Abstract
Background
Premenopausal risk-reducing salpingo-oophorectomy (RRSO) in women at high familial risk of ovarian cancer leads to immediate menopause. Although early natural menopause is associated with increased cardiovascular disease risk, evidence on long-term cardiovascular disease risk after early surgical menopause is scarce.
Objectives
We sought to determine the long-term influence of the timing of RRSO on the development of coronary artery calcium (CAC), an established marker for cardiovascular disease risk.
Methods
We conducted a cross-sectional study (N = 733) nested in a nationwide cohort of women at high familial risk of ovarian cancer. In women aged 60-70 years (n = 328), we compared CAC scores between women with a premenopausal RRSO (age ≤45 years) and women with a postmenopausal RRSO (age ≥54 years), using multivariable Poisson analyses. Within the premenopausal RRSO group (n = 498), we also examined the effect of age at RRSO. In addition, we compared the premenopausal RRSO group with an external reference cohort (n = 5,226).
Results
Multivariable analyses showed that the prevalence rates of any CAC (CAC >0), at least moderate CAC (CAC >100), and severe CAC (CAC >400) were comparable between the premenopausal and postmenopausal RRSO groups (relative risk [RR]: 0.93; 95% CI: 0.75-1.15 for any CAC; RR: 0.71; 95% CI: 0.43-1.17 for at least moderate CAC; RR: 0.81; 95% CI: 0.30-2.13 for severe CAC). There was no difference in CAC between the premenopausal RRSO group and a similar aged reference cohort. Timing of premenopausal RRSO (early premenopausal RRSO [<41 years] vs late premenopausal RRSO [41-45 years]) did not affect the outcomes.
Conclusions
Our results do not show a long-term adverse effect of surgical menopause on the development of CAC.
Key Words: BRCA, CAC, cardiovascular disease, ovarian cancer, RRSO, surgical menopause
Central Illustration
Available screening methods for early detection of ovarian cancer remain ineffective.1 Therefore, current guidelines for women at high familial risk for ovarian cancer, such as carriers of BRCA1/2 germline pathogenic variants (GPV), recommend risk-reducing salpingo-oophorectomy (RRSO) to prevent ovarian cancer. RRSO is advised after completion of childbearing, ideally between the ages of 35 and 40 years for BRCA1 GPV carriers, and between 40 and 45 years for BRCA2 GPV carriers.2 Although RRSO reduces the risk of ovarian cancer by 96%, it also induces early surgical menopause.3,4
Early menopause (≤45 years) has been associated with various long-term adverse effects, including an increased risk of cardiovascular disease, lowered bone mineral density, reduced quality of life, and cognitive impairment.4 There is ample evidence that early natural menopause increases the risk of cardiovascular disease in later life. Recent studies show especially increased risks of stroke and ischemic heart disease (IHD) after early natural menopause due to premature ovarian insufficiency (POI).5, 6, 7, 8 This increased risk is commonly attributed to the decreased production of endogenous estrogens.9 However, whether cardiovascular disease risk is similarly increased after surgical menopause has been less frequently investigated, with inconsistent results.5,6,10
Coronary artery calcium (CAC) measured by computed tomography is an established method for assessing individual cardiovascular disease risk in asymptomatic individuals, even at relatively young ages.11, 12, 13, 14, 15 In addition, a recent study showed that CAC is an excellent predictor of cardiovascular disease in asymptomatic postmenopausal women who experienced an early natural menopause.16 However, no studies have yet assessed CAC scores in relation to the timing of surgical menopause.
We aimed to investigate the long-term effect of a premenopausal RRSO (age ≤45 years) on the presence of CAC in a cross-sectional study of 733 women at high familial risk for ovarian cancer. We compared women who underwent a premenopausal RRSO (≤45 years) with women who underwent a postmenopausal RRSO (≥54 years), and we examined the effect of timing of RRSO within the premenopausal group. Additionally, we compared the premenopausal RRSO group with an external reference cohort.
Methods
Study cohort
The HARMOny (Health After eaRly Menopause Due to Oophorectomy) study is a Dutch multicenter cross-sectional study investigating the long-term effects of RRSO on cardiovascular disease, bone health, cognition, and quality of life. The study design of the HARMOny study (NCT03835793) has been described in detail previously and was approved in writing by the Medical Ethics Committee of the Antoni van Leeuwenhoek/Netherlands Cancer Institute (AVL/NKI).17 Women were recruited from the HEBON (Hereditary Breast and Ovarian cancer study Netherlands), a nationwide cohort of women at high familial risk of breast and/or ovarian cancer recruited from all 8 Dutch University Medical Centers and the Netherlands Cancer Institute.18
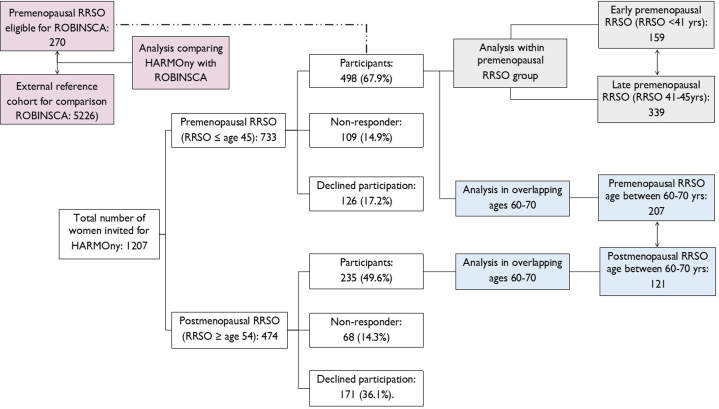
Between 2018 and 2022, 1,207 women were invited to participate in the study: 733 women who underwent a premenopausal RRSO (age ≤45 years) and were at least 55 years old at inclusion, and 474 women who underwent a postmenopausal RRSO (age ≥54 years) (Figure 1). Exclusion criteria included a history of ovarian cancer, age older than 80 years, therapy-induced menopause more than 5 years before RRSO, metastatic disease, or a prior intervention interfering with the assessment of CAC, such as a percutaneous coronary intervention or mechanical cardiac valve. A history of cancer, other than ovarian cancer, was not a reason for exclusion.
Figure 1.
