ABSTRACT
Background
Proenkephalin A 119–159 (penKid) is a novel blood biomarker for real-time assessment of kidney function and was found to be independently associated with worsening kidney function and mortality. A novel penKid-based estimated glomerular filtration rate equation (eGFRPENK-Crea), outperforms current creatinine-based eGFR equations in predicting iohexol or iothalamate plasma clearance-based measured GFR. In this study, we aimed to evaluate the predictive value of penKid and eGFRPENK-Crea for all-cause mortality in stable patients at high cardiovascular risk.
Methods
Circulating penKid levels were assessed in 615 stable patients hospitalized at the Department of Cardiology at University Hospital Aachen, Germany. The endpoint was all-cause mortality; follow up was 3 years.
Results
penKid levels were higher in 46 non-survivors [58.8 (IQR 47.5–85.0) pmol/l] compared to 569 survivors [43.8 (IQR 34.0–58.0) pmol/l; P < .0001]. Univariable Cox regression analyses found penKid and eGFRPENK-Crea to be associated with all-cause mortality (C index 0.703, χ2 33.27, P < .00001; C index 0.716, χ2 36.51, P < .00001). This association remained significant after adjustment for significant baseline parameters including age, smoking, chronic heart failure, use of diuretics, leucocytes, body mass index, sex, and creatinine (C index 0.799, χ2 72.06, P < .00001). Importantly, penKid provided significant added value on top of eGFRCKD-EPI 2021 (eGFRCKD-EPI 2021: C index 0.716, χ2 34.21; eGFRCKD-EPI 2021 + penKid: C index 0.727, χ2: 40.02; Delta χ2 5.81; all P < .00001) for all-cause mortality prediction in our cohort.
Conclusions
penKid levels and eGFRPENK-Crea is associated with all-cause mortality within a 3-year follow-up period and the addition of penKid on top of eGFRCKD-EPI 2021 provided significant added value in mortality prediction.
Keywords: high cardiovascular risk, mortality prediction, proenkephalin A 119-159, study
KEY LEARNING POINTS.
What was known:
PenKid is a novel blood biomarker for real-time assessment of kidney function and was found to be independently associated with worsening kidney function and mortality.
This study adds:
PenKid and the novel eGFRPENK-Crea are strong biomarkers for all-cause mortality in stable patients with high cardiovascular risk. The addition of penKid on top of creatinine or eGFRCKD-EPI 2021 provides significant add-on value in mortality prediction.
Potential impact:
Future studies in larger cohorts with external validation are needed to investigate the impact of routine penKid measurement on clinical decision-making in stable patients at high cardiovascular risk.
INTRODUCTION
Proenkephalin A 119–159 (penKid) is a novel blood biomarker for real-time assessment of kidney function [1]. Endogenous opioids, including enkephalins (i.e. met-enkephalin, leu-enkephalin), exert various physiological functions when binding to delta opioid receptors. Enkephalins and the stable surrogate marker penKid originate from proteolytic cleavage of its precursor preproenkephalin A. In addition to neuronal, cardiac, and intestinal tissue, delta opioid receptors are highly prevalent in the kidney and are discussed to stimulate kidney function when activated [2–4]. The predictive value and performance of penKid as a biomarker was evaluated in various cohorts for clinical outcomes. As penKid measurements provide real-time assessment of kidney function, it detects acute kidney injury (AKI) within 48 hours and 7 days, whereas current creatinine-based estimations [3] show a 24–48-hour delay in detection of deteriorating kidney function. In patients after myocardial infarction, with acute heart failure or sepsis, or in critically ill burn patients, penKid was found to be independently associated with worsening kidney function and mortality [5–9]. A novel penKid-based estimated glomerular filtration rate equation (eGFRPENK-Crea) that incorporates creatinine and age has been evaluated in a study and was superior to current creatinine-based eGFR equations such as MDRD or CKD-EPI 2009 (not superior to CKD-EPI 2021) in predicting iohexol or iothalamate plasma clearance-based measured GFR [10]. In this study, we aimed to evaluate the predictive value of penKid and eGFRPENK-Crea for all-cause mortality in stable patients at high cardiovascular risk.
