Abstract
Tumor-associated macrophages (TAMs) are important immune cells in the tumor micro-environment (TME) and play a key role in the occurrence and development of cervical cancer. Besides, targeting TAMs can significantly inhibit cervical cancer tumor growth, invasion, metastasis, and angiogenesis as well as affect immune regulation. This review summarizes the correlation between TAM and tumors, the mechanism of action of TAM in cervical cancer, and the potential application of TAM in the treatment of cervical cancer. Therefore, this study may provide new ideas and targets for the development of further treatment strategies for cervical cancer patients.
Keywords: Cervical cancer, tumor microenvironment, tumor-associated macrophages, polarization, immunotherapy
Introduction
Cervical cancer (CC) is the most common malignant tumor of the female reproductive system, with increasing incidence and a younger age of onset [1]. More than 600,000 new cases of cervical cancer and 340,000 deaths are reported yearly worldwide [2]. Studies have shown that persistent infection with human papillomavirus (HPV-16 and HPV-18) is a key risk factor for cervical cancer, accounting for about 80% to 90% [3]. In addition, some studies have shown that the incidence of cervical cancer may be related to early marriage, multiple births, dietary habits, poor local hygiene, oral contraceptives, etc. These factors mainly promote the occurrence and development of cervical cancer by affecting the tumor microenvironment.
Although there are currently five treatments for cervical cancer (surgery, radiotherapy, chemotherapy, targeted therapy, and immunotherapy), the treatment effect of advanced and recurrent cervical cancer is poor. Therefore, further studies should assess how to improve the treatment strategy for cervical cancer, reduce the recurrence rate, and improve the five-year survival rate are.
Tumor microenvironment (TME) has become one of the hot topics in the current research field in recent years. Studies have demonstrated that tumor-associated macrophages (TAMs) are immune cells in the TME, accounting for 30% to 50% of TME cells [3]. TAMs can promote tumor development, invasion, metastasis, immunosuppression, and angiogenesis [4-6]. TAMs also play a key role in the occurrence and development of cervical cancer and are a potential target for immunotherapy in patients with cervical cancer. However, the role of TAMs in cervical cancer is complex, where TAM clearance or polarization of related phenotypes may contribute to tumor immunotherapy [7,8]. This review focuses on the correlation between TAMs and tumors, the potential functional mechanisms of TAMs in cervical cancer, and the potential applications of TAMs in the treatment of cervical cancer, in order to provide new ideas for the immunotherapy of cervical cancer.
Correlation between TAM and tumor
The main sources and differentiation of TAMs
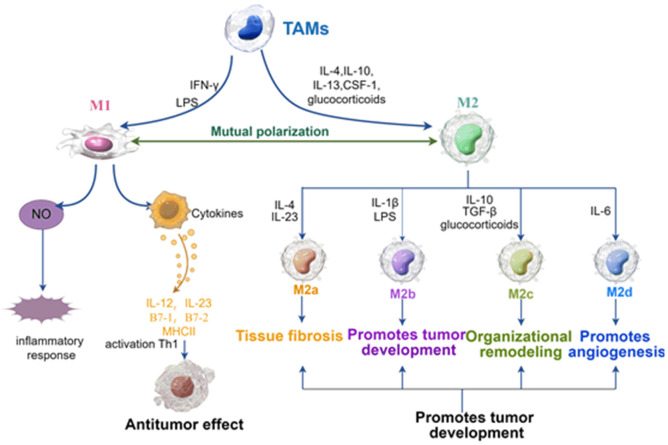
Tumor-associated macrophages (TAMs) are important components of the TME [9]. TAMs can promote tumor development, invasion, metastasis, immunosuppression, and angiogenesis by secreting cytokines and chemokines as well as coordinating inflammatory mechanisms [4-6]. TAMs also act as immunomodulators in the TME, and a potential target for immunotherapy in cancer patients [10]. Some TAMs are derived from embryos and maintain their activities through continuous self-renewal, such as Kupffer cells in the liver, spleen, and lymph nodes; osteoclasts in bone tissue; microglia in nerve tissue; Langerhans cells in skin tissue and alveolar macrophages in the lungs [3]. In addition, most TAMs originate from peripheral blood monocytes in the bone marrow [11]. Many pro-inflammatory mediators recruit circulating monocytes to tumors, inflamed or infected tissues, and differentiate them into macrophages [12]. Studies have shown that the spleen significantly impacts monocyte-derived TAMs, which play an important role in inflammatory responses. Monocyte-derived TAMs are also an important extramedullary site that can continuously provide monocytes to developing tumors [13]. Notably, macrophages differentiate into two functional phenotypes (classically activated macrophages (M1) and alternatively activated macrophages (M2)) under the influence of different signals in TME [14]. Specifically, macrophages can differentiate into M1 macrophages under the induction of lipopolysaccharides (LPS) or interferon-γ (IFN-γ) in vitro, which mainly secrete nitric oxide and inflammatory cytokines, such as interleukin-12 (IL-12), interleukin-23 (IL-23), histocompatibility complex II (MHCII) and B7 family members (B7-1 (CD80) and B7-2 (CD86)) [15]. M1 macrophages mainly participate in the immune response, play an antigen presentation function, enhance the inflammatory response, and play an anti-tumor role by activating helper T cell 1 (Th1). However, monocytes differentiate into M2 macrophages in the presence of colony-stimulating factor 1 (CSF-1), IL-4, IL-10, IL-13, glucocorticoids, and immune complexes induced by IL-1R ligands [16]. M2 macrophages mainly inhibit immune response by secreting transforming growth factor β (TGF-β), IL-10, etc., secretes IL-1 chemokine receptor antagonist matrix metalloproteinase (MMP) and upregulates MHC to participate in tissue remodeling and immune tolerance [17]. Notably, M2 macrophages stimulate tumor invasion, growth, and metastasis, thus inhibiting the anti-tumor effect of T cells. In addition, the latest studies have shown that M2 macrophages are divided into four subtypes according to different stimuli: M2a (induced by IL-4 and IL-23); M2b (induced by immune complexes containing IL-1β or LPS); M2c (induced by IL-10, TGF-β and glucocorticoids) and M2d (induced by IL-6) [18]. Although M2 macrophage subtypes play a common anti-inflammatory and immunomodulatory role, they have some different functions. For example, the M2a subtype is mainly involved in tissue fibrosis, the M2b subtype mainly promotes tumor development, the M2c subtype is mainly responsible for tissue remodeling, and the M2d subtype mainly promotes angiogenesis [19] (Figure 1).
Figure 1.
Phenotypic polarization of TAMs (by figdraw). TAMs may be polarized into classically activated M1 macrophages and alternatively activated M2 macrophages. M1 macrophages are activated by LPS and INF-γ, while M2 macrophages are activated by IL-4, IL-10, IL-13, CFS-1, and glucocorticoids. M2 macrophages are further subdivided into M2a, M2b, M2c, and M2d based on different microenvironmental stimuli, leading to tissue fibrosis, tumor development, tissue remodeling, and angiogenesis, thereby promoting tumor development. IL, interleukin; LPS, lipopolysaccharide; IC, immune complex; CSF, colony-stimulating factor.