Flowchart of Participation in the HARMOny Study
The colors represent the 3 different statistical comparisons made: blue illustrates coronary artery calcium (CAC) scores of the HARMOny (Health After eaRly Menopause Due to Oophorectomy) premenopausal risk-reducing salpingo-oophorectomy (RRSO) group vs the postmenopausal RRSO group (aged 60-70 years at the study visit); grey shows CAC scores of the HARMOny early premenopausal RRSO group (age ≤41 years) vs the late premenopausal RRSO group (age 41-45 years); and pink compares CAC scores of the premenopausal RRSO group from HARMOny eligible for the ROBINSCA (Risk or Benefit in Screening for Cardiovascular Disease) cohort vs the ROBINSCA external reference cohort (aged 55-70 years at the study visit). This latter comparison is indicated by a dashed line.
External reference cohort ROBINSCA
We used an external reference cohort from the ROBINSCA (Risk or Benefit in Screening for Cardiovascular Disease) study, which was recruited from the Dutch general population in 3 different regions. Eligibility criteria required participants to have no history of cardiovascular disease but at least 1 cardiovascular disease risk factor.19 In the ROBINSCA study, CAC scores and cardiovascular disease risk factors were available for 5,226 women aged 55 to 70 years.
Study assessments
Participation in the HARMOny study involved completing an extensive online questionnaire and attending a clinical visit.17 The questionnaire covered traditional and female-specific cardiovascular disease risk factors, medical history, and medication use, including menopausal hormone therapy (MHT). The clinical visit included a CAC score measurement by computed tomography, blood sampling, and an outpatient clinic visit with a research physician for anthropometric measurements (height, weight, heart rate, blood pressure, and waist and hip circumference).
CAC scores were calculated by experienced cardiovascular radiologists at the participating medical centers using the standardized Agatston scoring method, which is known for its excellent interscanner and interrater reliability.20, 21, 22 Percentiles of the CAC score were determined using the MESA (Multi-Ethnic Study of Atherosclerosis) score.23,24 Blood samples were taken to analyze non-fasting levels of lipids, glucose, HbA1c, high-sensitivity C-reactive protein, and high-sensitivity cardiac troponin. If a participant had undergone radiotherapy for breast cancer, the radiotherapy records were evaluated for internal mammary chain (IMC) irradiation, a known risk factor for IHD.25 According to the study protocol, the results of all measurements were shared with participants, and a letter detailing the results was sent to their general practitioners.
Statistical analyses
Continuous data were presented as the mean ± SD for normally distributed variables and as median with 25th-75th percentiles (Q1-Q3) for skewed distribution. Categorical data were presented as counts with percentages. Characteristics of women in the premenopausal RRSO (age ≤45 years) and postmenopausal RRSO (age ≥54 years) groups were compared using the independent samples t-test or Wilcoxon rank sum test for continuous data, and the Fisher exact test or chi-square test for categorical data. A 2-sided P value of <0.05 was considered statistically significant. Normality of data was assessed using the Shapiro-Wilk test.
According to the HARMOny study protocol, we attempted to match the premenopausal RRSO (age ≤45 years) and postmenopausal RRSO (age ≥54 years) groups on age at the study visit.17 However, during the inclusion period, we observed a substantial age difference (median 10.1 years) between the 2 groups. This age difference was attributed to the change in the 2007 guidelines for the management of ovarian cancer risk in BRCA1/2 GPV carriers, which led to a significant increase in the prevalence of premenopausal RRSO.2 Therefore, in the current study, we restricted the comparison of CAC scores between the premenopausal and postmenopausal RRSO groups to women who were between 60 and 70 years old at the time of the study visit (Figure 1).
In addition, within the entire premenopausal RRSO group, we evaluated CAC scores in women who had an early premenopausal RRSO (age ≤41 years) and those who had a late premenopausal RRSO (age 41-45 years). We performed sensitivity analyses in women with and without MHT use and women without a history of breast cancer. Finally, we compared the CAC scores of women in our premenopausal RRSO group who met the eligibility criteria for ROBINSCA with the CAC scores of similarly aged women in the ROBINSCA study.
To evaluate whether the timing of premenopausal RRSO affects CAC scores later in life, we estimated relative risks (RRs) with 95% CIs for various CAC score cutoff points using multivariable Poisson regression analysis. The assumptions of the Poisson model were assessed through the deviance and Pearson goodness-of-fit tests. The outcome categories analyzed were any CAC (CAC >0), at least moderate CAC (CAC >100), and severe CAC (CAC >400).
The variables assessed as possible confounders included age at study entry, current or ever smoking, alcohol use, use of menopausal MHT, history of breast cancer, history of IMC irradiation, body mass index (BMI), diabetes mellitus (defined as the use of antidiabetic medication for type 1 or 2 diabetes), hypertension (defined as the use of antihypertensive medication, a systolic blood pressure >140 mm Hg, or a diastolic blood pressure >90 mm Hg), and dyslipidemia (defined as the use of lipid-lowering medication or LDL cholesterol >4.0 mmol/L). A variable was considered a confounder if the RR estimate for the association of interest was changed by more than 10% when added to the model. All statistical analyses were performed using STATA version 15.1 software (StataCorp).
Results
Participant characteristics of the entire HARMOny study population are provided in Supplemental Table 1.
Participant characteristics of women aged 60 to 70 years at study visit
We included 328 women who were aged 60 to 70 years at the time of the study visit (207 in the premenopausal RRSO group [age ≤45 years] and 121 in the postmenopausal RRSO group [age ≥54 years]). The median age at the study visit was 64.5 years (61.9-67.0 years). The median time since RRSO was 21.0 years in the premenopausal group (18.3-23.3 years) and 10.7 years in the postmenopausal group (9.6-11.9 years) (Table 1). Both groups were comparable in terms of BRCA1/2 GPV carrier status (overall 66.7%) and history of breast cancer (overall 61.8%).
Table 1.
