Abstract
Background:
Cell-free DNA (cfDNA) sequence analysis to screen for fetal aneuploidy can incidentally detect maternal cancer. Additional data are needed to identify DNA sequencing patterns and other biomarkers that can distinguish women who are most likely to have cancer and to determine the best approach for maternal follow-up.
Methods:
Pregnant or postpartum individuals who did not perceive signs or symptoms of malignancy underwent a uniform cancer screening protocol after receiving unusual or nonreportable clinical cfDNA-sequencing results from one of 12 different commercial laboratories in North America. Rapid whole-body magnetic resonance imaging (WB-MRI), laboratory tests and standardized research cfDNA sequencing using a genome-wide platform were performed.
Results:
Cancer was present in 52/107 (48.6%) participants. The sensitivity and specificity of WB-MRI in detecting occult malignancies were 98.0% and 88.5%. Physical examination and laboratory tests had limited utility in identifying participants with cancer. Research sequencing showed that 49 participants had a combination of sub-chromosomal and/or whole chromosome copy number gains and losses across multiple (≥3) chromosomes. Cancer was present in 47/49 (95.9%) participants with this sequencing pattern. cfDNA-sequencing patterns in which there were only chromosomal gains (multiple trisomies) or only chromosomal losses (one or more monosomies) were found in women with non-malignant conditions, such as fibroids.
Conclusions:
In this study, 48.6% of patients who received unusual or nonreportable clinical cfDNA-sequencing results had an occult malignancy. Further study of DNA sequencing patterns in screening for occult malignancies during prenatal screening is warranted.
Introduction:
Sequencing of circulating cell-free DNA (cfDNA) in maternal plasma has had a substantial impact on prenatal screening for fetal aneuploidy. Owing to its superior accuracy compared to serum biochemical and nuchal translucency screening,1,2 as of October 2020, cfDNA sequencing is now routinely offered to all pregnant women.3 It has resulted in a 50-70% global reduction of invasive diagnostic procedures, such as amniocentesis, demonstrating its clinical utility.4
As with many new genomic technologies, the potential for unexpected results has been realized. Retrospective studies from large commercial or national laboratories have reported an association between unusual sequencing results, such as those with multiple aneuploidies or an autosomal monosomy, and maternal malignancies.5–10 During a typical pregnancy, circulating cfDNA derives from the placenta (~10%) and the maternal hematopoietic system (~90%). The sequenced cfDNA in the test sample is compared to a reference sample, and gains or losses across the genome are used in bioinformatics algorithms to establish ratios. If a maternal tumor is present, it can also shed cfDNA into the circulation, distorting the expected ratios for a euploid or aneuploid fetus. In the United States (US), this most often triggers a nonreportable sequencing result since the fetal aneuploidy status cannot be assessed.
Although various approaches have been suggested,9,11–13 there is currently insufficient evidence to inform the subsequent management of pregnant women who receive nonreportable or unusual sequencing results. Additional data are needed to better understand the ability of cfDNA sequencing to detect maternal malignancies and to determine the optimal evaluation.3,14 To fill this knowledge gap, we launched the Incidental Detection of matErnal Neoplasia Through non-Invasive cell-Free DNA analYsis (IDENTIFY) study. The goals of the study were to identify DNA sequencing patterns and other biomarkers that could distinguish the subset of asymptomatic women who are likely to have malignancy and determine the best approach for diagnostic work-up of pregnant women who receive these results.
Methods:
Study Oversight
IDENTIFY is an ongoing natural history study being conducted at the National Institutes of Health (NIH) in Bethesda, Maryland. We are enrolling women who underwent cfDNA sequencing during their routine obstetrical care and received unexpected results for which maternal malignancy was included in the differential diagnosis. Here we present results from the initial cohort of IDENTIFY participants. Participants were informed of the option to participate in the IDENTIFY study by the sequencing laboratory or their health care provider. The NIH institutional review board approved the study protocol. All participants provided written informed consent. The study was designed by the second and last authors. All authors were involved in the collection, analysis, and/or interpretation of the data. The first author wrote the first draft of the manuscript, which was then revised by the last author. All authors have reviewed the manuscript, vouch for the completeness and accuracy of the data, and made the decision to submit the manuscript for publication, according to the protocol (available at NEJM.org).
Study Participants
Eligible participants were at least 18 years of age, pregnant or up to two years postpartum, and had received sequencing results from one of 12 commercial laboratories (Table S1) that were either (1) abnormal and inconsistent with a viable fetus on sonogram, (2) abnormal and discordant with the fetal karyotype or chromosome microarray analysis, or (3) nonreportable. Our knowledge of the initial cfDNA-sequencing result was limited to what was documented on the written laboratory report (Figure S1) and/or communicated by the referring provider. These eligibility criteria reflect the current clinical landscape in the US, where multiple commercial laboratories offer either targeted or genome-wide prenatal cfDNA sequencing and have variable reporting practices.12 Patients were ineligible if their sequencing results were nonreportable due to insufficient fetal fraction or other technical or sample-related issues. The final protocol specified enrollment of 120 women without known cancer, based on operational rather than scientific considerations.