Phenotypic changes of TAMs during tumorigenesis and progression
The polarization state of macrophages is very complex. TME has macrophages with M1 characteristics, M2 characteristics, and those with neither. The polarization process of TAMs is directly affected by cancer cells within the TME. Besides, TME changes or therapeutic interventions can alter the polarization process [20], indicating that macrophages may be potential therapeutic targets. M1 macrophages in TME initiate immune responses, destroy tissue integrity, and initiate anti-tumor responses in the early stages of tumorigenesis [21]. Some studies have indicated that M1 macrophages present antigens to T cell receptors (TCR) [20], tumor-derived chemokines CXCL9, CXCL10, and CXCL11 [22]. The antigens are then secreted into the TME to recruit and activate NK cells [23], thereby achieving the anti-tumor effect. Therefore, high expression of NK cell cytokines (IFN-γ and TNF-α) and chemokines (CCL4, CCL5 and CCL23) can recruit more immune cells and send anti-tumor signals [20-23]. However, factors released in the TME (IL-10, IL-4, and IL-13) during tumor development polarize M1 macrophages into M2 macrophages, thus exhibiting various characteristics, such as pro-angiogenesis, pro-fibrosis, and suppression of immune activity, thereby promoting tumor growth and metastasis [24]. Studies have also shown that TAMs express M1 markers in the early stages of lung cancer. Notably, the macrophage phenotype changes from M1 to M2 during lung cancer progression [25]. M1 and M2 macrophages play a central role in the occurrence and development of breast cancer. Clark et al. found that the cell microenvironment constructed by breast cancer cells can inhibit the expression of inflammatory response factors in breast cancer and adjacent tissues [26], leading to the accumulation of M2 cells, transdifferentiation of M0/M1 cells into M2 cells, and the formation of TAM. In addition, many studies have revealed that M2 macrophage polarization is associated with tumor development. For example, Circ-ITGB6 accelerates cisplatin resistance in ovarian cancer by inhibiting macrophage M2 polarization [27]; MiR-423-3p suppresses the development of cervical cancer by inhibiting macrophage M2 polarization [28]. Therefore, further studies should explore the mechanism of action of tumor-associated macrophages in cervical cancer and provide new strategies for the treatment of cervical cancer.
Novel TAM subpopulations based on single-cell RNA sequencing
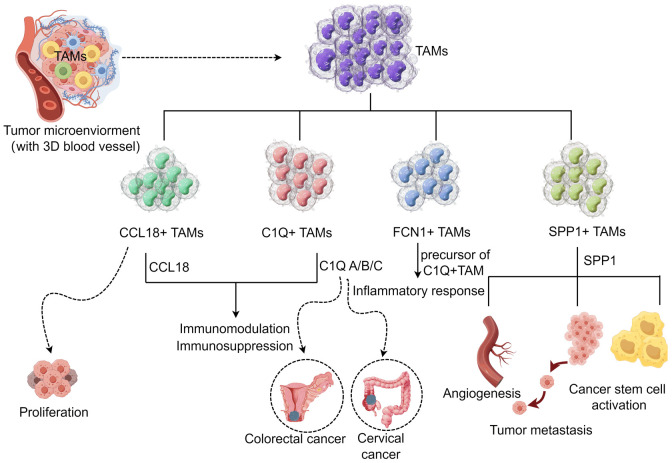
Single-cell RNA sequencing (scRNA-seq) can discover the relationship between genes and reveal the subpopulations of rare cell populations. scRNA-seq divides TAMs in solid tumors into four subgroups based on core gene characteristics (C1Q+, SPP1+, FCN1+, and CCL18+TAM subgroups) [13]. These TAM subgroups have high phenotypic plasticity and tumor heterogeneity [29]. The C1Q+TAM subgroup expresses C1QA/B/C, especially in colorectal cancer [30] and cervical cancer [31]. Cervical cancer patients with high C1QC expression have a better prognosis than those with low C1QC expression. The C1Q+TAM subgroup mainly induces immunomodulation and immunosuppression effects. This subgroup relies on the recognition molecule C1Q of the classical complement pathway to bind to the immune complex in the TAM for the functions [13]. In addition, C1Q can interact with endothelial cells through cell surface receptors, thereby promoting the formation of new blood vessels [32]. The SPP1+TAM subpopulation is mainly identified by the specific expression of the SPP1 gene. The SPP1+TAM subpopulation plays many roles in tumors, including tumor metastasis, angiogenesis, and tumor stem cell activation [13]. The promotion of tumor cell invasion is related to the high expression of MMP9, MMP12, MMP14, and MMP19 in SPP1+ macrophages in tumor tissues [33]. Besides, the promotion of angiogenesis and tumor metastasis is related to the main metabolic pathway of SPP1+ macrophages (glycolysis) [34]. Li et al. found that cervical cancer patients with low SPP1 expression have the best prognosis, while patients with high SPP1 expression have the worst prognosis [31]. FCN1+TAM subpopulation is the precursor of C1Q+TAM, mainly derived from inflammatory monocytes and involved in inflammatory response [34]. A recent study showed [35] that FCN1 is almost exclusively expressed in macrophages infiltrating the colonic mucosa of children with inflammatory bowel disease, indicating that it may be a novel and promising diagnostic biomarker for inflammatory bowel disease in children. The CCL18+TAM subgroup is characterized by the expression of the core gene CCL18, which is mainly related to the immunosuppression of the TAM. CCL18 is in an immunosuppressive state in tumor tissues [36]. Studies have shown that the CCL18+TAM subgroup can affect tumor cell proliferation, migration induction, invasion, angiogenesis, and vasculogenesis [37], indicating that CCL18 may become a potential target for anticancer therapy. Although scRNA-seq data have suggested that TAM subgroups have different functions, further studies should clarify the functions of these different subgroups for guiding tumor treatment (Figure 2).
Figure 2.
Potential function of the novel TAM subpopulation in TAM (by figdraw). According to the characteristics of core genes, the TAM in solid tumors was divided into four subgroups. The CCL18+TAM subgroup and the C1Q+TAM subgroup mainly played immunosuppressive functions, and the C1Q+TAM subgroup mostly existed in colorectal cancer and cervical cancer. FCN1+TAM subgroup is the precursor of C1Q+TAM, which is mainly involved in inflammatory response. The SPP1+TAM subpopulation plays a variety of roles, including involvement in tumor metastasis, angiogenesis, and activation of tumor stem cells.
The mechanism of action of TAM in cervical cancer
TAMs promote the proliferation, invasion and metastasis of cervical cancer cells
TAMs and tumor cells promote cancer cell proliferation in TME mainly by secreting cytokines. Besides, the interaction of different types of stromal protumor cells, including TAMs promotes tumor invasion and metastasis [5]. In addition, the ECM-degrading enzymes (serine proteases, metalloproteinases, and cathepsins) released by TAMs relax the fibrous connective tissue around the tumor, followed by the detaching of tumor cells from the solid tumor tissue, which then enter the systemic circulation, resulting in distant metastasis [38]. Some scholars [39] have found that M2 TAM can upregulate PD-L1 in cervical cancer cells through the PI3K/AKT pathway and enhance their migration and invasion capabilities, thus affect tumor progression. Nuclear factor of activated T cells 1 (NFATc1) is mainly expressed in the TME, especially in macrophages. M2 TAM can regulate the function of macrophages, thereby increasing tumor invasive activity and promoting tumor development. Tan et al. showed that NFATc1-induced M2 macrophages can promote the proliferation, migration, and invasion of cervical cancer cells. Furthermore, they showed that NFATc1 knockout can significantly inhibit the tumor-promoting function of TAMs [40]. FGD5 antisense RNA1 (FGD5-AS1) can directly target miR-129-5p, mainly by inducing bone marrow stromal cell antigen 2 (BST2) in cervical cancer, promoting M2 macrophage polarization, thereby leading to the development of cervical cancer [41]. Shen Lin et al. [8] found that astragaloside IV (AS-IV) can significantly inhibit M2 polarization of macrophages, mainly manifested as reduced expression of CD163, IL-10, TGFβ, and CD206. AS-IV inactivates TGF-β/Smad2/3 signal transduction, thereby preventing the migration and epithelial-mesenchymal transition of cervical cancer cells. Similarly, Jin et al. found that SPI1-related protein (SPIB) can inhibit cervical cancer cell progression and macrophage migration in cervical cancer [42]. In addition, co-culture system of cancer cells and macrophages, has shown that IL-17A overexpression can promote the polarization of M2 macrophages in cervical cancer. Notably, Oct4 (a homeodomain transcription factor of the POU family) binds to cervical cancer cells and transcriptionally activates IL-17A, thereby upregulating IL-17A and promoting the proliferation, migration and invasion of cervical cancer cells [43]. This discovery has the potential to be a therapeutic target cervical cancer treatment.