MATERIALS AND METHODS
Study population
In this cohort study, 956 hemodynamically stable patients hospitalized due to different internal medical diseases/causes at high cardiovascular risk with or without type 2 diabetes (T2D), coronary artery disease (CAD), chronic kidney disease (CKD), and/or chronic heart failure (CHF) were recruited from the Department of Cardiology at University Hospital Aachen, RWTH Aachen University, Germany, from February 2012 to June 2016. Inclusion criterion was age ≥18 years. Exclusion criteria were critical illness (e.g. hemodynamically unstable patients) and failure to give written informed consent. Personal history, including cardiovascular diseases, comorbidities, cardiovascular risk factors, and medication, was obtained from all patients at baseline. Human biosamples were processed and stored by the RWTH centralized Biomaterial Bank (RWTH cBMB, Aachen, Germany) and provided in accordance with the regulations of the RWTH cBMB. Centralized biobanking was approved by the Ethics Committee of the Medical Faculty Aachen (EK 206/09). The primary endpoint of the study was all-cause mortality, and the follow-up period lasted for a duration of 3 years. Follow up was performed by structural questionnaire. The study protocol complies with the ethical guidelines of the Declaration of Helsinki (Hong Kong Amendment) and Good Clinical Practice (European guidelines) and was approved by the local ethics committee (EK 206/09). All patients provided written informed consent.
Laboratory measurements
Serum chemistry data including hematology, lipid profile, glucose metabolism, estimated glomerular filtrated rate (eGFRCKD-EPI 2021), and NT-proBNP were obtained at hospital admission. PenKid was measured in EDTA plasma samples using the immunoluminometric sphingotest® assay (SphingoTec GmbH, Hennigsdorf, Germany) as described previously [11]. The laboratory performing of the biomarker measurement was blinded to clinical and demographic data of the patients. The 97.5th percentile in healthy adult subjects is 89 pmol/l (90% CI 85–118 pmol/l). The upper normal range (89 pmol/l) is also the clinical cut-off for diagnosis of AKI. For estimation of glomerular filtration rate eGFRPENK-Crea (ml/min/1.73 m2) the following algorithm incorporating age, penKid, and creatinine levels, as developed by Beunders et al. [10], was used:
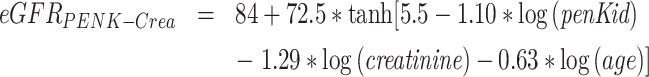 |
Statistical analysis
After exclusion of 341 patients (missing follow up and missing values of penKid and creatinine), 615 patients were analyzed. Values are expressed as medians and interquartile ranges (IQR), or counts and percentages, as appropriate. Group comparisons of continuous variables were performed using the Kruskal–Wallis test. Categorical data were compared using Pearson's chi-squared (χ2) test for count data. Biomarker data were log-transformed. The predictive value of each model was assessed by the model likelihood ratio chi-square statistic. The concordance index (C index) is given as an effect measure. Survival curves plotted by the Kaplan–Meier method were used for illustrative purposes, and log-rank P values computed if pre-specified cut points were applied. All statistical tests were two-tailed and a two-sided P value of .05 was considered significant. The statistical analyses were performed using R version 4.2.2 (http://www.r-project.org, library rms, Hmisc, ROCR) and Statistical Package for the Social Sciences (SPSS) version 22.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
Baseline characteristics are shown in Table 1. The median age of study participants was 66 (IQR 56–74) years, 76.3% were male, with a median BMI of 27.3 (IQR 24.6–30.3) kg/m2 and a median eGFRCKD-EPI 2021 of 83.1 (IQR 67.6–96.1) ml/min/1.73 m2. Of the patients in our cohort, 81.3% have been previously diagnosed with CAD, a history of hypertension in 71%, T2D in 27.3%, current smoking in 24%, and presence of CHF in 59.7% of all patients. Patients had median low density lipoprotein cholesterol levels of 78 (IQR 49–117) mg/dl.
