Abstract
Aims
Causes of death remain largely unexplored in the atrial fibrillation (AF) population. We aimed to (i) thoroughly assess causes of death in patients with AF, especially those associated with sudden cardiac death (SCD) and (ii) evaluate the potential association between AF and SCD.
Methods and results
Linked primary and secondary care United Kingdom Clinical Practice Research Datalink dataset comprising 6 529 382 individuals aged ≥18. We identified 214 222 patients with newly diagnosed AF, and an equivalent number of non-AF patients matched for age, sex and primary care practice. The underlying primary cause of death for each patient was assessed in the form of International Classification of Diseases Tenth Revision (ICD-10) codes and also as part of broader disease categories (i.e. ICD-10 chapters).
Findings
Over a median follow-up of 2.7 (interquartile range: 0.7–6.0) years, 124 781 (58.25%) patients with AF died. Sudden cardiac death occurred in 13 923 patients with AF [6.50% patients with AF vs. 2.01% non-AF patients; odds ratio (OR) = 3.38, 95% confidence interval (CI): 3.27–3.50, P < 0.0001], contributing to 11.05% of all AF mortality. Diseases of the circulatory system, neoplasms and respiratory diseases explained 45% of AF mortality. Sudden cardiac death occurred more frequently in males (OR = 1.87, 95% CI: 1.80–1.93, P < 0.0001), and females with AF died more often of diseases of the circulatory, respiratory, digestive, and genitourinary system and less often of neoplastic disorders.
Interpretation
Conditions of the circulatory system are the main driver of mortality in the AF population. Females with AF experience higher cardiovascular and respiratory mortality but die less frequently of neoplasms. The risk of SCD is higher in the AF population, occurring more frequently in males.
Keywords: Mortality, Arrhythmia, Sudden Cardiac Death, Cardiovascular, Women, EHR-WAS
Key Learning Points.
-
What is already known:
Patients with atrial fibrillation (AF) have higher mortality and risk of sudden cardiac death (SCD) than the general population.
Females with AF have higher mortality, but a detailed assessment on causes of death contributing for that risk excess is currently lacking.
No nationwide data on AF mortality and SCD are available for the UK or other European countries.
-
What this study adds:
We identified 2432 different primary causes of death among 259 391 study participants who died and listed the top 100 primary causes of death in the AF population.
We demonstrated that diseases of the circulatory system, neoplasms, and respiratory diseases explained 45% of AF mortality.
Sudden cardiac death occurs more frequently in males with AF, and females with AF die more often of diseases of the circulatory, respiratory, digestive and genitourinary system and less often of neoplastic disorders.
Background
Atrial fibrillation (AF) is recognized as the most prevalent cardiac arrhythmia globally and based on data from the Global Burden of Disease it may affect over 50 million worldwide.1 Atrial fibrillation is associated with many cardiovascular and non-cardiovascular comorbidities,2 and new-onset AF has been associated with an increased mortality and morbidity from stroke, heart failure, dementia, and hospitalizations.3
A nationwide Korean study showed that compared with the general population, patients with AF have higher mortality risk from cardiovascular and non-cardiovascular causes.4 Stroke is one of the known and most feared AF-related complications5 but is responsible for only 7% of mortality.3,6 Importantly, heart failure, infections, and cancer,6 as well as ischaemic heart disease3 are thought to be the main contributors to AF mortality. Nevertheless, a detailed assessment of the most frequent primary causes of death in the AF population utilizing an electronic health records-wide association study (EHR-WAS) is currently lacking.
Sudden cardiac death (SCD) is defined as an unexpected fatal event occurring in a short time period (usually within an hour of symptom onset) in individuals with known or unknown cardiac disease.7 In Western countries, SCD contributes to 15–20% of all fatalities, with ischaemic heart disease, cardiomyopathies/heart failure, inherited arrhythmias, and valvular heart disease as the main contributing disease substrates.8 The Department of Health estimates that annually 100 000 people in the UK die suddenly due to cardiac causes, with ischaemic heart disease involved in most cases.9,10
A meta-analysis of observational studies and randomized controlled trials from Odutayo et al.2 showed that individuals with AF have a 46% higher risk of all-cause mortality, and 88% higher risk of SCD. However, no nationwide data are available for the UK or other European countries, and a better understanding of the substrate for the increased risk of SCD in patients with AF is required.
Among patients with AF, females have higher mortality and AF-related complications, such as stroke,3,4,11 although recent data suggest this sex difference AF-related stroke is less apparent.12 A lower risk of SCD has been reported for females across all age groups in a nationwide Danish study.13 Whether or not this occurs in the AF population, and what are the potential drivers for this difference, remains to be explained.
We aimed to (i) thoroughly assess the causes of death in patients with AF utilizing an EHR-WAS approach; (ii) evaluate the potential associations between AF and SCD, and (iii) investigate potential differences in causes of death between males and females with AF, utilizing a nationwide UK dataset.
Methods
We investigated the causes of death in patients with AF compared to their age, gender, and primary care-practice matched controls without AF, as well as the association of AF with SCD, in a UK nationwide electronic health record dataset. To achieve the goal, we identified people with newly diagnosed AF from 1 January 1998 to 31 May 2016, and subsequently, matched them with patients not afflicted with AF.
Data sources, study population, and design
Our linked data included data from primary care (Clinical Practice Research Datalink, CPRD), secondary care (Hospital Episodes Statistic, HES), and death registry data (Office for National Statistics, ONS), comprising a total of 6 529 382 participants aged 18 years or older with complete data.3 This dataset comprises data from ∼10% of primary care practices across the UK, including information from England, Scotland, Wales, and Northern Ireland. Data on the distribution in terms of age, gender, socioeconomic diversity, geographic location, prevalence of various health conditions, and their management accurately mirrors the overall population of the UK, and validation on accuracy, reliability, and completeness are available.14–16
For each incident AF patient, a matched non-AF control was selected within the same primary care practice, according to the sex and age at the index date, utilizing the exact matching method from NHS-R Community.17 The index date was defined as the date of initial AF diagnosis for individuals with AF, and as the earliest of the following for controls: the date of the participant's registration or the start date of data collection by the primary care-practice. The end of the study was established by the earliest date among the following circumstances: the patient died, or the date of study exit (31 May 2016).
Study variables, including baseline comorbidities, were derived based on the phenotyping algorithms of HDR-UK Phenotype platform.18,19 These included sociodemographic factors, comorbidities, lifestyle measurements, and prescribed medications and were selected based on expert opinion, and availability within CPRD dataset. Atrial fibrillation was diagnosed and utilized the I48 International Classification of Diseases Tenth Revision (ICD-10) code from HES, and Read codes G573200, G573400, G573500, 3272.00, G573000, G573300, G573.00, G573z00, 3273.00, 793M100, 793M300, and G573100 from CPRD.20 Sudden cardiac death diagnoses included ICD-10 codes I461 and I469 from HES and ONS as well as Read code G575100, as part of a previously validated SCD phenotype.21
Death occurrences, precise death dates, and primary causes of death were extracted from the records of the ONS. With regards to cause of death, certificates in the UK provide information on the primary cause of death (designated as 1a; ‘the disease of condition immediately causing the death’), the underlying conditions that led to the primary cause (listed as 1b, 1c, and, eventually, 1d, as a sequence of events), and contributory factors (listed as 2, and defined as other significant conditions that contributed to the death but were not part of the direct causal sequence). For this study, the immediate cause of death (i.e. 1a) for each patient was assessed in the form of ICD-10 codes and also as part of broader disease categories (i.e. ICD-10 chapters).
Statistical analyses
Baseline characteristics of patients with AF and non-AF controls were summarized as number and percentage of people in each category, alongside with means and standard deviations for continuous variables. The differences between variables were evaluated via χ2 and t-tests.
Binary logistic regressions were implemented, and odds ratios (ORs) with 95% confidence intervals (CIs) were estimated for occurrence of specific causes of death for the AF group vs. the non-AF group. A P-value threshold of <0.05 was defined for assessing all statistical significances.
We performed an EHR-WAS, determining the top 100 associations with AF, utilizing the previously described approach.22 Due to numerous logistic regressions being carried through simultaneously when assessing causes of death, we applied the Bonferroni correction to adjust for multiple comparisons and adjusted the significance level of the P-value threshold to minimize chances of Type I error. The adjusted significance level was the P-value of 0.05 divided by the number of statistical tests executed—2.039 × 10−5 for primary diagnosis of cause of death (i.e. 0.05/2432, due to 2432 different primary diagnoses) or 0.0025 for ICD-10 chapters (i.e. 0.05/20, due to 20 different ICD-10 chapters).
Kaplan–Meier curves were utilized to visualize time-to-event, SCD, in AF vs. non-AF patients, and males vs. females with AF, and the respective hazard ratios were estimated (unadjusted, and with adjustment for age and sex). Iris plots were utilized to visualize OR of identified ICD-10 code associations, grouped by ICD-10 chapter colour.
All analyses were conducted through the R (version 4.3.0) software and statistical software for data science (STATA/MP 17.0 edition) within the UCL’s Data Safe Haven platform in compliance with the Information Governance training.
The study and access to dataset were approved by the Medicines and Healthcare products Regulatory Agency Independent Scientific Advisory Committee [17_205].
