Abstract
Objective: To analyze the clinical characteristics and molecular biomarkers of adult T-cell lymphoblastic lymphoma (T-LBL) to identify prognostic factors, and to evaluate the efficacy of different chemotherapy regimens, providing a basis for optimizing treatment strategies for T-LBL. Methods: A total of 89 Patients aged 18-72 years with T-LBL, confirmed via histopathological examination of lymph nodes, extranodal tissues, or bone marrow, were retrospectively included. Clinical data, treatment details, and mutational profiles were collected. Prognostic factors were assessed based on clinical and molecular characteristics, and the efficacy and safety of two chemotherapy regimens were compared. Descriptive statistics were used to analyze the disease spectrum. Results: Most patients (84.00%) presented with advanced disease (stages III-IV). Mediastinal invasion was observed in 63 patients (70.80%), and 59 patients (66.30%) exhibited B symptoms. Bone marrow involvement occurred in 19 patients (21.20%), and bulky mediastinum (>10 cm) was present in 50 patients (56.18%). Mutations were detected in 29 patients, with NOTCH1 being the most frequently mutated gene, followed by PHF-6, JAK-1, JAK-3, IL-7R, and TP53. The complete response (CR) rate was 51.69%. The 3-year overall survival (OS) and progression-free survival (PFS) rates were 74.9% and 58.80%, respectively. Multivariate analysis identified female sex, lack of CR, and elevated lactate dehydrogenase (LDH) levels (>2× normal) as independent predictors of poor OS (58.25%). Chemotherapy regimens, LDH levels, and sex were independent prognostic factors for PFS (21.24%). Conclusion: T-LBL is characterized by high-frequency gene mutations across multiple signaling pathways. Mediastinal invasion (70.80%) and extranodal involvement (39.33%) were prevalent in Chinese patients and were associated with poor prognosis. Combined assessment of clinical and molecular features allows for improved prognostic stratification and facilitates the development of targeted therapies for high-risk patients.
Keywords: Lymphoblastic lymphoma, T cells, clinical features, prognosis, gene mutation
Introduction
T-cell lymphoblastic lymphoma (T-LBL) is a rare and aggressive precursor T-cell tumor, accounting for approximately 2% of all non-Hodgkin’s lymphoma (NHL) cases. T-LBL and acute lymphoblastic leukemia (ALL) are lymphoblastic tumors originating from lymphocytes, sharing similarities in clinical, morphological, and immunophenotypic characteristics. These tumors are part of the same disease spectrum and are primarily distinguished by the degree of bone marrow (BM) involvement.
The treatment of T-LBL has shifted from NHL-like to ALL-like regimens, but large-scale case studies remain scarce, and no standardized treatment protocol has been established. Traditional CHOP-like regimens show limited efficacy in T-LBL/ALL, particularly for high-risk patients and those with disease progression or relapse. Currently, high-intensity, short-course, ALL-like regimens are commonly used for induction chemotherapy in T-LBL/ALL, yielding improved outcomes [1,2]. The overall remission rate is approximately 60.00%, and the 3-5-year disease-free survival (DFS) rate ranges from 30.00% to 50.00% [3,4]. However, whether T-LBL and T-ALL are distinct diseases remains a topic of debate.
The rarity of T-LBL and challenges in obtaining adequate research materials have limited the understanding of its molecular and prognostic characteristics. Gaining insights into the clinical and molecular features of T-LBL is crucial for developing effective risk stratification strategies and identifying targeted therapies to improve outcomes. Therefore, this study retrospectively analyzed the clinical characteristics, treatment regimens, and prognostic factors of 89 patients with T-LBL.
Materials and methods
Materials
On February 25, 2024, clinical data were collected from patients aged 18-72 years diagnosed with T-LBL who visited Shanxi Province Cancer Hospital between January 2010 and November 2023.
Inclusion and exclusion criteria
Patients were included if they aged 18-72 years and had a definitive diagnosis of T-LBL based on histopathology, histochemistry, immunophenotyping, molecular biology, and cytogenetics, according to the WHO classification criteria for lymphohematopoietic tissue tumors. T-LBL was diagnosed when tumor involvement in blood and bone marrow (BM) was minimal or absent (primitive and naive lymphocyte percentage ≤25.00% in BM). Patients were excluded if they were under 18 years old, had a BM blast cell percentage >25.00%, or had incomplete clinical and disease-related data.
Diagnostic and staging methods
Classification and differential diagnosis were performed using cellular immunophenotyping via multiparameter flow cytometry [5]. T-LBL cells expressed the T-cell-specific antigen cluster of differentiation 3 (CD3), along with markers such as CD38, CD7, CD99, cytoplasmic terminal deoxynucleotide transferase (cTdT), and CD2. Additionally, CD1a, CD2, CD4, CD5, and CD8 were expressed to varying degrees, with co-expression of CD4 and CD8 observed. Myeloid markers CD13 and CD33 were found in 20.00%-30.00% of T-ALL/LBL cells. The Ann Arbor staging system was used to classify lymphomas. BM involvement was defined as a blast cell percentage >5.00% and <25.00%, while ALL was diagnosed when BM lymphoblasts exceeded 25%.
Genetic testing
Of the 89 patients included, 37 underwent next-generation sequencing (NGS) to detect genetic mutations (51 or 302 genes). These included mutations in N/K-RAS, NOTCH1/FBXW7 (N/F), PTEN, and T-cell receptor (TCR) genes. Specimens were derived from lymph nodes, BM fluid, or BM tissue. Genomic DNA was extracted from BM or peripheral blood samples using commercial kits at Sino-US Diagnostics Laboratories, Tianjin, China. DNA libraries were constructed using Kapa Biosystems’ library construction kit (Wilmington, MA), and somatic mutations were identified using the “Lymphoblastic Leukemia Gene Mutation Detection Panel (113 genes)” on the Illumina NextSeq550 platform.
Clinical evaluation and ethics
Patient general status was assessed using the Eastern Cooperative Oncology Group Performance Status (ECOG-PS) criteria. All participants provided informed consent, and the study was approved by the Ethics Committee of Shanxi Province Cancer Hospital (Approval No.: IIT-2024-015). The study adhered to the ethical guidelines outlined in the Declaration of Helsinki.
