Abstract
This study aims to identify factors influencing aesthetic outcomes following facial basal cell carcinoma (BCC) plastic surgery to enhance post-operative satisfaction and cosmetic results. A retrospective cohort study was conducted on 303 patients who underwent facial BCC plastic surgery between June 2021 and June 2023. Data on demographics, blood tests, SF-12, and Skindex-16 scores were analyzed. Patients were categorized into satisfactory and unsatisfactory outcome groups based on post-operative assessments. The training set of patients was sourced from the Third Affiliated Hospital of Soochow University, while the testing set of patients was sourced from the First Affiliated Hospital of Soochow University. Of 209 patients, 116 were in the satisfactory group, 93 in the unsatisfactory. Factors enhancing positive outcomes included reconstruction methods (P < 0.001) and smaller tumor diameters (P = 0.006). Higher pre-op 12-item Short Form Survey (SF-12) scores correlated with better outcomes (P = 0.005). Lower Skindex-16 scores were noted in the satisfactory group (P < 0.001). Logistic regression highlighted reconstruction method, aging signs, SF-12 scores, and Skindex-16 as key predictors. A random forest model achieved an area under the curve (AUC) of 0.984. External validation confirmed similar associations with satisfactory outcomes (AUC = 0.870). Aesthetic outcomes in facial BCC plastic surgery are influenced by reconstruction method and tumor diameter, patient health status (SF-12), and skin-related quality of life (Skindex-16). Personalized surgical planning and comprehensive care are essential for optimizing outcomes.
Keywords: Basal cell carcinoma, plastic surgery, aesthetic outcome, 12-item Short Form Survey, Skindex-16, tumor diameter, reconstruction methods
Introduction
Basal cell carcinoma (BCC) is the most prevalent form of skin cancer, accounting for approximately 80% of all non-melanoma skin cancers [1]. As a malignancy primarily affecting the skin, BCC often occurs on sun-exposed areas of the body, with the face being one of the most common sites [2]. The high incidence of facial BCC poses significant challenges, not only due to its local invasiveness but also the critical importance of facial aesthetics and function [3,4]. Hence, surgical excision remains the gold standard treatment, aiming to completely remove tumor while preserving cosmetic and functional integrity [5]. However, outcomes vary considerably depending on multiple factors including patient demographics, tumor characteristics, and surgical techniques employed [6].
Several studies have identified factors that influence outcomes following BCC excision and reconstruction, such as primary closure, local flap reconstruction, and skin grafting [7-9]. Each method carries distinct advantages and limitations based on the defect size, location, and patient-specific factors [10]. Direct suturing and local flaps are often favored for their superior aesthetic results, attributed to better color and texture matching with surrounding tissues and enhanced vascularization that promotes healing [11]. In contrast, skin grafting, while effective for larger defects, frequently results in aesthetic discrepancies and a higher rates of complications [12].
Health-related quality of life (HRQoL) is a critical measure in evaluating the success of facial BCC surgery. Beyond the physical removal of the tumor, the psychosocial implications of surgical outcomes significantly influence patient satisfaction [13]. Tools like the 12-item Short Form Survey (SF-12) capture both physical and mental health dimensions, providing a comprehensive evaluation of postoperative outcomes [14]. Dermatology-specific instruments like the Skindex-16 delve into the impact of skin disease on quality of life, encompassing functional, emotional, and symptomatic domains [15].
Emerging evidence suggests that systemic and local inflammatory responses may influence surgical outcomes and healing processes in skin cancer patients [16]. Biomarkers such as interleukin-6 (IL-6), interleukin-8 (IL-8), tumor necrosis factor-alpha (TNF-α), and C-reactive protein (CRP) are indicative of inflammatory states that could potentially impact wound healing and surgical recovery [17]. However, the specific relationship between preoperative inflammatory markers and cosmetic outcomes in BCC surgery remains to be clarified.
Given the complexity of influencing factors and the variability in outcomes, there is a need for a predictive model that can assist clinicians in selecting the most appropriate reconstructive strategy tailored to individual patients. The current study aims to construct such a model by analyzing a comprehensive dataset, including detailed preoperative, intraoperative, and postoperative parameters, to identify key predictors of patient satisfaction and develop a practical tool for clinical decision-making.
Materials and methods
Subjects
This retrospective cohort study included 303 patients who underwent facial BCC resection via plastic surgery between June 2021 and June 2023 at the Third Affiliated Hospital of Soochow University and the First Affiliated Hospital of Soochow University. Among these, 209 patients with complete preoperative and postoperative data were included in the training set for the predictive model, and 94 patients were included in the test set for external validation. Patient demographic information, including general information, complete blood count, serum inflammatory markers, SF-12 score and Skindex-16 score, was collected through the medical record system. The study was approved by the Ethical Review Committee of the Third Affiliated Hospital of Soochow University and the First Affiliated Hospital of Soochow University. Informed consent was waived due to the retrospective nature of this study. The sample size for this study was calculated to ensure sufficient statistical power to detect a clinically significant difference in the primary outcome measure, which was the proportion of patients achieving satisfactory aesthetic outcomes after facial BCC surgery. To ensure sufficient statistical power, a power analysis was conducted based on preliminary data indicating an expected effect size of d = 0.8 with α = 0.05 and power (1 - β) = 0.95. This analysis suggested a minimum sample size of 42 participants per group to detect a significant difference between the two groups. Considering an anticipated dropout rate, the final sample size was adjusted accordingly.
Inclusion Criteria: (1) Age > 18 years, no history of mental illness, normal cognitive function, and the ability to cooperate with various treatments and examinations; (2) Patients diagnosed with a single facial BCC with planned plastic surgery resection.
Exclusion Criteria: (1) Unstable vital signs, including heart rate, body temperature, and blood pressure; (2) Severe cognitive impairment (mini-mental state examination (MMSE) score < 24 points), visual or auditory dysfunction, history of mental illness, or inability to cooperate with treatment or examination; (3) Patients with failed follow-up or incomplete clinical data within one month after surgery.
Grouping criteria
The primary focus of this research is the aesthetic outcome post-repair. One month after surgery, cosmetic results were evaluated jointly by the chief surgeon, other departmental physicians, and patients themselves, based on five criteria [18]. (1) Symmetry: 3 points for bilateral symmetry, 2 points for slight asymmetry, and 1 point for severe asymmetry; (2) Organ traction deformation: 3 points denote no deformation, 2 points for mild deformation, and 1 point for obvious deformation; (3) Local flatness: 3 points for smoothness without protrusions or depressions, 2 points for minor protrusions or depressions, and 1 point for noticeable protrusions or depressions; (4) Color match: 3 points for matching surrounding tissue color, 2 points for some color difference, and 1 point for significant color disparity; (5) Texture: 3 points for soft texture, 2 points for medium texture, and 1 point for hard texture.