Results
Out of a total of 6 529 382 patients of age ≥18 years with valid linked data, 214 222 patients with newly diagnosed AF met study entry criteria (see Supplementary material online, Figure S1), and an equivalent number of non-AF matched patients were identified. Baseline characteristics at the study entry of AF individuals and their matched controls are displayed in Table 1. The mean age at diagnosis was 75 ± 13, and 49% were females. Patients with AF were more often drinking alcohol or smokers/ex-smokers, two thirds had hypertension, and when compared with controls, had significantly more cardiovascular and non-cardiovascular comorbidities at the time of diagnosis.
Table 1.
Population demographics and comorbidities at baseline
| Variables | AF (n = 214 222) | Non-AF (n = 214 222) | Male (n = 217 490) | Female (n = 210 954) |
|---|---|---|---|---|
| Age | 75.24 ± 12.98 | 75.08 ± 13.07 | 72.03 ± 13.16 | 78.39 ± 12.05 |
| Females | 105 477 (49.24) | 105 477 (49.24) | – | 210 954 (100) |
| Alcohol consumption | 80 897 (37.76) | 57 023 (26.62) | 70 743 (32.53) | 67 177 (31.84) |
| Current smokers | 28 449 (13.28) | 18 331 (8.56) | 29 673 (13.64) | 17 042 (8.11) |
| Ex-smokers | 58 346 (27.24) | 21 835 (10.19) | 50 990 (23.44) | 29 191 (13.84) |
| Non-smokers | 100 809 (47.06) | 76 888 (35.89) | 76 198 (35.04) | 101 499 (48.11) |
| Abdominal aortic aneurysm | 5098 (2.38) | 1715 (0.80) | 5201 (2.39) | 1612 (0.76) |
| Diabetes | 36 419 (17.00) | 18 260 (8.52) | 30 590 (14.07) | 24 089 (11.42) |
| Heart failure | 46 614 (21.76) | 10 232 (4.78) | 27 294 (12.55) | 29 552 (14.01) |
| Hypertension | 136 221 (63.59) | 72 643 (33.91) | 97 414 (44.79) | 111 450 (52.83) |
| Acute myocardial infarction | 29 008 (13.54) | 10 986 (5.13) | 25 488 (11.72) | 14 506 (6.88) |
| Peripheral arterial disease | 16 945 (7.91) | 6754 (3.15) | 14 032 (6.45) | 9667 (4.58) |
| Stroke | 23 941 (11.18) | 10 934 (5.10) | 16 972 (7.80) | 17 903 (8.49) |
| Dementia | 8011 (3.74) | 13 867 (6.47) | 7894 (3.63) | 13 984 (6.63) |
| Valvular heart disease | 14 092 (6.58) | 2397 (1.12) | 8048 (3.70) | 8441 (4.00) |
| Chronic kidney disease | 36 071 (16.84) | 8356 (3.90) | 19 867 (9.13) | 24 560 (11.64) |
| Oral anticoagulants | 14 812 (6.91) | 115 (0.05) | 8364 (3.85) | 6563 (3.11) |
| Died in community | 121 279 (56.61) | 112 154 (52.35) | 109 942 (50.55) | 123 491 (58.54) |
| Died in hospital | 3502 (1.63) | 6256 (2.92) | 4445 (2.04) | 5313 (2.52) |
Note: age at the index date is age at AF diagnosis for patients with AF and the date of study entry for controls. Oral anticoagulants prescription at the time of diagnosis.
When segregated by sex, females were significantly older on average (78 ± 12 vs. 72 ± 13, P < 0.0001) but were less frequently smokers or ex-smokers. Females were more frequently hypertensive and more likely to have history of stroke, dementia, and chronic kidney disease at baseline.
During a median follow-up period of 2.7 (interquartile range: 0.7–6.0) years, 124 781 (58.25%) patients with AF, and 118 410 (55.27%) non-AF patients died (P < 0.0001). Regardless of whether patients had AF, the majority of patients died in the community: 56.61% died in the community, and 1.63% died in hospital, for AF cases; 52.35% vs. 2.92% for non-AF controls.
AF vs. non-AF patients
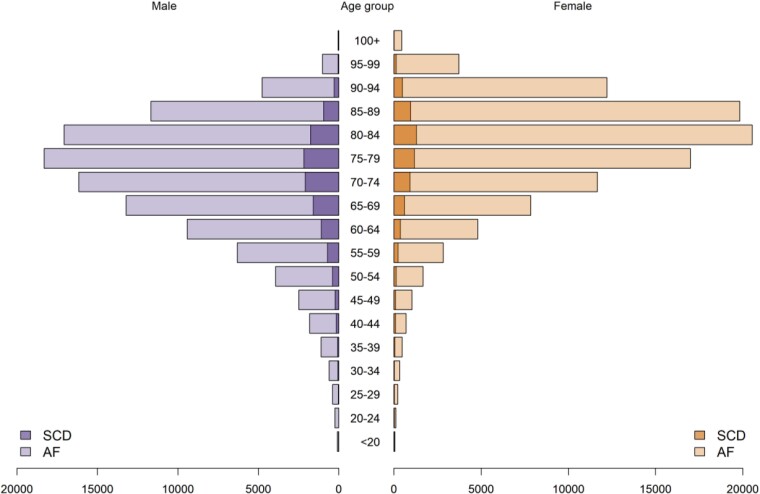
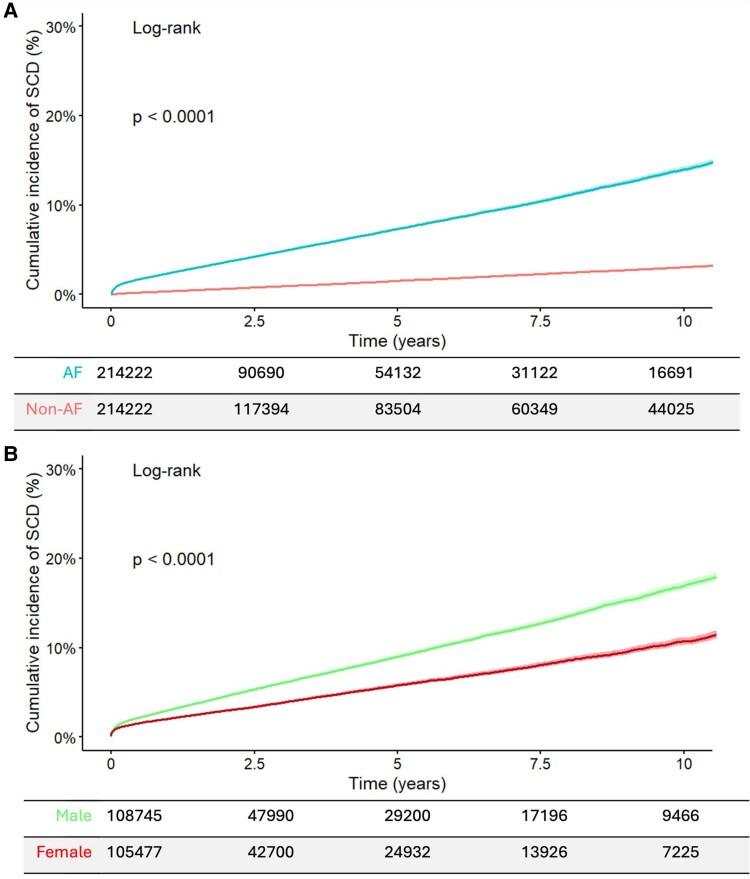
In total, 18 239 SCD events were identified among the 428 444 eligible participants. In the AF group SCD occurred in 13 923 and in non-AF patients 4316 had SCD (6.50% vs. 2.01%; OR = 3.38, 95% CI: 3.27–3.50, P < 0.0001). Excess in SCD mortality among patients with AF was observed both for the community (OR = 2.77, 95% CI: 2.66–2.88, P < 0.0001), and in hospital (OR = 1.72, 95% CI: 1.48–2.00, P < 0.0001). The age distribution of the AF population and SCD events is illustrated in Figure 1, and the cumulative incidence of SCD is represented in Figure 2A. Time-to-event analysis using hazard ratios confirmed an increased risk of SCD in patients with AF (unadjusted hazard ratio = 1.84, 95% CI: 1.78–1.91, and hazard ratio adjusted for age and sex = 1.85, 95% CI: 1.79–1.92). Sudden cardiac death contributed to 11.05% of all mortality cases in the AF population.
Figure 1.
Distribution of age and gender the study population by atrial fibrillation and sudden cardiac death.
Figure 2.
Cumulative incidence curves for sudden cardiac death in atrial fibrillation and non- atrial fibrillation groups (A), and atrial fibrillation males and atrial fibrillation females (B).
Among patients with AF, those who died from SCD were younger than those who died from other causes and more frequently males (Table 2). Alcohol consumption and smokers/ex-smokers were also more frequent in the SCD group. Patients with atrial fibrillation with known history of diabetes mellitus, myocardial infarction, heart failure, and valvular heart disease were more represented in the SCD group.
Table 2.
