better understand the rationality and superiority of the combination therapy strategy of DX126-262 in combination with Cisplatin and 5-FU.
Notably, the overlapping toxicities resulting from the combination of ADCs and chemotherapy is also a challenge deserving attention. For instance, in the DESTINY-Gastric03 trial, the combination of DS-8201a with 5-FU or Capecitabine could result in significant toxicities in patients with metastatic HER2-positive gastric cancer, including dose-limiting stomatitis, and a substantial increase in the incidence of grade ≥3 adverse events [25]. Additionally, T-DM1, another HER2-targeted ADC, exhibited dose-limiting toxicities (DLTs) and grade ≥3 adverse events in approximately 80% of patients when combined with Docetaxel (with or without Pertuzumab) for HER2-positive metastatic breast cancer [35]. These studies indicate a possibility of notable increase in toxicity when ADCs are combined with conventional chemotherapeutic agents, which could be due to the overlap of toxicities caused by the off-target and off-tumor effects of the ADC payloads [36]. In this study, no significant weight loss or other severe adverse events were observed in mice when different doses of DX126-262 (1.5, 3, 6.4 mg/kg) were combined with a fixed dose of Cisplatin (1 mg/kg) and 5-FU (15 mg/kg), suggesting that this triple combination strategy did not produce significant overlapping toxicities at least in NCI-N87 xenograft models. Due to interspecies differences, whether obvious additive toxicity will occur in the clinical application of combination therapy in patients will also be one of the critical safety considerations that researchers need to focus on in the future.
By now, DX126-262 is in the phase II clinical trial (CTR20211871), and the monotherapy has shown remarkable efficacy in the treatment of patients with HER2-positive advanced gastric cancer. Combination of DX126-262 with Cisplatin and 5-FU is expected to further enhance clinical efficacy and long-term benefits for patients in the future. Moving forward, we will further explore the potential efficacy and possibilities of combining immunotherapy with DX126-262 and chemotherapy, aiming to discover more effective combination strategies to treat HER2-positive gastric cancer.
In conclusion, the present study provides the first preclinical evidence that DX126-262 in combination with Cisplatin and 5-FU is a potential strategy to improve the clinical efficacy of HER2-positive gastric cancer, without increased drug toxicity. It also suggests that the HER2-targeted combination still plays an irreplaceable role as an anti-gastric cancer therapy.
Acknowledgements
We are indebted to the Core Facility of Hangzhou DAC Biotechnology Co., Ltd. and its staff for assisting a variety of in vitro and in vivo analyses. We thank Huihui Guo, Xiangfei Kong, Yongxiang Chen and You Zhou for helpful discussion and technical assistance. X.H.F. was partly supported by grants from the National Natural Science Foundation of China (U21A20356, 32321002, 31730057), the National Key Research and Development Program of China (2022YFC3401500), the Natural Science Foundation of Zhejiang Province (LD21C070001), Hangzhou West Lake Pearl Project Leading Innovative Youth Team Project (TD2023017), and the Fundamental Research Funds for the Central Universities. X.Z.Y. was supported by grant from the Natural Science Foundation of Zhejiang Province (LQ20H160039).
Disclosure of conflict of interest
R.Y.Z. is the President of Hangzhou DAC Biotechnology Co., Ltd., which holds the intellectual property right of the product DX126-262. X.C., M.C., and Q.Y. are employees of Hangzhou DAC Biotechnology Co., Ltd.