Treatment
The first-line treatment options included the German Multicenter Study Group for Adult ALL (GMALL) 07/2003 protocol or the hyper-CVAD A/B protocol. Additionally, 23 patients received local intensity-modulated radiation therapy (IMRT), 12 underwent allogeneic hematopoietic stem cell transplantation (HSCT), and 5 received sequential autologous hematopoietic stem cell transplantation (ASCT) as consolidation therapy. The median number of chemotherapy cycles was 6 (range: 3-11).
After completing treatment, patients were followed up every 3 months during the first 2 years, every 6 months from years 3 to 5, and annually thereafter. Follow-up assessments included blood cell counts and serum lactate dehydrogenase (LDH) levels [6].
Evaluation of short-term treatment efficacy
At diagnosis, the treatment efficacy at lesion invasion sites was assessed. Patients with BM involvement underwent BM examination during each treatment cycle. Complete response (CR) was defined as a neutrophil count ≥1000/μL, a platelet count ≥100,000/μL, a BM blast cell percentage <5.00%, and the absence of extramedullary lesions. Relapse was defined as a BM leukemic blast percentage ≥5.00% and/or the reappearance of extramedullary lesions.
Treatment efficacy was evaluated using fluorodeoxyglucose positron emission tomography (18F-FDG PET/CT) or computed tomography (CT) after every two chemotherapy cycles, based on the revised 2014 Lugano Efficacy Evaluation Criteria for lymphomas. Efficacy was categorized as CR, partial response (PR), or no response (NR). The overall response rate (ORR) was calculated as the sum of CR and PR rates.
The cut-off date for follow-up was November 1, 2023. The endpoint for follow-up was the time of death. Overall survival (OS) was defined as the time from diagnosis to death or the last follow-up, while progression-free survival (PFS) was defined as the time from initial disease progression or recurrence to death.
Statistical analysis
Sample size calculations were based on the primary endpoint. Data were processed using R software (version 4.3.0, released April 21, 2023) and Storm software (www.medsta.cn/software). Patients were grouped based on chemotherapy regimens, and data were analyzed using the t-test and chi-square (χ2) test. Survival analyses were performed using the Kaplan-Meier method and Cox regression analysis, with comparisons made using the log-rank test. A P-value <0.05 was considered statistically significant.
Results
Clinical characteristics
This study included 89 patients with T-LBL. Among them, 63 patients (70.80%) presented with mediastinal invasion, with primary symptoms including dyspnea, chest pain, cough, pleural-pericardial effusion, and superior vena cava syndrome. CNS and BM involvement were relatively rare. The median age was 30.44 years (range: 18-72), with a male-to-female ratio of 69:20.
Most patients (84.00%) presented with highly aggressive, advanced-stage disease (stages III-IV) at onset, and 59 patients (66.29%) exhibited B symptoms. BM involvement was noted in 9 patients (10.11%), and serous cavity effusion (e.g., pleural or pericardial effusion) was observed in 40 patients (45.60%). Bulky mediastinum (>10 cm) was present in 50 patients (56.18%), extranodal lesions in 35 patients (39.33%), and CNS invasion in 11 patients (12.36%).
Elevated lactate dehydrogenase (LDH) levels were observed in 54 patients (60.67%), with 30 patients (33.71%) having LDH levels exceeding 1× the normal level and 24 patients exceeding 2× the normal level. Additionally, 63 patients (70.79%) presented with anemia, and splenomegaly was observed in 49 patients (55.06%). Clinical characteristics are summarized in Table 1.
Table 1.
Clinical features of T-LBL
| Variable | Total (n=89) | GMALL07/2003 (n=58) | Hyper-CVAD (n=31) | Statistic | P |
|---|---|---|---|---|---|
| Age, Mean ± SD | 30.44 ± 12.15 | 29.84 ± 11.50 | 31.55 ± 13.42 | t=-0.628 | 0.532 |
| LDH, M (Q1, Q3) | 327.00 (188.00-587.00) | 310.00 (186.50-565.25) | 332.00 (200.50-661.50) | Z=0.396 | 0.692 |
| Sex, n (%) | χ2=0.000 | 0.986 | |||
| Female | 20 (22.47) | 13 (22.41) | 7 (22.58) | ||
| Male | 69 (77.53) | 45 (77.59) | 24 (77.42) | ||