Cancer Screening Protocol
The cancer screening protocol included rapid whole-body magnetic resonance imaging (WB-MRI), blood tests, serum tumor markers, fecal occult blood test, family and medical history intake, and physical examination with oncologic symptom review. Participants who were not up to date with their cervical cancer screening underwent a pap smear with human papillomavirus testing.
Blood tests included complete blood count with differential, complete metabolic panel with liver and renal function tests, and serum vitamin B12 levels. The serum tumor markers analyzed7 were CA-125 (cancer antigen-125), CA 15-3, CA 27.29, CA 19-9, and CEA (carcinoembryonic antigen).
Various approaches to imaging have been suggested when cfDNA-sequencing results raise concern for maternal malignancy.9,15,16 We selected WB-MRI because of its safety during pregnancy and its proven effectiveness at detecting occult malignancies in other high-risk patient populations.17 Non-pregnant participants were studied with and without gadolinium contrast, whereas in pregnant participants no contrast was used (Table S2).
All participants met with a medical oncologist. If cancer was detected, the oncologist provided referral for biopsy and subsequent management in the participant’s locale. If cancer was not detected, placental biopsies were collected at the time of delivery to evaluate for confined placental mosaicism (Supplementary Methods).
Standardized Research Cell-Free DNA Sequencing
Research cfDNA sequencing was performed on all participants as a fee-for-service by a CAP/CLIA certified contract laboratory. Peripheral blood (10 mL) was collected in Streck® tubes (Omaha, NE), subjected to plasma separation and DNA extraction, library preparation, and genome-wide massively parallel sequencing using previously described methods.18
Study Outcome
The primary outcome was the presence of cancer in each participant following the initial clinical evaluation.
Secondary Analysis
To explore whether the sequencing data could distinguish women with malignancies from those with non-malignant findings, two investigators (AET and DWB) performed an exploratory analysis of the 50-kb sequencing traces. All 50-kb traces were blindly inspected and visually stratified to establish different groups of sequencing patterns. Any differences in stratification were resolved through discussion and further review of the chromosome ideogram data. Once the groups were established, the clinical outcome information was reviewed (Figure S2). To evaluate the test performance of the standardized cancer screening protocol, we calculated the sensitivity, specificity, and positive and negative predictive values with 95% confidence intervals and estimated the area under the nonparametric receiver-operator-characteristic (ROC) curve with 95% confidence intervals using the R package caret.19
Results
Study Participants
From December 23, 2019, to December 4, 2023, 117 participants without a known cancer diagnosis were enrolled, and 107/117 had complete data available for analysis. Ten women were excluded because they did not undergo cancer screening or had incomplete clinical data (Figure 1).
Figure 1.
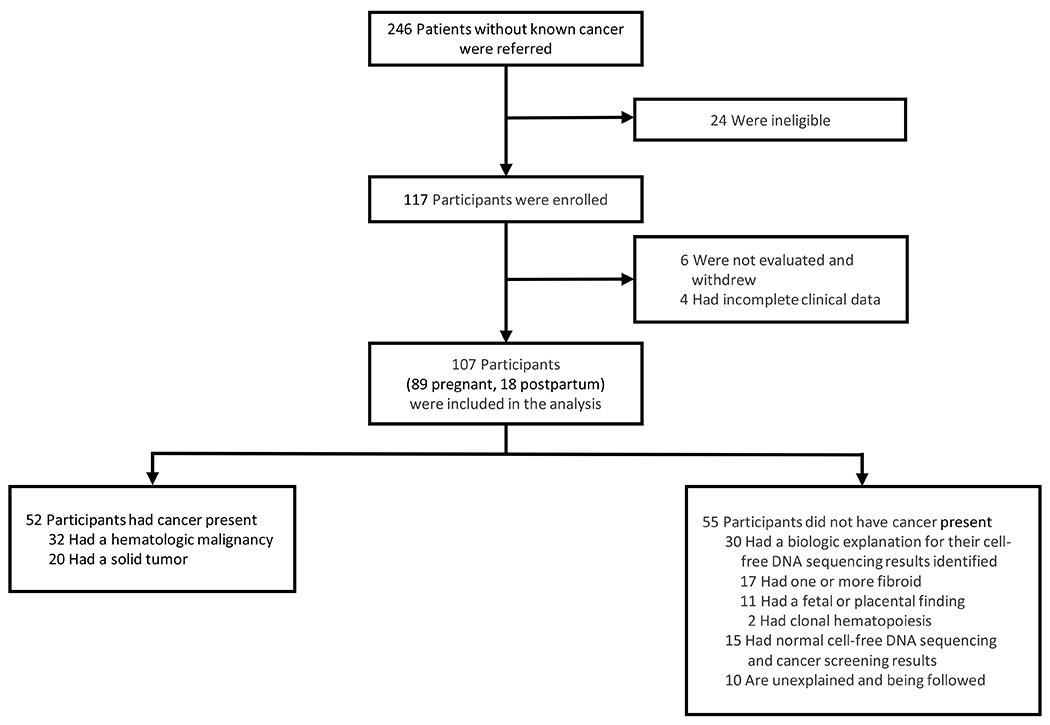
Enrollment and Outcomes
Participant characteristics are presented in Table 1. The mean maternal age was 32.7 years (range, 19-53 years). Eighty-nine participants were pregnant at the time of their cancer screening, and the mean gestational age was 22.2 weeks (range, 13.3-36 weeks). Most participants (90/107) were referred with nonreportable or abnormal results from genome-wide cfDNA sequencing (Figure S1).