TAMs regulate involved in angiogenesis in cervical cancer cells
Angiogenesis is crucial in TME. Angiogenesis forms new blood vessels from existing blood vessels, and provides rich nutrients for tumor growth. Therefore, anti-angiogenic therapy plays an important role in cancer treatment. Studies have shown that TAM participates in tumor angiogenesis by secreting pro-angiogenic factors (VEGF-A, EGF, PlGF, TGF-β, TNF-α, IL-1β, IL-8, CCL2, CXCL8, and CXCL12) [44]. Studies have found [45] that macrophages induced by anaerobic digestive streptococci stimulate angiogenesis in cervical cancer mainly by secreting vascular endothelial growth factor (VEGF), thereby promoting the development of cervical cancer. Cytoskeleton-associated protein 2 (CKAP2) is a microtubule-stabilizing tubulin that regulates aneuploidy, cell cycle, apoptosis, and cell senescence in a p53/TP53-dependent manner. CKAP2 also plays an important role in controlling cell division [46]. Guo et al. [47] found that CKAP2 can regulate the proliferation of endothelial cells in TME and promote the development of cervical cancer with the help of TAM. CKAP2 mainly regulates TME through NF-κB signaling, thus inducing cervical cancer progression. In addition, studies have shown [48] that macrophages (TEM) expressing tyrosine kinase containing immunoglobulin and epidermal growth factor homology domain 2 (TIE2) can promote the development of cervical cancer. Notably, TIE2 protein is directly transferred from TIE2-high-expressing cervical cancer cells to monocytes and macrophages through exosomes, thereby increasing tumor-infiltrating TEM and tumor microvascular intensity. Besides, in vitro and in vitro experiments have confirmed the mechanism. In addition, TAMs increase tumor angiogenesis by inducing pro-inflammatory mediators, such as IL-1 and IL-6, thereby upregulating the production of pro-angiogenic factors [44]. These findings provide new insights into the angiogenic mechanisms of cervical cancer and may provide new ideas for improving anti-angiogenic therapy.
TAMs regulate autophagy and immune escape in cervical cancer cells
Autophagy is a conserved process that occurs in tumor cells. Autophagy levels are upregulated due to the rapid proliferation, nutrient deprivation, and hypoxia of tumor cells. Studies have shown that autophagy in cancer cells promotes immunosuppression and tumor growth [49]. Besides, stud autophagy can promote antigen presentation and immune activation [50]. Yang et al. found that IFN-γ significantly increases the autophagy levels of HeLa and SiHa cells by promoting the expression of indoleamine-2,3-dioxygenase-1 (IDO1). Notably, IFN-γ-IDO1 axis may inhibit the progression of cervical cancer by promoting the accumulation of kynurenine in cells, enhancing the phagocytosis of macrophages, and further upregulating the autophagy level of cervical cancer cells [51]. The latest research reports have shown that autophagy can regulate. TAM plasticity and functional polarization in TME, mainly by regulating antigen presentation, LC3-related phagocytosis, cytokine secretion, inflammasome. TAM recruitment, differentiation and polarization, etc. [52]. TAMs can regulate the killing effect of T cells and NK cells on tumor cells, and directly inhibit the proliferation of CD8+ T cells through the metabolism of L-arginine by arginase 1, oxygen free radicals, or nitrogen substances [53]. Studies have reported [54] that the interaction between M0 macrophages and naive CD4+ T cells in the TME of cervical cancer helps prevent tumor escape. Cancer cells evade the immune system through the expression of CD47, a cell surface protein that interacts with signal regulatory protein α (SIRPα) on the surface of macrophages to prevent phagocytosis [55,56]. Chen et al. found that oncogenic transcription factor 1 (ZEB1) can activate the transcription of the CD47 gene in cervical squamous cell carcinoma (CSCC) under hypoxic conditions, thereby promoting immune evasion in cervical cancer cells by enhancing the CD47-SIRPα axis. They also found that endogenous ZEB1 is enriched in exosomes derived from hypoxic CSCC cells. ZEB1 are transferred to macrophages through these exosomes [57]. As a result, TAM has become a novel target for the treatment of cervical cancer.
Other mechanisms of action of TAM in cervical cancer
High mobility group protein 1 (HMGB1) is a multifunctional redox-sensitive protein primarily involved in various intracellular (such as chromatin remodeling, and transcription, autophagy) and extracellular (inflammation and autoimmunity) processes. A recent study demonstrated [58] that targeting extracellular HMGB1 can significantly remodel the tumor immune microenvironment. Notably, inhibiting HMGB1 leads to a significant decrease in monocyte/granulocyte myeloid suppressor cells (MDSC) and regulatory T lymphocytes, an increase in the macrophage M1/M2 ratio, and an increased in activation of dendritic cells (DC) and plasmacytoid dendritic cells and (pDC), thereby achieving effective cancer treatment. In addition, studies have found [59] a new lymphatic pattern in the TME, where lymphatic vessels (LV) are wrapped by tumor-associated macrophages (LVEM) to form an interconnected network. This is common in cervical cancer lymphatic metastasis and actively participates in lymphatic metastasis. In addition, Sp1-highly expressed lymphatic endothelial cells can selectively recruit M2 macrophages, forming a unique metastasis-promoting mode. Sp1-highly expressed lymphatic endothelial cells promote the transcriptional activation of C-C motif chemokine ligand 1 (CCL1), which promotes the recruitment of TAMs and tumor cells, thereby forming a positive feedback loop to enhance LVEM formation. This shows that the identification of LVEM and its regulatory mechanism provides a new target for the development of anti-metastasis therapy for cervical cancer. This also provides a basis for selecting specific patient groups that may benefit from certain molecular-targeted drugs.
Potential application of TAM in the treatment of cervical cancer
Polarization of TAM clearance or related phenotypes
Several studies have shown that an increase in the number of M1 macrophages indicates a good prognosis, while an increase in the number of M2 macrophages indicates a poor prognosis. This suggests that regulating polarization between macrophages may control tumor progression [60,61]. A study found that the antifungal agent itraconazole (ITZ) can repolarize M2 macrophages to M1 type, reduce the expression of CD163 and chemokine ligand 18 (CCL18), then inhibit the growth of cervical cancer cells (CaSki), showing a good anti-tumor effect [62]. Similarly, Chen et al. [63] found that topical Nocardia rubrum cell wall skeleton (Nr-CWS), as an immunotherapeutic agent, may regulate the polarization of TAMs from M2 to M1 through the STAT1/STAT6 pathway, improving local immune responses and reversing immunosuppression, thereby exerting anti-cervical cancer activity. Liu et al. [7] reported that low-dose naltrexone (LDN) can inhibit the epithelial-mesenchymal transition of cervical cancer cells in vitro, mainly by inhibiting the proliferation, migration, and invasion of Hela cells and promoting their apoptosis. Meanwhile, LDN can also indirectly reduce the number of M2 macrophages in the serum of nude mice and reduce the expression of the anti-inflammatory factor IL-10. These findings indicate that LDN is a potential treatment option for cervical cancer. In addition, Shen Ling et al. [8] found that astragaloside IV (AS-IV) can significantly inhibit the M2 polarization of macrophages and reduce CD163, IL-10. TGF-β, CD206 expression. In conclusion, AS-IV mainly prevents the migration and epithelial-mesenchymal transition of cervical cancer cells by inactivating TGF-β/Smad2/3 signaling, thereby achieving anti-tumor effects. Similarly, some studies have found that curcumin can repolarize tumor-associated macrophages into M1 macrophages, while recruiting activated natural killer cells and I-Tc cells into tumor cells. This has been verified using a mouse cervical cancer model [64]. However, the inhibitory activity of chalcone 16, as a widely studied anticancer agent, on cervical cancer HeLa cells may also affect macrophage polarization, thereby a significantly increasing the levels of M1 type-specific pro-inflammatory cytokines IL1-β and TNF-α [65]. SOCS2, belonging to the suppressor of cytokine signaling (SOCS) protein family, is closely related to the occurrence and development of various cancers [66]. Li et al. found that the SOCS2 is significantly down-regulated in cervical cancer tissues. SOCS2 overexpression can inhibit the polarization of M2 macrophages and show anti-tumor effects, indicating that it may be a new therapeutic target and inhibitory factor for cervical cancer [67]. In addition, some researchers [68] have identified a new type of cervical cancer vaccine (a vaccine containing HPV16E743-77 peptide and adjuvant unmethylated cytosine phosphate guanosine oligodeoxynucleotides). The vaccination can induce an increase in systemic and local CD4+ and CD8+ T cells, and reduce immunosuppressive cells, such as myeloid suppressor cells, regulatory T cells, and M2 tumor-associated macrophages in a mouse model of cervical cancer, inducing a strong anti-tumor immune response, thereby effectively controlling established implanted tumors. These findings provide some theoretical support for exploring new treatments for cervical cancer.