Descriptive characteristics of the patients with atrial fibrillation (n = 214 222)
| Variables | AF patients with SCD (n = 13 923) | AF patients who died (n = 125 993) | AF patients who survived (n = 88 229) | P-value |
|---|---|---|---|---|
| Age | 71.99 ± 12.95 | 80.21 ± 9.87 | 68.13 ± 13.56 | <0.001a |
| Females | 4887 (35.10) | 66 885 (53.09) | 37 174 (42.13) | <0.001a |
| Alcohol consumption | 5580 (40.08) | 44 852 (35.60) | 36 045 (40.85) | <0.001b |
| Current smokers | 2598 (18.66) | 16 343 (12.97) | 12 106 (13.72) | <0.001a |
| Ex-smokers | 4123 (29.61) | 33 622 (26.69) | 24 724 (28.02) | <0.001a |
| Non-smokers | 5653 (40.60) | 57 719 (45.81) | 43 090 (48.84) | <0.001a |
| Abdominal aortic aneurysm | 523 (3.76) | 3715 (2.95) | 1383 (1.57) | <0.001a |
| Diabetes | 3070 (22.05) | 23 099 (18.33) | 13 320 (15.10) | <0.001a |
| Heart failure | 5276 (37.89) | 36 372 (28.87) | 10 242 (11.61) | <0.001a |
| Hypertension | 9264 (66.54) | 84 100 (66.75) | 52 121 (59.07) | <0.001a |
| Acute myocardial infarction | 4391 (31.54) | 19 912 (15.80) | 9096 (10.31) | <0.001a |
| Peripheral arterial disease | 1624 (11.66) | 12 903 (10.24) | 4042 (4.58) | <0.001a |
| Stroke | 1323 (9.50) | 17 559 (13.94) | 6382 (7.23) | <0.001a |
| Dementia | 261 (1.87) | 7090 (5.63) | 921 (1.04) | <0.001a |
| Valvular heart disease | 1400 (10.06) | 8096 (6.43) | 5996 (6.80) | <0.001a |
| Chronic kidney disease | 2696 (19.36) | 24 034 (19.08) | 12 037 (13.64) | <0.001a |
| Oral anticoagulants | 1244 (8.93) | 9061 (7.19) | 5751 (6.52) | <0.001a |
| Died in community | 9329 (96.39) | 121 279 (96.26) | – | – |
| Died in hospital | 349 (2.51) | 3502 (2.78) | – | – |
aNote: P-values satisfying Bonferroni correction threshold 0.05/38 = 0.0015, for the 34 comparisons of AF patients with SCD vs. AF patients who survived, and AF patients who died vs. AF patients who survived.
b P-values satisfying Bonferroni correction threshold only for comparison of AF patients who died vs. AF patients who survived.
Diseases of the circulatory system were the predominant common cause of death, when comparing the causes of deaths by ICD chapter: 50 379 deaths in patients with AF (23.52% of all AF-deaths) and 42.221 deaths in non-AF patients. This was followed by neoplasms, accounting for 20% of deaths, and diseases of respiratory at 17%. Comparing the relative frequency of mortality explained by each ICD-10 chapter, patients with AF died more frequently due to diseases of the circulatory system (23.52% vs. 19.71% in non-AF controls; OR = 1.25, 95% CI: 1.23–1.27, P < 0.0001), and less frequently from neoplasms (11.06% vs. 13.71%, respectively; OR = 0.78, 95% CI: 0.77–0.80, P < 0.0001; see Supplementary material online, Table S1 and Supplementary material online, Figure S2).
There were 2432 different primary causes of death recognized among the 259 391 people who died (adjusted significance level for multiple comparisons: 0.05/2432, P < 2.039 × 10−5). Among the top primary causes of death listed by absolute frequency in descending order, over half fell under the chapter on circulatory system diseases. The most frequent cause of death (n = 15 592) was ‘chronic ischaemic heart disease, unspecified’, which was twice more prevalent in the AF group (4.77% vs. 2.51%, OR = 1.94, 95% CI: 1.88–2.01, P < 0.0001) (Tables 3 and 4). This was followed by deaths due to ‘stroke, not specified as haemorrhage or infarction’ (n = 13 694) and ‘acute myocardial infarction, unspecified’ (n = 12 513), both (OR = 1.08, 95% CI: 1.05–1.12, P < 0.0001). Primary causes of death with large ORs were included in the top five, such as ‘myotonic disorders’ [0.01% (n = 26) vs. 0% (n = 1), OR = 26.00, 95% CI: 3.53–191.35], and ‘ventricular fibrillation and flutter’ [0.01% (n = 18) vs. 0% (n = 1), OR = 18.00, 95% CI: 2.41–143.66; Table 4]. However, due to the relatively small number of patients with deaths caused by those causes, none crossed the adjusted P value threshold for multiple comparisons.
Table 3.
Top primary causes of death by descending order of absolute frequencies
| Causes of death | AF n (%) | Non-AF n (%) |
|---|---|---|
| Chronic ischaemic heart disease, unspecified (I25.9) | 10 213 (4.77) | 5379 (2.51) |
| Stroke, not specified as haemorrhage or infarction (I64) | 7115 (3.32) | 6579 (3.07) |
| Acute myocardial infarction, unspecified (I21.9) | 6499 (3.03) | 6014 (2.81) |
| Unspecified dementia (F03) | 5549 (2.59) | 10126 (4.73) |
| Malignant neoplasm: bronchus or lung, unspecified (C34.9) | 4949 (2.31) | 4510 (2.11) |
| Bronchopneumonia, unspecified (J18.0) | 4249 (1.98) | 5723 (2.67) |
| Pneumonia, unspecified (J18.9) | 4163 (1.94) | 2736 (1.28) |
| Chronic obstructive pulmonary disease, unspecified (J44.9) | 4014 (1.87) | 3219 (1.5) |
| Chronic obstructive pulmonary disease with acute lower respiratory infection (J44.0) | 2362 (1.1) | 998 (0.47) |
| Cerebrovascular disease, unspecified (I67.9) | 2324 (1.08) | 3195 (1.49) |
| Senility (R54) | 2004 (0.94) | 4803 (2.24) |
| Congestive heart failure (I50.0) | 1989 (0.93) | 1048 (0.49) |
| Malignant neoplasm of prostate (C61) | 1945 (0.91) | 2739 (1.28) |
| Alzheimer disease, unspecified (G30.9) | 1282 (0.6) | 3262 (1.52) |
| Cerebral infarction, unspecified (I63.9) | 1217 (0.57) | 961 (0.45) |
| Malignant neoplasm: breast, unspecified (C50.9) | 1209 (0.56) | 1627 (0.76) |
| Other specified respiratory disorders (J98.8) | 1169 (0.55) | 1773 (0.83) |
| Aortic (valve) stenosis (I35.0) | 1085 (0.51) | 474 (0.22) |
| Malignant neoplasm without specification of site (C80) | 1063 (0.5) | 1718 (0.8) |
| Malignant neoplasm: colon, unspecified (C18.9) | 1029 (0.48) | 1284 (0.6) |
| Intracerebral haemorrhage, unspecified (I61.9) | 981 (0.46) | 728 (0.34) |
| Malignant neoplasm: pancreas, unspecified (C25.9) | 888 (0.41) | 1117 (0.52) |
| Peripheral vascular disease, unspecified (I73.9) | 875 (0.41) | 499 (0.23) |
| Malignant neoplasm: bladder, unspecified (C67.9) | 869 (0.41) | 1197 (0.56) |
| Other interstitial pulmonary diseases with fibrosis (J84.1) | 844 (0.39) | 657 (0.31) |
| Parkinson disease (G20) | 823 (0.38) | 1981 (0.92) |
| Unspecified acute lower respiratory infection (J22) | 792 (0.37) | 573 (0.27) |
| Sepsis, unspecified (A41.9) | 653 (0.3) | 364 (0.17) |
| Heart failure, unspecified (I50.9) | 627 (0.29) | 321 (0.15) |
| Malignant neoplasm: stomach, unspecified (C16.9) | 600 (0.28) | 859 (0.4) |
| Abdominal aortic aneurysm, ruptured (I71.3) | 600 (0.28) | 940 (0.44) |
| Chronic obstructive pulmonary disease with acute exacerbation, unspecified (J44.1) | 585 (0.27) | 195 (0.09) |
| Multiple myeloma (C90.0) | 578 (0.27) | 383 (0.18) |
| Hypertensive heart disease with (congestive) heart failure (I11.0) | 564 (0.26) | 266 (0.12) |
| Exposure to unspecified factor causing other and unspecified injury (X59.9) | 491 (0.23) | 668 (0.31) |
| Chronic ischaemic heart disease NOS (414.9) | 471 (0.22) | 1170 (0.55) |
| Myocardial degeneration (I51.5) | 455 (0.21) | 699 (0.33) |
| Acute myocardial infarction (410) | 399 (0.19) | 1695 (0.79) |
| Unspecified fall—Home (W19.0) | 398 (0.19) | 274 (0.13) |
| Bronchiectasis (J47) | 371 (0.17) | 186 (0.09) |
| Endocarditis, valve unspecified (I38) | 352 (0.16) | 68 (0.03) |
| Exposure to unspecified factor (X59.0) | 347 (0.16) | 239 (0.11) |
| Dilated cardiomyopathy (I42.0) | 333 (0.16) | 34 (0.02) |
| Malignant neoplasm, primary site unknown, so stated (C80.0) | 288 (0.13) | 178 (0.08) |
| Aortic valve disorder, unspecified (I35.9) | 254 (0.12) | 47 (0.02) |
| Cellulitis, unspecified (L03.9) | 251 (0.12) | 107 (0.05) |
| Mitral valve disease, unspecified (I05.9) | 234 (0.11) | 36 (0.02) |