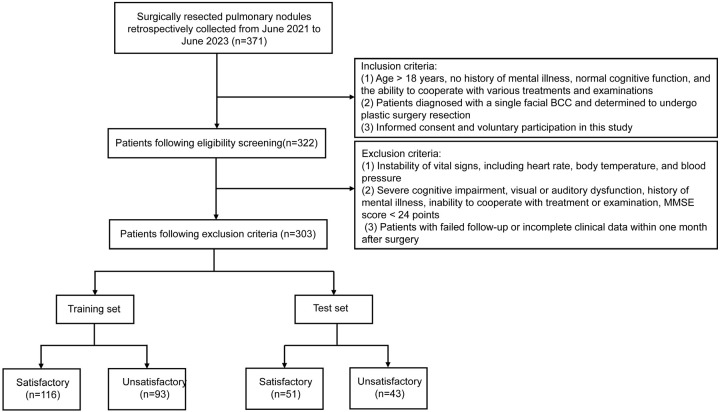
Patients were classified into two groups based on their total aesthetic effect scores post-repair. A score of 27 or higher indicated a satisfactory outcome (satisfactory group, n = 116), whereas scores below 27 were considered unsatisfactory (unsatisfactory group, n = 93). A total of 209 patients were included in the training set for this study. In addition, an internal validation was conducted using a 10-fold cross-validation method to ensure the stability and reliability of the model. For external validation, 94 patients who met the same inclusion criteria were also evaluated. Based on the overall aesthetic score after repair, these patients were similarly divided into a satisfactory group (n = 51) and an unsatisfactory group (n = 43). The training set of patients was sourced from the Third Affiliated Hospital of Soochow University, while the testing set of patients was sourced from the First Affiliated Hospital of Soochow University. The study design flow chart for this research is shown in Figure 1.
Figure 1.
Study design flowchart.
Blood testing
Fasting venous blood (5 ml) was collected from each patient before 8 am. DxH800 blood analyzer (Beckman Coulter, Inc., Brea, CA, USA) was utilized to measure complete blood count, including red blood cells, white blood cells, neutrophils, lymphocytes, eosinophils, basophils, hemoglobin, and platelets. CRP level was assessed using the BECKMAN Synchronx20 fully automatic biochemical analyzer (Beckman Coulter, Inc., Brea, CA, USA) with rate scattering turbidimetry. Whole blood samples were anticoagulated with ethylenediaminetetraacetic acid (EDTA), and the erythrocyte sedimentation rate (ESR) was measured using the TEST 1 automated analyzer (ALIFAX, Inc., Italy). The sample was centrifuged at 3000 revolutions per minute for 5 minutes to extract the supernatant. Tumor necrosis factor-alpha (TNF-α) (ab181421, Abcam, USA), interleukin-6 (IL-6) (ab178013, Abcam, USA), and interleukin-8 (IL-8) (ab185986, Abcam, USA) levels were measured using enzyme-linked immunosorbent assay (ELISA).
Surgical methods
Surgeons carry out operations under either local infiltration anesthesia or general anesthesia for tracheal intubation, depending on the patient’s condition. Using methylene blue, the incision line was demarcated according to the tumor’s type and location, with a margin of 0.4-2.0 cm from the tumor boundary. The affected tissue was excised completely along the marked line and sent for immediate frozen pathological assessment. If the pathology report indicated a positive margin, the resection boundary was extended. Once a negative margin was confirmed, surgical repair was initiated based on the dimensions of the wound.
The primary repair techniques include direct suturing, local skin flaps, free skin grafts, and free skin flaps. Direct suturing involves sequentially reducing tension and closing the wound in layers. Local skin flaps may involve various methods such as advancement, rotation, kite, dual triangle, and expansion flaps, depending on the site’s anatomy, to preserve aesthetic tissue morphology. During flap repair, the flap was designed slightly larger than the wound to ensure adequate blood circulation at the pedicle. For free skin grafting, donor skin closely match the facial skin color was chosen to minimize post-operative color mismatch, and nylon thread was used for suturing to reduce visible scarring, particularly near the eyes. For free flap procedures, meticulous attention was given to blood vessel anastomosis and maintaining good pedicle blood supply during post-operative bandaging.
Health status, SF-12 score
The SF-12 survey is a 12-question instrument used to assess an individual’s health status across eight dimensions, aiming to identify health problems and evaluate life functions. The overall score is calculated by summing the weighted scores of each dimension. Higher total scores indicates better health status. The SF-12 is divided into two components: Mental Health Component (MCS) and Physical Health Component (PCS). The instrument shows good internal consistency, as evidenced by Cronbach’s alpha values of 0.707 for MCS and 0.743 for PCS [19]. The SF-12 scores were obtained at least one month prior to surgery to avoid the influence of surgical outcomes on the results.
Skin-related QOL, Skindex-16 subscale
Skindex-16 serves as an instrument for evaluating skin health, consisting of 16 questions that address various aspects. The assessment is divided into three primary dimensions: symptoms, emotions, and function. Higher scores on this scale indicate a more significant impact of skin issues on the individual assessed. The internal consistency of the Skindex-16 is demonstrated by Cronbach’s alpha values: 0.867 for symptoms, 0.930 for emotions, and 0.888 for functioning, demonstrating strong reliability for each domains [20]. The Skindex-16 scores were also obtained at least one month before the surgery to ensure that they were not influenced by the surgical outcomes.
Statistical method
Measurement data were presented as either the mean ± standard deviation (SD) or the median with interquartile range (IQR), depending on whether the data conform to a normal distribution. Categorical data were reported as frequencies and percentages. Continuous variables were compared between two groups using unpaired t-tests. Univariate and multivariate logistic regression analyses were performed to calculate the odds ratio (OR) and 95% confidence interval (CI) for each parameter when treated as a continuous variable. A P value of less than 0.05 indicates statistical significance. All statistical analyses were performed using SPSS software version 19 (SPSS Inc., Chicago, IL, USA) and the R software package version 3.0.2 (Free Software Foundation, Inc., Boston, MA, USA).
Results
Comparison of general information between two groups of patients
Demographic and clinical characteristics of the study participants are summarized in Table 1. There were no significant differences between the Satisfactory group (n = 116) and the Unsatisfactory group (n = 93) for most parameters. However, the reconstruction method showed significant differences between groups, with the Satisfactory group having higher proportions of direct stitching and local skin flaps, whereas the Unsatisfactory group had a higher usage of skin grafting (P < 0.001).
Table 1.