TAMs influence immune checkpoints
PD-L1 is expressed on the tumor surface and binds to PD-1 on T cells, resisting the killing effect of T cells in TME, leading to tumor immune escape [69]. TAMs mainly inhibit the recruitment and function of T cells through cytokines, surface immune checkpoint ligands, and exosomes. Besides, TAMs play a key role in regulating PD-1/PD-L1 immunosuppression [70]. Several studies have found that the combination of targeted TAMs therapy and anti-PD-1/PD-L1 therapy can improve the efficacy of cancer treatment. Targeted TAMs therapies mainly include TGF-β inhibitors and colony-stimulating factor 1 receptor (CSF-1R) inhibitors [71,72]. Studies have shown [73] that the combination of TGF-β inhibitor tranilast and anti-PD-1/PD-L1 can enhance the infiltration of M1 TAMs in tumor tissues, thereby improving the efficacy of anti-PD-L1. Furthermore, the CSF-1R inhibitor PLX3397 (pecidatinib) combined with anti-PD-1 can increase CD8+ T cell infiltration, thus improving anti-tumor efficacy [74]. Therefore, the regulation effect of TAMs on PD-L1 expression in tumor cells should be clarified for combined therapy. Besides, studies have shown that secretions or exosomes of tumor cells transform TAMs from an M1-like phenotype to an M2-like phenotype [75,76]. The infiltration of M2-type TAMs in various cancers is significantly associated with adverse clinical outcomes, such as poor prognosis and tumor progression [77]. Some studies have found that N6-methyladenosine (m6A) may affect the polarization or function of macrophages in the tumor microenvironment. A study found that high expression of METTL14 (methyltransferase 14) in cervical cancer tissues can increase the proportion of PD-1-positive tumor-associated macrophages, thereby leading to impaired ability of macrophages to phagocytize tumor cells. This was verified using C57BL/6J and BALB/c nude mice [78]. A NiCOL Phase 1 trial (NCT03298893) [79] found that patients without progression have higher levels of PD-1 on tumor-infiltrating CD4+ T cells and tumor-associated macrophages, showing active immune infiltration and enrichment of IFN-related pathways. This indicated that concurrent chemoradiotherapy (CRT) and blockade of the PD-1 pathway can enhance immune-mediated tumor control by increasing phagocytosis, cell death, and antigen presentation. However, further studies should validate the findings using locally advanced cervical cancer with pre-existing adaptive immune activation.
Nano-drug delivery system regulates and kills tumors by targeting TAM
The rapid development of nano-drug delivery systems provides a potential platform for tumor therapy. Nanoparticles, such as ferrous oxide [80], zinc oxide [81], and copper oxide [82], have been widely used in clinical tumor treatment and diagnosis. Nanoparticle-based delivery systems can target solid tumors by targeting tumor cells, and stimulating or reprogramming immune cells. These systems can also reshape the TME and change the immune response, thereby producing effective anti-tumor immune effects [83]. Many studies have shown that the nano-drug delivery system can target TAM, polarize M2 macrophages, and affect their mediated phagocytosis, thus regulating tumor growth, metastasis, invasion, and other processes [84]. Li et al. [85] synthesized a novel nanodrug delivery system using porous hollow iron oxide nanoparticles (PHNPs) to load PI3Kγ small molecule inhibitors (3-methyladenine), and further modified the system using mannose to target TAMs. This system could reshape TME by repolarizing TAMs into pro-inflammatory M1 macrophages. Meanwhile, the system could reduce the proportion of Treg cells and the secretion of immunosuppressive factors, achieving highly efficient inhibition of tumor growth. Therefore, combining iron oxide nanoparticles with the PI3Kγ/Akt signaling pathway to convert TAM phenotype is a promising alternative for targeted TAM therapy. Similarly, some researchers [86] developed a new smart nanocarrier, polyethylenimine-modified dendritic mesoporous silica nanoparticles loaded with microRNA-125a (DMSN-PEI@125a), and showed that the system could repolarized TAMs into M1 macrophages, thus inhibiting tumor growth in the TC-1 mouse model in a synergistic manner, showing significant anti-tumor effect. Kim et al. [87] proposed a nanoemulsion (NE)-based immunotherapy platform that could induce the recruitment and activation of innate immune cells and the infiltration of lymphocytes in a cervical cancer model. The platform could also polarize M2 macrophages, thereby inhibiting tumor growth and prolonging survival in primary and re-aggression tumor models. In addition, a recent study [88] found that membrane biomimetic nanoparticles, such as sophorolipid-bound membrane-biomimetic phosphorylcholine-polylactic acid-glycolic acid hybrid nanoparticles (SDPN), can improve the efficiency of oral administration and promote the transformation of M2 TAM to M1 phenotype, thereby effectively reducing tumor metastasis. These findings indicate that the nano-drug delivery system can improve the targeting and anti-tumor effects of cervical cancer treatment, providing strong theoretical support for the treatment of cervical cancer.
Summary and outlook
Targeted therapy has been widely used in the treatment of various cancers. The role and regulatory mechanism of TAM in cervical cancer have been a research hotspot in recent years. Studies have shown that targeting TAM can inhibit cervical cancer tumor growth, invasion and metastasis, and angiogenesis, thus affecting immune regulation. Although a series of nano-drug delivery systems have been developed to target TAMs and kill tumors, there are still some concerns: 1) Identifying the phenotypic polarization and diversity of M2 macrophages; 2) Achieving specific targeted therapy; 3) Reducing the toxic side effects of drugs. This review summarizes the correlation between TAM and tumors, the mechanism of action of TAM in cervical cancer, and the potential application of TAM in the treatment of cervical cancer. In summary, targeting TAMs has great potential for developing more effective cervical cancer treatments. These strategies, combined with surgical treatment, chemoradiotherapy, targeted therapy, and immunotherapy, can provide more precise and effective treatment options for cervical cancer. Nonetheless, further studies are needed to provide more effective and safer treatment methods for cervical cancer.
Acknowledgements
The present study was supported by a grant from the National Natural Science Foundation of China (grant No. 82260557) and New Cancer Supportive Treatment Research Project of Education (cphcf-2022-012).
Disclosure of conflict of interest
None.