Abbreviations
- 5-FU
5-Fluorouracil
- ADC
Antibody-drug conjugate
- CCK-8
Cell counting kit-8
- CI
Combination index
- CR
Complete response
- DAR
Drug antibody ratio
- DOR
Duration of response
- HER2
Human epidermal growth factor receptor 2
- iv
Intravenous injection
- MFI
Mean fluorescence intensity
- ORR
Objective response rate
- OS
overall survival
- PFS
Progression-free survival
- SOC
Standard-of-care
- TGI
Tumor growth inhibition
Supporting Information
References
- 1.Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73:17–48. doi: 10.3322/caac.21763. [DOI] [PubMed] [Google Scholar]
- 2.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 3.He F, Wang S, Zheng R, Gu J, Zeng H, Sun K, Chen R, Li L, Han B, Li X, Wei W, He J. Trends of gastric cancer burdens attributable to risk factors in China from 2000 to 2050. Lancet Reg Health West Pac. 2024;44:101003. doi: 10.1016/j.lanwpc.2023.101003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhu Y, Zhu X, Wei X, Tang C, Zhang W. HER2-targeted therapies in gastric cancer. Biochim Biophys Acta Rev Cancer. 2021;1876:188549. doi: 10.1016/j.bbcan.2021.188549. [DOI] [PubMed] [Google Scholar]
- 5.Arteaga CL, Sliwkowski MX, Osborne CK, Perez EA, Puglisi F, Gianni L. Treatment of HER2-positive breast cancer: current status and future perspectives. Nat Rev Clin Oncol. 2011;9:16–32. doi: 10.1038/nrclinonc.2011.177. [DOI] [PubMed] [Google Scholar]
- 6.Yan M, Schwaederle M, Arguello D, Millis SZ, Gatalica Z, Kurzrock R. HER2 expression status in diverse cancers: review of results from 37,992 patients. Cancer Metastasis Rev. 2015;34:157–164. doi: 10.1007/s10555-015-9552-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abrahao-Machado LF, Scapulatempo-Neto C. HER2 testing in gastric cancer: an update. World J Gastroenterol. 2016;22:4619–4625. doi: 10.3748/wjg.v22.i19.4619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Cutsem E, Bang YJ, Feng-Yi F, Xu JM, Lee KW, Jiao SC, Chong JL, López-Sanchez RI, Price T, Gladkov O, Stoss O, Hill J, Ng V, Lehle M, Thomas M, Kiermaier A, Rüschoff J. HER2 screening data from ToGA: targeting HER2 in gastric and gastroesophageal junction cancer. Gastric Cancer. 2015;18:476–484. doi: 10.1007/s10120-014-0402-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang D, Lu N, Fan Q, Sheng W, Bu H, Jin X, Li G, Liu Y, Li X, Sun W, Zhang H, Li X, Zhou Z, Yan M, Wang X, Sha W, Ji J, Cheng X, Zhou Z, Xu J, Du X. HER2 status in gastric and gastroesophageal junction cancer assessed by local and central laboratories: Chinese results of the HER-EAGLE study. PLoS One. 2013;8:e80290. doi: 10.1371/journal.pone.0080290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sheng WQ, Huang D, Ying JM, Lu N, Wu HM, Liu YH, Liu JP, Bu H, Zhou XY, Du X. HER2 status in gastric cancers: a retrospective analysis from four Chinese representative clinical centers and assessment of its prognostic significance. Ann Oncol. 2013;24:2360–2364. doi: 10.1093/annonc/mdt232. [DOI] [PubMed] [Google Scholar]
- 11.Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T, Aprile G, Kulikov E, Hill J, Lehle M, Ruschoff J, Kang YK ToGA Trial Investigators. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–697. doi: 10.1016/S0140-6736(10)61121-X. [DOI] [PubMed] [Google Scholar]
- 12.Wang FH, Zhang XT, Tang L, Wu Q, Cai MY, Li YF, Qu XJ, Qiu H, Zhang YJ, Ying JE, Zhang J, Sun LY, Lin RB, Wang C, Liu H, Qiu MZ, Guan WL, Rao SX, Ji JF, Xin Y, Sheng WQ, Xu HM, Zhou ZW, Zhou AP, Jin J, Yuan XL, Bi F, Liu TS, Liang H, Zhang YQ, Li GX, Liang J, Liu BR, Shen L, Li J, Xu RH. The Chinese Society of Clinical Oncology (CSCO): clinical guidelines for the diagnosis and treatment of gastric cancer, 2023. Cancer Commun (Lond) 2024;44:127–172. doi: 10.1002/cac2.12516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guan WL, He Y, Xu RH. Gastric cancer treatment: recent progress and future perspectives. J Hematol Oncol. 2023;16:57. doi: 10.1186/s13045-023-01451-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakamura Y, Kawazoe A, Lordick F, Janjigian YY, Shitara K. Biomarker-targeted therapies for advanced-stage gastric and gastro-oesophageal junction cancers: an emerging paradigm. Nat Rev Clin Oncol. 2021;18:473–487. doi: 10.1038/s41571-021-00492-2. [DOI] [PubMed] [Google Scholar]