Comparison of general information between two groups
| Parameters | Satisfactory group (n = 116) | Unsatisfactory group (n = 93) | t/χ2 | P |
|---|---|---|---|---|
| Age (years) | 60.40 ± 27.50 | 62.86 ± 28.2 | 0.636 | 0.526 |
| BMI (kg/m2) | 24.73 ± 3.30 | 25.02 ± 3.12 | 0.666 | 0.506 |
| Education Level (years) | 13.36 ± 3.62 | 13.32 ± 2.85 | 0.082 | 0.935 |
| Gender [n (%)] | 0.039 | 0.844 | ||
| Male | 75 (64.66%) | 58 (62.37%) | ||
| Female | 41 (35.34%) | 35 (37.63%) | ||
| Employment, work for pay [n (%)] | 66 (56.90%) | 40 (43.01%) | 3.446 | 0.063 |
| Hypertension [n (%)] | 0.071 | 0.79 | ||
| Yes | 38 (32.76%) | 33 (35.48%) | ||
| No | 78 (67.24%) | 60 (64.52%) | ||
| Diabetes Mellitus [n (%)] | 0.226 | 0.635 | ||
| Yes | 45 (38.79%) | 40 (43.01%) | ||
| No | 71 (61.21%) | 53 (56.99%) | ||
| Smoking history [n (%)] | 49 (42.24%) | 42 (45.16%) | 0.08 | 0.777 |
| Drinking history [n (%)] | 52 (44.83%) | 47 (50.54%) | 0.465 | 0.495 |
| History of previous NMSC [n (%)] | 64 (55.17%) | 48 (51.61%) | 0.139 | 0.709 |
| Aging signs [n (%)] | 84 (72.41%) | 83 (89.25%) | 8.091 | 0.004 |
| Anatomic location of BCC [n (%)] | None | 0.981 | ||
| Forehead unit | 34 (29.31%) | 25 (26.88%) | ||
| Nasal unit | 38 (32.76%) | 31 (33.33%) | ||
| Eyelid units | 16 (13.79%) | 13 (13.98%) | ||
| Cheek units | 23 (20.17%) | 21 (22.58%) | ||
| Upper/lower lips | 5 (4.31%) | 3 (3.23%) | ||
| Reconstruction method [n (%)] | None | P < 0.001 | ||
| Direct stitching | 22 (19.05%) | 6 (6.45%) | ||
| Local skin flap | 76 (65.52%) | 33 (35.48%) | ||
| Skin grafting | 15 (12.93%) | 51 (54.84%) | ||
| Free skin flap | 3 (2.59%) | 3 (3.23%) | ||
| Treating clinician [n (%)] | 0.213 | 0.899 | ||
| Attending physician | 61 (52.59%) | 46 (49.46%) | ||
| Resident | 31 (26.72%) | 26 (27.96%) | ||
| Nurse practitioner | 24 (20.69%) | 21 (22.58%) |
Note: BMI: body mass index; NMSC: Non-melanoma Skin Cancer; BCC: basal cell carcinoma.
Comparison of preoperative blood routine test between two groups of patients
The comparison of preoperative blood routine test results between two groups did not reveal any significant differences in terms of ESR, Red blood cell counts, white blood cell counts, neutrophil counts, lymphocyte counts, eosinophil counts, basophil counts, hemoglobin levels, and platelet counts (Table 2).
Table 2.
Comparison of preoperative blood routine test between two groups of patients
| Parameters | Satisfactory group (n = 116) | Unsatisfactory group (n = 93) | t | P |
|---|---|---|---|---|
| ESR (mm/h) | 35.83 ± 5.36 | 34.76 ± 4.98 | 1.488 | 0.138 |
| Red blood cell (1×106/μL) | 5.44 ± 1.59 | 5.32 ± 1.67 | 0.51 | 0.61 |
| White blood cell (1×103/μL) | 7.38 ± 1.62 | 7.26 ± 1.67 | 0.513 | 0.609 |
| Neutrophil (1×103/μL) | 4.32 ± 1.06 | 4.37 ± 1.08 | 0.379 | 0.705 |
| Lymphocyte (1×103/μL) | 2.03 ± 0.68 | 2.09 ± 0.71 | 0.64 | 0.523 |
| Eosinophil (1×102/μL) | 0.28 ± 0.03 | 0.28 ± 0.03 | 1.566 | 0.119 |
| Basophil (1×102/μL) | 0.09 ± 0.03 | 0.09 ± 0.03 | 0.129 | 0.897 |
| Hemoglobin (g/L) | 149.4 ± 24.8 | 149.8 ± 25.3 | 0.114 | 0.909 |
| Platelet (1×103/μL) | 215.8 ± 121.8 | 215.6 ± 120.6 | 0.012 | 0.99 |
Note: ESR: erythrocyte sedimentation rate.
Comparison of serum inflammatory factors between two groups of patients
The comparison of serum inflammatory factors between the satisfactory and unsatisfactory groups before treatment did not reveal any significant differences in terms of IL-6, IL-8, TNF-α, and CRP levels (all P > 0.05, Table 3).
Table 3.
Comparison of serum inflammatory factors between two groups of patients before treatment
| Parameters | Satisfactory group (n = 116) | Unsatisfactory group (n = 93) | t | P |
|---|---|---|---|---|
| IL-6 (ng/L) | 28.28 ± 1.15 | 28.25 ± 1.12 | 0.22 | 0.826 |
| IL-8 (μg/L) | 36.51 ± 1.81 | 36.52 ± 1.89 | 0.024 | 0.981 |
| TNF-α (pg/ml) | 18.26 ± 1.88 | 18.25 ± 1.79 | 0.038 | 0.969 |
| CRP (mg/L) | 16.18 ± 3.14 | 15.74 ± 3.77 | 0.895 | 0.372 |
Note: IL: interleukin; TNF: tumor necrosis factor; CRP: C-reactive protein.
Comparison of tumor characteristics between two groups of patients
The comparison of tumor characteristics between the satisfactory and unsatisfactory groups revealed a significant difference in tumor diameter (Table 4). A greater proportion of patients in the satisfactory group had tumors with a diameter of 10 mm or less compared to the unsatisfactory group (68 vs. 36; P = 0.006). However, the presence of histologic risk factors for recurrence did not significantly differ between the two groups (P = 0.339). Additionally, there was no significant difference in the progression time (P = 0.777).
Table 4.
Comparison of tumor characteristics between two groups of patients
| Parameters | Satisfactory group (n = 116) | Unsatisfactory group (n = 93) | t/χ2 | P |
|---|---|---|---|---|
| Tumor diameter [n (%)] | 7.409 | 0.006 | ||
| ≤ 10 mm | 68 (58.62%) | 36 (38.71%) | ||
| > 10 mm | 48 (41.38%) | 57 (61.29%) | ||
| Histologic risk factor for recurrence (presence/absence) | 19/97 | 21/72 | 0.913 | 0.339 |
| Progression (months) | 12.35 ± 3.12 | 12.47 ± 3.20 | 0.283 | 0.777 |
Comparison of health status (SF-12 score) between two groups of patients
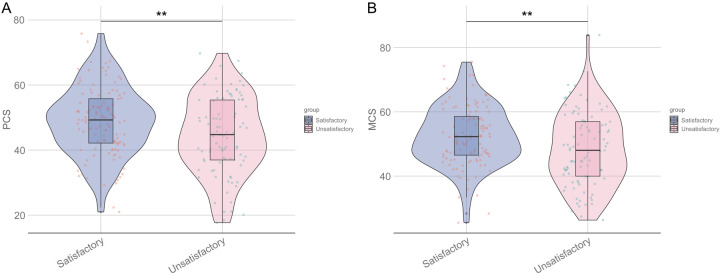
SF-12 scores were significantly higher in the satisfactory group for both PCS (48.87 ± 10.61 vs. 44.35 ± 11.93; P = 0.005) and MCS (52.46 ± 9.28 vs. 48.32 ± 11.47; P = 0.005) (Figure 2).