References
- 1.Zhou X, Lian H, Li H, Fan M, Xu W, Jin Y. Nanotechnology in cervical cancer immunotherapy: therapeutic vaccines and adoptive cell therapy. Front Pharmacol. 2022;13:1065793. doi: 10.3389/fphar.2022.1065793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 3.Liu Y, Li L, Li Y, Zhao X. Research progress on tumor-associated macrophages and inflammation in cervical cancer. Biomed Res Int. 2020;2020:6842963. doi: 10.1155/2020/6842963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ge Z, Ding S. The crosstalk between tumor-associated macrophages (TAMs) and tumor cells and the corresponding targeted therapy. Front Oncol. 2020;10:590941. doi: 10.3389/fonc.2020.590941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang Q, Guo N, Zhou Y, Chen J, Wei Q, Han M. The role of tumor-associated macrophages (TAMs) in tumor progression and relevant advance in targeted therapy. Acta Pharm Sin B. 2020;10:2156–2170. doi: 10.1016/j.apsb.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Malekghasemi S, Majidi J, Baghbanzadeh A, Abdolalizadeh J, Baradaran B, Aghebati-Maleki L. Tumor-associated macrophages: protumoral macrophages in inflammatory tumor microenvironment. Adv Pharm Bull. 2020;10:556–565. doi: 10.34172/apb.2020.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu N, Ma M, Qu N, Wang R, Chen H, Hu F, Gao S, Shan F. Low-dose naltrexone inhibits the epithelial-mesenchymal transition of cervical cancer cells in vitro and effects indirectly on tumor-associated macrophages in vivo. Int Immunopharmacol. 2020;86:106718. doi: 10.1016/j.intimp.2020.106718. [DOI] [PubMed] [Google Scholar]
- 8.Shen L, Li Y, Hu G, Song X, Wang X, Li X, Xu X. Astragaloside IV suppresses the migration and EMT progression of cervical cancer cells by inhibiting macrophage M2 polarization through TGFβ/Smad2/3 signaling. Funct Integr Genomics. 2023;23:133. doi: 10.1007/s10142-023-01017-z. [DOI] [PubMed] [Google Scholar]
- 9.Guo F, Kong W, Zhao G, Cheng Z, Ai L, Lv J, Feng Y, Ma X. The correlation between tumor-associated macrophage infiltration and progression in cervical carcinoma. Biosci Rep. 2021;41:BSR20203145. doi: 10.1042/BSR20203145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xiang X, Wang J, Lu D, Xu X. Targeting tumor-associated macrophages to synergize tumor immunotherapy. Signal Transduct Target Ther. 2021;6:75. doi: 10.1038/s41392-021-00484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Storz P. Roles of differently polarized macrophages in the initiation and progression of pancreatic cancer. Front Immunol. 2023;14:1237711. doi: 10.3389/fimmu.2023.1237711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Q, Steger A, Mahner S, Jeschke U, Heidegger H. The formation and therapeutic update of tumor-associated macrophages in cervical cancer. Int J Mol Sci. 2019;20:3310. doi: 10.3390/ijms20133310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang J, Zhu N, Su X, Gao Y, Yang R. Novel tumor-associated macrophage populations and subpopulations by single cell RNA sequencing. Front Immunol. 2023;14:1264774. doi: 10.3389/fimmu.2023.1264774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gunassekaran GR, Poongkavithai Vadevoo SM, Baek MC, Lee B. M1 macrophage exosomes engineered to foster M1 polarization and target the IL-4 receptor inhibit tumor growth by reprogramming tumor-associated macrophages into M1-like macrophages. Biomaterials. 2021;278:121137. doi: 10.1016/j.biomaterials.2021.121137. [DOI] [PubMed] [Google Scholar]
- 15.Chen S, Saeed AFUH, Liu Q, Jiang Q, Xu H, Xiao GG, Rao L, Duo Y. Macrophages in immunoregulation and therapeutics. Signal Transduct Target Ther. 2023;8:207. doi: 10.1038/s41392-023-01452-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xia Y, Rao L, Yao H, Wang Z, Ning P, Chen X. Engineering macrophages for cancer immunotherapy and drug delivery. Adv Mater. 2020;32:e2002054. doi: 10.1002/adma.202002054. [DOI] [PubMed] [Google Scholar]
- 17.Bao Z, Zeng W, Zhang D, Wang L, Deng X, Lai J, Li J, Gong J, Xiang G. SNAIL induces EMT and lung metastasis of tumours secreting CXCL2 to promote the invasion of M2-type immunosuppressed macrophages in colorectal cancer. Int J Biol Sci. 2022;18:2867–2881. doi: 10.7150/ijbs.66854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang Q, Sioud M. Tumor-associated macrophage subsets: shaping polarization and targeting. Int J Mol Sci. 2023;24:7493. doi: 10.3390/ijms24087493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang Z, Leng Z, Kang L, Yan X, Shi J, Ji Y, Guo C, Fang K, Wang Z, Li Z, Sun M, Zhao Z, Feng A, Chen Z, Zhang S, Wan D, Chen T, Xu M. Alcohol inducing macrophage M2b polarization in colitis by modulating the TRPV1-MAPK/NF-κB pathways. Phytomedicine. 2024;130:155580. doi: 10.1016/j.phymed.2024.155580. [DOI] [PubMed] [Google Scholar]
- 20.Boutilier AJ, Elsawa SF. Macrophage polarization states in the tumor microenvironment. Int J Mol Sci. 2021;22:6995. doi: 10.3390/ijms22136995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kerneur C, Cano CE, Olive D. Major pathways involved in macrophage polarization in cancer. Front Immunol. 2022;13:1026954. doi: 10.3389/fimmu.2022.1026954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luan X, Lei T, Fang J, Liu X, Fu H, Li Y, Chu W, Jiang P, Tong C, Qi H, Fu Y. Blockade of C5a receptor unleashes tumor-associated macrophage antitumor response and enhances CXCL9-dependent CD8+ T cell activity. Mol Ther. 2024;32:469–489. doi: 10.1016/j.ymthe.2023.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Susek KH, Karvouni M, Alici E, Lundqvist A. The role of CXC chemokine receptors 1-4 on immune cells in the tumor microenvironment. Front Immunol. 2018;9:2159. doi: 10.3389/fimmu.2018.02159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang H, Tian T, Zhang J. Tumor-associated macrophages (TAMs) in colorectal cancer (CRC): from mechanism to therapy and prognosis. Int J Mol Sci. 2021;22:8470. doi: 10.3390/ijms22168470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sedighzadeh SS, Khoshbin AP, Razi S, Keshavarz-Fathi M, Rezaei N. A narrative review of tumor-associated macrophages in lung cancer: regulation of macrophage polarization and therapeutic implications. Transl Lung Cancer Res. 2021;10:1889–1916. doi: 10.21037/tlcr-20-1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clark NM, Bos PD. Tumor-associated macrophage isolation and in vivo analysis of their tumor-promoting activity. Methods Mol Biol. 2019;1884:151–160. doi: 10.1007/978-1-4939-8885-3_10. [DOI] [PubMed] [Google Scholar]