| Clinical Response, n (%) | χ2=4.220 | 0.121 | |||
| CR | 46 (51.69) | 26 (44.83) | 20 (64.52) | ||
| PR | 18 (20.22) | 15 (25.86) | 3 (9.68) | ||
| NR | 25 (28.09) | 17 (29.31) | 8 (25.81) | ||
| Bone Marrow Involvement, n (%) | χ2=0.073 | 0.788 | |||
| No | 80 (89.89) | 53 (91.38) | 27 (87.10) | ||
| Yes | 9 (10.11) | 5 (8.62) | 4 (12.90) | ||
| Clinical Stages, n (%) | - | 0.911 | |||
| 1 | 1 (1.12) | 1 (1.72) | 0 (0.00) | ||
| 2 | 4 (4.49) | 3 (5.17) | 1 (3.23) | ||
| 3 | 5 (5.62) | 4 (6.90) | 1 (3.23) | ||
| 4 | 79 (88.76) | 50 (86.21) | 29 (93.55) | ||
| Whether stage 4, n (%) | χ2=0.480 | 0.489 | |||
| 0 | 10 (11.24) | 8 (13.79) | 2 (6.45) | ||
| 1 | 79 (88.76) | 50 (86.21) | 29 (93.55) | ||
| B symptoms, n (%) | χ2=0.465 | 0.495 | |||
| No | 30 (33.71) | 21 (36.21) | 9 (29.03) | ||
| Yes | 59 (66.29) | 37 (63.79) | 22 (70.97) | ||
| LDH abnormal level, n (%) | χ2=0.136 | 0.934 | |||
| 0 | 35 (39.33) | 22 (37.93) | 13 (41.94) | ||
| 1 time | 30 (33.71) | 20 (34.48) | 10 (32.26) | ||
| 2 times | 24 (26.97) | 16 (27.59) | 8 (25.81) | ||
| β2MG Abnormal, n (%) | χ2=1.740 | 0.187 | |||
| No | 61 (68.54) | 37 (63.79) | 24 (77.42) | ||
| Yes | 28 (31.46) | 21 (36.21) | 7 (22.58) | ||
| ECOG, n (%) | - | 0.971 | |||
| 0 | 4 (4.49) | 3 (5.17) | 1 (3.23) | ||
| 1 | 49 (55.06) | 31 (53.45) | 18 (58.06) | ||
| 2 | 27 (30.34) | 17 (29.31) | 10 (32.26) | ||
| 3 | 8 (8.99) | 6 (10.34) | 2 (6.45) | ||
| 4 | 1 (1.12) | 1 (1.72) | 0 (0.00) | ||
| ECOG level, n (%) | χ2=0.060 | 0.807 | |||
| ≥2 | 36 (40.45) | 24 (41.38) | 12 (38.71) | ||
| 0-1 | 53 (59.55) | 34 (58.62) | 19 (61.29) | ||
| IPI, n (%) | - | 0.770 | |||
| 0 | 4 (4.49) | 3 (5.17) | 1 (3.23) | ||
| 1 | 24 (26.97) | 17 (29.31) | 7 (22.58) | ||
| 2 | 35 (39.33) | 23 (39.66) | 12 (38.71) | ||
| 3 | 21 (23.6) | 11 (18.97) | 10 (32.26) | ||
| 4 | 3 (3.37) | 2 (3.45) | 1 (3.23) | ||
| 5 | 2 (2.25) | 2 (3.45) | 0 (0.00) | ||
| IPI level, n (%) | χ2=0.904 | 0.342 | |||
| 0 | 63 (70.79) | 43 (74.14) | 20 (64.52) | ||
| 1 | 26 (29.21) | 15 (25.86) | 11 (35.48) | ||
| Central invasion, n (%) | χ2=0.000 | 1.000 | |||
| No | 78 (87.64) | 51 (87.93) | 27 (87.10) | ||
| Yes | 11 (12.36) | 7 (12.07) | 4 (12.90) | ||
| Anemia, n (%) | χ2=0.267 | 0.605 | |||
| No | 26 (29.21) | 18 (31.03) | 8 (25.81) | ||
| Yes | 63 (70.79) | 40 (68.97) | 23 (74.19) | ||
| Large Mass, n (%) | χ2=4.226 | 0.040 | |||
| No | 39 (43.82) | 30 (51.72) | 9 (29.03) | ||
| Yes | 50 (56.18) | 28 (48.28) | 22 (70.97) | ||
| Splenomegaly, n (%) | χ2=0.174 | 0.677 | |||
| No | 40 (44.94) | 27 (46.55) | 13 (41.94) | ||
| Yes | 49 (55.06) | 31 (53.45) | 18 (58.06) | ||
| Extranodal invasion, n (%) | χ2=0.136 | 0.713 | |||
| No | 54 (60.67) | 36 (62.07) | 18 (58.06) | ||
| Yes | 35 (39.33) | 22 (37.93) | 13 (41.94) | ||
| Lymphocytosis, n (%) | χ2=0.052 | 0.820 | |||
| 0 | 33 (37.08) | 22 (37.93) | 11 (35.48) | ||
| 1 | 56 (62.92) | 36 (62.07) | 20 (64.52) | ||
| Age group, n (%) | χ2=0.021 | 0.885 | |||
| 1 | 44 (49.44) | 29 (50.00) | 15 (48.39) | ||
| 2 | 45 (50.56) | 29 (50.00) | 16 (51.61) | ||
| LDH 2X abnormal, n (%) | χ2=0.032 | 0.857 | |||
| 0 | 65 (73.03) | 42 (72.41) | 23 (74.19) | ||
| 1 | 24 (26.97) | 16 (27.59) | 8 (25.81) | ||
| CR or not, n (%) | χ2=3.136 | 0.077 | |||
| CR | 46 (51.69) | 26 (44.83) | 20 (64.52) | ||
| notCR | 43 (48.31) | 32 (55.17) | 11 (35.48) |
Immunophenotyping
Immunophenotyping and immunohistochemical analysis are essential diagnostic tools for T-LBL, especially in cases where tissue biopsy specimens are unavailable. For patients with mediastinal masses and pleural-pericardial invasive lesions, tumor cell immunophenotyping from pleural or pericardial effusions facilitates rapid diagnosis [3].
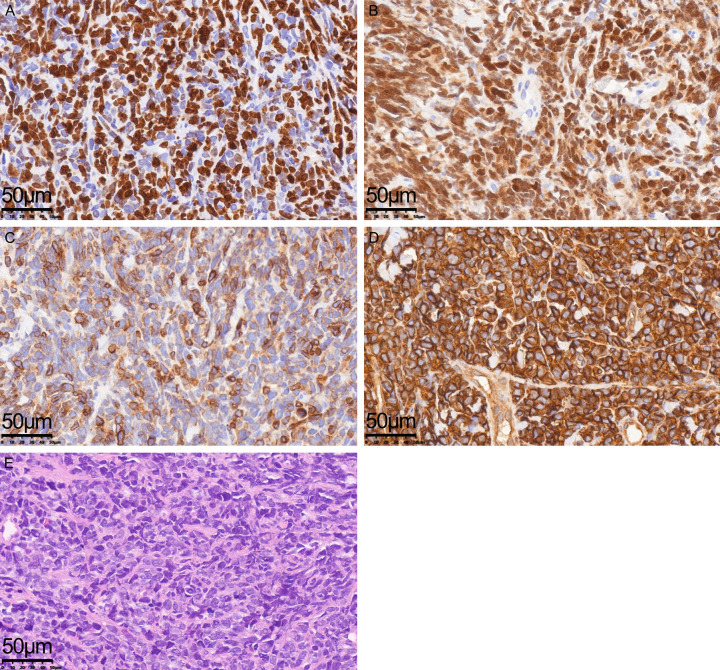
Among 89 patients, 15 with mediastinal masses were diagnosed with T-LBL via immunophenotyping of pleural or pericardial effusions and immunohistochemical analysis (Figure 1). Terminal deoxynucleotidyl transferase (TdT), a critical marker for small round cell tumors, including LBL, was expressed in 79 patients. Combined expression of TdT with CD99 and CD10 supported the identification of blast cell differentiation.