Participant Characteristics
| Age 60-70 y at Study Visit (n = 328a) |
Premenopausal RRSO (n = 498a) |
|||||
|---|---|---|---|---|---|---|
| Premenopausal RRSO, Age ≤45 y (n = 207) |
Postmenopausal RRSO, Age ≥54 y (n = 121) |
P Value | Early Premenopausal RRSO, Age ≤41 y (n = 159) |
Late Premenopausal RRSO, Age 41-45 y (n = 339) |
P Value | |
| Age at study visit, y | 62.4 (61.0-64.4) | 67.2 (65.6-68.5) | <0.001 | 58.8 (57.2-61.6) | 59.0 (57.8-62.3) | 0.033 |
| Time since RRSO, y | 21.0 (18.3-23.3) | 10.7 (9.6-11.9) | <0.001 | 20.9 (19.1-23.3) | 16.6 (14.3-19.5) | <0.001 |
| Age at menopause, y | 42.0 (40.0-44.0) | 51.0 (50.0-54.0) | <0.001 | 39.0 (37.0-40.0) | 43.0 (42.0-44.0) | <0.001 |
| BRCA GPV carrier status | <0.001 | <0.001 | ||||
| BRCA1 | 106 (51.2) | 36 (29.8) | 96 (60.4) | 144 (42.5) | ||
| BRCA2 | 37 (17.9) | 44 (36.4) | 23 (14.5) | 73 (21.5) | ||
| Noncarrier | 64 (30.9) | 41 (33.9) | 40 (25.2) | 122 (36.0) | ||
| MHT use | 61 (29.5) | 8 (6.6) | <0.001 | 74 (46.5) | 68 (20.1) | <0.001 |
| Breast cancer history | 126 (60.9) | 71 (58.7) | 0.70 | 81 (50.9) | 217 (64.0) | 0.006 |
| Chemotherapy | 90 (43.5) | 45 (37.2) | 0.26 | 57 (37.1) | 170 (50.2) | 0.006 |
| IMC radiotherapy | 18 (8.7) | 3 (2.5) | 0.024 | 11 (6.9) | 31 (9.1) | 0.47 |
| Endocrine therapy | 41 (19.8) | 26 (21.5) | 0.72 | 19 (12.0) | 97 (28.6) | <0.001 |
| Smoking | 0.16 | 0.77 | ||||
| Current smoker | 18 (8.7) | 6 (5.0) | 15 (9.4) | 34 (10.0) | ||
| Former smoker | 108 (52.2) | 56 (46.3) | 62 (39.0) | 142 (41.9) | ||
| Never | 81 (39.1) | 59 (48.8) | 82 (51.6) | 163 (48.1) | ||
| Alcohol >2 drinks daily | 100 (48.3) | 60 (49.6) | 0.82 | 81 (50.9) | 187 (55.2) | 0.38 |
| Family member with MIb | 71 (34.3) | 40 (33.1) | 0.84 | 42 (26.4) | 117 (34.5) | 0.068 |
| BMI, kg/m2 | 25.1 (22.7-28.8) | 25.3 (23.2-28.7) | 0.97 | 24.5 (22.5-29.1) | 25.5 (22.8-29.0) | 0.31 |
| Systolic blood pressure, mm Hg | 135.8 (17.6) | 143.7 (15.9) | <0.001 | 132.7 (17.4) | 134.1 (17.2) | 0.40 |
| Diastolic blood pressure, mm Hg | 77.5 (12.1) | 81.0 (11.5) | 0.011 | 76.4 (11.5) | 77.7 (12.3) | 0.24 |
| Total cholesterol, mmol/L | 5.6 (1.1) | 5.6 (1.4) | 0.72 | 5.6 (1.1) | 5.6 (1.0) | 0.61 |
| LDL cholesterol, mmol/L | 3.3 (1.0) | 3.4 (1.2) | 0.47 | 3.3 (0.9) | 3.3 (0.9) | 0.99 |
| HDL cholesterol, mmol/L | 1.8 (0.4) | 1.8 (0.6) | 0.70 | 1.7 (0.6) | 1.7 (0.4) | 0.93 |
| Antihypertensive medication | 57 (27.5) | 22.3% | 0.30 | 32 (20.1) | 65 (19.2) | 0.80 |
| Lipid-lowering medication | 37 (17.9) | 23 (19.0) | 0.80 | 18 (11.3) | 41 (12.1) | 0.80 |
| Diabetes mellitus, any type | 20 (9.7) | 10 (8.3) | 0.66 | 9 (5.7) | 25 (7.4) | 0.47 |
| Hypertensionc | 110 (53.1) | 79 (65.3) | 0.031 | 66 (41.5) | 155 (45.7) | 0.38 |
| Dyslipidemiad | 81 (39.1) | 59 (48.8) | 0.090 | 61 (38.4) | 120 (35.4) | 0.52 |
| MESA 10-y CHD riske | 2.6 (1.6-6.6) | 3.8 (1.9-7.4) | 0.036 | 2.1 (1.3-4.7) | 2.2 (1.5-4.9) | 0.26 |
| CAC score | 1 (0-74) | 13 (0-136) | 0.088 | 0 (0-28) | 0 (0-39) | 0.26 |
| CAC >0 | 107 (51.7) | 71 (58.7) | 0.22 | 65 (40.9) | 155 (45.7) | 0.31 |
| CAC >100 | 40 (19.3) | 32 (26.5) | 0.13 | 17 (10.7) | 54 (15.9) | 0.12 |
| CAC >400 | 11 (5.3) | 16 (13.2) | 0.015 | 6 (3.8) | 16 (4.7) | 0.63 |
| MESA score | 57 (0-80) | 58 (0-80) | 0.65 | 0 (0-77) | 0 (0-81) | 0.30 |
| MESA >75% | 65 (31.4) | 34 (28.1) | 0.53 | 46 (28.9) | 103 (30.4) | 0.74 |
Values are median (Q1-Q3), n (%). Values that are mean ± SD have their respective measure units described directly after the variable (eg, total cholesterol, mmol/L). The variables are: BMI, systolic blood pressure, diastolic blood pressure, total cholesterol, LDL cholesterol and HDL cholesterol. The P value was calculated using independent samples t test, chi-square test, and Wilcoxon rank sum test.
BMI = body mass index; GPV = pathogenic variant; HDL = high-density lipoprotein; IMC = internal mammary chain; MHT = menopausal hormone therapy; RRSO = risk-reducing salpingo-oophorectomy.
See Figure 1.
Myocardial infarction (MI) first- or second-degree family member before the age of 65 years.
Hypertension defined as either the use of antihypertensive medication, a systolic blood pressure >140 mm Hg, or a diastolic blood pressure >90 mm Hg.