- 27.Li H, Luo F, Jiang X, Zhang W, Xiang T, Pan Q, Cai L, Zhao J, Weng D, Li Y, Dai Y, Sun F, Yang C, Huang Y, Yang J, Tang Y, Han Y, He M, Zhang Y, Song L, Xia JC. CircITGB6 promotes ovarian cancer cisplatin resistance by resetting tumor-associated macrophage polarization toward the M2 phenotype. J Immunother Cancer. 2022;10:e004029. doi: 10.1136/jitc-2021-004029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yan X, Zhang S, Jia J, Yang J, Song Y, Duan H. Exosomal MiR-423-3p inhibits macrophage M2 polarization to suppress the malignant progression of cervical cancer. Pathol Res Pract. 2022;235:153882. doi: 10.1016/j.prp.2022.153882. [DOI] [PubMed] [Google Scholar]
- 29.Sun BY, Zhou C, Guan RY, Liu G, Yang ZF, Wang ZT, Gan W, Zhou J, Fan J, Yi Y, Qiu SJ. Dissecting intra-tumoral changes following immune checkpoint blockades in intrahepatic cholangiocarcinoma via single-cell analysis. Front Immunol. 2022;13:871769. doi: 10.3389/fimmu.2022.871769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang L, Li Z, Skrzypczynska KM, Fang Q, Zhang W, O’Brien SA, He Y, Wang L, Zhang Q, Kim A, Gao R, Orf J, Wang T, Sawant D, Kang J, Bhatt D, Lu D, Li CM, Rapaport AS, Perez K, Ye Y, Wang S, Hu X, Ren X, Ouyang W, Shen Z, Egen JG, Zhang Z, Yu X. Single-cell analyses inform mechanisms of myeloid-targeted therapies in colon cancer. Cell. 2020;181:442–459. e29. doi: 10.1016/j.cell.2020.03.048. [DOI] [PubMed] [Google Scholar]
- 31.Li X, Zhang Q, Chen G, Luo D. Multi-omics analysis showed the clinical value of gene signatures of C1QC+ and SPP1+ TAMs in cervical cancer. Front Immunol. 2021;12:694801. doi: 10.3389/fimmu.2021.694801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roumenina LT, Daugan MV, Noé R, Petitprez F, Vano YA, Sanchez-Salas R, Becht E, Meilleroux J, Clec’h BL, Giraldo NA, Merle NS, Sun CM, Verkarre V, Validire P, Selves J, Lacroix L, Delfour O, Vandenberghe I, Thuilliez C, Keddani S, Sakhi IB, Barret E, Ferré P, Corvaïa N, Passioukov A, Chetaille E, Botto M, de Reynies A, Oudard SM, Mejean A, Cathelineau X, Sautès-Fridman C, Fridman WH. Tumor cells hijack macrophage-produced complement C1q to promote tumor growth. Cancer Immunol Res. 2019;7:1091–1105. doi: 10.1158/2326-6066.CIR-18-0891. [DOI] [PubMed] [Google Scholar]
- 33.Hao B, Zhang Z, Lu Z, Xiong J, Fan T, Song C, He R, Zhang L, Pan S, Li D, Meng H, Lin W, Luo B, Yang J, Li N, Geng Q. Single-cell RNA sequencing analysis revealed cellular and molecular immune profiles in lung squamous cell carcinoma. Transl Oncol. 2023;27:101568. doi: 10.1016/j.tranon.2022.101568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang Q, Zhang H, Wei T, Lin A, Sun Y, Luo P, Zhang J. Single-cell RNA sequencing reveals the heterogeneity of tumor-associated macrophage in non-small cell lung cancer and differences between sexes. Front Immunol. 2021;12:756722. doi: 10.3389/fimmu.2021.756722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen X, Gao Y, Xie J, Hua H, Pan C, Huang J, Jing M, Chen X, Xu C, Gao Y, Li P. Identification of FCN1 as a novel macrophage infiltration-associated biomarker for diagnosis of pediatric inflammatory bowel diseases. J Transl Med. 2023;21:203. doi: 10.1186/s12967-023-04038-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu Y, Yang S, Ma J, Chen Z, Song G, Rao D, Cheng Y, Huang S, Liu Y, Jiang S, Liu J, Huang X, Wang X, Qiu S, Xu J, Xi R, Bai F, Zhou J, Fan J, Zhang X, Gao Q. Spatiotemporal immune landscape of colorectal cancer liver metastasis at single-cell level. Cancer Discov. 2022;12:134–153. doi: 10.1158/2159-8290.CD-21-0316. [DOI] [PubMed] [Google Scholar]
- 37.Korbecki J, Olbromski M, Dzięgiel P. CCL18 in the progression of cancer. Int J Mol Sci. 2020;21:7955. doi: 10.3390/ijms21217955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Belli C, Trapani D, Viale G, D’Amico P, Duso BA, Della Vigna P, Orsi F, Curigliano G. Targeting the microenvironment in solid tumors. Cancer Treat Rev. 2018;65:22–32. doi: 10.1016/j.ctrv.2018.02.004. [DOI] [PubMed] [Google Scholar]
- 39.Guo F, Kong W, Li D, Zhao G, Anwar M, Xia F, Zhang Y, Ma C, Ma X. M2-type tumor-associated macrophages upregulated PD-L1 expression in cervical cancer via the PI3K/AKT pathway. Eur J Med Res. 2024;29:357. doi: 10.1186/s40001-024-01897-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tan J, Yang L, Zhao H, Ai Y, Ren L, Zhang F, Dong W, Shi R, Sun D, Feng Y. The role of NFATc1/c-myc/PKM2/IL-10 axis in activating cervical cancer tumor-associated M2 macrophage polarization to promote cervical cancer progression. Exp Cell Res. 2022;413:113052. doi: 10.1016/j.yexcr.2022.113052. [DOI] [PubMed] [Google Scholar]
- 41.Liu G, Du X, Xiao L, Zeng Q, Liu Q. Activation of FGD5-AS1 promotes progression of cervical cancer through regulating BST2 to inhibit macrophage M1 polarization. J Immunol Res. 2021;2021:5857214. doi: 10.1155/2021/5857214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jin J, Du X, Zhou L, Yao D, Zou Q. SPI1-related protein inhibits cervical cancer cell progression and prevents macrophage cell migration. J Obstet Gynaecol Res. 2022;48:2419–2430. doi: 10.1111/jog.15336. [DOI] [PubMed] [Google Scholar]
- 43.Bian Z, Wu X, Chen Q, Gao Q, Xue X, Wang Y. Oct4 activates IL-17A to orchestrate M2 macrophage polarization and cervical cancer metastasis. Cancer Immunol Immunother. 2024;73:73. doi: 10.1007/s00262-023-03596-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fu LQ, Du WL, Cai MH, Yao JY, Zhao YY, Mou XZ. The roles of tumor-associated macrophages in tumor angiogenesis and metastasis. Cell Immunol. 2020;353:104119. doi: 10.1016/j.cellimm.2020.104119. [DOI] [PubMed] [Google Scholar]
- 45.Zhou G, Zhou F, Gu Y, Zhang M, Zhang G, Shen F, Hua K, Ding J. Vaginal microbial environment skews macrophage polarization and contributes to cervical cancer development. J Immunol Res. 2022;2022:3525735. doi: 10.1155/2022/3525735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McAlear TS, Bechstedt S. The mitotic spindle protein CKAP2 potently increases formation and stability of microtubules. Elife. 2022;11:e72202. doi: 10.7554/eLife.72202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guo L, Li C, Guo J, Qiu J, Hua K. CKAP2 promotes cervical cancer progression by modulating the tumor microenvironment via NF-κB signaling. Am J Cancer Res. 2023;13:2376–2391. [PMC free article] [PubMed] [Google Scholar]
- 48.Du S, Qian J, Tan S, Li W, Liu P, Zhao J, Zeng Y, Xu L, Wang Z, Cai J. Tumor cell-derived exosomes deliver TIE2 protein to macrophages to promote angiogenesis in cervical cancer. Cancer Lett. 2022;529:168–179. doi: 10.1016/j.canlet.2022.01.005. [DOI] [PubMed] [Google Scholar]