Table 1.
Selected Characteristics of Participants at Baseline
| Characteristic | Value (n = 107) |
|---|---|
| Mean maternal age (range) – yr | 32.7 (19–53) |
| Mean gestational age (range) at time of initial clinical sequencing – wk | 13 (9–33.1) |
| Self-reported race, ethnicity – no. (%) | |
| Asian | 7 (6.5) |
| Black | 7 (6.5) |
| Multiple race | 2 (1.9) |
| White, Hispanic | 16 (15.0) |
| White, non-Hispanic | 75 (70.1) |
| Geographic Region – no. (%) | |
| Canada | 4 (3.7) |
| Israel | 1 (0.9) |
| United States | |
| Northeast | 19 (17.8) |
| Southeast | 44 (41.1) |
| Midwest | 15 (14.0) |
| Southwest | 9 (8.4) |
| West | 15 (14.0) |
| Pregnancy status at time of cancer screening and research sequencing – no. (%) | |
| Pregnant | 89 (83.2) |
| Mean gestational age (range) – wk | 22.2 (13.3–36.0) |
| Postpartum | 18 (16.8) |
| Median time elapsed (range) between initial clinical sequencing and cancer screening – wk | 9.6 (2–114.6) |
| Pregnancy by assisted reproductive technology – no. (%) | 10 (9.3) |
| Diagnostic testing of fetus or baby– no. (%) | |
| No | 50 (46.7) |
| Yes | 57 (53.2) |
| Prenatal | 51 (47.7) |
| Postnatal | 5 (4.7) |
| Genetic testing on products of conception following miscarriage | 1 (0.9) |
Primary Outcome
Cancer was present in 52/107 participants (48.6%) (Figure 1). Fifty-one participants underwent biopsies to confirm their diagnoses (Table S5). Lymphoma was the most common diagnosis (31/52, 59.6%), followed by colorectal cancer (9/52, 17.3%) and breast cancer (4/52, 7.7%). Additional malignancies included two cases of cholangiocarcinoma and one case each of adrenocortical carcinoma, non-small cell lung cancer, pancreatic cancer, Ewing sarcoma, and renal carcinoma. Of the 31 confirmed lymphoma cases, 20 were Hodgkin lymphoma and 11 were non-Hodgkin lymphoma. Of the 20 solid tumor cases, two participants had stage 1 disease, five participants had stage 2 or 3 disease, and 13 participants had stage 4 disease. Thirteen of 20 participants were eligible for potentially curative treatment, including five participants with colon cancer and one with Ewing sarcoma who had metastases to a single area (Figure S3). Of the participants with cancer, 29/52 (55.8%) were asymptomatic and 13/52 (25%) had symptoms that were ascribed to pregnancy or other etiologies, such as epigastric pain attributed to reflux in a patient with pancreatic cancer. In 10/52 participants (19.2%), clinical symptoms were either not recognized by the patient, or when worked up, revealed unconcerning results (Table S3).
The remaining 55/107 (51.4%) participants were not diagnosed with cancer. In 30/107 (28.0%) cases, there was an explanation that did not involve cancer for the initial clinical sequencing result, including fibroids (17/107, 15.9%), confined placental mosaicism (8/107, 7.5%), a fetal finding (3/107, 2.8%), or clonal hematopoiesis (2/107, 1.9%). In 25/107 (23.4%) cases, the initial clinical sequencing result was unexplained, and in 10 of these participants, there is ongoing concern for malignancy (Figure 1). Participants will be followed for up to five years.
Secondary Analyses
Standardized Research cfDNA Sequencing
Research cfDNA sequencing was performed in all participants; results were unavailable in two participants (one with cancer, one with fibroids) because their samples failed to amplify. Our blinded inspection of the sequencing data revealed there were six patterns represented: normal (no copy number gain/loss present) (n=28); chromosomal gains and losses (sub-chromosomal and/or whole chromosome copy number gains and losses across multiple (≥3) chromosomes) (n=49); chromosomal gains only (one or more duplications or whole chromosome trisomies) (n=11); chromosomal losses only (one or more deletions or whole chromosome monosomies) (n=4); maternal copy number variants (n=2); or borderline abnormal (no clear copy number abnormalities but borderline z-scores across multiple chromosomes) (n=11) (Figure 2).