Dyslipidemia defined as either the use of lipid lowering medication or low-density lipoprotein (LDL) cholesterol >4.0 mmol/L.
MESA (Multi-Ethnic Study of Atherosclerosis) estimated 10-year risk of coronary heart disease (CHD) event, including coronary artery calcium (CAC) score.
Compared with the postmenopausal RRSO group, the premenopausal RRSO group had significantly higher rates of IMC radiotherapy (8.7% vs 2.5%) and a more frequent history of MHT use (29.5% vs 6.6%). Hypertension was significantly less prevalent in the premenopausal RRSO group compared with the postmenopausal RRSO group (53.1% vs 65.3%).
Participant characteristics of women with a premenopausal RRSO
Within the entire premenopausal group (n = 498), we compared women who had an early premenopausal RRSO (age ≤41 years) (n = 159) with those who had a late premenopausal RRSO (age 41-45 years) (n = 339). Compared with the late premenopausal RRSO group, women in the early premenopausal RRSO group were significantly more likely to be BRCA1/2 GPV carriers (74.8% vs 64.0%) and were less likely to have a history of breast cancer (50.9% vs 64.0%), chemotherapy (37.1% vs 50.2%), and endocrine therapy (12.0% vs 28.6%).
CAC scores after premenopausal vs postmenopausal RRSO in women aged 60 to 70 years at study visit
Univariable analyses showed a higher prevalence of increased CAC scores in the postmenopausal RRSO (age ≥54 years) group compared with the premenopausal RRSO (age ≤45 years) group. For instance, severe CAC (CAC >400) was observed in 13.2% vs 5.3% of women, respectively. The MESA score was comparable between both groups (median 57; Q1-Q3: 0-80 vs median 58; Q1-Q3: 0-80).
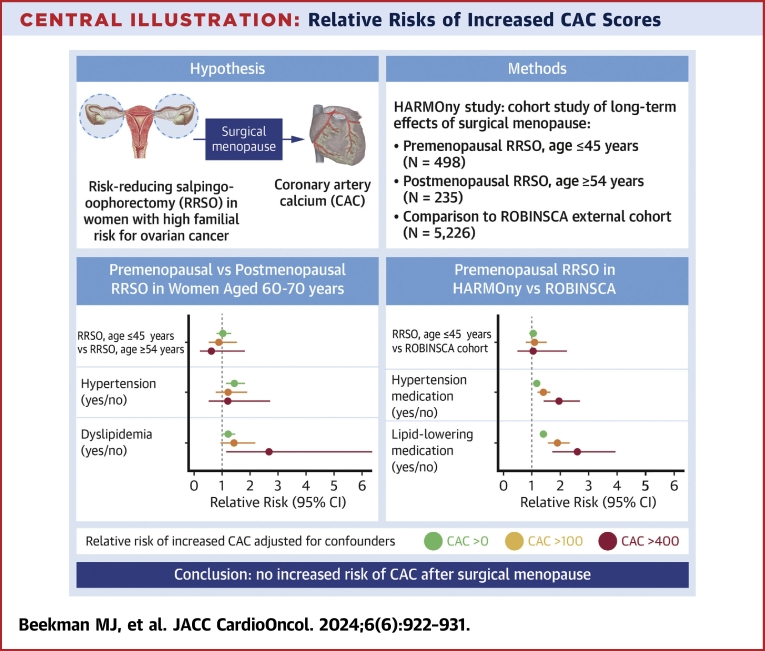
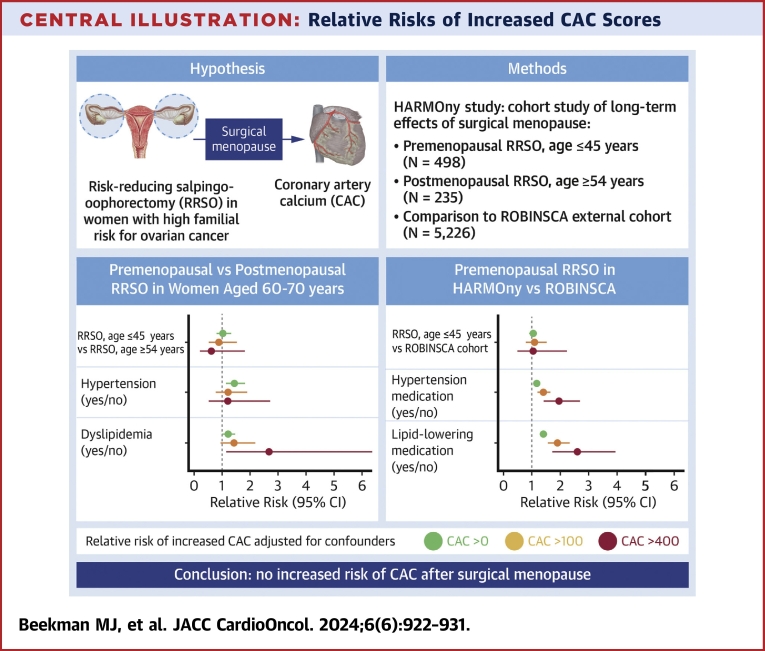
After adjustment for age, hypertension, and dyslipidemia, there was no statistically significant difference in CAC scores between the premenopausal and postmenopausal RRSO groups among women aged 60 to 70 years (Table 2, Central Illustration). The prevalence rates of any CAC (CAC >0), at least moderate CAC (CAC >100), and severe CAC (CAC >400) were not higher in the premenopausal RRSO group compared with the postmenopausal RRSO group (RR: 1.07; 95% CI: 0.83-1.37 for any CAC; RR: 0.89; 95% CI: 0.52-1.52 for CAC >100; RR: 0.61; 95% CI: 0.21-1.74 for CAC >400). The prevalence rates of participants with a MESA percentile score above 75% were also comparable in both groups (RR: 1.13; 95% CI: 0.72-1.80).
Table 2.