- 49.Sil P, Suwanpradid J, Muse G, Gruzdev A, Liu L, Corcoran DL, Willson CJ, Janardhan K, Grimm S, Myers P, Degraff LM, MacLeod AS, Martinez J. Noncanonical autophagy in dermal dendritic cells mediates immunosuppressive effects of UV exposure. J Allergy Clin Immunol. 2020;145:1389–1405. doi: 10.1016/j.jaci.2019.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 50.Zhou WJ, Chang KK, Wu K, Yang HL, Mei J, Xie F, Li DJ, Li MQ. Rapamycin synergizes with cisplatin in antiendometrial cancer activation by improving IL-27-stimulated cytotoxicity of NK cells. Neoplasia. 2018;20:69–79. doi: 10.1016/j.neo.2017.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang SL, Tan HX, Niu TT, Liu YK, Gu CJ, Li DJ, Li MQ, Wang HY. The IFN-γ-IDO1-kynureine pathway-induced autophagy in cervical cancer cell promotes phagocytosis of macrophage. Int J Biol Sci. 2021;17:339–352. doi: 10.7150/ijbs.51241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kuo WT, Chang JM, Chen CC, Tsao N, Chang CP. Autophagy drives plasticity and functional polarization of tumor-associated macrophages. IUBMB Life. 2022;74:157–169. doi: 10.1002/iub.2543. [DOI] [PubMed] [Google Scholar]
- 53.Pan Y, Yu Y, Wang X, Zhang T. Tumor-associated macrophages in tumor immunity. Front Immunol. 2020;11:583084. doi: 10.3389/fimmu.2020.583084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen H, Ma R, Zhou B, Yang X, Duan F, Wang G. Integrated immunological analysis of single-cell and bulky tissue transcriptomes reveals the role of interactions between M0 macrophages and naïve CD4+ T cells in the immunosuppressive microenvironment of cervical cancer. Comput Biol Med. 2023;163:107151. doi: 10.1016/j.compbiomed.2023.107151. [DOI] [PubMed] [Google Scholar]
- 55.Lequeux A, Noman MZ, Xiao M, Sauvage D, Van Moer K, Viry E, Bocci I, Hasmim M, Bosseler M, Berchem G, Janji B. Impact of hypoxic tumor microenvironment and tumor cell plasticity on the expression of immune checkpoints. Cancer Lett. 2019;458:13–20. doi: 10.1016/j.canlet.2019.05.021. [DOI] [PubMed] [Google Scholar]
- 56.Feng M, Jiang W, Kim BYS, Zhang CC, Fu YX, Weissman IL. Phagocytosis checkpoints as new targets for cancer immunotherapy. Nat Rev Cancer. 2019;19:568–586. doi: 10.1038/s41568-019-0183-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen XJ, Guo CH, Wang ZC, Yang Y, Pan YH, Liang JY, Sun MG, Fan LS, Liang L, Wang W. Hypoxia-induced ZEB1 promotes cervical cancer immune evasion by strengthening the CD47-SIRPα axis. Cell Commun Signal. 2024;22:15. doi: 10.1186/s12964-023-01450-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hubert P, Roncarati P, Demoulin S, Pilard C, Ancion M, Reynders C, Lerho T, Bruyere D, Lebeau A, Radermecker C, Meunier M, Nokin MJ, Hendrick E, Peulen O, Delvenne P, Herfs M. Extracellular HMGB1 blockade inhibits tumor growth through profoundly remodeling immune microenvironment and enhances checkpoint inhibitor-based immunotherapy. J Immunother Cancer. 2021;9:e001966. doi: 10.1136/jitc-2020-001966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen XJ, Wei WF, Wang ZC, Wang N, Guo CH, Zhou CF, Liang LJ, Wu S, Liang L, Wang W. A novel lymphatic pattern promotes metastasis of cervical cancer in a hypoxic tumour-associated macrophage-dependent manner. Angiogenesis. 2021;24:549–565. doi: 10.1007/s10456-020-09766-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rakaee M, Busund LR, Jamaly S, Paulsen EE, Richardsen E, Andersen S, Al-Saad S, Bremnes RM, Donnem T, Kilvaer TK. Prognostic value of macrophage phenotypes in resectable non-small cell lung cancer assessed by multiplex immunohistochemistry. Neoplasia. 2019;21:282–293. doi: 10.1016/j.neo.2019.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cheng H, Wang Z, Fu L, Xu T. Macrophage polarization in the development and progression of ovarian cancers: an overview. Front Oncol. 2019;9:421. doi: 10.3389/fonc.2019.00421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Takimoto Y, Tsubamoto H, Taniguchi R, Sakata K, Takada Y, Adachi J, Tomonaga T, Ueda T, Nakagawa K, Narita S, Wakimoto YU, Shibahara H. Itraconazole repolarizes tumor-associated macrophages and suppresses cervical cancer cell growth. Anticancer Res. 2023;43:569–580. doi: 10.21873/anticanres.16193. [DOI] [PubMed] [Google Scholar]
- 63.Chen W, Guo Q, Zhang Y, Liu Q, Zhang Y, Zhao C, Li X, Bai X, Zhang L, Shao S. Nocardia rubra cell wall skeleton regulates tumour-associated macrophage polarization by reprogramming M2 macrophages into M1 macrophages via STAT1/STAT6 pathways. Scand J Immunol. 2023;98:e13320. doi: 10.1111/sji.13320. [DOI] [PubMed] [Google Scholar]
- 64.Baidoo JNE, Mukherjee S, Kashfi K, Banerjee P. A new perspective on cancer therapy: changing the treaded path? Int J Mol Sci. 2021;22:9836. doi: 10.3390/ijms22189836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Horta B, Freitas-Silva J, Silva J, Dias F, Teixeira AL, Medeiros R, Cidade H, Pinto M, Cerqueira F. Antitumor effect of chalcone derivatives against human prostate (LNCaP and PC-3), cervix HPV-positive (HeLa) and lymphocyte (Jurkat) cell lines and their effect on macrophage functions. Molecules. 2023;28:2159. doi: 10.3390/molecules28052159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sobah ML, Liongue C, Ward AC. SOCS proteins in immunity, inflammatory diseases, and immune-related cancer. Front Med (Lausanne) 2021;8:727987. doi: 10.3389/fmed.2021.727987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li D, Huang Y, Wei M, Chen B, Lu Y. Overexpression of SOCS2 inhibits EMT and M2 macrophage polarization in cervical cancer via IL-6/JAK2/STAT3 pathway. Comb Chem High Throughput Screen. 2024;27:984–995. doi: 10.2174/1386207326666230818092532. [DOI] [PubMed] [Google Scholar]
- 68.Che Y, Yang Y, Suo J, An Y, Wang X. Induction of systemic immune responses and reversion of immunosuppression in the tumor microenvironment by a therapeutic vaccine for cervical cancer. Cancer Immunol Immunother. 2020;69:2651–2664. doi: 10.1007/s00262-020-02651-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pu Y, Ji Q. Tumor-associated macrophages regulate PD-1/PD-L1 immunosuppression. Front Immunol. 2022;13:874589. doi: 10.3389/fimmu.2022.874589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tichet M, Wullschleger S, Chryplewicz A, Fournier N, Marcone R, Kauzlaric A, Homicsko K, Deak LC, Umaña P, Klein C, Hanahan D. Bispecific PD1-IL2v and anti-PD-L1 break tumor immunity resistance by enhancing stem-like tumor-reactive CD8+ T cells and reprogramming macrophages. Immunity. 2023;56:162–179. e6. doi: 10.1016/j.immuni.2022.12.006. [DOI] [PubMed] [Google Scholar]