| Mitral (valve) insufficiency (I34.0) | 221 (0.1) | 27 (0.01) |
| Alcoholic liver disease, unspecified (K70.9) | 221 (0.1) | 98 (0.05) |
| Essential (primary) hypertension (I10) | 216 (0.1) | 348 (0.16) |
| Unspecified diabetes mellitus—with peripheral circulatory complications (E14.5) | 200 (0.09) | 120 (0.06) |
| Malignant neoplasm, primary site unspecified (C80.9) | 198 (0.09) | 93 (0.04) |
| Mal neo bronch/lung NOS (162.9) | 182 (0.08) | 781 (0.36) |
| Cholangitis (K83.0) | 173 (0.08) | 101 (0.05) |
| Motor neuron disease (G12.2) | 172 (0.08) | 289 (0.13) |
| Calculus of gallbladder without cholecystitis (K80.2) | 171 (0.08) | 96 (0.04) |
| Chr airway obstruct NEC (496) | 169 (0.08) | 712 (0.33) |
| Ischaemic cardiomyopathy (I25.5) | 164 (0.08) | 30 (0.01) |
| Sequelae of other and unspecified cerebrovascular diseases (I69.8) | 164 (0.08) | 291 (0.14) |
| Alzheimer disease with late onset (G30.1) | 159 (0.07) | 395 (0.18) |
| Cardiomegaly (I51.7) | 154 (0.07) | 79 (0.04) |
| Cardiomyopathy, unspecified (I42.9) | 137 (0.06) | 18 (0.01) |
| Cellulitis of other parts of limb (L03.1) | 134 (0.06) | 57 (0.03) |
| Hypertensive heart and renal disease with both (congestive) heart failure and renal failure (I13.2) | 122 (0.06) | 50 (0.02) |
| Type 2 diabetes mellitus—with peripheral circulatory complications (E11.5) | 121 (0.06) | 55 (0.03) |
| Acute pancreatitis, unspecified (K85.9) | 115 (0.05) | 45 (0.02) |
| Unspecified diabetes mellitus—with renal complications (E14.2) | 109 (0.05) | 53 (0.02) |
| Other specified degenerative diseases of nervous system (G31.8) | 104 (0.05) | 177 (0.08) |
| Acute and subacute infective endocarditis (I33.0) | 104 (0.05) | 30 (0.01) |
| CHF NOS (428.0) | 98 (0.05) | 221 (0.1) |
| Other secondary pulmonary hypertension (I27.2) | 94 (0.04) | 9 (0) |
| Cholecystitis, unspecified (K81.9) | 89 (0.04) | 32 (0.01) |
| Aortic aneurysm of unspecified site, ruptured (I71.8) | 88 (0.04) | 161 (0.08) |
| Generalized and unspecified atherosclerosis (I70.9) | 74 (0.03) | 188 (0.09) |
| Alcoholic hepatic failure (K70.4) | 72 (0.03) | 27 (0.01) |
| Other ill-defined heart diseases (I51.8) | 71 (0.03) | 14 (0.01) |
| Organ-limited amyloidosis (E85.4) | 67 (0.03) | 22 (0.01) |
| Disorders of both mitral and aortic valves (I08.0) | 60 (0.03) | 13 (0.01) |
| Mitral stenosis (I05.0) | 56 (0.03) | 11 (0.01) |
| Primary pulmonary hypertension (I27.0) | 51 (0.02) | 8 (0) |
| Unspecified diabetes mellitus—with multiple complications (E14.7) | 50 (0.02) | 11 (0.01) |
| Tricuspid insufficiency (I07.1) | 42 (0.02) | 7 (0) |
| Other obesity (E66.8) | 41 (0.02) | 4 (0) |
| Cerebral atherosclerosis (I67.2) | 40 (0.02) | 90 (0.04) |
| Other forms of systemic sclerosis (M34.8) | 39 (0.02) | 4 (0) |
| Arthrosis, unspecified (M19.9) | 37 (0.02) | 93 (0.04) |
| Mal neo oesophagus NOS (150.9) | 34 (0.02) | 160 (0.07) |
| Cerebral artery occlusion, unspecified (434.9) | 32 (0.01) | 118 (0.06) |
| Diabetes mellitus without mention of complication (250.0) | 31 (0.01) | 149 (0.07) |
| Peripheral vascular disesase NOS (443.9) | 31 (0.01) | 117 (0.05) |
| Degenerative disease of nervous system, unspecified (G31.9) | 29 (0.01) | 81 (0.04) |
| Urinary tract infection NOS (599.0) | 27 (0.01) | 131 (0.06) |
| Malaise and fatigue (R53) | 26 (0.01) | 83 (0.04) |
| Traumatic amputation of arm and hand (complete) (partial) (887) | 24 (0.01) | 80 (0.04) |
| Left heart failure (428.1) | 20 (0.01) | 72 (0.03) |
| Other lymphomas (202.8) | 19 (0.01) | 89 (0.04) |
| Pneumococcal pneumonia (481) | 18 (0.01) | 70 (0.03) |
| Unspecified (402.9) | 17 (0.01) | 67 (0.03) |
| Osteoporosis (733.0) | 12 (0.01) | 56 (0.03) |
| Emphysema (492) | 11 (0.01) | 52 (0.02) |
Table 4.
Top primary causes of death, as measured by OR magnitude in descending order
| Variables | OR (95% CI) | P-value |
|---|---|---|
| Myotonic disorders (G71.1) | 25.98 (3.53, 191.35) | 0.001 |
| Ventricular fibrillation and flutter (I49.0) | 18.00 (2.41, 134.66) | 0.005 |
| Disorders of both mitral and tricuspid valves (I08.1) | 16.00 (2.12, 120.49) | 0.007 |
| Alcoholic hepatitis (K70.1) | 14.00 (1.84, 106.32) | 0.011 |
| Chronic constrictive pericarditis (I31.1) | 13.00 (1.7, 99.24) | 0.013 |
| Alcoholic cardiomyopathy (I42.6) | 12.50 (2.96, 52.78) | <0.001 |
| Codes for research or others (U99) | 10.99 (2.59, 46.78) | 0.001 |
| Other secondary pulmonary hypertension (I27.2) | 10.45 (5.27, 20.7) | <0.001a |
| Other obesity (E66.8) | 10.25 (3.67, 28.62) | <0.001a |
| Sleep apnoea (G47.3) | 10.00 (1.28, 78.01) | 0.028 |
| Dilated cardiomyopathy (I42.0) | 9.81 (6.89, 13.96) | <0.001a |
| Other forms of systemic sclerosis (M34.8) | 9.75 (3.48, 27.29) | <0.001a |
| Other hypertrophic cardiomyopathy (I42.2) | 9.33 (2.84, 30.69) | <0.001 |
| Mitral (valve) insufficiency (I34.0) | 8.19 (5.49, 12.22) | <0.001a |
| Autoimmune hepatitis (K75.4) | 8.00 (1, 63.97) | 0.049 |
| Disease of biliary tract, unspecified (K83.9) | 8.00 (1, 63.97) | 0.049 |
| Ventricular tachycardia (I47.2) | 8.00 (1, 63.97) | 0.049 |
| Cardiomyopathy, unspecified (I42.9) | 7.62 (4.66, 12.45) | <0.001a |
| Other restrictive cardiomyopathy (I42.5) | 7.50 (1.72, 32.8) | 0.007 |
| Osteomyelofibrosis (D47.4) | 7.33 (2.2, 24.49) | 0.001 |
| Mitral valve disease, unspecified (I05.9) | 6.51 (4.58, 9.24) | <0.001a |
| Primary pulmonary hypertension (I27.0) | 6.38 (3.03, 13.43) | <0.001a |
| Tricuspid insufficiency (I07.1) | 6.00 (2.7, 13.36) | <0.001a |
| Sepsis due to unspecified staphylococcus (A41.2) | 6.00 (1.77, 20.36) | 0.004 |
| Aneurysm and dissection of unspecified site (I72.9) | 5.99 (1.34, 26.81) | 0.019 |
| Surgical operation with anastomosis, bypass or graft (Y83.2) | 5.99 (1.34, 26.81) | 0.019 |
| Other and unspecified kyphosis (M40.2) | 5.50 (1.22, 24.82) | 0.027 |
| Ischaemic cardiomyopathy (I25.5) | 5.47 (3.71, 8.07) | <0.001a |
| Aortic valve disorder, unspecified (I35.9) | 5.41 (3.96, 7.38) | <0.001a |
| Mitral (valve) prolapse (I34.1) | 5.4 (2.08, 14.02) | <0.001 |
| Rheumatic heart disease, unspecified (I09.9) | 5.33 (1.55, 18.3) | 0.008 |
| Endocarditis, valve unspecified (I38) | 5.18 (4, 6.72) | <0.001a |
| Mitral stenosis (I05.0) | 5.09 (2.67, 9.72) | <0.001a |
| Other ill-defined heart diseases (I51.8) | 5.07 (2.86, 9) | <0.001a |
| Sarcoidosis, unspecified (D86.9) | 5.00 (1.91, 13.06) | 0.001 |
| Pericardial effusion (noninflammatory) (I31.3) | 5.00 (1.1, 22.82) | 0.038 |
| Aneurysm of heart (I25.3) | 5.00 (1.1, 22.82) | 0.038 |
| Acute subendocardial myocardial infarction (I21.4) | 5.00 (1.1, 22.82) | 0.038 |
| Other medical procedures (Y84.8) | 4.67 (1.34, 16.23) | 0.015 |
| Disorders of both mitral and aortic valves (I08.0) | 4.62 (2.53, 8.41) | <0.001a |
| Type 2 diabetes mellitus—with multiple complications (E11.7) | 4.56 (2.21, 9.37) | <0.001 |
| Unspecified diabetes mellitus—with multiple complications (E14.7) | 4.55 (2.37, 8.73) | <0.001a |
| Obstructive hypertrophic cardiomyopathy (I42.1) | 4.50 (1.52, 13.3) | 0.007 |
| Thyrotoxicosis, unspecified (E05.9) | 4.13 (1.91, 8.93) | <0.001 |
| Chronic myelomonocytic leukaemia (C93.1) | 4.00 (1.5, 10.66) | 0.006 |
| Acute nephritic syndrome—unspecified (N00.9) | 3.80 (1.42, 10.18) | 0.008 |
| Embolism and thrombosis of unspecified artery (I74.9) | 3.75 (1.24, 11.3) | 0.019 |
| Obesity, unspecified (E66.9) | 3.70 (1.84, 7.44) | <0.001 |
| Infective myositis (M60.0) | 3.67 (1.02, 13.14) | 0.046 |
| Anoxic brain damage, not elsewhere classified (G93.1) | 3.60 (1.79, 7.25) | <0.001 |
| Systemic sclerosis, unspecified (M34.9) | 3.60 (1.34, 9.7) | 0.011 |