Figure 2. Distinct Cell-Free DNA Sequencing Patterns Associated with Malignant and Non-Malignant Findings.
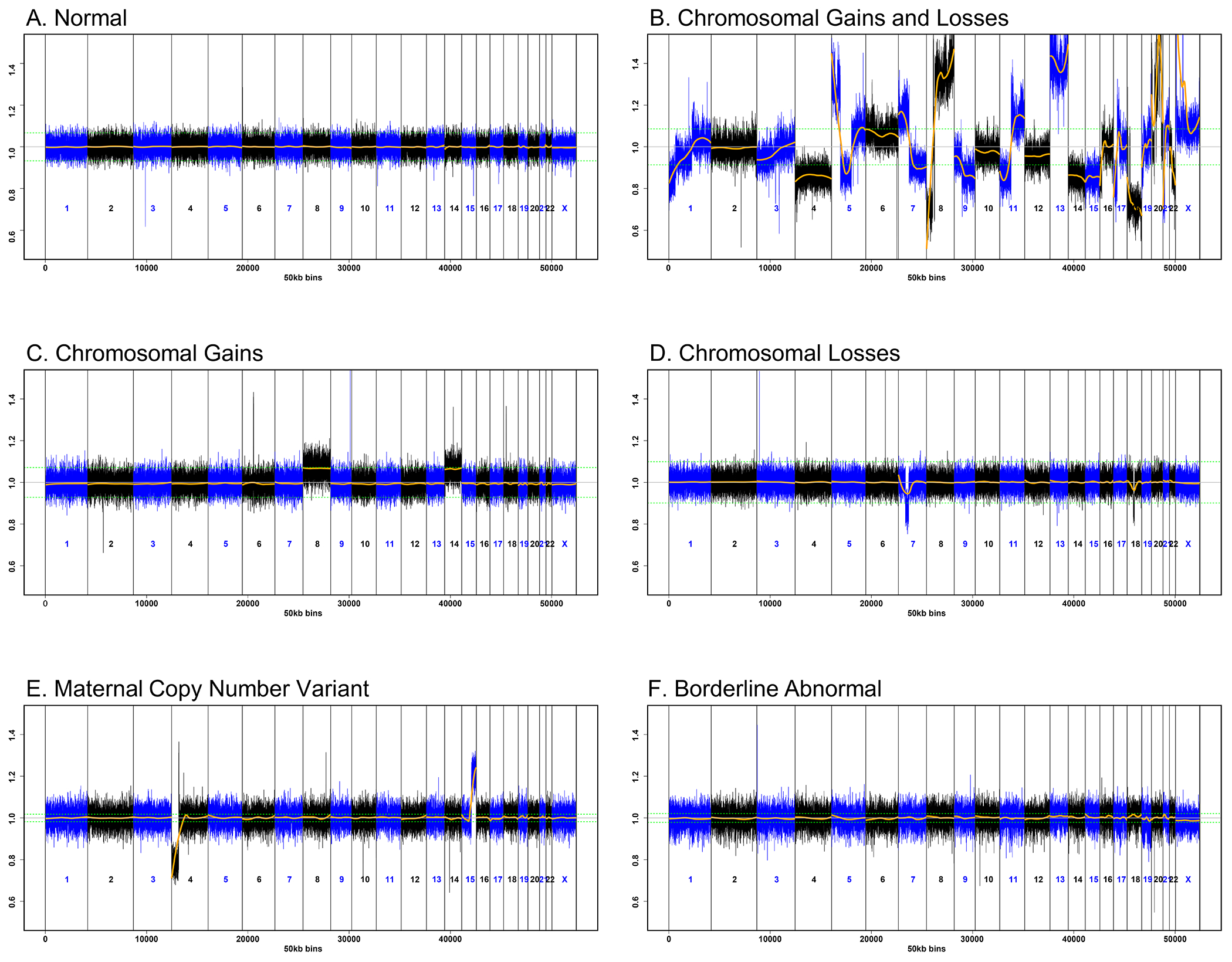
50kb sequencing traces from research whole genome massively parallel sequencing of cell-free DNA (cfDNA) from maternal plasma. The x-axis indicates genomic location. The y-axis represents the normalized segment representation for each chromosome, centered at 1.0. The green dotted lines show the upper and lower bounds expected from a euploid fetus. The orange line depicts the normalized segment representation as calculated from the sequencing data. A. Genome-wide cfDNA profile consistent with a euploid fetus in a participant without malignancy. B. Genome-wide sub-chromosomal and whole chromosome copy number gains and losses in a participant with colon cancer. C. Whole chromosome trisomies of chromosomes 8 and 14 in a case of confined placental mosaicism detected in placental biopsies. D. Sub-chromosomal losses at chromosomes 7 and 18 in a participant with multiple submucosal fibroids. E. A maternal chromosome translocation (4;15)(p16;q24) in a participant with clonal hematopoiesis of indeterminate potential. F. Borderline z-scores across multiple chromosomes that prevents assessment of fetal aneuploidy status in a participant with breast cancer.