- 71.Mariathasan S, Turley SJ, Nickles D, Castiglioni A, Yuen K, Wang Y, Kadel EE III, Koeppen H, Astarita JL, Cubas R, Jhunjhunwala S, Banchereau R, Yang Y, Guan Y, Chalouni C, Ziai J, Şenbabaoğlu Y, Santoro S, Sheinson D, Hung J, Giltnane JM, Pierce AA, Mesh K, Lianoglou S, Riegler J, Carano RAD, Eriksson P, Höglund M, Somarriba L, Halligan DL, van der Heijden MS, Loriot Y, Rosenberg JE, Fong L, Mellman I, Chen DS, Green M, Derleth C, Fine GD, Hegde PS, Bourgon R, Powles T. TGFβ attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature. 2018;554:544–548. doi: 10.1038/nature25501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gomez-Roca CA, Italiano A, Le Tourneau C, Cassier PA, Toulmonde M, D’Angelo SP, Campone M, Weber KL, Loirat D, Cannarile MA, Jegg AM, Ries C, Christen R, Meneses-Lorente G, Jacob W, Klaman I, Ooi CH, Watson C, Wonde K, Reis B, Michielin F, Rüttinger D, Delord JP, Blay JY. Phase I study of emactuzumab single agent or in combination with paclitaxel in patients with advanced/metastatic solid tumors reveals depletion of immunosuppressive M2-like macrophages. Ann Oncol. 2019;30:1381–1392. doi: 10.1093/annonc/mdz163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Panagi M, Voutouri C, Mpekris F, Papageorgis P, Martin MR, Martin JD, Demetriou P, Pierides C, Polydorou C, Stylianou A, Louca M, Koumas L, Costeas P, Kataoka K, Cabral H, Stylianopoulos T. TGF-β inhibition combined with cytotoxic nanomedicine normalizes triple negative breast cancer microenvironment towards anti-tumor immunity. Theranostics. 2020;10:1910–1922. doi: 10.7150/thno.36936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shi G, Yang Q, Zhang Y, Jiang Q, Lin Y, Yang S, Wang H, Cheng L, Zhang X, Li Y, Wang Q, Liu Y, Wang Q, Zhang H, Su X, Dai L, Liu L, Zhang S, Li J, Li Z, Yang Y, Yu D, Wei Y, Deng H. Modulating the tumor microenvironment via oncolytic viruses and CSF-1R inhibition synergistically enhances anti-PD-1 immunotherapy. Mol Ther. 2019;27:244–260. doi: 10.1016/j.ymthe.2018.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sarode P, Zheng X, Giotopoulou GA, Weigert A, Kuenne C, Günther S, Friedrich A, Gattenlöhner S, Stiewe T, Brüne B, Grimminger F, Stathopoulos GT, Pullamsetti SS, Seeger W, Savai R. Reprogramming of tumor-associated macrophages by targeting β-catenin/FOSL2/ARID5A signaling: a potential treatment of lung cancer. Sci Adv. 2020;6:eaaz6105. doi: 10.1126/sciadv.aaz6105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yin C, Han Q, Xu D, Zheng B, Zhao X, Zhang J. SALL4-mediated upregulation of exosomal miR-146a-5p drives T-cell exhaustion by M2 tumor-associated macrophages in HCC. Oncoimmunology. 2019;8:1601479. doi: 10.1080/2162402X.2019.1601479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sumitomo R, Hirai T, Fujita M, Murakami H, Otake Y, Huang CL. PD-L1 expression on tumor-infiltrating immune cells is highly associated with M2 TAM and aggressive malignant potential in patients with resected non-small cell lung cancer. Lung Cancer. 2019;136:136–144. doi: 10.1016/j.lungcan.2019.08.023. [DOI] [PubMed] [Google Scholar]
- 78.Wang B, Mao Z, Ye J, Jiao X, Zhang T, Wang Q, Han S, Zhang Y, Wang C, Dong T, Cui B. Glycolysis induced by METTL14 is essential for macrophage phagocytosis and phenotype in cervical cancer. J Immunol. 2024;212:723–736. doi: 10.4049/jimmunol.2300339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rodrigues M, Vanoni G, Loap P, Dubot C, Timperi E, Minsat M, Bazire L, Durdux C, Fourchotte V, Laas E, Pouget N, Castel-Ajgal Z, Marret G, Lesage L, Meseure D, Vincent-Salomon A, Lecompte L, Servant N, Vacher S, Bieche I, Malhaire C, Huchet V, Champion L, Kamal M, Amigorena S, Lantz O, Chevrier M, Romano E. Nivolumab plus chemoradiotherapy in locally-advanced cervical cancer: the NICOL phase 1 trial. Nat Commun. 2023;14:3698. doi: 10.1038/s41467-023-39383-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Man S, Li M, Zhou J, Wang H, Zhang J, Ma L. Polyethyleneimine coated Fe3O4 magnetic nanoparticles induce autophagy, NF-κB and TGF-β signaling pathway activation in HeLa cervical carcinoma cells via reactive oxygen species generation. Biomater Sci. 2020;8:201–211. doi: 10.1039/c9bm01563a. [DOI] [PubMed] [Google Scholar]
- 81.Thomas S, Gunasangkaran G, Arumugam VA, Muthukrishnan S. Synthesis and characterization of zinc oxide nanoparticles of solanum nigrum and its anticancer activity via the induction of apoptosis in cervical cancer. Biol Trace Elem Res. 2022;200:2684–2697. doi: 10.1007/s12011-021-02898-6. [DOI] [PubMed] [Google Scholar]
- 82.Chen H, Feng X, Gao L, Mickymaray S, Paramasivam A, Abdulaziz Alfaiz F, Almasmoum HA, Ghaith MM, Almaimani RA, Aziz Ibrahim IA. Inhibiting the PI3K/AKT/mTOR signalling pathway with copper oxide nanoparticles from Houttuynia cordata plant: attenuating the proliferation of cervical cancer cells. Artif Cells Nanomed Biotechnol. 2021;49:240–249. doi: 10.1080/21691401.2021.1890101. [DOI] [PubMed] [Google Scholar]
- 83.Chen Y. Nanotechnology for next-generation cancer immunotherapy: state of the art and future perspectives. J Control Release. 2023;356:14–25. doi: 10.1016/j.jconrel.2023.02.016. [DOI] [PubMed] [Google Scholar]
- 84.Zhang Y, Chen Y, Li J, Zhu X, Liu Y, Wang X, Wang H, Yao Y, Gao Y, Chen Z. Development of toll-like receptor agonist-loaded nanoparticles as precision immunotherapy for reprogramming tumor-associated macrophages. ACS Appl Mater Interfaces. 2021;13:24442–24452. doi: 10.1021/acsami.1c01453. [DOI] [PubMed] [Google Scholar]
- 85.Li K, Lu L, Xue C, Liu J, He Y, Zhou J, Xia Z, Dai L, Luo Z, Mao Y, Cai K. Polarization of tumor-associated macrophage phenotype via porous hollow iron nanoparticles for tumor immunotherapy in vivo. Nanoscale. 2020;12:130–144. doi: 10.1039/c9nr06505a. [DOI] [PubMed] [Google Scholar]
- 86.Yang L, Li F, Cao Y, Liu Q, Jing G, Niu J, Sun F, Qian Y, Wang S, Li A. Multifunctional silica nanocomposites prime tumoricidal immunity for efficient cancer immunotherapy. J Nanobiotechnology. 2021;19:328. doi: 10.1186/s12951-021-01073-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kim SY, Kim S, Kim JE, Lee SN, Shin IW, Shin HS, Jin SM, Noh YW, Kang YJ, Kim YS, Kang TH, Park YM, Lim YT. Lyophilizable and multifaceted toll-like receptor 7/8 agonist-loaded nanoemulsion for the reprogramming of tumor microenvironments and enhanced cancer immunotherapy. ACS Nano. 2019;13:12671–12686. doi: 10.1021/acsnano.9b04207. [DOI] [PubMed] [Google Scholar]
- 88.Gu X, Zhang R, Sun Y, Ai X, Wang Y, Lyu Y, Wang X, Wu Y, Wang Z, Feng N, Liu Y. Oral membrane-biomimetic nanoparticles for enhanced endocytosis and regulation of tumor-associated macrophage. J Nanobiotechnology. 2023;21:206. doi: 10.1186/s12951-023-01949-5. [DOI] [PMC free article] [PubMed] [Google Scholar]