| Acute and subacute infective endocarditis (I33.0) | 3.47 (2.31, 5.21) | <0.001a |
| Wegener granulomatosis (M31.3) | 3.33 (1.34, 8.3) | 0.01 |
| Aortic (valve) insufficiency (I35.1) | 3.31 (1.78, 6.15) | <0.001 |
| Removal of other organ (partial) (total) (Y83.6) | 3.25 (1.06, 9.97) | 0.039 |
| Fall involving bed (W06.0) | 3.25 (1.06, 9.97) | 0.039 |
| Sequelae of respiratory and unspecified tuberculosis (B90.9) | 3.25 (1.06, 9.97) | 0.039 |
| Diverticulum of oesophagus, acquired (K22.5) | 3.17 (1.26, 7.93) | 0.014 |
| Organ-limited amyloidosis (E85.4) | 3.05 (1.88, 4.93) | <0.001a |
| Chronic obstructive pulmonary disease with acute exacerbation, unspecified (J44.1) | 3.01 (2.56, 3.53) | <0.001a |
| Muscular dystrophy (G71.0) | 2.80 (1.01, 7.77) | 0.048 |
| Cholecystitis, unspecified (K81.9) | 2.78 (1.86, 4.17) | <0.001a |
| Amyloidosis, unspecified (E85.9) | 2.78 (1.3, 5.95) | 0.009 |
| Surgical operation with implant of artificial internal device (Y83.1) | 2.75 (1.22, 6.18) | 0.014 |
| Amputation of limb(s) (Y83.5) | 2.71 (1.14, 6.46) | 0.024 |
| Arteritis, unspecified (I77.6) | 2.69 (1.51, 4.77) | <0.001 |
| Alcoholic hepatic failure (K70.4) | 2.67 (1.71, 4.15) | <0.001a |
| Cardiac arrhythmia, unspecified (I49.9) | 2.67 (1.37, 5.18) | 0.004 |
| Calculus of bile duct with cholangitis (K80.3) | 2.67 (1.04, 6.82) | 0.04 |
| Disease of pericardium, unspecified (I31.9) | 2.67 (1.04, 6.82) | 0.04 |
| Other surgical procedures (Y83.8) | 2.63 (1.16, 5.93) | 0.02 |
| Acute pancreatitis, unspecified (K85.9) | 2.56 (1.81, 3.61) | <0.001a |
| Polycystic kidney, unspecified (Q61.3) | 2.56 (1.18, 5.52) | 0.017 |
| Intracerebral haemorrhage, intraventricular (I61.5) | 2.50 (1.28, 4.88) | 0.007 |
| Hypertensive heart and renal disease with both (congestive) heart failure and renal failure (I13.2) | 2.44 (1.76, 3.39) | <0.001a |
| Chronic obstructive pulmonary disease with acute lower respiratory infection (J44.0) | 2.38 (2.21, 2.57) | <0.001a |
| Pulmonary heart disease, unspecified (I27.9) | 2.36 (1.17, 4.78) | 0.017 |
| Cellulitis of other parts of limb (L03.1) | 2.35 (1.72, 3.21) | <0.001a |
| Cellulitis, unspecified (L03.9) | 2.35 (1.87, 2.94) | <0.001a |
| Intracerebral haemorrhage in cerebellum (I61.4) | 2.32 (1.35, 3.97) | 0.002 |
| Fatty (change of) liver, not elsewhere classified (K76.0) | 2.31 (1.29, 4.16) | 0.005 |
| Aortic (valve) stenosis (I35.0) | 2.30 (2.06, 2.56) | <0.001a |
| Alcoholic liver disease, unspecified (K70.9) | 2.26 (1.78, 2.86) | <0.001a |
| Disease of intestine, unspecified (K63.9) | 2.23 (1.16, 4.29) | 0.016 |
| Type 2 diabetes mellitus—with peripheral circulatory complications (E11.5) | 2.20 (1.6, 3.03) | <0.001a |
| Pyothorax without fistula (J86.9) | 2.19 (1.39, 3.45) | <0.001 |
| Malignant neoplasm, primary site unspecified (C80.9) | 2.13 (1.66, 2.73) | <0.001a |
| Hypertensive heart disease with (congestive) heart failure (I11.0) | 2.12 (1.84, 2.46) | <0.001a |
| Peritoneal adhesions (K66.0) | 2.07 (1.09, 3.92) | 0.025 |
| Unspecified diabetes mellitus—With renal complications (E14.2) | 2.06 (1.48, 2.86) | <0.001a |
| Pneumonia due to Streptococcus pneumoniae (J13) | 2.00 (1.05, 3.8) | 0.034 |
| Bronchiectasis (J47) | 2.00 (1.67, 2.38) | <0.001a |
| Type 2 diabetes mellitus—with renal complications (E11.2) | 1.96 (1.4, 2.74) | <0.001 |
| Heart failure, unspecified (I50.9) | 1.96 (1.71, 2.24) | <0.001a |
| Cardiomegaly (I51.7) | 1.95 (1.49, 2.56) | <0.001a |
| Chronic ischaemic heart disease, unspecified (I25.9) | 1.94 (1.88, 2.01) | <0.001a |
| Congestive heart failure (I50.0) | 1.91 (1.77, 2.05) | <0.001a |
| Acute lymphoblastic leukaemia [ALL] (C91.0) | 1.89 (1.07, 3.34) | 0.029 |
| Diffuse large B-cell lymphoma (C83.3) | 1.84 (1.24, 2.73) | 0.002 |
| Acute ischaemic heart disease, unspecified (I24.9) | 1.84 (1.36, 2.5) | <0.001 |
| Unspecified diabetes mellitus—with ketoacidosis (E14.1) | 1.83 (1.03, 3.26) | 0.039 |
| Surgical procedure, unspecified (Y83.9) | 1.81 (1.06, 3.08) | 0.029 |
| Pneumoconiosis due to asbestos and other mineral fibres (J61) | 1.81 (1.16, 2.8) | 0.008 |
| Alcoholic cirrhosis of liver (K70.3) | 1.80 (1.29, 2.51) | <0.001 |
| Sepsis, unspecified (A41.9) | 1.80 (1.58, 2.04) | <0.001a |
| Calculus of gallbladder without cholecystitis (K80.2) | 1.78 (1.39, 2.29) | <0.001a |
| Peripheral vascular disease, unspecified (I73.9) | 1.76 (1.57, 1.96) | <0.001a |
| Sepsis due to other Gram-negative organisms (A41.5) | 1.74 (1.12, 2.71) | 0.014 |
| Cerebral infarction due to embolism of cerebral arteries (I63.4) | 1.74 (1.14, 2.65) | 0.01 |
| Cholangitis (K83.0) | 1.71 (1.34, 2.19) | <0.001a |
| Unspecified diabetes mellitus—with peripheral circulatory complications (E14.5) | 1.67 (1.33, 2.09) | <0.001a |
| Interstitial pulmonary disease, unspecified (J84.9) | 1.65 (1.24, 2.18) | <0.001 |
| Malignant neoplasm, primary site unknown, so stated (C80.0) | 1.62 (1.34, 1.95) | <0.001a |
| Other forms of chronic ischaemic heart disease (I25.8) | 1.61 (1.06, 2.44) | 0.025 |
| Perforation of intestine (nontraumatic) (K63.1) | 1.58 (1.28, 1.96) | <0.001 |
| Acute vascular disorders of intestine (K55.0) | 1.58 (1.25, 1.98) | <0.001 |
| Gastroenteritis and colitis of unspecified origin (A09.9) | 1.57 (1.16, 2.13) | 0.003 |
| Unspecified fall—school, other institution and public administrative area (W19.2) | 1.57 (1.04, 2.37) | 0.033 |
| Acute cholecystitis (K81.0) | 1.56 (1.07, 2.26) | 0.021 |
| Ulcer of lower limb, not elsewhere classified (L97) | 1.54 (1.21, 1.95) | <0.001 |
| Pneumonia, unspecified (J18.9) | 1.53 (1.46, 1.61) | <0.001a |
| Intracranial haemorrhage (nontraumatic), unspecified (I62.9) | 1.51 (1.2, 1.9) | <0.001 |
| Multiple myeloma (C90.0) | 1.51 (1.33, 1.72) | <0.001a |
| Subdural haemorrhage (acute)(nontraumatic) (I62.0) | 1.49 (1.2, 1.86) | <0.001 |
| Chronic kidney disease (N18.0) | 1.48 (1.02, 2.15) | 0.041 |
| Unspecified fall—Home (W19.0) | 1.45 (1.25, 1.7) | <0.001a |
| Exposure to unspecified factor—Home (X59.0) | 1.45 (1.23, 1.71) | <0.001a |
| Unspecified fall—unspecified place (W19.9) | 1.42 (1.08, 1.87) | 0.013 |
| Left ventricular failure (I50.1) | 1.38 (1.18, 1.62) | <0.001 |
| Unspecified acute lower respiratory infection (J22) | 1.38 (1.24, 1.54) | <0.001a |
| Other and unspecified cirrhosis of liver (K74.6) | 1.38 (1.13, 1.69) | 0.002 |
| Cardiovascular disease, unspecified (I51.6) | 1.36 (1.01, 1.82) | 0.04 |
| Intracerebral haemorrhage, unspecified (I61.9) | 1.35 (1.23, 1.49) | <0.001a |
| Hypertensive heart disease without (congestive) heart failure (I11.9) | 1.32 (1.09, 1.61) | 0.005 |
| Acute renal failure, unspecified (N17.9) | 1.30 (1.04, 1.62) | 0.022 |
| Other interstitial pulmonary diseases with fibrosis (J84.1) | 1.29 (1.16, 1.42) | <0.001a |
| Cerebral infarction, unspecified (I63.9) | 1.27 (1.16, 1.38) | <0.001a |
| Acute myeloblastic leukaemia [AML] (C92.0) | 1.26 (1.08, 1.47) | 0.003 |
| Chronic obstructive pulmonary disease, unspecified (J44.9) | 1.25 (1.19, 1.31) | <0.001a |
| Rheumatoid arthritis, unspecified (M06.9) | 1.24 (1.01, 1.53) | 0.04 |
| Chronic lymphocytic leukaemia of B-cell type (C91.1) | 1.22 (1.01, 1.48) | 0.035 |
| Enterocolitis due to Clostridium difficile (A04.7) | 1.20 (1.04, 1.38) | 0.012 |
| Malignant neoplasm of rectosigmoid junction (C19) | 1.19 (1, 1.42) | 0.05 |
| Malignant neoplasm: bronchus or lung, unspecified (C34.9) | 1.10 (1.06, 1.15) | <0.001a |
| Stroke, not specified as haemorrhage or infarction (I64) | 1.08 (1.05, 1.12) | <0.001a |
| Acute myocardial infarction, unspecified (I21.9) | 1.08 (1.05, 1.12) | <0.001a |
a P-values satisfied adjusted threshold of 2.039 × 10−5.