RRs of Increased CAC Scores According to Timing of RRSO in Women Aged 60-70 Years
| CAC >0a | CAC >100a | CAC >400a | MESA >75%b | |
|---|---|---|---|---|
| Timing of RRSO | ||||
| Postmenopausal RRSO, age ≥54 y | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| Premenopausal RRSO, age ≤45 y | 1.07 (0.83-1.37) | 0.89 (0.52-1.52) | 0.61 (0.21-1.74) | 1.13 (0.72-1.80) |
| Hypertension | 1.55 (1.23-1.95) | 1.36 (0.88-2.11) | 1.19 (0.54-2.61) | 1.51 (1.05-2.17) |
| Dyslipidemia | 1.21 (0.99-1.46) | 1.48 (0.98-2.24) | 2.80 (1.20-6.52) | 1.52 (1.09-2.11) |
Values are adjusted relative risk (95% CI).
Abbreviations as in Table 1.
Risk of having any, moderate, severe CAC. Relative risks (RRs) were multivariably adjusted for age, hypertension, dyslipidemia, and timing of RRSO.
Risk of having a MESA score above 75%. Relative risk (RRs) were multivariably adjusted for hypertension, dyslipidemia, and timing of RRSO.
Central Illustration.
Relative Risks of Increased CAC Scores
Relative risks of increased coronary artery calcium (CAC) scores according to the timing of risk-reducing salpingo-oophorectomy (RRSO) and other risk factors are shown. After a median follow-up of 21.0 years, women who underwent premenopausal RRSO did not have an elevated risk of increased CAC scores, adjusted for age, hypertension ,and dyslipidemia, when compared with women who underwent postmenopausal RRSO or to the general population. Hypertension and dyslipidemia were independent risk factors for increased CAC scores. HARMOny = Health After eaRly Menopause Due to Oophorectomy.
Participants with hypertension and/or dyslipidemia had significantly higher CAC scores and MESA percentiles compared with women without these risk factors. Including MHT use, current smoking, BMI, history of breast cancer, diabetes mellitus, and IMC radiotherapy in the analyses did not change the outcomes (Supplemental Table S2).
CAC scores according to timing of premenopausal RRSO
The prevalence rates of any CAC, at least moderate CAC, and severe CAC were comparable between the early and late premenopausal groups (RRs adjusted for age, hypertension, and dyslipidemia; RR: 0.93; 95% CI: 0.75-1.15 for any CAC; RR: 0.71; 95% CI: 0.43-1.17 for CAC >100; RR: 0.81; 95% CI: 0.30-2.13 for CAC >400) (Table 3). The prevalence rates of participants with a MESA score above 75% percentile were also comparable between the 2 groups (RR: 0.96; 95% CI: 0.72-1.28). Participants with hypertension and/or dyslipidemia had significantly higher CAC scores and MESA percentiles than those without these risk factors. Including MHT use, current smoking, BMI, history of breast cancer, diabetes mellitus, and IMC radiotherapy in the analyses did not affect the results.
Table 3.
RRs of Increased CAC Scores According to Timing of RRSO in Women With a Premenopausal RRSO
| CAC >0a | CAC >100a | CAC >400a | MESA >75%b | |
|---|---|---|---|---|
| Timing of RRSO | ||||
| Late premenopausal RRSO, age 41-45 y | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| Early premenopausal RRSO, age <41 y | 0.93 (0.75-1.15) | 0.71 (0.43-1.17) | 0.81 (0.34-2.13) | 0.96 (0.72-1.28) |
| Hypertension | 1.43 (1.16-1.75) | 1.33 (0.86-2.06) | 1.30 (0.59-2.84) | 1.42 (1.08-1.86) |
| Dyslipidemia | 1.13 (0.93-1.37) | 1.68 (1.10-2.56) | 4.35 (1.81-10.45) | 1.33 (1.02-1.73) |
Values are adjusted relative risk (95% CI).
Risk of having any, moderate, or severe CAC. RRs were multivariably adjusted for age, hypertension, dyslipidemia, and timing of RRSO.
Risk of having a MESA score above 75%. RRs were multivariably adjusted for hypertension, dyslipidemia, and timing of RRSO.
Sensitivity analyses
Sensitivity analyses conducted in women with and without MHT use (Supplemental Tables 2 to 4) and in women with and without breast cancer (Supplemental Tables 5 and 6) yielded similar results.
CAC scores in the premenopausal RRSO group compared with an external reference cohort
In total, 270 women in the premenopausal RRSO (age ≤45 years) group met the eligibility criteria for the ROBINSCA study (Supplemental Table 7). Women in the premenopausal RRSO group were significantly younger and had a significantly higher BMI and a higher prevalence of any type of diabetes mellitus compared with women in the ROBINSCA study in the same age group (55-70 years; n = 5,226). Other measured cardiovascular disease risk factors were comparable between the 2 groups.
The prevalence rates of increased CAC scores were comparable between the 2 groups for any CAC, CAC >100, and CAC >400. Multivariable Poisson analyses showed no significant differences between the premenopausal RRSO group and the ROBINSCA reference group for the different CAC outcomes (analyses adjusted for age, hypertension medication, and lipid-lowering medication) (Table 4). Including BMI or the prevalence of diabetes mellitus in the analyses did not change the outcomes.
Table 4.
RRs of Increased CAC Scores in the Premenopausal RRSO Group Compared With the ROBINSCA Cohort
| CAC >0a | CAC >100a | CAC >400a | |
|---|---|---|---|
| Timing of RRSO | |||
| ROBINSCA, age 55-70 y | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| Premenopausal RRSO, age ≤45 y | 1.05 (0.92-1.21) | 1.11 (0.80-1.53) | 1.05 (0.50-2.20) |
| Antihypertensive medication | 1.18 (1.11-1.26) | 1.43 (1.23-1.67) | 1.92 (1.41-2.61) |
| Lipid-lowering medication | 1.48 (1.37-1.59) | 2.12 (1.76-2.55) | 2.61 (1.77-3.85) |
Values are adjusted relative risk (95% CI).
ROBINSCA = Risk or Benefit in Screening for Cardiovascular Disease study; other abbreviations as in Tables 1 and 2.
Risk of having any, moderate, or severe CAC. RRs were multivariably adjusted for age, antihypertensive medication, lipid-lowering medication, and timing of RRSO.
Discussion
Twenty-one years after surgical menopause, we did not observe increased CAC scores in women who underwent a premenopausal RRSO (age ≤45 years), either when compared with women who underwent postmenopausal RRSO (age ≥54 years) or with an external reference population. Furthermore, an early premenopausal RRSO (age ≤41 years), compared with a late premenopausal RRSO (age 41-45 years), was not associated with increased CAC scores.