Figure 2.
Comparison of SF-12 scores between two groups of patients. A: PCS; B: MCS. PCS: physical health factors; MCS: mental health factors; **: P < 0.01.
Comparison of skin-related QOL (Skindex-16 subscale score) between two groups of patients
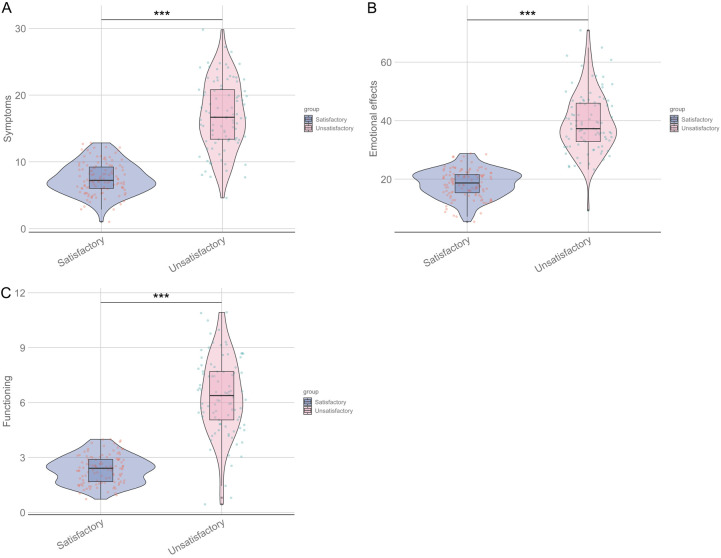
Skindex-16 subscale scores were significantly lower in the satisfactory group across all subscales (symptoms: 7.56 ± 2.38 vs. 17.03 ± 5.17; P < 0.001; emotional effects: 18.47 ± 4.81 vs. 39.32 ± 10.26; P < 0.001; functioning: 2.35 ± 0.76 vs. 6.35 ± 2.11; P < 0.001) compared to the unsatisfactory group (Figure 3).
Figure 3.
Comparison of Skindex-16 subscale scores between two groups of patients. Symptoms (A), Emotional effects (B), Functioning Score (C). ***: P < 0.001.
Univariate correlation analysis of factors affecting the outcomes of plastic surgery
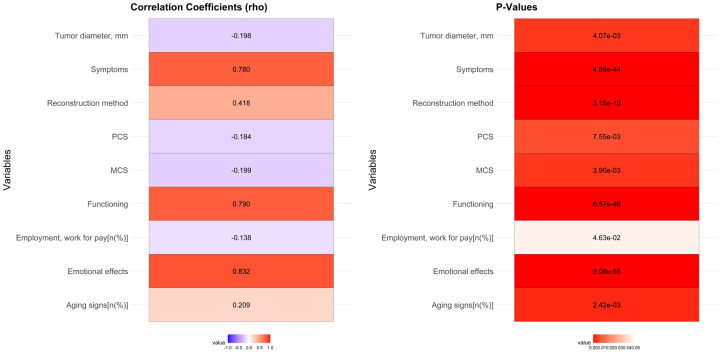
Univariate correlation analysis identified several factors significantly correlated with unsatisfactory outcomes: employment status (rho = -0.138, P = 0.046), aging signs (rho = 0.209, P = 0.002), reconstruction method (rho = 0.418, P < 0.001), tumor diameter (rho = -0.198, P = 0.004), and Skindex-16 subscales (symptoms: rho = 0.780, P < 0.001; emotional effects: rho = 0.832, P < 0.001; functioning: rho = 0.790, P < 0.001) (Figure 4).
Figure 4.
Univariate correlation analysis between unsatisfactory outcome and various factors of plastic surgery.
Multivariate logistic regression analysis of factors affecting the outcomes of plastic surgery
Multivariate logistic regression analysis identified aging signs (OR, 3.162; 95% CI, 1.507-7.158; β = 1.151; P = 0.003), reconstruction method (OR, 3.579; 95% CI, 2.265-5.903; β = 1.275; P < 0.001), tumor diameter (OR, 1.004; 95% CI, 0.254-0.775; β = -0.808; P = 0.004), SF-12 scores (PCS: OR, 0.965; 95% CI, 0.94-0.989; β = -0.036; P = 0.005; MCS: OR, 0.962; 95% CI, 0.935-0.988; β = -0.039; P = 0.005), and Skindex-16 subscales (symptoms: OR, 2.005; 95% CI, 1.68-2.512; β = 0.696; P < 0.001; emotional effects: OR, 1.66; 95% CI, 1.439-2.015; β = 0.507; P < 0.001; functioning: OR, 6.735; 95% CI, 4.09-12.931; β = 1.907; P < 0.001) as significant predictors of unsatisfactory outcomes (Table 5).
Table 5.
Multivariate logistic regression analysis of factors affecting satisfactory outcome
| Parameters | Odds ratio | 95% CI | Beta | P Value |
|---|---|---|---|---|
| Aging signs | 3.162 | 1.507-7.158 | 1.151 | 0.003 |
| Reconstruction method | 3.579 | 2.265-5.903 | 1.275 | < 0.001 |
| Tumor diameter (mm) | 1.004 | 0.254-0.775 | -0.808 | 0.004 |
| PCS | 0.965 | 0.94-0.989 | -0.036 | 0.005 |
| MCS | 0.962 | 0.935-0.988 | -0.039 | 0.005 |
| Symptoms | 2.005 | 1.68-2.512 | 0.696 | < 0.001 |
| Emotional effects | 1.66 | 1.439-2.015 | 0.507 | < 0.001 |
| Functioning | 6.735 | 4.09-12.931 | 1.907 | < 0.001 |
Note: PCS: Physical Health Components; MCS: Mental Health Components.