In 49 samples, the research cfDNA-sequencing results showed chromosomal gains and losses (Figure 2B). In most cases, the abnormalities were genome-wide and profound, but in some, the abnormalities were subtle or affected only a few autosomes (Figure S4). Of the 49 samples, 47 (95.9%) were in women with cancer. There is ongoing concern for malignancy in the other two participants. There were four participants who had cancer but did not have the suspicious pattern of chromosomal gains and losses: two participants (one with stage 1 breast cancer and one with recurrent Hodgkin lymphoma) had borderline abnormal results; two participants (one with stage 1 primary mediastinal large B-cell lymphoma and one with stage 1 renal carcinoma) had normal results (Figure S2).
The other research cfDNA-sequencing patterns (Figure 2, panels C–F) had a biologic source other than cancer. In 11 samples there were only chromosomal gains: 10 showed whole chromosome trisomies of two to four autosomes, and one sample showed trisomy 13. These cases were explained by the fetus (n=3) and/or placenta (n=8) (Figure S2, Table S4). In four samples, there was one or more sub-chromosomal and/or whole-chromosome monosomy present; these occurred in women with fibroids detected by WB-MRI (Figure S2). Eleven participants with fibroids were referred with multiple monosomies (n=5) or nonreportable results (n=6) on clinical sequencing, but we detected no abnormalities in these women on research sequencing. Three participants with fibroids also had cancer; all three had a combination of chromosomal gains and losses on research sequencing (Table S5).
Two samples showed a maternal copy number variant that was determined to reflect clonal hematopoiesis (Supplementary Methods). In 11 samples, the sequencing results were borderline abnormal; two of these were in women with cancer, and one was in a participant with fibroids. The other eight cases were unexplained, and these participants are being followed. Of the 15 participants with normal research cfDNA and cancer screening results, seven were postpartum; the abnormalities detected on the original clinical sequencing may have been confined to the fetus or placenta, and therefore were not present in the postpartum blood sample.
Standardized Cancer Screening Protocol
Results of the test performance calculations are shown in Table 2. Rapid WB-MRI showed suspicion for malignancy in 48/101 participants, all of whom were confirmed to have cancer (Figure 3, Table S6). The single false negative result was in a participant who was diagnosed with stage 1 breast cancer three months after her evaluation at NIH. There were six indeterminate WB-MRI findings that required follow-up: two liver lesions that could not be fully characterized without contrast, one breast lesion, one thyroid lesion, one axillary nodule, and one lung lesion. Through subsequent imaging, all were determined to be benign. The AUC for WB-MRI was 93.2% (95% CI 88.4 to 98.0) (Table 2).
Table 2.
Test Performance*
| Clinical Test | Whole-body MRI (N=101)A | Serum Tumor Markers§ (N=103)B | CEA, CA 15-3, CA 19-9₸ (N=103)B | Fecal Occult Blood (N=80)C | Physical Examination (N=107) |
|---|---|---|---|---|---|
| True positive (n) | 48 | 34 | 24 | 4 | 9 |
| True negative (n) | 46 | 39 | 48 | 71 | 55 |
| False positive (n) | 6 | 14 | 5 | 1 | 0 |
| False negative (n) | 1 | 16 | 26 | 4 | 43 |
| Area Under the ROC Curve (AUC) (%) (95% CI) | 93.2 (88.4–98.0) | 70.8 (61.9–79.7) | 69.3 (61.2–77.3) | 74.3 (55.7–92.9) | 58.7 (53.5–63.8) |
| Sensitivity (%) (95% CI) | 98.0 (89.1–99.9) | 68.0 (53.3–80.5) | 48.0 (33.7–62.6) | 50.0 (15.7–84.3) | 17.3 (8.2–30.3) |
| Specificity (%) (95% CI) | 88.5 (76.6–95.6) | 73.6 (59.7–84.7) | 90.6 (79.3–96.9) | 98.6 (92.5–100.0) | 100.0 (93.5–100.0) |
| Positive predictive value (%) (95% CI) | 88.9 (77.4–95.8) | 70.8 (55.9–83.0) | 82.8 (64.2–94.6) | 80.0 (28.4–99.5) | 100.0 (66.4–100.0) |
| Negative predictive value (%) (95% CI) | 97.9 (88.7–99.9) | 70.9 (57.1–82.4) | 64.9 (52.9–75.6) | 94.7 (86.9–98.5) | 56.1 (45.7–66.1) |
A true positive result was defined as any radiologic or laboratory finding that was suspicious for malignancy in a person who was ultimately diagnosed with cancer. A false positive result was defined as any radiologic or laboratory finding that was suspicious for malignancy and/or required additional follow-up evaluation, such as further imaging, in a person who did not have cancer. True negative results were unremarkable radiologic or laboratory investigations in a person who did not have cancer. False negative results were unremarkable radiologic or laboratory findings in a person diagnosed with cancer.