Figure 1.
Hematoxylin- and eosin-stained T-LBL tissue sections (×400) and results of immunohistochemical analysis (×400). A: A high proliferation rate as indicated by proliferation cell nuclear antigen (Ki-67); B: Terminal deoxynucleotidyl transferase (TdT) is an important marker for the differential diagnosis of T-LBL. The nuclei of tumor cells exhibited a positive reaction for TdT; C: Tumor cell membrane/cytoplasm of tumor cells showed specific positivity for CD3; D: The cell membrane was positive for CD99 expression; E: Representative images of hematoxylin- and eosin-stained sections (×400).
The immunophenotypes of T-LBL cells were predominantly CD2+, CD4+, CD8+, CD1α+/-, and CD7+. Notably, CD7 and CD43 alone are insufficient as T-lymphocyte markers. Cortical CD1α+ expression was detected in 23 patients. An early T phenotype (), characterized by the lack of CD1α, CD8, and CD5 (CD5-negative or dim), was observed in only 5 patients. Additionally, 13 patients co-expressed stem cell and myeloid markers, indicating a mixed phenotype.
Gene expression frequency and characteristics
Gene mutation analysis
Genetic mutation testing via NGS was performed on 37 patients, covering 143 genes. Mutations were detected in 29 patients, with the most frequently mutated gene being NOTCH1, followed by PHF6, JAK1, JAK3, IL7R, and TP53.
The analyzed genes were predominantly involved in key signaling pathways, including the JAK-STAT, NOTCH, Ras/protein phosphatase/MAPK, and transcription factor regulation pathways. Mutation frequencies are detailed in Table 2.
Table 2.
T-LBL gene expression
| Signaling Pathways | Gene | Incidence Rate (%) |
|---|---|---|
| JAK-STAT | IL-7R, JAK1, JAK3, SH2B3 | 27.50 |
| NOTCH | NOTCH1, FBXW7 | 65.70 |
| RAS/Protein phosphate/MARK/PI3K | TP53 | 17.10 |
| Transcription factors/regulation | PAX5, CREBBP, PHF6, PTEN | 6.20 |
| Tyrosine kinase recetor/non-receptor | FLT3 | 3.20 |
| Other | NT5C2 | 1.00 |
Efficacy and survival analysis
All 89 patients received treatment after the diagnosis of T-LBL was confirmed, with no induction-related deaths reported. Patients were divided into two groups based on the treatment protocol: hyper-CVAD A/B and GMALL07/2003.
Hyper-CVAD A/B Group: This regimen (8 courses) included Scheme A (cyclophosphamide [Baxter Oncology GmbH, H20160467], vincristine [Shenzhen Wanle Pharmaceutical Co., Ltd., H44021772], idarubicin [Nanjing Chia Tai Tianqing Pharmaceutical Co., Ltd., H20203344], and dexamethasone [Henan Runhong Pharmaceutical Co., Ltd., H41020330]; Scheme B: high-dose methotrexate [Pfizer (Perth) Pty Ltd., H20140206] and cytarabine [PHARMACIA Ital, X20010272]).
GMALL07/2003 Group: This protocol included VDCLP induction therapy (vincristine, daunorubicin, cyclophosphamide, L-asparaginase, and prednisone), dose-intensive consolidation, late intensification, 2-year maintenance therapy, and CNS prophylaxis with intrathecal chemotherapy and cranial irradiation.
A total of 23 patients received local intensity-modulated radiation therapy (IMRT) and 17 patients underwent hematopoietic stem cell transplantation (HSCT) as consolidation therapy. Among these, 12 received allogeneic HSCT (allo-HSCT) and 5 received autologous HSCT (auto-HSCT).
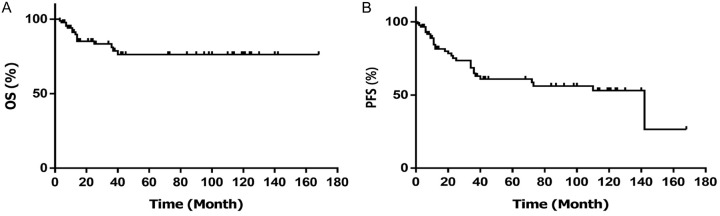
The CR rate was 64.52% and 44.83% for the hyper-CVAD A/B and GMALL07/2003 groups, respectively. However, the difference in CR rates was not statistically significant (χ2=3.136, P=0.077). Kaplan-Meier analysis revealed 3-year OS and PFS rates of 74.90% and 58.80%, respectively (Figure 2).
Figure 2.
The 3-year clinical outcomes of 89 T-LBL patients. (A) Overall survival (OS), (B) Progression-free survival (PFS).
Prognostic factors
Seventeen potential prognostic factors were analyzed. Univariate analysis identified sex, IPI scores, CNS invasion, splenomegaly, LDH levels, and treatment efficacy as significant factors affecting OS (P<0.05). Additional potential factors included ECOG scores, extranodal invasion, and chemotherapy regimen. However, age, BM invasion, B symptoms, disease stage, tumor mass, β2 microglobulin levels, CR rate, and increased peripheral blood lymphocyte counts were not significantly associated with OS.