Establishment of combined predictive model
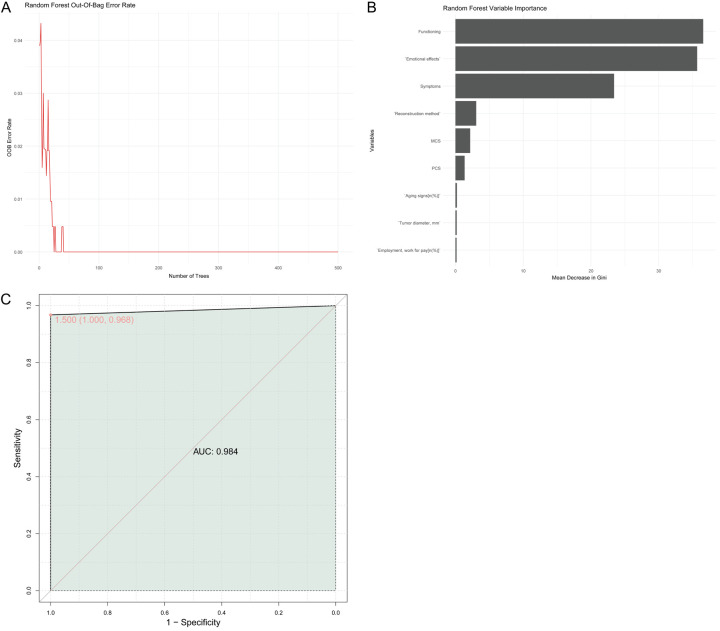
A random forest model incorporating all independent factors showed high predictive accuracy (AUC = 0.984) (Figure 5A). Variable importance analysis indicated that “function”, “emotional effect”, and “symptom” were the most influential variables (Figure 5B). The ROC curve demonstrated excellent discriminative ability (Figure 5C).
Figure 5.
Establishment of combined predictive model. A: Out-of-bag error rate plot; B: Random forest variable importance; C: Receiver Operating Characteristic (ROC) curve. AUC: area under the curve.
External validation of the predictive model
Table 6 presents the comparison of parameters between the satisfactory and unsatisfactory groups in the external validation set. There were no significant differences between the two groups in terms of age (P = 0.683), BMI (P = 0.657), education level (P = 0.952), gender (P = 0.996), employment (P = 0.333), hypertension (P = 0.905), diabetes mellitus (P = 0.626), smoking history (P = 0.457), drinking history (P = 0.743), history of previous non-melanoma skin cancer (NMSC) (P = 0.891), anatomic location of BCC (P = 0.989), and treating clinician (P = 0.925). The presence of aging signs was more prevalent in the unsatisfactory group compared to the satisfactory group (P = 0.036). The reconstruction method had a significant impact on the outcome, with a higher proportion of direct stitching and local skin flaps in the satisfactory group and a higher proportion of skin grafting in the unsatisfactory group (P = 0.043). Tumor diameter was also a significant factor, with smaller tumors (≤ 10 mm) more frequently associated with satisfactory outcomes (P = 0.003). Higher pre-operative SF-12 physical component summary (PCS) scores (50.21 ± 10.43 vs. 45.12 ± 11.58, P = 0.027) and mental component summary (MCS) scores (53.16 ± 9.12 vs. 47.92 ± 11.21, P = 0.014) were observed in the satisfactory group. Skindex-16 subscale scores were significantly lower in the satisfactory group for symptoms (10.06 ± 5.21 vs. 14.47 ± 5.03, P < 0.001), emotional effects (26.23 ± 6.71 vs. 30.51 ± 8.08, P = 0.006), and functioning (4.01 ± 1.73 vs. 5.19 ± 2.05, P = 0.003). In this external validation dataset, these results indicate that the significant differences between the two groups in tumor diameter, PCS score, MCS score, symptom subscale score, emotional impact subscale score, and functional subscale score are consistent with the results of the test set.
Table 6.
Comparison of parameters between satisfactory and unsatisfactory groups in the external validation set
| Parameters | Satisfactory group (n = 51) | Unsatisfactory group (n = 43) | t/χ2 | P |
|---|---|---|---|---|
| Age (years) | 61.22 ± 26.78 | 63.51 ± 27.32 | 0.410 | 0.683 |
| BMI (kg/m2) | 24.71 ± 3.29 | 25.01 ± 3.11 | 0.445 | 0.657 |
| Education Level (years) | 13.35 ± 3.61 | 13.31 ± 2.84 | 0.06 | 0.952 |
| Gender [n (%)] | 0 | 0.996 | ||
| Male | 32 (62.75%) | 27 (62.79%) | ||
| Female | 19 (37.25%) | 16 (37.21%) | ||
| Employment, work for pay [n (%)] | 30 (58.82%) | 21 (48.84%) | 0.937 | 0.333 |
| Hypertension [n (%)] | 0.014 | 0.905 | ||
| Yes | 16 (31.37%) | 13 (30.23%) | ||
| No | 35 (68.63%) | 30 (69.77%) | ||
| Diabetes Mellitus [n (%)] | 0.237 | 0.626 | ||
| Yes | 20 (39.22%) | 19 (44.19%) | ||
| No | 31 (60.78%) | 24 (55.81%) | ||
| Smoking history [n (%)] | 21 (41.18%) | 21 (48.84%) | 0.554 | 0.457 |
| Drinking history [n (%)] | 22 (43.14%) | 20 (46.51%) | 0.107 | 0.743 |
| History of previous NMSC [n (%)] | 28 (54.90%) | 23 (53.49%) | 0.019 | 0.891 |
| Aging signs [n (%)] | 36 (70.59%) | 38 (88.37%) | 4.405 | 0.036 |
| Anatomic location of BCC [n (%)] | 0.022 | 0.989 | ||
| Forehead unit | 17 (%) | 12 (%) | ||
| Nasal unit | 16 (%) | 13 (%) | ||
| Eyelid units | 8 (%) | 7 (%) | ||
| Cheek units | 9 (%) | 9 (%) | ||
| Upper/lower lips | 1 (%) | 2 (%) | ||
| Reconstruction method [n (%)] | 4.087 | 0.043 | ||
| Direct stitching | 10 (19.61%) | 3 (6.98%) | ||
| Local skin flap | 33 (64.71%) | 18 (41.86%) | ||
| Skin grafting | 7 (13.73%) | 20 (46.51%) | ||
| Free skin flap | 1 (1.96%) | 2 (4.65%) | ||
| Treating clinician [n (%)] | 0.898 | 0.925 | ||
| Attending physician | 26 (51.02%) | 22 (51.16%) | ||
| Resident | 16 (31.37%) | 13 (30.23%) | ||
| Nurse practitioner | 9 (17.65%) | 8 (18.60%) | ||
| Tumor diameter [n (%)] | 14.196 | 0.003 | ||
| ≤ 10 mm | 32 (62.75%) | 18 (41.86%) | ||
| > 10 mm | 19 (37.25%) | 25 (58.14%) | ||
| PCS (scores) | 50.21 ± 10.43 | 45.12 ± 11.58 | 2.242 | 0.027 |
| MCS (scores) | 53.16 ± 9.12 | 47.92 ± 11.21 | 2.501 | 0.014 |
| Symptoms (scores) | 10.06 ± 5.21 | 14.47 ± 5.03 | 4.152 | < 0.001 |
| Emotional effects (scores) | 26.23 ± 6.71 | 30.51 ± 8.08 | 2.804 | 0.006 |
| Functioning (scores) | 4.01 ± 1.73 | 5.19 ± 2.05 | 3.034 | 0.003 |
Note: BMI: body mass index; NMSC: Non-melanoma Skin Cancer; BCC: basal cell carcinoma; PCS: physical health factors; MCS: mental health factors.