Serum tumor markers included CA-125 (cancer antigen-125), CA 15-3, CA 27.29, CA 19-9, and CEA (carcinoembryonic antigen). Results were classified as malignancy suspicious when at least one tumor marker exceeded the normal reference values.
CEA, CA 19-9, and CA 15-3 were assessed separately because these tumor markers are not affected significantly by pregnancy.
Whole-body magnetic resonance imaging (WB-MRI) was not performed in six participants. Three participants with cancer had targeted imaging. Three participants without cancer (one fetal finding, one placental finding, one false positive sequencing result) could not tolerate the WB-MRI.
Serum tumor markers were not analyzed in two participants with cancer and two participants without cancer.
27 participants, including one person with colon cancer, were unable to provide a fecal sample for testing.
Figure 3. Whole-Body Magnetic Resonance Images.

Case 1. A 30-year-old pregnant (28 weeks, 6 days) participant with stage 4 primary mediastinal large B-cell lymphoma. Image 1A: Composite coronal short tau inversion recovery (STIR) of the whole body demonstrates conglomerate mediastinal adenopathy or mass (red arrow) and lung nodules (yellow arrow). Image 1B: Axial STIR image of the chest demonstrates anterior mediastinal adenopathy/mass (red arrow) and multiple lung nodules bilaterally. Yellow arrow identifies one of several nodules in the right and left lungs. Image 1C: Axial STIR demonstrates a mass in or adjacent to the tail of the pancreas (red arrow). Image 1D: Axial STIR demonstrates a mass in the posterior right kidney (red arrow). Case 2. A 40-year-old pregnant (14 weeks, 6 days) participant with stage 3 breast cancer. Image 2A: Subtle lateral 1.5 cm right breast mass (red arrow) noted on axial STIR image of the chest. Image 2B: Lateral right breast mass more conspicuous on axial b-800 diffusion image (red arrow). Image 2C: Right infraclavicular adenopathy (yellow arrow) noted on axial STIR image of the chest. Image 2D: Right infraclavicular and axillary adenopathy (yellow arrow) noted on composite coronal STIR image of the neck, chest, and upper abdomen. Case 3. A 30-year-old pregnant (22 weeks) participant with stage 3 adrenocortical carcinoma. Image 3A: Composite coronal STIR of the whole body demonstrates a right adrenal mass (red arrow). Image 3B: Axial STIR image demonstrates a 6 x 6.3 x 5.8 cm right adrenal mass (red arrow). Axial b-800 (image 3C) and axial apparent diffusion coefficient (ADC) (image 3D) demonstrate diffusion restriction (red arrows). Image 3E: Axial b-800 image demonstrates retroperitoneal adenopathy (yellow arrows). Case 4. A 34-year-old pregnant (24 weeks) participant with a 17 x 15 x 13 cm intramural fibroid. Image 4A: Composite coronal STIR image of the lower chest, abdomen, pelvis, and thighs demonstrates a large left-sided intramural uterine fibroid (red arrow). Adjacent intrauterine fetus noted. Fetal head identified (yellow arrow).
Pertinent medical history and clinical details are presented in Table S7 and Figure S5. Physical examination was performed in all participants and was abnormal in 9/52 participants with cancer, a sensitivity of 17.3% (95% CI 8.2 to 30.3). Screening blood tests did not show concern for malignancy in any participant. Serum tumor marker results were available in 103 participants. Of the 52 participants with cancer, 34 had at least one abnormal serum tumor marker (true positives), a sensitivity of 68.0% (95% CI 53.3 to 80.5). Fourteen participants without cancer had abnormal serum tumor marker results, a false positive rate of 26.4% (95% CI 15.3 to 46.7). Assessing only CA 19-9, CA 15-3, and CEA, which are not typically affected by pregnancy,20 did not improve accuracy. Of the nine participants with colorectal cancer, eight underwent fecal occult blood testing, and only four had abnormal results (Table 2).
Discussion
Using a standardized sequencing and cancer screening protocol, we detected cancer in 48.6% of women who initially received nonreportable or unusual cfDNA-sequencing results from 12 commercial laboratories in North America. All participants were referred with concern for malignancy. Our cohort is not representative of the general pregnant population (Table S8). The US differs from other countries in that clinical laboratories use different sequencing technologies, proprietary bioinformatics algorithms, and test-reporting practices. This is confusing for obstetric providers and poses challenges to the identification of women at highest risk for cancer.21 Our results support WB-MRI and the further investigation of certain cfDNA-sequencing patterns in the evaluation of cancer in persons who receive nonreportable or unusual cfDNA-sequencing results.
