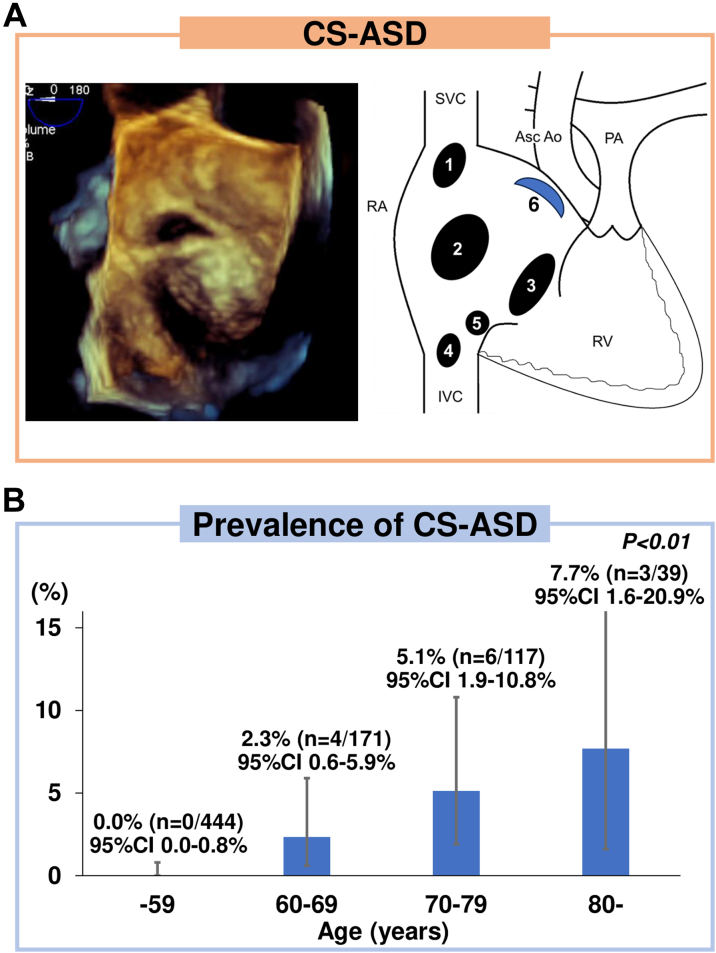
In patients with atrial septal defect (ASD), we have observed a unique crescent-like morphology on 3-dimensional transesophageal echocardiography (TEE). A crescent-shaped atrial septal defect (CS-ASD) is found between septum secundum adjacent to aortic wall and septum primum. Because of its location and morphology, a CS-ASD is traditionally considered a stretched patent foramen ovale (PFO) (Figure 1). This study aimed to elucidate characteristics of CS-ASDs.
Figure 1.
CS-ASD and Prevalence of Patients With CS-ASD
(A) A representative case of crescent-shaped arterial septal defect (CS-ASD) on 3-dimensional (3D) transesophageal echocardiography (TEE) and a schema of CS-ASD. Numbers 1-5 show the conventional classification of arterial septal defect (ASD), and number 6 shows the location of a CS-ASD: (1) superior sinus venous defect; (2) regularly shaped secundum ASD; (3) primum ASD; (4) inferior sinus venous defect; (5) unroofed coronary sinus; and (6) CS-ASD. The CS-ASD is located between the septum secundum adjacent to the aortic wall and the septum primum, and it is visually described as having a crescent-shaped morphology on 3D TEE. (B) CS-ASDs are rarely seen on 3D TEE. The prevalence of CS-ASD among all patients analyzed in this study increased significantly with age. AO = aorta; ASC = ascending; IVC = inferior vena cava; PA = pulmonary artery; RA = right atrium; RV = right ventricle; SVC = superior vena cava.
A total of 983 patients who underwent transcatheter ASD closure at Okayama University Hospital were reviewed. Indications for transcatheter ASD closure was assessed using universal guidelines.1,2 Exclusion criteria for transcatheter ASD closure were defect diameter >38 mm or insufficient rim except towards aorta.1,2 Patients with other coexisting congenital heart disease, prior surgical ASD closure, age <15 years, multiple ASDs, and missing TEE images were excluded. Finally, 771 patients were analyzed, of whom 13 (1.7% [n = 13/771]): 6 women (46.2% [n = 6 of 13]) were identified as having CS-ASDs. Of the remaining 758 patients with regularly shaped (circular or oval) ASDs,3 13 were selected as the control group using propensity score (PS) matching. This study adhered to tenets of Declaration of Helsinki. Written, informed consent was obtained from all patients. The study was approved by Institutional Review Board of Okayama University Graduate School of Medicine.