ROC analysis of the predictive model in validation set
The external validation ROC curve provides additional insights into the performance of the predictive model (Figure 6). The AUC value of 0.870 indicates a strong ability of the model to distinguish between satisfactory and unsatisfactory outcomes in the external dataset. The curve itself follows a similar pattern to the internal validation curve, suggesting consistent performance across different datasets. The point marked on the curve with coordinates (0.633, 0.941) and its CI of 0.651 further supports the robustness of the model in predicting outcomes accurately. Overall, the external validation ROC curve reinforces the reliability of the developed model in predicting aesthetic outcomes following plastic surgery for facial basal cell carcinoma.
Figure 6.
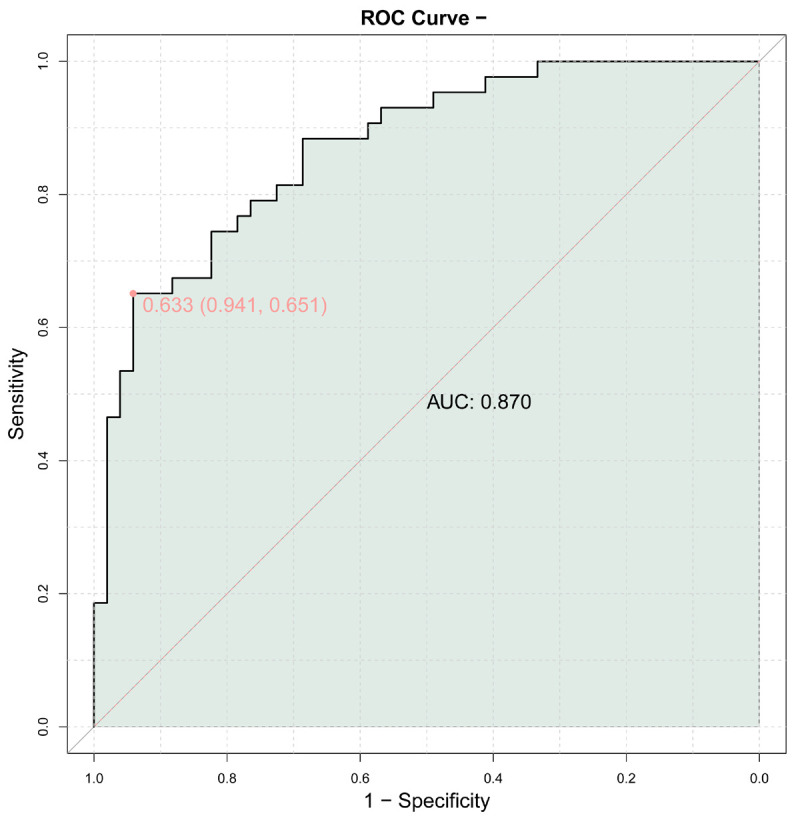
External validation ROC curve. ROC: Receiver Operating Characteristic; AUC: area under the curve.
Discussion
In this study, we sought to construct a predictive model for evaluating the effectiveness of plastic surgery and repair in patients with facial basal cell carcinoma (BCC). The findings of this work contribute valuable insights into the factors influencing successful surgical outcomes and the overall health and quality of life of patients undergoing these procedures.
A significant finding in our study is the differential impact of reconstruction methods on surgical outcomes. Specifically, direct suturing and local skin flaps were associated with higher satisfaction rates, whereas skin grafting was associated with poorer outcomes. This discrepancy can be attributed to several intrinsic and extrinsic factors related to tissue physiology and surgical technique. Direct suturing and local skin flaps preserve local tissue characteristics such as skin color, texture, and vascularization, which allow for better integration with the surrounding facial tissue, promoting more natural aesthetic outcomes [21,22]. In contrast, skin grafting involves transplanting skin from a different body region, which can lead to mismatched color, texture, and potential scarring - factors negatively impacting cosmetic satisfaction [23]. Furthermore, skin grafts lack the original vascularization found in local flaps, which may contribute to suboptimal healing and increased complication rates, thereby affecting patient satisfaction [24,25].
Another significant predictor of surgical satisfaction was tumor diameter. Larger tumor sizes were correlated with poorer outcomes, highlighting the technical complexities and aesthetic challenges posed by larger resections. Tumors exceeding 10 mm in diameter require more extensive tissue removal, which consequently requires more complex reconstructive techniques [26]. As the resected area increases, it becomes more challenging to achieve symmetrical and aesthetically pleasing results. Moreover, larger defects may impede the use of simpler repair methods like direct suturing, often necessitating skin grafts or complex flap techniques, which - as noted - are associated with higher dissatisfaction rates [26,27]. These findings underscore the importance of early detection and prompt surgical intervention in BCC to limit tumor growth and simplify reconstructive efforts.
The HRQoL, as measured by the SF-12 survey, revealed that patients with higher PCS and MCS scores experienced better surgical outcomes. These findings suggest that patients in better overall health, both physically and mentally, have a more favorable recovery trajectory and less postoperative distress. From a physiological perspective, better physical health are associated with more efficient wound healing and reduced risk of postoperative complications, which can significantly affect cosmetic outcomes [28]. Psychologically, a higher MCS score likely reflects better mental resilience and coping mechanisms, allowing patients to manage postoperative pain and stress more effectively [29,30]. These factors together may contribute to patients perceiving their surgical outcomes more favorably [31]. Therefore, incorporating preoperative interventions to improve both physical and mental health could be beneficial to enhance surgical satisfaction.
Skin-related quality of life, as assessed by the Skindex-16 subscale, emerged as another critical determinant of patient satisfaction. Lower scores in symptoms, emotional effects, and functioning subscales were strongly associated with satisfactory outcomes. These components of the Skindex-16 survey capture the direct impact of skin conditions on daily life, emotional wellbeing, and functional capabilities. Patients who report fewer symptoms and emotional effects are inherently more satisfied with their physical appearance post-surgery, as fewer issues translate to lesser interference with their quality of life [32,33]. Also, patients experiencing fewer skin-related symptoms are likely to have less postoperative discomfort and better cosmetic results, as persistent symptoms like itching, pain, or dryness can detract from the aesthetic outcomes [34]. Emotional and functional dimensions further suggest that patients who perceive less impact on their daily lives and social interactions are more likely to report higher satisfaction, indicating the importance of holistic patient care that addresses both physical and psychosocial needs [34].
The random forest model validated the critical importance of several factors, notably the Skindex-16 subscales and reconstruction method, in predicting surgical outcomes. The high discriminative capability of our predictive model, with an AUC of 0.984, underscores its robustness and clinical applicability in preoperative planning. By integrating these critical variables, this model can offer personalized predictive assessments, thereby guiding surgeons in making informed decisions about the most appropriate reconstructive approach and preparing patients with realistic expectations regarding their surgical outcomes.