In the research sequencing results, the combination of sub-chromosomal and/or whole chromosome copy number gains and losses across multiple chromosomes was observed in 47/51 (92.2%) participants with cancer and available sequencing data; patients with this sequencing pattern appear to have the highest risk of cancer and should be identified on the written laboratory report so that timely cancer screening can be pursued. Other cfDNA-sequencing patterns appeared to not be associated with malignancy. For example, cancer was not present in participants with multiple trisomies or one or more monosomies. Although prior studies have suggested that multiple aneuploidies or an autosomal monosomy is suggestive of cancer, we found that multiple whole-chromosome trisomies, commonly double aneuploidies, were more likely to be explained by anomalies in the fetus and/or placenta, and sub-chromosomal and/or whole chromosome monosomies to be explained by uterine fibroids.
Although fibroids are a well-documented etiology for nonreportable or discordant cfDNA-sequencing results,6,22 their presence does not preclude a co-existing malignancy. In this study, the three participants with fibroids and cancer had research cfDNA results showing multiple copy-number gains and losses, whereas the 16 women with only fibroids had a different pattern (Table S5). The DNA sample from one woman with multiple sub-serosal fibroids failed to amplify (Table S5). Further work on whether and to what extent sequencing patterns can prioritize patients with both fibroids and occult cancer for WB-MRI is warranted.
In some countries other than the US, whole-body imaging is routinely performed when cfDNA-sequencing results suggest maternal malignancies.9,15 Current practice in the US is to consider imaging on a case-by-case basis depending on the patient’s medical and family histories, results of physical examination and laboratory tests, and, ultimately, insurance coverage.3,12 Suggested work-ups in the literature have prioritized targeted imaging, such as chest radiographs, over whole body approaches.11,12 We found that patient history, self-reported symptoms, physical examination, and laboratory tests had limited utility in identifying which patients had cancer or its location. A lack of overt symptoms and/or pregnancy are not reasons to delay imaging.
WB-MRI was safe, efficient, and the most effective method for detecting cancer. The false positive rate of WB-MRI screening was 11.5% (95% CI, 4.4 to 23.4), considerably lower than that reported in other high-risk patient populations screened with WB-MRI.23 The effectiveness of cfDNA sequencing in identifying patients with an existing cancer likely explains this lower false positive rate.
The median time between participants’ initial clinical sequencing and their cancer screening evaluation was 9.6 weeks (range, 2 to 114.6 weeks). Factors contributing to delays in referral to the IDENTIFY study included confusion about the significance of the initial sequencing results, providers ordering repeat sequencing and/or pursuing fetal diagnostic testing prior to maternal follow-up, and participants’ ambivalence about pursuing cancer screening.24 The importance of prompt cancer screening in patients who receive cfDNA-sequencing results suggestive of maternal malignancy is highlighted by the five women in this study who had stage 2 or 3 solid tumors identified, and the six women with stage 4 cancers with limited metastatic involvement that were eligible for potentially curative treatment.
Strengths of our study include that participants had no known diagnosis at the time of study entry, a uniform sequencing and cancer screening protocol was performed, and eligibility criteria reflected current US clinical practice and the challenges clinicians face in identifying patients at risk for malignancy.21 Study limitations include subjective grouping of the cfDNA-sequencing patterns as an exploratory analysis, lack of pre-specification of hypotheses or analyses, lack of statistical justification of the sample size, our inability to directly compare the research and original clinical sequencing results, the need to exclude some participants due to incomplete clinical data, and variable follow-up periods for participants with ongoing concerns for malignancy. We may have underestimated the proportion of participants with cancer. And finally, the sequencing patterns described here are novel and require prospective validation in an independent population adequately sized to estimate diagnostic accuracy.
Supplementary Material
Acknowledgments
We thank our participants, the clinical laboratories, and clinicians who made referrals to the study, Mireya Gomez, B.A., and Jessica Presentacion, B.A., for patient care coordination, Kevin Conlon, M.D., Bernard Parker, M.D., and Brooke Solarz, M.S.N. for their clinical evaluation of some of the participants, Samantha Caldwell, M.S., Eyad Almasri, Ph.D., and Tong Liu, Ph.D. for their assistance with the sequencing data, Darryl Leja, M.F.A., for his assistance with the figures, and Michael Wierzbicki, Ph.D., for his statistical input.
Funding
This study was supported by the NIH Intramural Research Programs, 1ZIA HG200400-07 and 1ZIA BC011584-10.