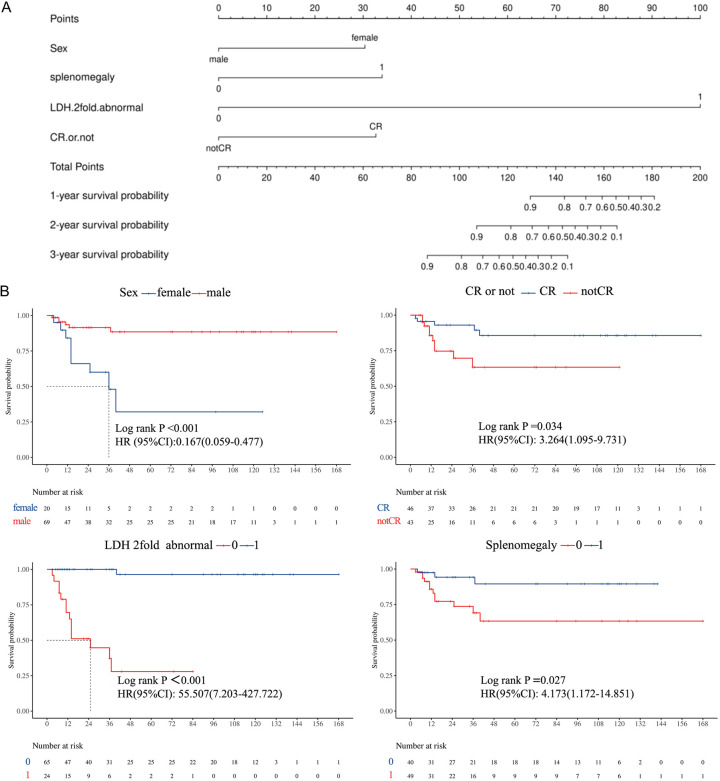
Multivariate Cox regression analysis identified 9 independent prognostic factors (Table 3). A nomogram incorporating these factors (Figure 3A) highlighted that LDH levels exceeding twice the normal value contributed the most to prognosis, followed by splenomegaly, CR rate, and sex. Female sex, failure to achieve CR, and elevated LDH levels were independent predictors of poor OS (Figure 3B).
Table 3.
Multivariate cox regression of the training set
| Variables | Beta | S.E | Z | P | HR (95% CI) |
|---|---|---|---|---|---|
| Sex | |||||
| Male | Ref | ||||
| Female | 1.79 | 0.53 | 3.34 | <.001 | 5.98 (2.10-17.05) |
| Splenomegaly | |||||
| 1 | Ref | ||||
| 0 | -1.43 | 0.65 | -2.21 | 0.027 | 0.24 (0.07-0.85) |
| LDH 2X abnormal | |||||
| 0 | Ref | ||||
| 1 | 4.02 | 1.04 | 3.86 | <.001 | 55.51 (7.20-427.72) |
| CR or not | |||||
| notCR | Ref | ||||
| CR | -1.18 | 0.56 | -2.12 | 0.034 | 0.31 (0.10-0.91) |
Ref= Reference group; HR= Hazard Ratio; CI= Confdence Interval; CR= complete response; LDH= serum lactate dehydrogenase.
Figure 3.
The results of multivariate cox regression. A: Nomogram for predicting 1-year, 2-year and 3-year survival probability of T-cell lymphoblastic lymphoma (T-LBL patients). To use the nomogram, an individual patient’s value is located on each variable axis, and a vertical line is drawn upward to determine the number of points corresponding to each variable value. The sum of these numbers is located on the Total Points axis, and a line is drawn downward to the survival axes to determine the survival probability. B: The results showed female sex, lack of CR, serum lactate dehydrogenase (LDH) levels more than twice times normal, and independent adverse prognostic factors associated with overall survival (OS).
Univariate analysis identified sex, BM invasion, disease stage, B symptoms, IPI scores, CNS invasion, bulky disease, LDH levels, and chemotherapy regimen as potential factors influencing PFS. Among these, IPI scores, LDH levels, and chemotherapy regimen were statistically significant (all P<0.05).
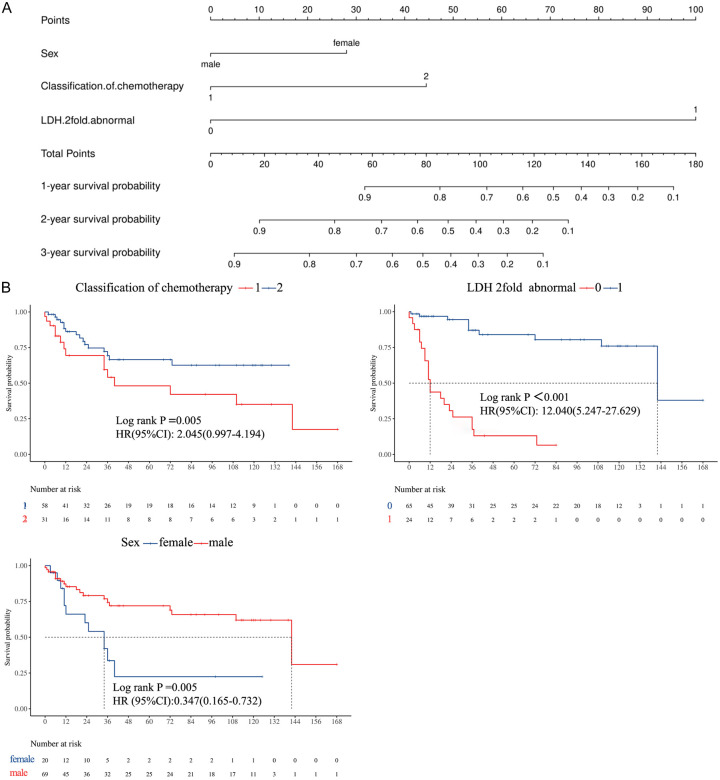
Multivariate Cox regression analysis confirmed LDH levels, chemotherapy regimen, and sex as independent factors associated with PFS (Table 4). A nomogram developed based on these factors (Figure 4A) demonstrated that LDH levels exceeding twice the normal value had the highest prognostic weight, followed by chemotherapy regimen and sex. The nomogram enabled calculation of total scores, which were mapped to PFS probabilities (Figure 4B).
Table 4.