From a mechanistic standpoint, the interaction between immunological factors and postoperative recovery, although not statistically significant in this study, remains a critical area for future exploration. The lack of significant differences in preoperative serum inflammatory markers like IL-6, IL-8, TNF-α, and CRP between two groups suggests that baseline systemic inflammation may not play a discernible role in predicting the immediate cosmetic outcomes of facial BCC surgery. However, localized inflammatory responses and wound microenvironment dynamics post-surgery might still significantly influence healing processes. Further investigations with more sensitive biomarkers or longitudinal post-surgical assessments could provide deeper insights.
Our study’s strengths lie in its comprehensive approach to evaluating multiple predictors of surgical outcome, but there are limitations that warrant consideration. The retrospective design might introduce selection bias and limits our ability to draw causal inferences. Additionally, while our cohort size was reasonably robust, larger multi-center studies are needed to generalize findings across diverse populations. Future research should also incorporate long-term follow-up data to understand the sustained impact of these factors on cosmetic outcomes and patient satisfaction.
Conclusion
In conclusion, our study confirms that reconstruction method selection, tumor size, general health status, and skin-related quality of life significantly influence the effectiveness of plastic surgery and repair in patients with facial BCC. These insights can aid in refining preoperative evaluations, optimizing surgical planning, and tailoring postoperative care to enhance patient satisfaction and quality of life. By leveraging constructed predictive models, clinicians can better anticipate surgical outcomes and provide more individualized, effective patient care.
Acknowledgements
I would like to express my gratitude to all those who helped me during the writing of this thesis. I acknowledge the help of my colleague Lijun Wu. They have offered me suggestions in academic studies. This study was supported by the Clinical Treatment Technology Innovation Project of the First Affiliated Hospital of Soochow University (2100201); Suzhou Basic Research Pilot Project (SSD2024052): Construction of key clinical specialties for the Suzhou Municipal “Strengthening Health through Science and Education” Funding Project; Hospital Research Fund (SDFEYBS1805, SDFEYGJ2013, XKTJ-HRC20210015); Suzhou Science and Technology Development Project (SKJY2021078 and 2022SS43); the Special Project of “Technological Innovation” Project of CNNC Medical Industry Co., Ltd. (ZHYLZD2021002); Project of State Key Laboratory of Radiation Medicine and Protection, Soochow University (GZK1202244); and the CNNC Elite Talent Program and Suzhou Medical Innovation Applied Research Project (SKYD2023104).
Disclosure of conflict of interest
None.
References
- 1.De Fré M, Vermeersch N, De F, Ulicki M, Smets K, Tondu T, Thiessen F. Giant basal cell carcinoma on the forehead and why we should prevent them - case report. Acta Chir Plast. 2020;61:24–27. [PubMed] [Google Scholar]
- 2.Calin MA, Parasca SV. Automatic detection of basal cell carcinoma by hyperspectral imaging. J Biophotonics. 2022;15:e202100231. doi: 10.1002/jbio.202100231. [DOI] [PubMed] [Google Scholar]
- 3.De Vera E, Magliano J, Bazzano C. Large cheek defect reconstruction with a rhomboid flap and Burow’s advancement flap after excision of a basal cell carcinoma. Actas Dermosifiliogr. 2023;114:722–724. doi: 10.1016/j.ad.2022.11.016. [DOI] [PubMed] [Google Scholar]
- 4.Borg M, Amatore F, Foletti JM, Bertrand B, Macagno N, Delaporte E, Richard MA. Predictors of initial incomplete resection of basal cell carcinoma of the face and neck. Ann Dermatol Venereol. 2023;150:284–285. doi: 10.1016/j.annder.2023.05.002. [DOI] [PubMed] [Google Scholar]
- 5.Aristokleous I, Schultz I, Vassilaki I, Krynitz B, Lapins J, Girnita A, Nilsson MF. Mohs micrographic surgery revisited: a multidisciplinary, collaborative approach for the treatment of aggressive and recurrent basal cell carcinoma on the head and neck. J Plast Reconstr Aesthet Surg. 2022;75:3373–3383. doi: 10.1016/j.bjps.2022.04.037. [DOI] [PubMed] [Google Scholar]
- 6.Bertrand N, Guerreschi P, Basset-Seguin N, Saiag P, Dupuy A, Dalac-Rat S, Dziwniel V, Depoortère C, Duhamel A, Mortier L. Vismodegib in neoadjuvant treatment of locally advanced basal cell carcinoma: first results of a multicenter, open-label, phase 2 trial (VISMONEO study): neoadjuvant vismodegib in locally advanced basal cell carcinoma. EClinicalMedicine. 2021;35:100844. doi: 10.1016/j.eclinm.2021.100844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mamsen FPW, Kiilerich CH, Hesselfeldt-Nielsen J, Saltvig I, Remvig CL, Trøstrup H, Schmidt VJ. Risk stratification of local flaps and skin grafting in skin cancer-related facial reconstruction: a retrospective single-center study of 607 patients. J Pers Med. 2022;12:2067. doi: 10.3390/jpm12122067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Faenza M, Molle M, Mazzarella V, Antonetti AM, Filosa FG, Pelella T, Nicoletti GF. Functional and aesthetic comparison between grafts and local flaps in non-melanoma skin cancer surgery of the face: a cohort study. JPRAS Open. 2024;42:97–112. doi: 10.1016/j.jpra.2024.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Winden MEC, Bronkhorst EM, Visch MB, Krekels GAM, van der Geer S, Damen GWJA, Amir A, Aben KKH, Gerritsen MJP, van de Kerkhof PCM, de Jong EMGJ, Lubeek SFK. Predictors of surgical treatment burden, outcomes, and overall survival in older adults with basal cell carcinoma: results from the prospective, multicenter BATOA cohort. J Am Acad Dermatol. 2022;86:1010–1019. doi: 10.1016/j.jaad.2021.05.041. [DOI] [PubMed] [Google Scholar]
- 10.Ben Slama N, Adnot J, Trost O. Management of an extended bone-invasive basal cell carcinoma of the median forehead. J Stomatol Oral Maxillofac Surg. 2020;121:296–299. doi: 10.1016/j.jormas.2019.07.007. [DOI] [PubMed] [Google Scholar]