Funded by the NIH Intramural Research Programs; Trial Reg NCT04049604
References
- 1.Bianchi DW, Parker RL, Wentworth J, et al. DNA sequencing versus standard prenatal aneuploidy screening. N Engl J Med 2014;370:799–808. [DOI] [PubMed] [Google Scholar]
- 2.Norton ME, Jacobsson B, Swamy GK, et al. Cell-free DNA analysis for noninvasive examination of trisomy. N Engl J Med 2015;372:1589–97. [DOI] [PubMed] [Google Scholar]
- 3.American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins—Obstetrics; Committee on Genetics; Society for Maternal-Fetal Medicine. Screening for fetal chromosomal abnormalities: ACOG Practice Bulletin, Number 226. Obstet Gynecol 2020;136:e48–e69. [DOI] [PubMed] [Google Scholar]
- 4.Hui L, Halliday J. A decade of non-invasive prenatal screening in Australia: national impact on prenatal screening and diagnostic testing. Aust N Z J Obstet Gynaecol 2023;63:264–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bianchi DW, Chudova D, Sehnert AJ, et al. Noninvasive prenatal testing and incidental detection of occult maternal malignancies. JAMA 2015;314:162–9. [DOI] [PubMed] [Google Scholar]
- 6.Dharajiya NG, Grosu DS, Farkas DH, et al. Incidental detection of maternal neoplasia in noninvasive prenatal testing. Clin Chem 2018;64:329–35. [DOI] [PubMed] [Google Scholar]
- 7.Ji X, Li J, Huang Y, et al. Identifying occult maternal malignancies from 1.93 million pregnant women undergoing noninvasive prenatal screening tests. Genet Med 2019;21:2293–302. [DOI] [PubMed] [Google Scholar]
- 8.Heesterbeek CJ, Aukema SM, Galjaard RH, et al. Noninvasive prenatal test results indicative of maternal malignancies: a nationwide genetic and clinical follow-up study. J Clin Oncol 2022;40:2426–35. [DOI] [PubMed] [Google Scholar]
- 9.Lenaerts L, Brison N, Maggen C, et al. Comprehensive genome-wide analysis of routine non-invasive test data allows cancer prediction: a single-center retrospective analysis of over 85,000 pregnancies. EClinicalMedicine 2021;35:100856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goldring G, Trotter C, Meltzer JT, et al. Maternal malignancy after atypical findings on single-nucleotide polymorphism-based prenatal cell-free DNA screening. Obstet Gynecol 2023;141:791–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carlson LM, Hardisty E, Coombs CC, Vora NL. Maternal malignancy evaluation after discordant cell-free DNA results. Obstet Gynecol 2018;131:464–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rink BD, Stevens BK, Norton ME. Incidental detection of maternal malignancy by fetal cell-free DNA screening. Obstet Gynecol 2022;140:121–31. [DOI] [PubMed] [Google Scholar]
- 13.Heesterbeek CJ, Lenaerts L, Tjan-Heijnen VCG, et al. Comprehensive recommendations for the clinical management of pregnant women with noninvasive prenatal test results suspicious of a maternal malignancy. JCO Oncol Pract 2024; April 12:OP2300594. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 14.Dungan JS, Klugman S, Darilek S, et al. Noninvasive prenatal screening (NIPS) for fetal chromosome abnormalities in a general-risk population: an evidence-based clinical guideline of the American College of Medical Genetics and Genomics (ACMG). Genet Med 2023;25:100336. [DOI] [PubMed] [Google Scholar]
- 15.Dow E, Freimund A, Smith K, et al. Cancer diagnoses following abnormal noninvasive prenatal testing: a case series, literature review, and proposed management model. JCO Precis Oncol 2021;5:1001–12. [DOI] [PubMed] [Google Scholar]
- 16.Jha P, Lenaerts L, Vermeesch J, et al. Noninvasive prenatal screening and maternal malignancy: role of imaging. Abdom Radiol (NY) 2023;48:1590–8. [DOI] [PubMed] [Google Scholar]
- 17.Petralia G, Zugni F, Summers PE, et al. Whole-body magnetic resonance imaging (WB-MRI) for cancer screening: recommendations for use. Radiol Med 2021;126:1434–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jensen TJ, Zwiefelhofer T, Tim RC, et al. High-throughput massively parallel sequencing for fetal aneuploidy detection from maternal plasma. PLoS One 2013;8:e57381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuhn M Building predictive models in R using the caret package. J Stat Softw 2008;28:1–26.27774042 [Google Scholar]
- 20.Sarandakou A, Protonotariou E, Rizos D. Tumor markers in biological fluids associated with pregnancy. Crit Rev Clin Lab Sci 2007;44:151–78. [DOI] [PubMed] [Google Scholar]
- 21.Turriff AE, Annunziata CM, Bianchi DW. Prenatal DNA sequencing for fetal aneuploidy also detects maternal cancer: importance of timely workup and management in pregnant women. J Clin Oncol 2022;40:2398–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scott F, Menezes M, Smet ME, et al. Influence of fibroids on cell-free DNA screening accuracy. Ultrasound Obstet Gynecol 2022;59:114–9. [DOI] [PubMed] [Google Scholar]
- 23.Mai PL, Khincha PP, Loud JT, et al. Prevalence of cancer at baseline screening in the National Cancer Institute Li-Fraumeni syndrome cohort. JAMA Oncol 2017;3:1640–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Turriff A, Miner SA, Annunziata CM, et al. Patients’ perspectives on prenatal screening results that suggest maternal cancer: a qualitative study. Prenat Diagn 2023;43:1101–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