Table 1:
penKid levels in 615 stable patients at high cardiovascular risk.
| n | penKid all patients (n = 615) | penKid <38.1 pmol/l (n = 207) | penKid > 38.1 and <54.0 pmol/l (n = 203) | penKid >54.0 pmol/l (n = 205) | P | |
|---|---|---|---|---|---|---|
| Age (years) | 615 | 66 [56–74] | 59 [52–67] | 66 [55–73] | 74 [66–78] | <.0001 |
| Male: no. (%) | 615 | 469 (76.3) | 175 (84.5) | 164 (80.8) | 130 (63.4) | <.0001 |
| BMI (kg/m²) | 607 | 27.3 [24.60–30.25] | 28.4 [25.3–31.6] | 27 [24.7–29.7] | 26.8 [24.1–29.5] | .0005 |
| Hypertension: no. (%) | 613 | 435 (71) | 136 (65.7) | 147 (72.8) | 152 (74.5) | .1137 |
| Smoking, current: no. (%) | 613 | 147 (24) | 59 (28.5) | 52 (25.7) | 36 (17.6) | .0001 |
| Type 2 diabetes: no. (%) | 615 | 168 (27.3) | 53 (25.6) | 58 (28.6) | 57 (27.8) | .7821 |
| Glycated hemoglobin: no. (%) | 457 | 5.7 [5.4–6.4] | 5.7 [5.4–6.5] | 5.7 [5.4–6.3] | 5.75 [5.40–6.32] | .8841 |
| History of CAD: no. (%) | 567 | 461 (81.3) | 158 (82.3) | 154 (81.1) | 149 (80.5) | .9039 |
| History of CHF: no. (%) | 566 | 338 (59.7) | 101 (52.6) | 106 (58.6) | 131 (67.9) | .0087 |
| Total cholesterol (mg/dl) | 448 | 180 [152.75–209.00] | 181 [153–215] | 173.5 [149–196] | 182 [155–206] | .2931 |
| LDL-C (mg/dl) | 436 | 78 [49–117] | 87 [53–120] | 73.5 [48.5–111.5] | 74 [48–117] | .3731 |
| HDL-C (mg/dl) | 430 | 63 [44–101] | 54 [41–92] | 64 [43–99] | 70 [46–103] | .1016 |
| Triglycerides (mg/dl) | 389 | 128 [94–183] | 132 [97–204] | 129 [94–183] | 119 [91.5–175.5] | .2257 |
| Leukocytes −/nl | 612 | 7.5 [6.20–9.03] | 7.8 [6.4–9.3] | 7.6 [6.3–9.2] | 7.2 [6.00–8.55] | .0304 |
| CRP (mg/l) | 543 | 5 [5–10.35] | 5 [5–13] | 5 [5–8] | 5 [5–11] | .0437 |
| NT-proBNP (pg/ml) | 476 | 423.5 [134–1270] | 209 [84.25–520.75] | 492 [141–1193] | 666 [254–2660] | <.0001 |
| Creatinine | 615 | 1 [0.81–1.10] | 0.9 [0.78–1.00] | 1 [0.9–1.1] | 1.1 [0.9–1.4] | <.0001 |
| eGFRMDRD (ml/min/1.73 m2) | 615 | 74 [62–86] | 84 [75–99] | 74 [65–85] | 61 [45–73] | <.0001 |
| eGFRCKD-EPI 2021 (ml/min/1.73 m2) | 615 | 83.13 [67.56–96.11] | 94.4 [84.73–102.08] | 82.49 [70.66–94.19] | 65.98 [48.82–82.20] | <.0001 |
| eGFRPENK-Crea (ml/min/1.73 m2) | 615 | 85.24 [69.89–98.52] | 102.46 [94.75–108.87] | 83.94 [77.14–90.97] | 63.42 [51.21–73.08] | <.0001 |
| Left ventricular systolic function − % | 506 | 52 [45.25–55.75] | 52 [50–56] | 52 [42–55] | 52 [43–55] | .0227 |
| Medication: no. (%) | ||||||
| Antiplatelets | 587 | 473 (80.6) | 157 (80.1) | 160 (82.1) | 156 (79.6) | .8103 |
| Oral anticoagulants | 587 | 186 (31.7) | 51 (26) | 62 (31.8) | 73 (37.2) | .0577 |
| Diuretics | 587 | 315 (53.7) | 83 (42.3) | 106 (54.4) | 126 (64.3) | .0001 |
| Statins | 587 | 457 (77.9) | 159 (81.1) | 150 (76.9) | 148 (75.5) | .3797 |
| Calcium channel blockers | 587 | 113 (19.3) | 34 (17.3) | 45 (23.1) | 34 (17.3) | .2528 |
| Beta blockers | 587 | 494 (84.2) | 164 (83.7) | 162 (83.1) | 168 (85.7) | .7552 |
| RAAS inhibitors | 587 | 488 (83.1) | 162 (82.7) | 162 (83.1) | 164 (83.7) | .9639 |
| Insulin | 587 | 48 (8.2) | 12 (6.1) | 17 (8.7) | 19 (9.7) | .4110 |
| Metformin | 587 | 80 (13.6) | 27 (13.8) | 31 (15.9) | 22 (11.2) | .4028 |
Values are expressed as medians and interquartile ranges (IQR), or counts and percentages. Significant P-values are highlighted in bold.