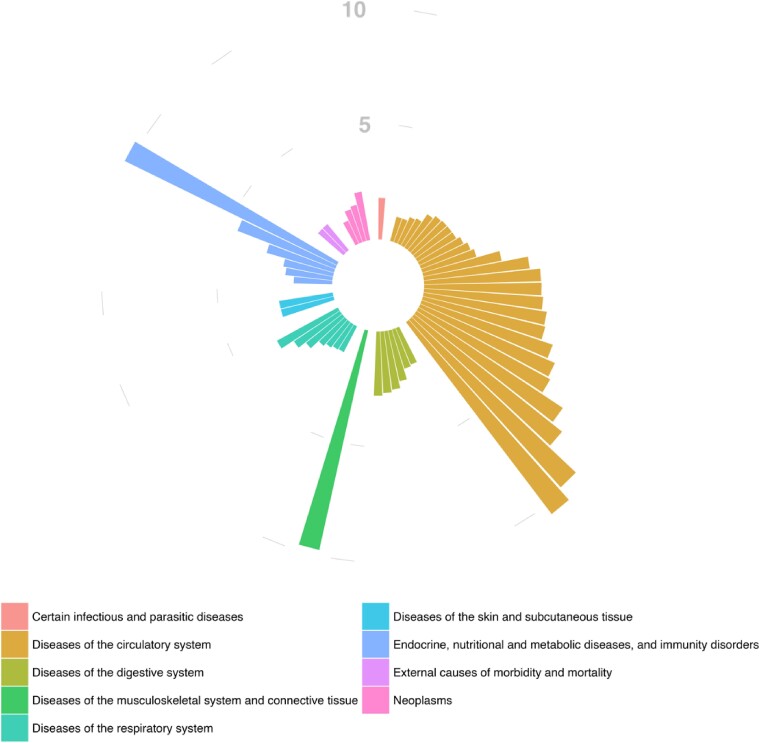
A total of 55 causes of death satisfied the criteria for the adjustment for multiple comparisons and were significantly more common in individuals with AF vs. non-AF (Figure 3, Table 4). Most of these causes of death were categorized under the diseases of circulatory system chapter (see Supplementary material online, Figures S3 and S4). Cardiovascular disorders associated with SCD like ‘dilated cardiomyopathy’, ‘other hypertrophic cardiomyopathy’, ‘cardiomyopathy unspecified’, and ‘ischaemic cardiomyopathy’ were more frequently causes of death in patients with AF (all with ORs >5, and meeting the adjusted P threshold for multiple comparisons). Besides the circulatory system conditions, other significant causes contributed to substantial odds of deaths in patients with AF, namely, ‘other obesity’ and ‘other forms of systemic sclerosis conditions’, contained the second and third largest ORs meeting the adjusted P-value threshold.
Figure 3.
Iris plot of odds ratios for the primary causes of death that met the adjusted P value for multiple comparisons.
AF females vs. AF males
Sudden cardiac death occurred more frequently in males (OR = 1.87, 95% CI: 1.80–1.93, P < 0.0001; Figure 2B). The excess of SCD mortality in males with AF was observed across different age strata (Figure 1), and also for setting, with SCD in the community (OR = 1.64, 95% CI: 1.57–1.71, P < 0.0001), and SCD occurring in hospital (OR = 1.26, 95% CI: 1.02–1.55, P = 0.0339) being more frequent in males. Time-to-event analysis using hazard ratios confirmed an increased risk of SCD in males with AF (unadjusted hazard ratio = 1.68, 95% CI: 1.62–1.74, and hazard ratio adjusted for age = 1.60, 95% CI: 1.53–1.65).
Among patients with AF, females experienced higher all-cause mortality than males (OR = 1.44, 95% CI: 1.41–1.46, P < 0.0001). Comparisons of cause of death by organ system or disease group, as assessed by ICD-10 chapters, showed that females with AF died more often of diseases of the circulatory (OR = 1.32, 95% CI: 1.29–1.35, P < 0.0001), respiratory (OR = 1.15, 95% CI: 1.11–1.19, P < 0.0001), digestive (OR = 1.40, 95% CI: 1.33–1.49, P < 0.0001) and genitourinary system (OR = 1.43, 95% CI: 1.33–1.55, P < 0.0001), and mental and behavioural disorders (OR = 1.84, 95% CI: 1.74–1.94, P < 0.0001), and less often of neoplastic disorders (OR = 0.71, 95% CI: 0.69–0.74, P < 0.0001) when compared with males (see Supplementary material online, Table S2).
A detailed analysis of cause of mortality in females with AF compared with males is presented in Supplementary material online, Table S3, with 33 causes of death meeting the adjusted P-value cut-off for multiple comparisons. Females with AF died more often than males of ‘stroke, not specified as haemorrhage or infarction’ (OR = 2.04, 95% CI: 1.90–2.16, P < 0.0001), ‘unspecified dementia’ (OR = 2.02, 95% CI: 1.90–2.16, P < 0.0001), ‘bronchopneumonia, unspecified’ (OR = 1.60, 95% CI: 1.49, 1.71, P < 0.0001), ‘congestive heart failure’ (OR = 1.61, 95% CI: 1.47–1.75, P < 0.0001), ‘heart failure, unspecified’ (OR = 1.37, 95% CI: 1.16–1.59, P < 0.0001), ‘hypertensive heart disease with congestive heart failure’ (OR = 1.37, 95% CI: 1.15–1.61, P < 0.0001), ‘aortic valve stenosis’ (OR = 1.43, 95% CI: 1.27–1.62, P < 0.0001), ‘mitral valve disease, unspecified’ (OR = 2.63, 95% CI: 1.98–3.49, P < 0.0001), ‘endocarditis, valve unspecified’ (OR = 1.81, 95% CI: 1.45–2.24, P < 0.0001), ‘bronchiectasis’ (OR = 1.72, 95% CI: 1.39–2.13, P < 0.0001), and ‘cellulitis’ (OR = 1.78, 95% CI: 1.38–2.31, P < 0.0001), among others.
Discussion
Utilizing nationwide UK electronic health records, our principal findings are as follows: (i) there was a 3.38-fold higher odds of SCD in patients with AF, contributing to over 10% of all deaths in this patient group; (ii) conditions of the circulatory system (ischaemic heart disease, stroke, and acute myocardial infarction being the most frequent primary diagnoses in death certificates) were the main driver of mortality in the AF population, causing over a third of all deaths, while neoplastic disorders and conditions of the respiratory system were the second and third main causes of mortality, causing a fifth and a sixth of mortality, respectively; (iii) ischaemic heart disease, stroke, and acute myocardial infarction were the most frequent primary diagnoses in death certificates. Important differences between females and males with AF were observed, with SCD being nearly twice as frequent in males, whilst females with AF tend to die more of diseases of the circulatory, respiratory, digestive, and genitourinary system, as well as more due to mental and behavioural disorders, and less often due to neoplastic disorders when compared to males.