Multivariate cox regression of the training set
| Variables | Beta | S.E | Z | P | HR (95% CI) |
|---|---|---|---|---|---|
| Sex | |||||
| Male | Ref | ||||
| Female | 1.06 | 0.38 | 2.78 | 0.005 | 2.88 (1.37-6.06) |
| Classification of chemotherapy | |||||
| 1 | Ref | ||||
| 2 | 0.72 | 0.37 | 1.95 | 0.051 | 2.04 (1.00-4.19) |
| LDH 2fold abnormal | |||||
| 1 | Ref | ||||
| 0 | -2.49 | 0.42 | -5.87 | <.001 | 0.08 (0.04-0.19) |
Ref= Reference group; HR= Hazard Ratio; CI= Confdence Interval; LDH= serum lactate dehydrogenase.
Figure 4.
The results of multivariate cox regression. A: The LDH 2fold as sharing the largest contribution to prognosis, followed by classification of chemotherapy and sex. B: Multivariate analysis showed that the chemotherapy regimen, serum lactate dehydrogenase (LDH) and gender were prognostic factors independently associated with progression-free survival (PFS).
While the hyper-CVAD A/B group exhibited a slightly higher CR rate, this group experienced worse PFS compared to the GMALL07/2003 group. Treatment protocol selection did not significantly affect OS, and achieving CR was identified as a protective factor for OS.
Discussion
Patients with T-LBL frequently present with a bulky mediastinum. Typical clinical manifestations include superior vena cava syndrome caused by a large anterior mediastinal mass, airway compression leading to cough and dyspnea, and, in some cases, pleural or pericardial effusion [7,8]. Approximately 15%-20% of newly diagnosed patients have BM or CNS invasion, and many exhibit elevated white blood cell counts and B symptoms at diagnosis.
Among the 89 patients included in this study, 63 (70.80%) had mediastinal invasion, 50 (56.18%) had a bulky mediastinum, and 11 (12.36%) exhibited CNS invasion at diagnosis, consistent with previous reports [1]. Additionally, 35 patients (39.33%) had extranodal lesions, commonly involving the pericardium and pleura (15/35), as well as other sites such as the liver, kidney, skin, spine, and muscles. Extranodal involvement, particularly in the pericardium and pleura, may contribute to the poor prognosis of T-LBL.
Despite improvements in treatment efficacy with ALL-like regimens, 30.00%-40.00% of T-LBL patients experience refractory relapse with poor prognosis and survival rates of only 10.00.0%-30.00% [3]. Identifying high-risk patients and employing more aggressive treatments or targeted therapies remain critical for improving outcomes.
The molecular features of T-LBL have gained attention in recent years, given their potential to enhance risk stratification and guide individualized treatment. Although T-LBL and T-ALL share overlapping genetic and molecular characteristics, T-ALL risk stratification strategies cannot be directly applied to T-LBL [7-9]. Recent studies have also highlighted genetic predisposition syndromes (e.g., Nijmegen breakage syndrome, constitutional mismatch repair deficiency, and ataxia telangiectasia) and environmental factors (e.g., ionizing radiation and viral infections such as HTLV-1, HHV-8, HIV, and HCV) as contributors to T-LBL/ALL development [10,11].
In this study, 37 of the 89 patients underwent genetic mutation testing, with mutations identified in 29 patients. The most frequently mutated pathway was NOTCH (65.70%), followed by the JAK-STAT pathway (27.50%). NOTCH1 was the most frequently mutated gene, followed by PHF6, JAK1, JAK3, IL7R, and TP53. NOTCH1 and FBXW7 mutations co-occurred in 13 patients, 11 of whom achieved CR after induction chemotherapy, with a 3-year OS rate of 86.20%. These findings align with previous studies suggesting better outcomes in patients with NOTCH1/FBXW7 mutations [12,13].
Univariate analysis revealed that sex, IPI scores, CNS invasion, splenomegaly, LDH levels, and treatment efficacy significantly affected OS. ECOG scores, extranodal invasion, and chemotherapy regimen emerged as potential prognostic factors but did not achieve statistical significance in this study. Multivariate analysis identified female sex, lack of CR, elevated LDH levels (>2× normal), and splenomegaly as independent factors associated with OS.
For PFS, univariate analysis identified sex, BM invasion, stage, B symptoms, IPI scores, CNS invasion, bulky disease, LDH levels, and chemotherapy regimen as potential factors, with sex, IPI scores, LDH levels, and chemotherapy regimen being statistically significant. Multivariate analysis confirmed that chemotherapy regimen, LDH levels, and sex were independent prognostic factors for PFS.
Although the hyper-CVAD A/B group had a slightly higher CR rate than the GMALL07/2003 group, PFS was worse in the hyper-CVAD A/B group. This suggests that treatment protocol selection did not significantly influence OS, with CR serving as a protective factor for survival.
T-LBL is a rare malignancy with a poor prognosis, and no standardized treatment regimen has been established. Historically, standard treatments for non-Hodgkin’s lymphoma yielded suboptimal outcomes [14,15]. The introduction of high-intensity, short-course, ALL-type multidrug regimens has significantly improved treatment efficacy [16,17].
Although the clinical characteristics of patients with LBL and ALL in China are similar to those observed in other countries, treatment outcomes differ. In the GMALL study [18,19], 45 T-LBL patients received two sequential ALL-targeted regimens, comprising an 8-drug induction protocol, a 6-drug reinduction regimen, and various consolidation treatments. Both groups underwent mediastinal and CNS irradiation alongside intrathecal chemoprophylaxis, achieving 7-year OS, CR, and DFS rates of 51.00%, 65.00%, and 62.00%, respectively [20].
In a subsequent trial, 149 patients (Cohort I: 101; Cohort II: 48) were treated with the ALL 07/2003 chemotherapy protocol without mediastinal radiotherapy [20,21]. However, similar outcomes were not replicated in Chinese cohorts. A study on 74 Chinese LBL patients reported overall response, CR, 3-year OS, 5-year OS, 3-year PFS, and 5-year PFS rates of 70.20%, 48.60%, 38.00%, 26.60%, 34.80%, and 23.20%, respectively. Subgroup analysis revealed worse outcomes in the T-LBL group, with a 5-year OS of only 22.70% among 57 patients [22].