BMI = body mass index, CKD-EPI 2021 = Chronic Kidney Disease Epidemiology Collaboration, HDL-C = high density lipoprotein cholesterol, CRP = c-reactive protein, LDL-C = low density lipoprotein cholesterol, MDRD = Modification of Diet Renal Disease, RAAS = renin-angiotensin-aldosterone system
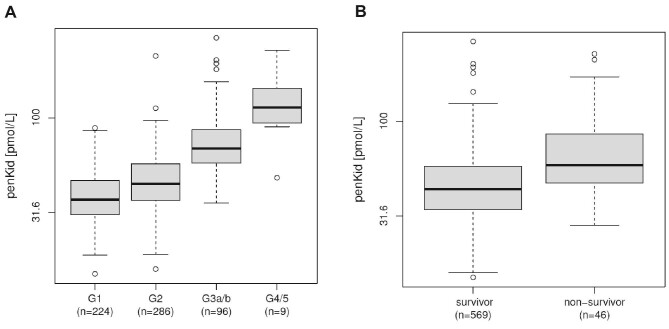
Median penKid levels were 44.5 (IQR 34.7–59.3) pmol/l and median eGFRPENK-Crea was 85.24 (69.9–98.5) ml/min/1.73 m2 in the 615 patients of our cohort at baseline. Highest penKid levels were found in patients with eGFRCKD-EPI 2021 <30 ml/min/1.73 m2 (G4/5) [113.4 (IQR 93.8–143.4) pmol/l]. Further, penKid levels were higher in patients with eGFRCKD-EPI 2021 30–60 ml/min/1.73 m2 (G3a/b) [68.75 (IQR 57.6–86.1) pmol/l] compared to patients with eGFRCKD-EPI 2021 60–90 ml/min/1.73 m2 (G2) [44.7 (IQR 36.5–57.1) pmol/l]. Lowest penKid levels were found in patients with eGFRCKD-EPI 2021 >90 ml/min/1.73 m2 (G1) [36.8 (IQR 30.6–46.4) pmol/l] (Fig. 1A; P < .001, ANOVA all comparisons).
Figure 1:
(A) PenKid levels (pmol/l) at baseline in patients with eGFR >90 ml/min/1.73 m2 (G1), between 60 and 90 ml/min/1.73 m2 (G2), between 30 and 60 ml/min/1.73 m2 (G3a/b), and <30 ml/min/1.73 m2 (G4/5), P < .001, ANOVA all comparisons. (B) PenKid levels (pmol/l) at baseline in survivors compared to non-survivors (all-cause mortality) within 3 years.