Our findings of increased risk of cardiovascular mortality and SCD among individuals with AF, are also consistent with reports from Taiwan.23 Lee et al.4 using nationwide data from Korea assessed mortality in 15 411 patients with AF and differences in males and females. Similarly to our study, Lee and colleagues described a higher mortality from diseases of the circulatory system in the AF population. A subsequent analysis of the Korean cohort showed a 3-fold higher risk of SCD in patients with AF but observed no sex interaction for that outcome.24
Kim et al.25 utilizing the Korean National Health Insurance Service database suggested that patients with AF were more likely to experience ventricular fibrillation, ventricular tachycardia and ventricular flutter. This aligns with our findings, with ‘ventricular fibrillation or flutter’ and ‘ventricular tachycardia’ being observed more frequently as primary causes of death in patients with AF (OR = 18 and OR = 8, respectively).
A systematic review of 30 cohort studies had previously shown a higher-risk of all-cause and cardiovascular mortality in females with AF.26 Our study reproduced these findings and provided further detail on the specific causes that affect females with AF more frequently than males: congestive heart failure, stroke, vascular dementia, aortic stenosis, and endocarditis. On the other hand, we observed a lower rate of SCD in females with AF, which merits further investigation. Despite previous suggestions of hormonal protection genetic and lifestyle factors, lower incidence of ischaemic heart disease, differences in cardiac structure, function or electrical activity, or earlier and more frequent healthcare utilization,27–30 as protective factors for SCD in females, a definitive answer is still warranted. Interestingly, females in our sample were less frequently smokers or ex-smokers and had lower prevalence of ischaemic heart disease at baseline, which corroborates some of the abovementioned hypotheses.
Waldmann et al.31 suggested that coronary heart disease and heart failure were the two most frequent substrates for SCD in the AF population. In our study, we observed higher mortality from cardiovascular diseases in females with AF, with comparable mortality for ischaemic heart disease vs. males, and higher mortality from congestive heart failure. Nevertheless, interpretation of these observations is complex and must be seen at light of competing risk: it is possible that males may die of SCD in earlier stages of heart failure and hence some do not evolve into more advanced stages or live long enough to die of pump failure. On the other hand, females are more likely to die during a congestive heart failure admission and hence do not live long enough to experience a SCD event.
The findings of the current study reinforce the need for aggressive cardiovascular risk factor control and management to tackle mortality in the AF population. Primary prevention efforts, such as strict blood pressure and cholesterol management, as well as the encouragement of lifestyle changes like quitting smoking and drinking, may contribute to a decrease the mortality. The increased risk of SCD in the AF population prompts the need for improvement of risk stratification in this patient group and further research to understand which patients are more likely to experience SCD and whether or not any intervention may lower this risk.
Indeed, current guidelines globally have advocated a more holistic or integrated care approach to AF management,32 given the improved outcomes seen with adherence to such an approach, including lower mortality.33
Strengths and limitations
Strengths of our investigation include a broad assessment of the top primary causes of death, and investigation of SCD with a previously validated definition in a large population representative of the UK.20 Our modified P-value threshold with adjustment for multiple comparisons reduced the chances of type I error.
However, the study limitations need to be highlighted. First, the utilization of ICD diagnosis codes for causes of death may fail to capture the complexity of events in some cases, and some variability may occur depending on the coding physician. Thus, some causes of death might be underestimated. Despite this, existing studies had proven the validation of the use of the terminology and phenotypes.3,21,34 Second, our study might not be able to assess all potential risk factors or diseases, but we attempted to minimize possible confounding by matching patients with AF with non-AF controls based on age, sex, and GP practice. Third, despite adjusting for age, we cannot rule out that age differences and survival bias may have played a role in some of the observed differences (e.g. was SCD observed more often in males because the women who would experience SCD died before they developed AF and/or entered the study?). Fourth, we did not perform a competing risk analysis and, as such, our findings need to be interpreted with caution. When assessing causes of death, it is accepted that if patients die from one cause, they are no longer at risk of dying from a different cause, thereby influencing the observed results for other causes of death. This might explain why for some observed associations, an OR of less than 1.0, suggesting that fewer patients with AF died of a specific cause, may in fact be a result of patients having died from a different cause instead, as reflected by a distinct ICD-10 code. Therefore, the presence of an OR less than 1.0 may not necessarily indicate a lower risk and can be a result of the competing risks not accounted for in the analysis. Fifth, for a broader scoping of the causes of mortality in patients with AF, we utilized an approach analogous to genome-wide association studies. Specifically, we conducted 2432 binary logistic regressions, one for each of the identified primary causes of death. Notably, we also performed two time-to-event analyses, and the resulting hazard ratios were consistent with the findings from the binary logistic regressions. Finally, this study does not allow to infer causality for the association between AF and SCD. It remains to be clarified if AF is driving SCD or if it reflects other comorbidities that associate with SCD (e.g. myocardial infarction and heart failure). Future studies utilizing Mendelian randomization may address this knowledge gap.
Conclusion
Conditions of the circulatory system are the main driver of mortality in the AF population and cause over a third of all deaths. Females with AF experience higher cardiovascular and respiratory mortality but die less frequently of neoplasms.
The risk of SCD is higher in the AF population, with 10% of patients with AF expected to suffer from SCD. Sudden cardiac death is nearly twice as frequent in males with AF.
Supplementary Material
Contributor Information
Yongtong Lai, Institute of Health Informatics Research, University College London, 222 Euston Road, London NW1 2DA, UK.
Hiroyuki Yoshimura, Institute of Health Informatics Research, University College London, 222 Euston Road, London NW1 2DA, UK.
Nadine Zakkak, Institute of Epidemiology and Health Care, University College London, 1-19 Torrington Pl, London WC1E 7HB, UK; Cancer Intelligence, Cancer Research UK, 2 Redman Place, London E20 1JQ, UK.
Eloi Marijon, Division of Cardiology, European Georges Pompidou Hospital, 20 Rue Leblanc, 75015 Paris, France.
Anwar Chahal, Department of Cardiovascular Medicine, Mayo Clinic, 200 First St. SW, Rochester, MN 55905, USA; Center for Inherited Cardiovascular Diseases, WellSpan Health, 30 Monument Rd, York, PA 17403, USA; Barts Heart Centre, St Bartholomew's Hospital, Barts Health NHS Trust, West Smithfield, London EC1A 7BE, UK.
Gregory Y H Lip, Liverpool Centre for Cardiovascular Science at University of Liverpool, Liverpool John Moores University and Liverpool Heart and Chest Hospital, Thomas Drive, Liverpool L14 3PE, UK; Danish Center for Health Services Research, Department of Clinical Medicine, Aalborg University, Aalborg University Hospital, Selma Lagerløfs Vej 249, 9260 Gistrup, Denmark.
Floriaan Schmidt, Institute of Cardiovascular Science, University College London, 62 Huntley St, London WC1E 6DD, UK; Department of Cardiology, Amsterdam Cardiovascular Sciences, Amsterdam University Medical Center, University of Amsterdam, Meibergdreef 9, 1105 AZ Amsterdam, The Netherlands.
Rui Providencia, Institute of Health Informatics Research, University College London, 222 Euston Road, London NW1 2DA, UK; Barts Heart Centre, St Bartholomew's Hospital, Barts Health NHS Trust, West Smithfield, London EC1A 7BE, UK.
Data availability
Data for this study were provided by United Kingdom’s Medicines and Healthcare products Regulatory Agency following approval by the Independent Scientific Advisory Committee [17_205], and can be made available to other researchers following application via the Clinical Practice Research Datalink (CPRD) website (https://www.cprd.com/).
Supplementary material
Supplementary material is available at European Heart Journal Open online.
Funding
R.P. is supported by the University College London British Heart Foundation Research Accelerator AA/18/6/34223, National Institute for Health and Care Research grant NIHR129463 and UK Research and Innovation/European Research Council/HORIZON 10103153 ARISTOTELES.