- 15.Peng Z, Liu T, Wei J, Wang A, He Y, Yang L, Zhang X, Fan N, Luo S, Li Z, Gu K, Lu J, Xu J, Fan Q, Xu R, Zhang L, Li E, Sun Y, Yu G, Bai C, Liu Y, Zeng J, Ying J, Liang X, Xu N, Gao C, Shu Y, Ma D, Dai G, Li S, Deng T, Cui Y, Fang J, Ba Y, Shen L. Efficacy and safety of a novel anti-HER2 therapeutic antibody RC48 in patients with HER2-overexpressing, locally advanced or metastatic gastric or gastroesophageal junction cancer: a single-arm phase II study. Cancer Commun (Lond) 2021;41:1173–1182. doi: 10.1002/cac2.12214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shitara K, Bang YJ, Iwasa S, Sugimoto N, Ryu MH, Sakai D, Chung HC, Kawakami H, Yabusaki H, Lee J, Saito K, Kawaguchi Y, Kamio T, Kojima A, Sugihara M, Yamaguchi K DESTINY-Gastric01 Investigators. Trastuzumab deruxtecan in previously treated HER2-positive gastric cancer. N Engl J Med. 2020;382:2419–2430. doi: 10.1056/NEJMoa2004413. [DOI] [PubMed] [Google Scholar]
- 17.Jiang M, Li Q, Xu B. Spotlight on ideal target antigens and resistance in antibody-drug conjugates: strategies for competitive advancement. Drug Resist Updat. 2024;75:101086. doi: 10.1016/j.drup.2024.101086. [DOI] [PubMed] [Google Scholar]
- 18.Tarantino P, Carmagnani Pestana R, Corti C, Modi S, Bardia A, Tolaney SM, Cortes J, Soria JC, Curigliano G. Antibody-drug conjugates: smart chemotherapy delivery across tumor histologies. CA Cancer J Clin. 2022;72:165–182. doi: 10.3322/caac.21705. [DOI] [PubMed] [Google Scholar]
- 19.Xiangming X, Friestad GK, Lei Y. Recent advances in the synthesis of tubulysins. Mini Rev Med Chem. 2013;13:1572–1578. doi: 10.2174/13895575113139990063. [DOI] [PubMed] [Google Scholar]
- 20.Kaur G, Hollingshead M, Holbeck S, Schauer-Vukasinovic V, Camalier RF, Domling A, Agarwal S. Biological evaluation of tubulysin A: a potential anticancer and antiangiogenic natural product. Biochem J. 2006;396:235–242. doi: 10.1042/BJ20051735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ogitani Y, Aida T, Hagihara K, Yamaguchi J, Ishii C, Harada N, Soma M, Okamoto H, Oitate M, Arakawa S, Hirai T, Atsumi R, Nakada T, Hayakawa I, Abe Y, Agatsuma T. DS-8201a, a novel HER2-targeting ADC with a novel DNA topoisomerase I inhibitor, demonstrates a promising antitumor efficacy with differentiation from T-DM1. Clin Cancer Res. 2016;22:5097–5108. doi: 10.1158/1078-0432.CCR-15-2822. [DOI] [PubMed] [Google Scholar]
- 22.Fuentes-Antras J, Genta S, Vijenthira A, Siu LL. Antibody-drug conjugates: in search of partners of choice. Trends Cancer. 2023;9:339–354. doi: 10.1016/j.trecan.2023.01.003. [DOI] [PubMed] [Google Scholar]
- 23.Moore KN, O’Malley DM, Vergote I, Martin LP, Gonzalez-Martin A, Malek K, Birrer MJ. Safety and activity findings from a phase 1b escalation study of mirvetuximab soravtansine, a folate receptor alpha (FRα)-targeting antibody-drug conjugate (ADC), in combination with carboplatin in patients with platinum-sensitive ovarian cancer. Gynecol Oncol. 2018;151:46–52. doi: 10.1016/j.ygyno.2018.07.017. [DOI] [PubMed] [Google Scholar]
- 24.Santin AD, Vergote I, Gonzalez-Martin A, Moore K, Oaknin A, Romero I, Diab S, Copeland LJ, Monk BJ, Coleman RL, Herzog TJ, Siegel J, Kasten L, Schlicker A, Schulz A, Kochert K, Walter AO, Childs BH, Elbi C, Bulat I. Safety and activity of anti-mesothelin antibody-drug conjugate anetumab ravtansine in combination with pegylated-liposomal doxorubicin in platinum-resistant ovarian cancer: multicenter, phase Ib dose escalation and expansion study. Int J Gynecol Cancer. 2023;33:562–570. doi: 10.1136/ijgc-2022-003927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Janjigian YY, Oh DY, Rha SY, Lee KW, Steeghs N, Chao Y, Di Bartolomeo M, Garcia MD, Haj Mohammad N, Stein A, McAdoo W, Winter M, Croydon L, Lee J. Dose-escalation and dose-expansion study of trastuzumab deruxtecan (T-DXd) monotherapy and combinations in patients (pts) with advanced/metastatic HER2+ gastric cancer (GC)/gastroesophageal junction adenocarcinoma (GEJA): DESTINY-Gastric03. J. Clin. Oncol. 2022;40:295. [Google Scholar]