Our nationwide study is the first to investigate CAC scores after premenopausal RRSO in women at high familial risk for ovarian cancer. Studies investigating cardiovascular disease risk after surgical menopause are scarce and inconclusive, primarily due to limited power and methodological issues, such as confounding by indication for surgical menopause.5,6,10,26 The most frequently reported indications for surgical menopause include RRSO, endometriosis, and benign cysts. Endometriosis has been associated with an increased risk of cardiovascular disease, regardless of a history of surgical menopause, while cardiovascular disease risk in women with cysts remains unclear.27,28 However, previous studies did not conduct subgroup analyses specifically among women with RRSO.
Our findings in women who underwent surgical menopause are consistent with a recent smaller study by Van Bommel et al,29 which found no association between time since RRSO and other measures of subclinical atherosclerosis, including pulse wave velocity and carotid intima thickness, in a cohort of women BRCA1/2 GPV carriers. Although surgical menopause does not appear to be associated with CAC, this does not entirely rule out the possibility of increased cardiovascular disease risk through other (less likely) pathways. Two recent studies also showed no differences in the prevalence of increased CAC levels after POI. However, the women included in these studies may have been too young (median ages 49.4 and 50 years, respectively) to detect differences in subclinical atherosclerosis.30, 31, 32
By contrast, 2 recent large meta-analyses convincingly showed increased cardiovascular disease risk after early natural menopause.7,8 Interestingly, a study by Krul et al33 showed no increase in cardiovascular disease risk after early iatrogenic menopause caused by chemotherapy-induced POI in Hodgkin lymphoma survivors. This supports our hypothesis that early natural menopause is associated with increased cardiovascular disease risk, whereas early surgical (or otherwise iatrogenic) menopause is not. This apparent discrepancy may be explained by the reverse causality hypothesis, which postulates that early natural menopause is the result of accelerated vascular aging, leading to a statistical (noncausal) association between early natural menopause and increased cardiovascular disease risk.34
It has been suggested that MHT may protect women against IHD after surgical menopause before the age of 45 years.26, 27, 28, 29, 30, 31, 32, 33,35 Therefore, we considered MHT as a potential confounder in our analyses. However, the prevalence of MHT use was relatively low in our study. Furthermore, we did not find a protective effect of MHT use, neither for ever use nor for the duration of use (Supplemental Tables 2 to 4 and 8 to 10), and MHT use was not a confounder in our analyses.
Strengths and limitations
The strengths of our nationwide study include the large sample size, which provided sufficient power for subgroup analyses, the long-term follow-up after premenopausal RRSO (age ≤45 years) and the use of a comparison group of women who also underwent RRSO, but at a later age. By excluding women who underwent RRSO between the ages of 46 and 54 years, we were able to make a more distinct evaluation of the differences in cardiovascular disease risk between the premenopausal and postmenopausal RRSO groups. Unlike other studies, the comparisons in our study are not affected by confounding due to the indication for bilateral oophorectomy.
Since the current standard of care for women at high familial risk of ovarian cancer is to undergo premenopausal RRSO, almost all women have the surgery before reaching menopause.2 Consequently, our study took advantage of a window of opportunity to recruit a large group of women who had undergone postmenopausal RRSO (age ≥54 years) years earlier. The participation rate of our study was strong (60.7%), considering the relatively long time since RRSO at the time of the study visit. In addition, our outcome measure, CAC, is an established predictor of cardiovascular disease risk in asymptomatic women, independent of other cardiovascular disease risk factors.11, 12, 13, 14 The CAC score has also been shown to be a reliable predictor of cardiovascular disease risk in women with an early menopause (before age 45 years).16
A limitation of our study is the age difference between the premenopausal RRSO (age ≤45 years) and postmenopausal RRSO (age ≥54 years) groups in the entire study population. However, we addressed this limitation by restricting our analysis to women aged 60 to 70 years at study enrollment. In addition, we used the entire premenopausal RRSO group to assess the association between timing of a premenopausal RRSO (age ≤41 years vs 41-45 years) and cardiovascular disease risk. Moreover, we had the unique opportunity to compare the CAC scores of our premenopausal RRSO group with those of similarly aged women in the ROBINSCA general population cohort, showing no differences.
When interpreting our results, it is important to note that 98% of the participants were Caucasian. Another potential limitation of this study is survival bias, as death related to cardiovascular disease after RRSO may have occurred before recruitment into the HARMOny study. Since our study was nested within the HEBON cohort, we had the opportunity to obtain causes of death from Statistics Netherlands for all women who were otherwise eligible for our study but died before possible inclusion.18 Only 1.9% of these women died from a cardiovascular event, whereas the most frequent cause of death was cancer (87.6%).
Selection bias may also have occurred due to differences in response rates between the premenopausal (68.0%) and postmenopausal groups (50.8%). We addressed this potential bias by using previously collected data from questionnaire surveys completed for the HEBON study.18 In these questionnaires, current nonresponders in the postmenopausal RRSO group did not report a lower or higher prevalence of cardiovascular disease than responders.
Conclusions
This study does not support a long-term adverse effect of surgical menopause on the development of CAC, a marker of cardiovascular disease risk. This is an important and reassuring message for women at high familial risk of ovarian cancer and may assist physicians in counseling these women. Our results may also have broader relevance for women who experience iatrogenic menopause after cancer treatment. Future studies should examine the risk of clinical cardiovascular disease after iatrogenic menopause.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: Women at high familial risk for ovarian cancer are recommended to undergo premenopausal RRSO to prevent ovarian cancer. However, data on long-term cardiovascular disease risk after surgical menopause are limited. The current study shows no long-term adverse effect of surgical menopause on the development of CAC. These results provide important and reassuring information for patients and health professionals involved in elective bilateral oophorectomy.
TRANSLATIONAL OUTLOOK: This study adds solid data to the growing body of evidence that surgical menopause does not increase markers of cardiovascular disease risk in women, in contrast to the available literature on early natural menopause. Further research to better understand these differences could provide more insight into the influence of menopause on cardiovascular disease risk.