Differences in clinical characteristics and prognosis between T-LBL and B-LBL were evident in the above studies [23-25]. In the present study, the CR rate among 89 T-LBL patients was 51.69% after 1-2 treatment courses. CR rates were 64.52% and 44.83% for the hyper-CVAD A/B and VDCLP groups, respectively, but the difference was not statistically significant (χ2=3.136, P=0.077). The 3-year OS and PFS rates were 74.90% and 58.80%, respectively. Adverse effect profiles between the two regimens were also comparable. Additionally, 23 patients received local IMRT, 12 underwent allo-HSCT, and 5 underwent auto-HSCT. In the cases included in this study, new drugs such as chidamide and venetoclax were not used in combination for high-risk patients. In future studies, we will further explore regimens involving the combination of these new drugs.
In conclusion, T-LBL is characterized by distinct molecular features, with high-frequency gene mutations across multiple signaling pathways. This study highlighted that mediastinal invasion and extranodal lesions were more prevalent in Chinese T-LBL patients and may contribute to poor prognosis. Integrating molecular characteristics into prognostic assessments can enhance the identification of high-risk patients, paving the way for improved therapeutic strategies using novel targeted agents.
Disclosure of conflict of interest
None.
References
- 1.Pagliaro L, Chen SJ, Herranz D, Mecucci C, Harrison CJ, Mullighan CG, Zhang M, Chen Z, Boissel N, Winter SS, Roti G. Acute lymphoblastic leukaemia. Nat Rev Dis Primers. 2024;10:41. doi: 10.1038/s41572-024-00525-x. [DOI] [PubMed] [Google Scholar]
- 2.Brown P, Inaba H, Annesley C, Beck J, Colace S, Dallas M, DeSantes K, Kelly K, Kitko C, Lacayo N, Larrier N, Maese L, Mahadeo K, Nanda R, Nardi V, Rodriguez V, Rossoff J, Schuettpelz L, Silverman L, Sun J, Sun W, Teachey D, Wong V, Yanik G, Johnson-Chilla A, Ogba N. Pediatric acute lymphoblastic leukemia, version 2.2020, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2020;18:81–112. doi: 10.6004/jnccn.2020.0001. [DOI] [PubMed] [Google Scholar]
- 3.Kroeze E, Loeffen JLC, Poort VM, Meijerink JPP. T-cell lymphoblastic lymphoma and leukemia: different diseases from a common premalignant progenitor? Blood Adv. 2020;4:3466–3473. doi: 10.1182/bloodadvances.2020001822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhu J, Ma J Union for China Lymphoma Investigators of Chinese Society of Clinical Oncology. Chinese society of clinical oncology (CSCO) diagnosis and treatment guidelines for malignant lymphoma 2021 (English version) Chin J Cancer Res. 2021;33:289–301. doi: 10.21147/j.issn.1000-9604.2021.03.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen C, Leng X, Zhang Y, Hu J, Wei D, Wang P, Wang X. Effects of platelets on characteristics of lymphocytes cultured in vitro and optimization of adoptive immunotherapy. Biocell. 2023;47:2661–2669. [Google Scholar]
- 6.Cao H, Xue Y, Wang F, Li G, Zhen Y, Guo J. Identification of prognostic molecular subtypes and model based on CD8+ T cells for lung adenocarcinoma. Biocell. 2024;48:473–490. [Google Scholar]
- 7.Morita K, Jain N, Kantarjian H, Takahashi K, Fang H, Konopleva M, El Hussein S, Wang F, Short NJ, Maiti A, Sasaki K, Garcia-Manero G, Konoplev S, Ravandi F, Khoury JD, Jabbour E. Outcome of T-cell acute lymphoblastic leukemia/lymphoma: focus on near-ETP phenotype and differential impact of nelarabine. Am J Hematol. 2021;96:589–598. doi: 10.1002/ajh.26144. [DOI] [PubMed] [Google Scholar]
- 8.Intermesoli T, Weber A, Leoncin M, Frison L, Skert C, Bassan R. Lymphoblastic lymphoma: a concise review. Curr Oncol Rep. 2022;24:1–12. doi: 10.1007/s11912-021-01168-x. [DOI] [PubMed] [Google Scholar]
- 9.Li Z, Song Y, Zhang M, Wei Y, Ruan H. Genomic landscape of T-cell lymphoblastic lymphoma. Chin J Cancer Res. 2022;34:83–94. doi: 10.21147/j.issn.1000-9604.2022.02.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tian XP, Su N, Wang L, Huang WJ, Liu YH, Zhang X, Huang HQ, Lin TY, Ma SY, Rao HL, Li M, Liu F, Zhang F, Zhong LY, Liang L, Lan XL, Li J, Liao B, Li ZH, Tang QL, Liang Q, Shao CK, Zhai QL, Cheng RF, Sun Q, Ru K, Gu X, Lin XN, Yi K, Shuang YR, Chen XD, Dong W, Sun C, Sang W, Liu H, Zhu ZG, Rao J, Guo QN, Zhou Y, Meng XL, Zhu Y, Hu CL, Jiang YR, Zhang Y, Gao HY, He WJ, Xia ZJ, Pan XY, Hai L, Li GW, Song LY, Kang TB, Xie D, Cai QQ. A CpG methylation classifier to predict relapse in adults with T-cell lymphoblastic lymphoma. Clin Cancer Res. 2020;26:3760–3770. doi: 10.1158/1078-0432.CCR-19-4207. [DOI] [PubMed] [Google Scholar]
- 11.Young KH. Hematopoietic stem cell transplantation for adults with T-cell lymphoblastic lymphoma: can we successfully step into the era of precision medicine? Leukemia. 2020;34:1213–1214. doi: 10.1038/s41375-019-0608-4. [DOI] [PubMed] [Google Scholar]