- 26.Janjigian YY, Viglianti N, Liu F, Mendoza-Naranjo A, Croydon L. A phase Ib/II, multicenter, open-label, dose-escalation, and dose-expansion study evaluating trastuzumab deruxtecan (T-DXd, DS-8201) monotherapy and combinations in patients with HER2-overexpressing gastric cancer (DESTINY-Gastric03) J. Clin. Oncol. 2024;39:261. [Google Scholar]
- 27.Malyutina A, Majumder MM, Wang W, Pessia A, Heckman CA, Tang J. Drug combination sensitivity scoring facilitates the discovery of synergistic and efficacious drug combinations in cancer. PLoS Comput Biol. 2019;15:e1006752. doi: 10.1371/journal.pcbi.1006752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chou TC. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res. 2010;70:440–446. doi: 10.1158/0008-5472.CAN-09-1947. [DOI] [PubMed] [Google Scholar]
- 29.Li XL, Yi SQ, Xu JM, Zhang Y, Yingying-Feng, Chen W, Song ST. The sequence-dependent cytotoxic effect of trastuzumab in combination with 5-Fluorouracil or cisplatin on gastric cancer cell lines. Cancer Invest. 2010;28:1038–1047. doi: 10.3109/07357907.2010.483512. [DOI] [PubMed] [Google Scholar]
- 30.Lee JH, Park YR, Jung M, Lim SG. Gene regulatory network analysis with drug sensitivity reveals synergistic effects of combinatory chemotherapy in gastric cancer. Sci Rep. 2020;10:3932. doi: 10.1038/s41598-020-61016-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ajani JA. Optimizing docetaxel chemotherapy in patients with cancer of the gastric and gastroesophageal junction: evolution of the docetaxel, cisplatin, and 5-fluorouracil regimen. Cancer. 2008;113:945–955. doi: 10.1002/cncr.23661. [DOI] [PubMed] [Google Scholar]
- 32.Al-Batran SE, Homann N, Pauligk C, Goetze TO, Meiler J, Kasper S, Kopp HG, Mayer F, Haag GM, Luley K, Lindig U, Schmiegel W, Pohl M, Stoehlmacher J, Folprecht G, Probst S, Prasnikar N, Fischbach W, Mahlberg R, Trojan J, Koenigsmann M, Martens UM, Thuss-Patience P, Egger M, Block A, Heinemann V, Illerhaus G, Moehler M, Schenk M, Kullmann F, Behringer DM, Heike M, Pink D, Teschendorf C, Löhr C, Bernhard H, Schuch G, Rethwisch V, von Weikersthal LF, Hartmann JT, Kneba M, Daum S, Schulmann K, Weniger J, Belle S, Gaiser T, Oduncu FS, Güntner M, Hozaeel W, Reichart A, Jäger E, Kraus T, Mönig S, Bechstein WO, Schuler M, Schmalenberg H, Hofheinz RD FLOT4-AIO Investigators. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. Lancet. 2019;393:1948–1957. doi: 10.1016/S0140-6736(18)32557-1. [DOI] [PubMed] [Google Scholar]
- 33.Mondaca S, Margolis M, Sanchez-Vega F, Jonsson P, Riches JC, Ku GY, Hechtman JF, Tuvy Y, Berger MF, Shah MA, Kelsen DP, Ilson DH, Yu K, Goldberg Z, Epstein AS, Desai A, Chung V, Chou JF, Capanu M, Solit DB, Schultz N, Janjigian YY. Phase II study of trastuzumab with modified docetaxel, cisplatin, and 5 fluorouracil in metastatic HER2-positive gastric cancer. Gastric Cancer. 2019;22:355–362. doi: 10.1007/s10120-018-0861-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Colombo R, Rich JR. The therapeutic window of antibody drug conjugates: a dogma in need of revision. Cancer Cell. 2022;40:1255–1263. doi: 10.1016/j.ccell.2022.09.016. [DOI] [PubMed] [Google Scholar]
- 35.Martin M, Fumoleau P, Dewar JA, Albanell J, Limentani SA, Campone M, Chang JC, Patre M, Strasak A, de Haas SL, Xu J, Garcia-Saenz JA. Trastuzumab emtansine (T-DM1) plus docetaxel with or without pertuzumab in patients with HER2-positive locally advanced or metastatic breast cancer: results from a phase Ib/IIa study. Ann Oncol. 2016;27:1249–1256. doi: 10.1093/annonc/mdw157. [DOI] [PubMed] [Google Scholar]
- 36.Wei Q, Li P, Yang T, Zhu J, Sun L, Zhang Z, Wang L, Tian X, Chen J, Hu C, Xue J, Ma L, Shimura T, Fang J, Ying J, Guo P, Cheng X. The promise and challenges of combination therapies with antibody-drug conjugates in solid tumors. J Hematol Oncol. 2024;17:1. doi: 10.1186/s13045-023-01509-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.