Funding Support and Author Disclosures
The Dutch Cancer Society (KWF) and the Maarten van der Weijden foundation funded this project, registered under grant 10164. The funding body had no role in the design of the study, collection, analysis or interpretation of data or in writing the paper. Dr van der Aalst has received an advanced research grant for ROBINSCA by the European Research Council. Dr Budde has received institutional support to the radiology department of the Erasmus Medical Center by Siemens and HeartFlow; payments to the Erasmus MC for lectures by Bayer and Siemens; and serves unpaid as board member of the European Society of Cardiovascular Radiology and the editorial boards of Radiology: Cardiothoracic Imaging, EHJ Digital Health, and Journal of Hybrid Imaging. Dr de Jong has received research support to the department of radiology of UMC Utrecht by Philips Healthcare. Dr Vliegenthart has received institutional research grants by Siemens Healthineers, Dutch Heart Foundation, Dutch Cancer Foundation, and Netherlands organization for Health Research and Development; speaker fees by Siemens Healthineers and Bayer Healthcare; participates in the strategic advisory board of the Institute for Cardiometabolism and Nutrition; and is president of the European Society of Cardiovascular Radiology. Dr Ausems has received institutional payments by AstraZeneca. Dr de Koning has received institutional grants for the 4-in-the-lung-run trial HORIZON 2020. Dr Maas has received speaker fees by Novartis, Organon and Omron. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Acknowledgments
The authors thank all the women who participated in this study and would also like to thank the Hereditary Breast and Ovarian cancer study Netherlands (HEBON) consortium for approving this study. The authors also thank Sandra Fase for her extensive administrative assistance. Lastly, the authors thank the employees at all participating hospitals for their help in facilitating the study visits.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental tables, please see the online version of this paper.
Appendix
References
- 1.Henderson J.T., Webber E.M., Sawaya G.F. Screening for ovarian cancer: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2018;319(6):595–606. doi: 10.1001/jama.2017.21421. [DOI] [PubMed] [Google Scholar]
- 2.Daly M.B., Pal T., Berry M.P., et al. Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2021;19(1):77–102. doi: 10.6004/jnccn.2021.0001. [DOI] [PubMed] [Google Scholar]
- 3.Domchek S.M., Friebel T.M., Singer C.F., et al. Association of risk-reducing surgery in BRCA1 or BRCA2 mutation carriers with cancer risk and mortality. JAMA. 2010;304(9):967–975. doi: 10.1001/jama.2010.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rebbeck T.R., Kauff N.D., Domchek S.M. Meta-analysis of risk reduction estimates associated with risk-reducing salpingo-oophorectomy in BRCA1 or BRCA2 mutation carriers. J Natl Cancer Inst. 2009;101(2):80–87. doi: 10.1093/jnci/djn442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dam V., van der Schouw Y.T., Onland-Moret N.C., et al. Association of menopausal characteristics and risk of coronary heart disease: a pan-European case-cohort analysis. Int J Epidemiol. 2019;48(4):1275–1285. doi: 10.1093/ije/dyz016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Honigberg M.C., Zekavat S.M., Aragam K., et al. Association of premature natural and surgical menopause with incident cardiovascular disease. JAMA. 2019;322(24):2411–2421. doi: 10.1001/jama.2019.19191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muka T., Oliver-Williams C., Kunutsor S., et al. Association of age at onset of menopause and time since onset of menopause with cardiovascular outcomes, intermediate vascular traits, and all-cause mortality: a systematic review and meta-analysis. JAMA Cardiol. 2016;1(7):767–776. doi: 10.1001/jamacardio.2016.2415. [DOI] [PubMed] [Google Scholar]
- 8.Roeters van Lennep J.E., Heida K.Y., Bots M.L., Hoek A. collaborators of the Dutch Multidisciplinary Guideline Development Group on Cardiovascular Risk Management after Reproductive Disorders. Cardiovascular disease risk in women with premature ovarian insufficiency: a systematic review and meta-analysis. Eur J Prev Cardiol. 2016;23(2):178–186. doi: 10.1177/2047487314556004. [DOI] [PubMed] [Google Scholar]
- 9.Mendelsohn M.E., Karas R.H. The protective effects of estrogen on the cardiovascular system. N Engl J Med. 1999;340(23):1801–1811. doi: 10.1056/NEJM199906103402306. [DOI] [PubMed] [Google Scholar]
- 10.Parker W.H., Broder M.S., Chang E., Feskanich D., Farquhar C., Liu Z., et al. Ovarian conservation at the time of hysterectomy and long-term health outcomes in the nurses' health study. Obstet Gynecol. 2009;113(5):1027–1037. doi: 10.1097/AOG.0b013e3181a11c64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoffmann U., Massaro J.M., Fox C.S., Manders E., O'Donnell C.J. Defining normal distributions of coronary artery calcium in women and men (from the Framingham Heart Study) Am J Cardiol. 2008;102(9):1136–1141.e1. doi: 10.1016/j.amjcard.2008.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McClelland R.L., Jorgensen N.W., Budoff M., et al. 10-Year coronary heart disease risk prediction using coronary artery calcium and traditional risk factors: derivation in the MESA (Multi-Ethnic Study of Atherosclerosis) with validation in the HNR (Heinz Nixdorf Recall) Study and the DHS (Dallas Heart Study) J Am Coll Cardiol. 2015;66(15):1643–1653. doi: 10.1016/j.jacc.2015.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oei H.H., Vliegenthart R., Hak A.E., et al. The association between coronary calcification assessed by electron beam computed tomography and measures of extracoronary atherosclerosis: the Rotterdam Coronary Calcification Study. J Am Coll Cardiol. 2002;39(11):1745–1751. doi: 10.1016/s0735-1097(02)01853-3. [DOI] [PubMed] [Google Scholar]