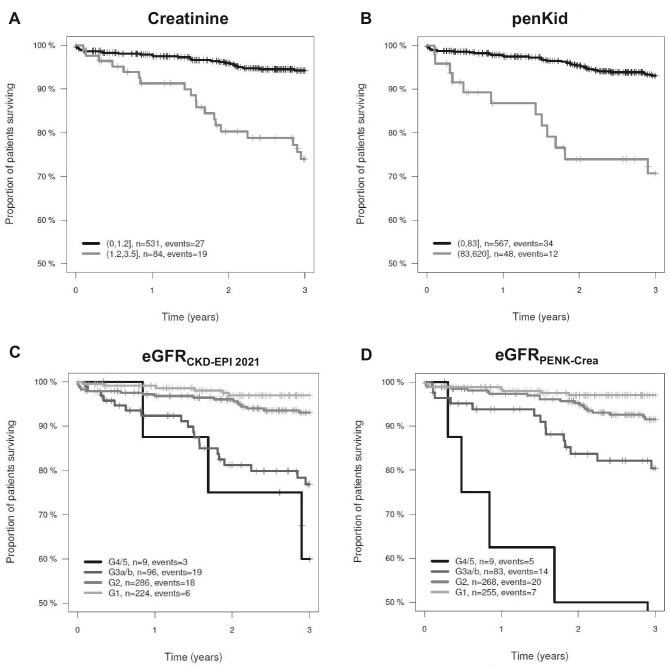
Over a 3-year follow-up period, a total of 46 patients died. PenKid levels were higher in 46 non-survivors [58.8 (IQR 47.5–85.0) pmol/l] compared to 569 survivors [43.8 (IQR 34.0–58.0) pmol/l; P < .0001] (Fig. 1B). Kaplan–Meier plots based on creatinine, penKid, eGFRCKD-EPI 2021, and eGFRPENK-Crea indicate the survival rate in our cohort of stable patients at high cardiovascular risk (Fig. 2A–D). The model likelihood ratio (LR) χ2 statistics and the C index indicate the predictive value of penKid and are summarized in Table 2. Univariable Cox regression analyses found penKid and eGFRPENK-Crea to be associated with all-cause mortality (C index 0.703, χ2 33.27, P < .00001; C index 0.716, χ2 36.51, P < .00001). This association remained significant after adjustment for significant baseline parameters including age, smoking, CHF, use of diuretics, leucocytes, body mass index, sex, and creatinine (C index 0.799, χ2 72.06, P < .00001). Importantly, penKid provided significant added value on top of eGFRCKD-EPI 2021 (eGFRCKD-EPI 2021: C index 0.716, χ2 34.21, P < .00001; eGFRCKD-EPI 2021 + penKid: C index 0.727, χ2 40.02, P < .00001) for all-cause mortality prediction in our cohort.
Figure 2:
Kaplan–Meier plots for creatinine (LR P = .00001) (A), penKid (LR P < .00001) (B), eGFRCKD-EPI 2021 (LR P < .00001) (C) and eGFRPENK-Crea (LR P < .00001) (D).
Table 2:
Uni- and multivariable Cox regression analysis for all-cause mortality within 3 years.
| Model | n | events | χ 2 test | d.f. | LR P value | C index [95 % CI] |
|---|---|---|---|---|---|---|
| Age | 615 | 46 | 34.99 | 1 | <.00 001 | 0.736 [0.675, 0.797] |
| penKid | 615 | 46 | 33.27 | 1 | <.00 001 | 0.703 [0.625, 0.780] |
| eGFRCKD-EPI 2021 | 615 | 46 | 34.21 | 1 | <.00 001 | 0.716 [0.640, 0.793] |
| eGFRPENK-Crea | 615 | 46 | 36.51 | 1 | <.00 001 | 0.716 [0.635, 0.797] |
| eGFRCKD-EPI 2021 + penKid | 615 | 46 | 40.02 | 2 | <.00 001 | 0.727 |
| Model 1 | 604 | 45 | 68.33 | 8 | <.00 001 | 0.795 |
| Model 1 + penKid | 604 | 45 | 72.06 | 9 | <.00 001 | 0.799 |
CKD-EPI 2021 = Chronic Kidney Disease Epidemiology Collaboration, d.f. = number of degrees of freedom, LR = likelihood ratio.
Model 1 includes age, smoking, CHF, use of diuretics, leucocytes, body mass index, sex, and creatinine.
DISCUSSION
Consequently, in this study penKid levels were associated with poor kidney function (eGFRCKD-EPI 2021) in stable patients at high cardiovascular risk. Moreover, we found penKid levels and eGFRPENK-Crea to be associated with all-cause mortality within a 3-year follow-up period. Importantly, the addition of penKid on top of eGFRCKD-EPI 2021 provided significant added value in mortality prediction.
To the best of our knowledge, this is the first study (i) demonstrating penKid levels to be associated with all-cause mortality providing added value in mortality prediction on top of eGFRCKD-EPI 2021 in stable patients at high cardiovascular risk and (ii) showing an association of the novel eGFRPENK-Crea equation with all-cause mortality in a real-world patient cohort.
This study has certain limitations. The follow-up period was limited to 3 years, which may not capture long-term outcomes. Furthermore, our cohort included only a few patients with severe CKD restricting the reliability of the results in this sub-population of patients with high cardiovascular risk. Moreover, the absence of comparison with cystatin C is an important limitation of our study. Future studies testing penKid should include measurements of cystatin C.