Patients underwent TEE using an iE33 with an X7-2t probe (Philips Medical Systems). The longest and shortest ASD diameters at end-systole on sweeping from 0° to 180° at 15° intervals on 2-dimensional TEE were defined as the maximal and minimal ASD diameters, respectively.4 ASD morphology was evaluated by 3-dimensional TEE. CS-ASD was defined as an ASD located between septum secundum adjacent to aortic wall and septum primum, which is visually described as a crescent-shaped morphology: an arcuate shape wider in the middle than at the ends as if composed of 2 different circular edges, throughout the cardiac cycle (Figure 1).
Amplatzer Septal Occluder (Abbott) or Occlutech Figulla Flex II (Occlutech GmbH) was used. Device size was selected according to the actual diameter measured in balloon sizing. If balloon sizing was not accurately performed due to the shape of CS-ASD, the device was selected based on following criteria, with reference to previous studies. When using Amplatzer Septal Occluder, a device that was 1-3 mm larger than the maximum diameter measured by TEE was selected. When using Occlutech Figulla Flex II, a device that was 3-5 mm larger than the maximum diameter measured by TEE was selected.4,5
Categorical variables are presented as numbers (%) and compared using the Fisher exact test. Continuous variables are presented as medians with IQRs and compared using the Mann-Whitney U test. To adjust for potential confounders (age, sex, body mass index, and presence of comorbidities [hypertension, diabetes mellitus, and prior stroke]), 1-to-1 PS matching was performed. A logistic regression model was used to derive PS for the presence of CS-ASD, with a caliper of 0.2 SDs of the logit of the PS. In CS-ASD patients, NYHA functional class and plasma B-type natriuretic peptide (BNP) levels were compared between before closure and one month later using Wilcoxon signed-rank test. Significance was set at P <0.05. These analyses were performed with R statistical package (version 3.6.3; R Foundation for Statistical Computing).
The prevalence of CS-ASD increased with age (Figure 1). Two CS-ASD patients underwent transthoracic echocardiography 6 or 9 years before the diagnosis of ASD, and their ASDs were not identified. Clinical indications for transcatheter ASD closure were right ventricular volume overload and pulmonary/systemic flow ratio >1.5 in 12 patients and platypnea-orthodeoxia due to a temporary right-to-left shunt in 1 patient. Eight CS-ASD patients (61.5% [n = 8 of 13]) had permanent atrial fibrillation (AF); 1 patient underwent electrical defibrillation but could not maintain sinus rhythm, and 7 patients underwent only anticoagulation therapy because electrical defibrillation and catheter ablation were inefficient considering their age and left atrial dilation. Five patients (38.5% [n = 5 of 13]) had sinus rhythm; 2 of them had a history of paroxysmal AF and underwent catheter ablation before closure.
Patients with CS-ASD were older (73 [Q1-Q3: 66-79] years) than those who had regularly shaped ASDs (55 [Q1-Q3: 38-67] years; P < 0.01). The prevalence of permanent AF was higher in patients with CS-ASD (61.5% [n = 8 of 13]) than patients with regularly shaped ASDs (6.3% [n = 48 of 758]; P < 0.01).
The control group had a median age of 73 years (P = 0.94 and standardized mean difference [SMD] = 0.05, compared with the CS-ASD group) and included 7 women (53.8% [n = 7 of 13]) (P = 1.00 and SMD = 0.15, compared with the CS-ASD group). The 2 groups were well balanced on all variables used in PS calculation after matching (SMD were ≤0.2) except for body mass index, whereas SMD was >0.2 in all variables before matching. The prevalence of permanent AF was higher in the CS-ASD group (61.5% [n = 8 of 13]) than the control group (15.4% [n = 2 of 13]; P < 0.05). The BNP level was higher in the CS-ASD group (223.2 [Q1-Q3: 108.4-335.5] pg/mL) than the control group (82.1 [Q1-Q3: 51.7-129.5] pg/mL; P < 0.05). The minimum ASD diameter was smaller in the CS-ASD group (7 [Q1-Q3: 5-9] mm) than the control group (11 [Q1-Q3: 10-14] mm; P < 0.01), whereas the maximum ASD diameter was not significantly different (13 [Q1-Q3: 11-16] mm and 16 [Q1-Q3: 14-18] mm; P = 0.22). The CS-ASD group had larger left atrial diameter (48.0 [Q1-Q3: 43.0-52.0] mm and 40.0 [Q1-Q3: 37.0-42.0] mm; P < 0.05) and left atrial volume index (66.5 [Q1-Q3: 58.0-82.0] mL/m2 and 50.0 [Q1-Q3: 37.5-55.5] mL/m2; P < 0.05). There were no significant differences in the pulmonary/systemic flow ratio (1.78 [Q1-Q3: 1.55-2.06] and 2.00 [Q1-Q3: 1.76-3.33]; P = 0.08), mean left atrial pressure (8 [Q1-Q3: 7-10.5] mm Hg and 8 [Q1-Q3: 6-9] mm Hg; P = 0.44), mean pulmonary artery pressure (19 [Q1-Q3: 15-20] mm Hg and 17 [Q1-Q3: 15-20] mm Hg; P = 0.68), systolic right ventricular pressure (36 [Q1-Q3: 31-39.5] mm Hg and 31.5 [Q1-Q3: 25-35] mm Hg; P = 0.29), and mean right atrial pressure (6 [Q1-Q3: 5-8] mm Hg and 5 [Q1-Q3: 4-9] mm Hg; P = 0.33).