- 11.Nierich J, Corten EML, de Jong T, Mureau MAM. Long-term patient-reported outcomes following oncological facial reconstructive surgery using the FACE-Q skin cancer module. JPRAS Open. 2024;39:262–270. doi: 10.1016/j.jpra.2024.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li X, Tan L, Kou H, Zhang J, Wang Y, Li G, Lu Y. Ocular preservation through limited tumor excision combined with ALA-PDT in patients with periocular basal cell carcinoma. Photodiagnosis Photodyn Ther. 2019;27:291–294. doi: 10.1016/j.pdpdt.2019.06.016. [DOI] [PubMed] [Google Scholar]
- 13.Derebaşınlıoğlu H, Özkaya NK. Analysis of basal cell carcinoma and squamous cell carcinoma according to nasal subunit location. Facial Plast Surg. 2021;37:407–410. doi: 10.1055/s-0041-1726024. [DOI] [PubMed] [Google Scholar]
- 14.Stankovic P, Bock R, Rudhart SA, Hoch S, Wilhelm T. Basal cell carcinoma of the head and neck-a retrospective single-centre comparison of the recurrence rate after R0 or R1 resection. Facial Plast Surg. 2023;39:155–159. doi: 10.1055/s-0042-1756466. [DOI] [PubMed] [Google Scholar]
- 15.Szabó Á, Brodszky V, Rencz F. A comparative study on the measurement properties of Dermatology Life Quality Index (DLQI), DLQI-Relevant and Skindex-16. Br J Dermatol. 2022;186:485–495. doi: 10.1111/bjd.20765. [DOI] [PubMed] [Google Scholar]
- 16.Cucurullo M, Colletti G. Basal cell carcinoma arising over a venous malformation of the forehead communicating with the endocranial veins. J Craniofac Surg. 2020;31:e92–e95. doi: 10.1097/SCS.0000000000005947. [DOI] [PubMed] [Google Scholar]
- 17.Wang X, Xie K, Guo X, Bi Z. Multiple facial basal cell carcinoma with xeroderma pigmentosum. J Craniofac Surg. 2023;34:e761–e762. doi: 10.1097/SCS.0000000000009642. [DOI] [PubMed] [Google Scholar]
- 18.Ofaiche J, Lopez R, Bérard E, André A, Bulai-Livideanu C, Méresse T, Vairel BB, Grolleau JL, Paul C, Meyer N. Surgical treatment of facial basal cell carcinoma: patient-based assessment of clinical outcome in a prospective cohort study. Dermatology. 2016;232:550–557. doi: 10.1159/000447354. [DOI] [PubMed] [Google Scholar]
- 19.Haddad C, Sacre H, Obeid S, Salameh P, Hallit S. Validation of the Arabic version of the “12-item short-form health survey” (SF-12) in a sample of Lebanese adults. Arch Public Health. 2021;79:56. doi: 10.1186/s13690-021-00579-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cárcano CBM, de Oliveira CZ, Paiva BSR, Paiva CE. The Brazilian version of Skindex-16 is a valid and reliable instrument to assess the health-related quality of life of patients with skin diseases. PLoS One. 2018;13:e0194492. doi: 10.1371/journal.pone.0194492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Edmondson M, Lonie S, Moir D, Shukla L. Sarcomatoid transformation of a primary basal cell carcinoma of the cheek. ANZ J Surg. 2023;93:1113–1115. doi: 10.1111/ans.18151. [DOI] [PubMed] [Google Scholar]
- 22.Greig A, Aloni K, Orchard G, Shams M, Craythorne E, Fassihi H. Treatment of multiple facial basal cell carcinomas in a child with xeroderma pigmentosum complementation group C with Mohs micrographic surgery. Br J Dermatol. 2021;184:e4. doi: 10.1111/bjd.19323. [DOI] [PubMed] [Google Scholar]
- 23.Gaitanis G, Spyridonos P, Seretis K, Moschovos V, Bassukas ID. Application of a burn scar assessment tool for the evaluation of visual scarring following immunocryosurgery for facial basal cell carcinoma. Eur J Dermatol. 2022;32:709–715. doi: 10.1684/ejd.2022.4354. [DOI] [PubMed] [Google Scholar]
- 24.Gaitanis G, Zampeta A, Tsintzou P, Fillis G, Seretis K, Feldmeyer L, Bassukas I. The feasibility of immunocryosurgery in the treatment of non-superficial, facial basal cell carcinoma that relapsed after standard surgical excision: an experience report from two centers. Curr Oncol. 2022;29:8475–8482. doi: 10.3390/curroncol29110668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bejinariu CG, Popescu S, Dragosloveanu CDM, Marinescu SA. Reconstruction of lower eyelid defects after the excision of basal cell carcinoma. Rom J Ophthalmol. 2020;64:414–418. doi: 10.22336/rjo.2020.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fu S, Panayi AC, Lu Q, Long H. Application of a “fish mouth flap” combined with an orbicularis oculi myocutaneous flap after surgical removal of basal cell carcinoma in the facial buccal region. Indian J Dermatol Venereol Leprol. 2019;85:649–652. doi: 10.4103/ijdvl.IJDVL_1003_18. [DOI] [PubMed] [Google Scholar]
- 27.Ahuja M, Mandal S, Singh M, Khurana N, Bhandari PS. Sebaceous carcinoma with apocrine differentiation arising in a known case of basal cell carcinoma: a rare entity. Indian J Pathol Microbiol. 2024;67:172–174. doi: 10.4103/ijpm.ijpm_972_21. [DOI] [PubMed] [Google Scholar]
- 28.Mohapatra DP, Friji MT, Dinesh Kumar S, Chittoria RK, Pathan I, Koliath S. Reconstruction of defects following excision of basal cell carcinoma of face: a subunit-based algorithm. J Cutan Aesthet Surg. 2023;16:1–13. doi: 10.4103/JCAS.JCAS_87_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thakurani S, Gupta S, Mohammad A, Escandón JM. Facial basal cell carcinoma: a study of causative factors and site-based algorithm for surgical reconstruction. J Cutan Aesthet Surg. 2022;15:275–283. doi: 10.4103/JCAS.JCAS_113_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mishra B, Mallik S, Agnihotry I, Behera J. Aesthetic reconstruction based on facial subunit principle for basal cell carcinoma of the face: a retrospective analysis. Cureus. 2024;16:e56826. doi: 10.7759/cureus.56826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang J, Lu Y, Zhang X, Yang Y, Kou H, Wang Y. Clinical efficacy of Mohs surgery combined with topical photodynamic therapy for facial basal cell carcinoma. J Cancer Res Ther. 2020;16:1051–1055. doi: 10.4103/jcrt.JCRT_987_19. [DOI] [PubMed] [Google Scholar]
- 32.Kim CS, Na YC. Basal cell nevus syndrome with excessive basal cell carcinomas. Arch Craniofac Surg. 2021;22:122–125. doi: 10.7181/acfs.2021.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qiu F, Lei S, Zhang L, Jiang X, Zuo C. A case of nevoid basal cell carcinoma syndrome dominated by facial basal cell carcinoma. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2022;47:384–389. doi: 10.11817/j.issn.1672-7347.2022.200925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Quazi SJ, Aslam N, Saleem H, Rahman J, Khan S. Surgical margin of excision in basal cell carcinoma: a systematic review of literature. Cureus. 2020;12:e9211. doi: 10.7759/cureus.9211. [DOI] [PMC free article] [PubMed] [Google Scholar]