In conclusion, our study highlights a significant association between elevated penKid levels and impaired kidney function in stable patients at high cardiovascular risk. Furthermore, penKid provides added value on top of eGFRCKD-EPI 2021 in prediction of all-cause mortality. Future studies in larger cohorts with external validation are needed to investigate the impact of routine penKid measurement on clinical decision-making in stable patients at high cardiovascular risk.
ACKNOWLEDGEMENTS
The authors thank the entire team of the RWTH centralized Biomaterial Bank (RWTH cBMB). RWTH cBMB is an operational unit of the Medical Faculty Aachen and is funded by the Faculty. The authors gratefully acknowledge the expert technical assistance of Gabriele Heuer, Hedwig Reichardt, Zakiya Coenen-Basmadjie and continuous support by Professor Müller-Wieland, employees of the University Hospital Aachen, Department of Internal Medicine I.
Contributor Information
Matthias Rau, Department of Internal Medicine I, University Hospital Aachen, RWTH Aachen University, Aachen, Germany.
Berkan Kurt, Department of Internal Medicine I, University Hospital Aachen, RWTH Aachen University, Aachen, Germany.
Oliver Hartmann, SphingoTec GmbH, Hennigsdorf, Germany.
Fábia Daniela Lobo de Sá, SphingoTec GmbH, Hennigsdorf, Germany.
Marvin Schwarz, Department of Internal Medicine I, University Hospital Aachen, RWTH Aachen University, Aachen, Germany.
Kirsten Thiele, Department of Internal Medicine I, University Hospital Aachen, RWTH Aachen University, Aachen, Germany.
Niels-Ulrik Korbinian Hartmann, Department of Internal Medicine I, University Hospital Aachen, RWTH Aachen University, Aachen, Germany.
Jens Spiesshoefer, Department of Pneumology and Intensive Care Medicine, University Hospital Aachen, RWTH Aachen University, Aachen, Germany.
Julia Möllmann, Department of Internal Medicine I, University Hospital Aachen, RWTH Aachen University, Aachen, Germany.
Mathias Hohl, Department of Internal Medicine III, Saarland University Hospital and Saarland University, Homburg/Saar, Germany.
Simina-Ramona Selejan, Department of Internal Medicine III, Saarland University Hospital and Saarland University, Homburg/Saar, Germany.
Emiel P C van der Vorst, Aachen-Maastricht Institute for CardioRenal Disease (AMICARE), Interdisciplinary Center for Clinical Research (IZKF), Institute for Molecular Cardiovascular Research (IMCAR), University Hospital Aachen, RWTH Aachen University, Aachen, Germany; Institute for Cardiovascular Prevention (IPEK), Ludwig-Maximilians-Universität München, München, Germany.
Edgar Dahl, RWTH cBMB at the Institute of Pathology, University Hospital Aachen, RWTH Aachen University, Aachen, Germany.
Nikolaus Marx, Department of Internal Medicine I, University Hospital Aachen, RWTH Aachen University, Aachen, Germany.
Florian Kahles, Department of Internal Medicine I, University Hospital Aachen, RWTH Aachen University, Aachen, Germany.
Michael Lehrke, Department of Internal Medicine I, University Hospital Aachen, RWTH Aachen University, Aachen, Germany.
FUNDING
F.K. was funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation, 520275106, Emmy Noether Research Group), the European Research Area Network on Cardiovascular Diseases (ERA-CVD and BMBF, Grant No. JTC-2019, MyPenPath–01KL2004) and the European Foundation for the Study of Diabetes (EFSD)/Novo Nordisk Foundation (NNF2OSA0066111). F.K. and E.V. were funded by the German Research Foundation (SFB TRR 219–322900939–M-07). This study was supported by grants from the Deutsche Forschungsgemeinschaft (SFB TRR 219–32900939–M-03, M-02) and LE 1350/9-1 to M.L. and the Marga und Walter Boll-Stiftung to M.L. N.M. was supported by grants from the Deutsche Forschungsgemeinschaft (SFB TRR 219–322900939–M-03, M-05) as well as the CORONA Stiftung, Germany.