All patients in the CS-ASD group successfully achieved closure. The size of device was 15.0 (Q1-Q3: 14.0-22.0) mm. During a median follow-up of 5.1 (Q1-Q3: 2.4-6.4) years, cardiovascular death, hospitalization for heart failure, cardiac erosion, or device dislodgement did not occur. The NYHA functional class improved from before closure (1, 9, 2, and 1 patients in functional classes I, II, III, and IV, respectively) to 1 month later (5, 7, 1, and 0 patients in functional classes I, II, III, and IV, respectively; P < 0.01). BNP levels decreased from before closure (233.2 [Q1-Q3: 108.4-33.5] pg/mL) to 1 month later (122.0 [Q1-Q3: 84.0-257.0] pg/mL; P < 0.05).
CS-ASDs were identified in elderly patients and increased with age. In addition, the ASDs of patients with CS-ASD were not identified on their previous transthoracic echocardiography. Thus, CS-ASDs may not be congenital, but rather appear in older age due to acquired factors that stretch the original PFO and develop into a permanent interatrial communication. The aortic side of the PFO is assumed to be arch-shaped, whereas the primary septum side is presumably stretched downwards, giving it a crescent shape.
This is the first study to evaluate the clinical features of CS-ASDs, but it was difficult to completely confirm the pathogenetic mechanism. Further investigations that will prospectively evaluate the time-course changes in the morphologies of ASDs are needed.
Although CS-ASDs are not large defects, the possibility of their presence must be considered in patients not previously diagnosed with an ASD because it is an acquired left-right shunt that can cause heart failure in elderly persons. Transcatheter closure can be performed safely and effectively.
Funding Support and Author Disclosures
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Acknowledgments
The authors thank FORTE Science Communications (https://www.forte-science.co.jp/) for language editing.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Stout K.K., Daniels C.J., Aboulhosn J.A., et al. 2018 AHA/ACC guideline for the management of adults with congenital heart disease: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. J Am Coll Cardiol. 2019;73(12):e81–e192. doi: 10.1016/j.jacc.2018.08.1029. [DOI] [PubMed] [Google Scholar]
- 2.Baumgartner H., De Backer J., Babu-Narayan S.V., et al. 2020 ESC guidelines for the management of adult congenital heart disease. Eur Heart J. 2021;42(6):563–645. doi: 10.1093/eurheartj/ehaa554. [DOI] [PubMed] [Google Scholar]
- 3.Johri A.M., Witzke C., Solis J., et al. Real-time three-dimensional transesophageal echocardiography in patients with secundum atrial septal defects: outcomes following transcatheter closure. J Am Soc Echocardiogr. 2011;24(4):431–437. doi: 10.1016/j.echo.2010.12.011. [DOI] [PubMed] [Google Scholar]
- 4.Takaya Y., Akagi T., Nakagawa K., et al. Feasibility of transcatheter closure for absent aortic rim in patients with atrial septal defect. Catheter Cardiovasc Interv. 2021;97(5):859–864. doi: 10.1002/ccd.29457. [DOI] [PubMed] [Google Scholar]
- 5.Haas N.A., Soetemann D.B., Ates I., et al. Closure of Secundum Atrial Septal Defects by Using the Occlutech Occluder Devices in More Than 1300 Patients: the IRFACODE project: a retrospective case series. Catheter Cardiovasc Interv. 2016;88(4):571–581. doi: 10.1002/ccd.26497. [DOI] [PubMed] [Google Scholar]