- 12.Bonn BR, Rohde M, Zimmermann M, Krieger D, Oschlies I, Niggli F, Wrobel G, Attarbaschi A, Escherich G, Klapper W, Reiter A, Burkhardt B. Incidence and prognostic relevance of genetic variations in T-cell lymphoblastic lymphoma in childhood and adolescence. Blood. 2013;121:3153–3160. doi: 10.1182/blood-2012-12-474148. [DOI] [PubMed] [Google Scholar]
- 13.Burkhardt B, Mueller S, Khanam T, Perkins SL. Current status and future directions of T-lymphoblastic lymphoma in children and adolescents. Br J Haematol. 2016;173:545–559. doi: 10.1111/bjh.14017. [DOI] [PubMed] [Google Scholar]
- 14.Lepretre S, Touzart A, Vermeulin T, Picquenot JM, Tanguy-Schmidt A, Salles G, Lamy T, Bene MC, Raffoux E, Huguet F, Chevallier P, Bologna S, Bouabdallah R, Benichou J, Briere J, Moreau A, Tallon-Simon V, Seris S, Graux C, Asnafi V, Ifrah N, Macintyre E, Dombret H. Pediatric-like acute lymphoblastic leukemia therapy in adults with lymphoblastic lymphoma: the GRAALL-LYSA LL03 study. J. Clin. Oncol. 2016;34:572–580. doi: 10.1200/JCO.2015.61.5385. [DOI] [PubMed] [Google Scholar]
- 15.Kantarjian H, Stein A, Gokbuget N, Fielding AK, Schuh AC, Ribera JM, Wei A, Dombret H, Foa R, Bassan R, Arslan O, Sanz MA, Bergeron J, Demirkan F, Lech-Maranda E, Rambaldi A, Thomas X, Horst HA, Bruggemann M, Klapper W, Wood BL, Fleishman A, Nagorsen D, Holland C, Zimmerman Z, Topp MS. Blinatumomab versus chemotherapy for advanced acute lymphoblastic leukemia. N Engl J Med. 2017;376:836–847. doi: 10.1056/NEJMoa1609783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Portell CA, Sweetenham JW. Adult lymphoblastic lymphoma. Cancer J. 2012;18:432–438. doi: 10.1097/PPO.0b013e31826b1232. [DOI] [PubMed] [Google Scholar]
- 17.Huguet F, Leguay T, Raffoux E, Thomas X, Beldjord K, Delabesse E, Chevallier P, Buzyn A, Delannoy A, Chalandon Y, Vernant JP, Lafage-Pochitaloff M, Chassevent A, Lheritier V, Macintyre E, Bene MC, Ifrah N, Dombret H. Pediatric-inspired therapy in adults with Philadelphia chromosome-negative acute lymphoblastic leukemia: the GRAALL-2003 study. J. Clin. Oncol. 2009;27:911–918. doi: 10.1200/JCO.2008.18.6916. [DOI] [PubMed] [Google Scholar]
- 18.Gökbuget N, Beck J, Brandt K, Brüggemann M, Burmeister T, Diedrich H, Faul C, Hüttmann A, Kondakci M, Kraemer D. Significant improvement of outcome in adolescents and young adults (AYAs) aged 15-35 years with acute lymphoblastic leukemia (ALL) with a pediatric derived adult all protocol; results of 1529 AYAs in 2 consecutive trials of the german multicenter study group for adult ALL (GMALL) Blood. 2013;122:839. [Google Scholar]
- 19.Huo W, Gao L, Song K, Huang J, Wang N, Cao L, Liu Y, Wang F, Li C, Zhu X, Wu X, Cao Y, Mo X, Hu X. Allogeneic haematopoietic stem cell transplantation for adult T-lymphoblastic lymphoma: a real-world multicentre analysis in China. Br J Haematol. 2024;204:2390–2399. doi: 10.1111/bjh.19481. [DOI] [PubMed] [Google Scholar]
- 20.Yu H, Mi L, Qi F, Wang X, Ye Y, Li M, Wang D, Ding N, Wang X, Song Y, Zhu J, Xie Y. Survival and prognostic analysis of T-cell lymphoblastic lymphoma patients treated with dose-adjusted BFM-90 regimen. Aging (Albany NY) 2022;14:3203–3215. doi: 10.18632/aging.204008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang X, Huang R, Zhang X, Zhang X. Current status and prospects of hematopoietic stem cell transplantation in China. Chin Med J (Engl) 2022;135:1394–1403. doi: 10.1097/CM9.0000000000002235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yuan Y, Zang L, Yue Z, Zhang Y, Wang X. Clinical features and prognosis of 74 cases of lymphoblastic lymphoma. Cancer Research on Prevention and Treatment. 2018;45:154–159. [Google Scholar]
- 23.Lu P, Liu Y, Yang J, Zhang X, Yang X, Wang H, Wang L, Wang Q, Jin D, Li J, Huang X. Naturally selected CD7 CAR-T therapy without genetic manipulations for T-ALL/LBL: first-in-human phase 1 clinical trial. Blood. 2022;140:321–334. doi: 10.1182/blood.2021014498. [DOI] [PubMed] [Google Scholar]
- 24.Zhang X, Yang J, Li J, Qiu L, Zhang J, Lu Y, Zhao YL, Jin D, Li J, Lu P. Analysis of 60 patients with relapsed or refractory T-cell acute lymphoblastic leukemia and T-cell lymphoblastic lymphoma treated with CD7-targeted chimeric antigen receptor-T cell therapy. Am J Hematol. 2023;98:1898–1908. doi: 10.1002/ajh.27094. [DOI] [PubMed] [Google Scholar]
- 25.Zhang M, Chen D, Fu X, Meng H, Nan F, Sun Z, Yu H, Zhang L, Li L, Li X, Wang X, Wang M, You F, Li Z, Chang Y, Zhou Z, Yan J, Li J, Wu X, Wang Y, Wang Y, Xiang S, Chen Y, Pan G, Xu H, Zhang B, Yang L. Autologous nanobody-derived fratricide-resistant CD7-CAR T-cell therapy for patients with relapsed and refractory T-cell acute lymphoblastic leukemia/lymphoma. Clin Cancer Res. 2022;28:2830–2843. doi: 10.1158/1078-0432.CCR-21-4097. [DOI] [PubMed] [Google Scholar]