References
- 1. Cheng S, He JZ, Han Y, Han S, Li P, Liao H, Guo J. Global burden of atrial fibrillation/atrial flutter and its attributable risk factors from 1990 to 2021. EP Europace 2024;26:euae195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Odutayo A, Wong CX, Hsiao AJ, Hopewell S, Altman DG, Emdin CA. Atrial fibrillation and risks of cardiovascular disease, renal disease, and death: systematic review and meta-analysis. BMJ 2016;354:i4482. [DOI] [PubMed] [Google Scholar]
- 3. Chung SC, Sofat R, Acosta-Mena D, Taylor JA, Lambiase PD, Casas JP, Providencia R. Atrial fibrillation epidemiology, disparity and healthcare contacts: a population-wide study of 5.6 million individuals. Lancet Reg Health Eur 2021;7:100157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lee E, Choi EK, Han KD, Lee H, Choe WS, Lee SR, Cha MJ, Lim WH, Kim YJ, Oh S. Mortality and causes of death in patients with atrial fibrillation: a nationwide population-based study. PLoS One 2018;13:e0209687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wolf PA, Dawber TR, Thomas HE Jr, Kannel WB. Epidemiologic assessment of chronic atrial fibrillation and risk of stroke: the Framingham Study. Neurology 1978;28:973–977. [DOI] [PubMed] [Google Scholar]
- 6. Marijon E, Le Heuzey JY, Connolly S, Yang S, Pogue J, Brueckmann M, Eikelboom J, Themeles E, Ezekowitz M, Wallentin L, Yusuf S; RE-LY Investigators . Causes of death and influencing factors in patients with atrial fibrillation: a competing-risk analysis from the randomized evaluation of long-term anticoagulant therapy study. Circulation 2013;128:2192–2201. [DOI] [PubMed] [Google Scholar]
- 7. Zipes DP, Wellens HJJ. Sudden cardiac death. Circulation 1998;98:2334–2351. [DOI] [PubMed] [Google Scholar]
- 8. Wong CX, Brown A, Lau DH, Chugh SS, Albert CM, Kalman JM, Sanders P. Epidemiology of sudden cardiac death: global and regional perspectives. Heart Lung Circ 2019;28:6–14. [DOI] [PubMed] [Google Scholar]
- 9. Department of Health . The National Service Framework (NSF) on Coronary Artery Disease. London: DH: 2005. Arrhythmias and sudden cardiac death. Chapter 8. Available at: https://www.c-r-y.org.uk/wp-content/uploads/2016/10/NSF-Chapter-8-1.pdf, last accessed 13th July 2024
- 10. National Health Service . Arrhythmias. 2021. Available at: https://www.nhs.uk/conditions/arrhythmia/, last accessed 13th July 2024 [Google Scholar]
- 11. Benjamin EJ, Wolf PA, D'Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation 1998;98:946–952. [DOI] [PubMed] [Google Scholar]
- 12. Teppo K, Airaksinen KEJ, Jaakkola J, Halminen O, Salmela B, Kouki E, Haukka J, Putaala J, Linna M, Aro AL, Mustonen P, Hartikainen J, Lip GYH, Lehto M. Ischaemic stroke in women with atrial fibrillation: temporal trends and clinical implications. Eur Heart J 2024;45:1819–1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lynge TH, Risgaard B, Banner J, Nielsen JL, Jespersen T, Stampe NK, Albert CM, Winkel BG, Tfelt-Hansen J. Nationwide burden of sudden cardiac death: a study of 54,028 deaths in Denmark. Heart Rhythm 2021;18:1657–1665. [DOI] [PubMed] [Google Scholar]
- 14. Gallagher AM, Dedman D, Padmanabhan S, Leufkens HGM, de Vries F. The accuracy of date of death recording in the clinical practice research datalink GOLD database in England compared with the office for national statistics death registrations. Pharmacoepidemiol Drug Saf 2019;28:563–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Herrett E, Thomas SL, Schoonen WM, Smeeth L, Hall AJ. Validation and validity of diagnoses in the general practice research database: a systematic review. Br J Clin Pharmacol 2010;69:4–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Padmanabhan S, Carty L, Cameron E, Ghosh RE, Williams R, Strongman H. Approach to record linkage of primary care data from clinical practice research datalink to other health-related patient data: overview and implications. Eur J Epidemiol 2019;34:91–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lewer D. Exact matching in R. NHS-R Community. 2019. Available at: https://nhsrcommunity.com/exact-matching-in-r/, last accessed 18th August 2024
- 18. Denaxas S, Gonzalez-Izquierdo A, Direk K, Fitzpatrick NK, Fatemifar G, Banerjee A, Dobson RJB, Howe LJ, Kuan V, Lumbers RT, Pasea L, Patel RS, Shah AD, Hingorani AD, Sudlow C, Hemingway H. UK phenomics platform for developing and validating electronic health record phenotypes: cALIBER. J Am Med Inform Assoc 2019;26:1545–1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Health Data Research UK . HDR UK Phenotype Library. 2024. available at: https://phenotypes.healthdatagateway.org, last accessed: 18th August 2024
- 20. Morley KI, Wallace J, Denaxas SC, Hunter RJ, Patel RS, Perel P, Shah AD, Timmis AD, Schilling RJ, Hemingway H. Defining disease phenotypes using national linked electronic health records: a case study of atrial fibrillation. PLoS One 2014;9:e110900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Whiteley WN, Ip S, Cooper JA, Bolton T, Keene S, Walker V, Denholm R, Akbari A, Omigie E, Hollings S, Di Angelantonio E, Denaxas S, Wood A, Sterne JAC, Sudlow C; CVD-COVID-UK consortium . Association of COVID-19 vaccines ChAdOx1 and BNT162b2 with major venous, arterial, or thrombocytopenic events: a population-based cohort study of 46 million adults in England. PLoS Med 2022;19:e1003926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chung SC, Schmit AF, Lip GYH, Providencia R. Electronic health record-wide association study for atrial fibrillation in a British cohort. Front Cardiovasc Med 2023;10:1204892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chao TF, Liu CJ, Tuan TC, Chen SJ, Chen TJ, Lip GYH, Chen SA. Risk and prediction of sudden cardiac death and ventricular arrhythmias for patients with atrial fibrillation—a nationwide cohort study. Sci Rep 2017;7:46445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kim YG, Jeong JH, Han KD, Roh SY, Lee HS, Choi YY, Shim J, Kim YH, Choi JI. Atrial fibrillation and risk of sudden cardiac arrest in young adults. Europace 2024;26:euae196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kim YG, Choi YY, Han KD, Min K, Choi HY, Shim J, Choi JI, Kim YH. Atrial fibrillation is associated with increased risk of lethal ventricular arrhythmias. Sci Rep 2021;11:18111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Emdin CA, Wong CX, Hsiao AJ, Altman DG, Peters SA, Woodward M, Odutayo AA. Atrial fibrillation as risk factor for cardiovascular disease and death in women compared with men: systematic review and meta-analysis of cohort studies. BMJ 2016;532:h7013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mendelsohn ME, Karas RH. Molecular and cellular basis of cardiovascular gender differences. Science 2005;308:1583–1587. [DOI] [PubMed] [Google Scholar]
- 28. Garcia M, Mulvagh SK, Merz CNB, Buring JE, Manson JE. Cardiovascular disease in women: clinical perspectives. Circ Res 2016;118:1273–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mosca L, Benjamin EJ, Berra K, Bezanson JL, Dolor RJ, Lloyd-Jones DM, Newby LK, Piña IL, Roger VL, Shaw LJ, Zhao D, Beckie TM, Bushnell C, D'Armiento J, Kris-Etherton PM, Fang J, Ganiats TG, Gomes AS, Gracia CR, Haan CK, Jackson EA, Judelson DR, Kelepouris E, Lavie CJ, Moore A, Nussmeier NA, Ofili E, Oparil S, Ouyang P, Pinn VW, Sherif K, Smith SC Jr, Sopko G, Chandra-Strobos N, Urbina EM, Vaccarino V, Wenger NK; American Heart Association . Effectiveness-based guidelines for the prevention of cardiovascular disease in women–2011 update: a guideline from the American Heart Association. J Am Coll Cardiol 2011;57:1404–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Marijon E, Bougouin W, Celermajer DS, Périer MC, Dumas F, Benameur N, Karam N, Lamhaut L, Tafflet M, Mustafic H, de Deus NM, Le Heuzey JY, Desnos M, Avillach P, Spaulding C, Cariou A, Prugger C, Empana JP, Jouven X. Characteristics and outcomes of sudden cardiac arrest during sports in women. Circ Arrhythm Electrophysiol 2013;6:1185–1191. [DOI] [PubMed] [Google Scholar]
- 31. Waldmann V, Jouven X, Narayanan K, Piot O, Chugh SS, Albert CM, Marijon E. Association between atrial fibrillation and sudden cardiac death: pathophysiological and epidemiological insights. Circ Res 2020;127:301–309. [DOI] [PubMed] [Google Scholar]
- 32. Writing Committee Members; Joglar JA, Chung MK, Armbruster AL, Benjamin EJ, Chyou JY, Cronin EM, Deswal A, Eckhardt LL, Goldberger ZD, Gopinathannair R, Gorenek B, Hess PL, Hlatky M, Hogan G, Ibeh C, Indik JH, Kido K, Kusumoto F, Link MS, Linta KT, Marcus GM, McCarthy PM, Patel N, Patton KK, Perez MV, Piccini JP, Russo AM, Sanders P, Streur MM, Thomas KL, Times S, Tisdale JE, Valente AM, Van Wagoner DR. 2023 ACC/AHA/ACCP/HRS guideline for the diagnosis and management of atrial fibrillation: a report of the American College of Cardiology/American Heart Association joint committee on clinical practice guidelines. J Am Coll Cardiol 2024;83:109–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Romiti GF, Guo Y, Corica B, Proietti M, Zhang H, Lip GYH; mAF-App II trial investigators . Mobile health-technology-integrated care for atrial fibrillation: a win ratio analysis from the mAFA-II randomized clinical trial. Thromb Haemost 2023;123:1042–1048. [DOI] [PubMed] [Google Scholar]
- 34. Bell S, Daskalopoulou M, Rapsomaniki E, George J, Britton A, Bobak M, Casas JP, Dale CE, Denaxas S, Shah AD, Hemingway H. Association between clinically recorded alcohol consumption and initial presentation of 12 cardiovascular diseases: population based cohort study using linked health records. BMJ 2017;356:j909. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data for this study were provided by United Kingdom’s Medicines and Healthcare products Regulatory Agency following approval by the Independent Scientific Advisory Committee [17_205], and can be made available to other researchers following application via the Clinical Practice Research Datalink (CPRD) website (https://www.cprd.com/).