DATA AVAILABILITY STATEMENT
The data underlying this article will be shared on a reasonable request to the corresponding author.
CONFLICT OF INTEREST STATEMENT
B.K. has received a travel reimbursement from SphingoTec/4TEEN4. F.K. has served as a speaker for Novo Nordisk, AstraZeneca, DGK-Akademie, consulted Novo Nordisk, Bayer, PricewaterhouseCoopers/Strategy&, received personal fees from Amgen, Novo Nordisk, Boehringer Ingelheim and Lilly and travel reimbursement from SphingoTec/4TEEN4. M.L. received grants and personal fees from Boehringer Ingelheim, grants and personal fees from MSD, grants and personal fees from Novo Nordisk, personal fees from Amgen, Sanofi, Astra Zeneca, Bayer, Lilly, Abiomed, Daiichi Sankyo. N.M. has served as speaker for Amgen, Bayer, Boehringer Ingelheim, Sanofi-Aventis, MSD, BMS, AstraZeneca, Lilly, Novo Nordisk. N.M. declines all personal compensation from pharma or device companies. M.S. received personal fees from Novartis, Pfizer, Berlin Chemie, Vantis, Daiichi Sankyo, AstraZeneca, Sanofi, Bayer. O.H. and F.D.L.S. are employed by SphingoTec GmbH, the company providing the assay for penKid measurements. SphingoTec GmbH had no influence on the design of the study. All other authors have no conflict of interest to declare.
REFERENCES
- 1. Beunders R, Struck J, Wu AHB et al. Proenkephalin (PENK) as a novel biomarker for kidney function. J Appl Lab Med 2017;2:400–12. 10.1373/jalm.2017.023598 [DOI] [PubMed] [Google Scholar]
- 2. Denning GM, Ackermann LW, Barna TJ et al. Proenkephalin expression and enkephalin release are widely observed in non-neuronal tissues. Peptides 2008;29:83–92. 10.1016/j.peptides.2007.11.004 [DOI] [PubMed] [Google Scholar]
- 3. Grossman A, Clement-Jones V. Opiate receptors: enkephalins and endorphins. Clin Endocrinol Metab 1983;12:31–56. 10.1016/S0300-595X(83)80028-0 [DOI] [PubMed] [Google Scholar]
- 4. Sezen SF, Kenigs VA, Kapusta DR. Renal excretory responses produced by the delta opioid agonist, BW373U86, in conscious rats. J Pharmacol Exp Ther 1998;287:238–45. [PubMed] [Google Scholar]
- 5. Ng LL, Sandhu JK, Narayan H et al. Proenkephalin and prognosis after acute myocardial infarction. J Am Coll Cardiol 2014;63:280–9. 10.1016/j.jacc.2013.09.037 [DOI] [PubMed] [Google Scholar]
- 6. Molvin J, Jujic A, Navarin S et al. Bioactive adrenomedullin, proenkephalin A and clinical outcomes in an acute heart failure setting. Open Heart 2019;6:e001048. 10.1136/openhrt-2019-001048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Matsue Y, Ter Maaten JM, Struck J et al. Clinical correlates and prognostic value of proenkephalin in acute and chronic heart failure. J Card Fail 2017;23:231–9. 10.1016/j.cardfail.2016.09.007 [DOI] [PubMed] [Google Scholar]
- 8. Caironi P, Latini R, Struck J et al. Circulating proenkephalin, acute kidney injury, and its improvement in patients with severe sepsis or shock. Clin Chem 2018;64:1361–9. 10.1373/clinchem.2018.288068 [DOI] [PubMed] [Google Scholar]
- 9. Dépret F, Polina A, Amzallag J et al. PenKid measurement at admission is associated with outcome in severely ill burn patients. Burns 2020;46:1302–9. 10.1016/j.burns.2020.01.002 [DOI] [PubMed] [Google Scholar]
- 10. Beunders R, Donato LJ, van Groenendael R et al. Assessing GFR with proenkephalin. Kidney Int Rep 2023;8:2345–55. 10.1016/j.ekir.2023.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Donato LJ, Meeusen JW, Lieske JC et al. Analytical performance of an immunoassay to measure proenkephalin. Clin Biochem 2018;58:72–77. 10.1016/j.clinbiochem.2018.05.010 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article will be shared on a reasonable request to the corresponding author.