- 14.Schmermund A., Mohlenkamp S., Stang A., et al. Assessment of clinically silent atherosclerotic disease and established and novel risk factors for predicting myocardial infarction and cardiac death in healthy middle-aged subjects: rationale and design of the Heinz Nixdorf RECALL Study. Risk factors, evaluation of coronary calcium and lifestyle. Am Heart J. 2002;144(2):212–218. doi: 10.1067/mhj.2002.123579. [DOI] [PubMed] [Google Scholar]
- 15.Dzaye O., Razavi A.C., Dardari Z.A., et al. Modeling the recommended age for initiating coronary artery calcium testing among at-risk young adults. J Am Coll Cardiol. 2021;78(16):1573–1583. doi: 10.1016/j.jacc.2021.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chu J.H., Michos E.D., Ouyang P., Vaidya D., Blumenthal R.S., Budoff M.J., et al. Coronary artery calcium and atherosclerotic cardiovascular disease risk in women with early menopause: the Multi-Ethnic Study of Atherosclerosis (MESA) Am J Prev Cardiol. 2022;11 doi: 10.1016/j.ajpc.2022.100362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Terra L., Hooning M.J., Heemskerk-Gerritsen B.A.M., et al. Long-term morbidity and health after early menopause due to oophorectomy in women at increased risk of ovarian cancer: protocol for a nationwide cross-sectional study with prospective follow-up (HARMOny study) JMIR Res Protoc. 2021;10(1) doi: 10.2196/24414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brohet R.M., Velthuizen M.E., Hogervorst F.B., et al. Breast and ovarian cancer risks in a large series of clinically ascertained families with a high proportion of BRCA1 and BRCA2 Dutch founder mutations. J Med Genet. 2014;51(2):98–107. doi: 10.1136/jmedgenet-2013-101974. [DOI] [PubMed] [Google Scholar]
- 19.van der Aalst C.M., Denissen S., Vonder M., et al. Screening for cardiovascular disease risk using traditional risk factor assessment or coronary artery calcium scoring: the ROBINSCA trial. Eur Heart J Cardiovasc Imaging. 2020;21(11):1216–1224. doi: 10.1093/ehjci/jeaa168. [DOI] [PubMed] [Google Scholar]
- 20.Agatston A.S., Janowitz W.R., Hildner F.J., Zusmer N.R., Viamonte M., Jr., Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15(4):827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 21.Ghadri J.R., Goetti R., Fiechter M., et al. Inter-scan variability of coronary artery calcium scoring assessed on 64-multidetector computed tomography vs. dual-source computed tomography: a head-to-head comparison. Eur Heart J. 2011;32(15):1865–1874. doi: 10.1093/eurheartj/ehr157. [DOI] [PubMed] [Google Scholar]
- 22.Takx R.A., de Jong P.A., Leiner T., et al. Automated coronary artery calcification scoring in non-gated chest CT: agreement and reliability. PLoS One. 2014;9(3) doi: 10.1371/journal.pone.0091239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Joshi P.H., Patel B., Blaha M.J., et al. Coronary artery Calcium predicts Cardiovascular events in participants with a low lifetime risk of Cardiovascular disease: the Multi-Ethnic Study of Atherosclerosis (MESA) Atherosclerosis. 2016;246:367–373. doi: 10.1016/j.atherosclerosis.2016.01.017. [DOI] [PubMed] [Google Scholar]
- 24.McClelland R.L., Chung H., Detrano R., Post W., Kronmal R.A. Distribution of coronary artery calcium by race, gender, and age: results from the Multi-Ethnic Study of Atherosclerosis (MESA) Circulation. 2006;113(1):30–37. doi: 10.1161/CIRCULATIONAHA.105.580696. [DOI] [PubMed] [Google Scholar]
- 25.Boekel N.B., Jacobse J.N., Schaapveld M., et al. Cardiovascular disease incidence after internal mammary chain irradiation and anthracycline-based chemotherapy for breast cancer. Br J Cancer. 2018;119(4):408–418. doi: 10.1038/s41416-018-0159-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rivera C.M., Grossardt B.R., Rhodes D.J., et al. Increased cardiovascular mortality after early bilateral oophorectomy. Menopause. 2009;16(1):15–23. doi: 10.1097/gme.0b013e31818888f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mu F., Rich-Edwards J., Rimm E.B., Spiegelman D., Missmer S.A. Endometriosis and risk of coronary heart disease. Circ Cardiovasc Qual Outcomes. 2016;9(3):257–264. doi: 10.1161/CIRCOUTCOMES.115.002224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Teede H.J., Misso M.L., Costello M.F., et al. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Hum Reprod. 2018;33(9):1602–1618. doi: 10.1093/humrep/dey256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Bommel M.H.D., de Jong M.A., Steenbeek M.P., et al. No signs of subclinical atherosclerosis after risk-reducing salpingo-oophorectomy in BRCA1/2 mutation carriers. J Cardiol. 2021;77(6):570–575. doi: 10.1016/j.jjcc.2020.11.005. [DOI] [PubMed] [Google Scholar]
- 30.Gunning M.N., Meun C., van Rijn B.B., et al. Coronary artery calcification in middle-aged women with premature ovarian insufficiency. Clin Endocrinol (Oxf) 2019;91(2):314–322. doi: 10.1111/cen.14003. [DOI] [PubMed] [Google Scholar]
- 31.Freaney P.M., Petito L., Colangelo L.A., et al. Association of premature menopause with coronary artery calcium: the CARDIA study. Circ Cardiovasc Imaging. 2021;14(11) doi: 10.1161/CIRCIMAGING.121.012959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mortensen M.B., Gaur S., Frimmer A., et al. Association of age with the diagnostic value of coronary artery calcium score for ruling out coronary stenosis in symptomatic patients. JAMA Cardiol. 2022;7(1):36–44. doi: 10.1001/jamacardio.2021.4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krul I.M., Opstal-van Winden A.W.J., Janus C.P.M., et al. Cardiovascular disease risk after treatment-induced premature ovarian insufficiency in female survivors of Hodgkin lymphoma. J Am Coll Cardiol. 2018;72(25):3374–3375. doi: 10.1016/j.jacc.2018.10.030. [DOI] [PubMed] [Google Scholar]
- 34.Kok H.S., van Asselt K.M., van der Schouw Y.T., et al. Heart disease risk determines menopausal age rather than the reverse. J Am Coll Cardiol. 2006;47(10):1976–1983. doi: 10.1016/j.jacc.2005.12.066. [DOI] [PubMed] [Google Scholar]
- 35.Lokkegaard E., Jovanovic Z., Heitmann B.L., Keiding N., Ottesen B., Pedersen A.T. The association between early menopause and risk of ischaemic heart disease: influence of hormone therapy. Maturitas. 2006;53(2):226–233. doi: 10.1016/j.maturitas.2005.04.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.