Abstract
Leaves are specialized organs characterized by defined developmental destiny and determinate growth. The overexpression of Knotted1-like homeobox genes in different species has been shown to alter leaf shape and development, but a definite role for this class of genes remains to be established. Transgenics that overexpress Knotted1-like genes present some traits that are characteristic of altered cytokinin physiology. Here we show that lettuce (Lactuca sativa) leaves that overexpress KNAT1, an Arabidopsis kn1-like gene, acquire characteristics of indeterminate growth typical of the shoot and that this cell fate change is associated with the accumulation of specific types of cytokinins. The possibility that the phenotypic effects of KNAT1 overexpression may arise primarily from the modulation of local ratios of different cytokinins is discussed.
Homeodomain-containing transcription factors (homeobox) control the expression of specific target genes and are thought to trigger important differentiation processes in organisms ranging from animals to plants (Gehring, 1987; Kessel and Gruss, 1990; Vollbrecht et al., 1991). At least five distinct homeobox families have been identified in plants (for review, see Chan et al., 1998; Reiser et al., 2000) based on general protein features, including similarities outside the homeodomain and the presence of associated motifs.
The KNAT1 gene from Arabidopsis belongs to the Knotted1 (Kn1)-like class of homeobox genes that appear to be involved in different aspects of the control of cell fate determination in shoot meristems. The first homeobox gene Kn1 was isolated by transposon tagging as a dominant leaf mutant in maize (Vollbrecht et al., 1991; Smith et al., 1992). In kn1 mutants the ectopic expression of the Kn1 gene product has been correlated with the mutant leaf phenotype. Moreover, the overexpression of Kn1 in several plant species has been demonstrated to modify leaf shape and plant architecture (Sinha et al., 1993; Lincoln et al., 1994; Hareven et al., 1996; Tamaoki et al., 1997). The transgenic tobacco phenotypes range from abnormal leaf shape to the formation of ectopic shoots on leaf surfaces, suggesting that Kn1 participates in the switch from indeterminate to determinate cell fates (Sinha et al., 1993). Ectopic Kn1 expression in tomato has been shown to produce “super compound” leaves, further suggesting a different function in compound developmental programs (Hareven et al., 1996). The Arabidopsis KNAT1 gene was found to be expressed in the shoot apical meristem and was down-regulated before leaf initiation (Lincoln et al., 1994). Overexpression of KNAT1 in Arabidopsis induces lobed leaves with ectopic meristems initiating in their sinuses in the close vicinity of veins (Lincoln et al., 1994). These phenotypes recall those observed in several transgenic plants overexpressing the bacterial gene isopentenyl transferase (ipt) involved in the production of active cytokinins (Hewelt et al., 1994; Faiss et al., 1997; Roeckel et al., 1997; McKenzie et al., 1998). Thus, the involvement of cytokinins in the knox gene signaling cascade has been hypothesized (Lincoln et al., 1994). However, target genes of Kn1-like proteins have not been isolated so far and pathways in which these proteins can be involved and act to influence the status of meristem cells are completely unknown. The maize Kn1 was recently expressed under the control of a senescence-activated promoter in tobacco plants (Ori et al., 1999), and a delay in senescence, accompanied by an increased cytokinin content in older leaves, was observed. In cultured tobacco tissues, ectopic expression of maize Kn1 resulted in cytokinin-autotrophic growth associated with an increase in cytokinins endogenous levels (Hewelt et al., 2000). Preliminary results from our laboratory showed that the overexpression of KNAT1, an Arabidopsis homologue of Kn1, in the aerial tissues of lettuce (Lactuca sativa), induces profound changes in the plant architecture (Frugis et al., 1999a). Here we show that the overexpression of KNAT1 induces leaves (organs with determinate growth) to acquire properties of indefinite growth characteristic of the shoot. Since we found that KNAT1 overexpression is associated with an overproduction of specific types of cytokinins, a possible involvement of knox genes in the control of cell fate through modification of cytokinin metabolism is discussed.
RESULTS
PetE:KNAT1 Lettuce Exhibit Altered Leaf Morphology and Plant Architecture
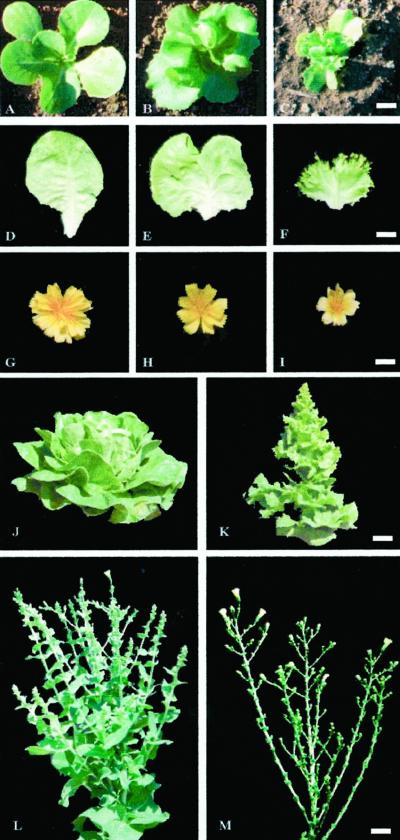
Twenty lettuce independent lines carrying the KNAT1 cDNA under the control of the pea plastocyanin promoter PetE (Helliwell et al., 1997) were obtained by cocultivation of lettuce cotyledon explants with Agrobacterium tumefaciens. Although phenotypic variability among primary transformants was very high, due to in vitro culture effect, 15 out of 20 KNAT1 plants showed peculiar leaf traits such as a reduction in midvein elongation, a decreased blade expansion, and a dramatic margin alteration. These plants were analyzed for the transgene copy number and two transformed lettuce plants (p173 and p177), derived from a single insertion event, were selected for further analysis (data not shown). Seventy seeds from each of the two self-pollinated KNAT1 primary transformants were germinated in pots and subsequently analyzed. Seed germination percentage, cotyledon morphology, first leaf emergence timing, and phyllotaxy was not altered compared with wild type. It is most interesting that 75% of plants from both progenies exhibited alterations of leaf morphology from the first leaf on. The alterations became more marked as later leaves formed. The T1 plants were placed in three phenotypic categories based on leaf morphology (normal, mild, and severe; Fig. 1, A–F). Plants with normal phenotype did not show any alteration with respect to wild-type plants (Fig. 1, A and D). Mild phenotype plants had leaf shortened midvein and slight alteration of margins, resembling the parental phenotype (Fig. 1, B and E). In the severe phenotype plants, the whole leaf vein structure was altered: the main vein was extremely short and leaf margins presented protruding substructures resembling secondary and tertiary leaflets (Fig. 1, C and F). The time interval between the production of successive leaf primordia (plastochron) was not altered, even in the plants exhibiting the severe phenotype. However, a mild or strong reduction in size was observed in the intermediate and severe phenotypes, respectively. Fresh and dry weight of organs of the PetE:KNAT1 plants and leaf expansion were reduced accordingly (data not shown).
Figure 1.
Phenotypical alterations of PetE::KNAT1 lettuce T1 plants. PetE::KNAT1 T1 plants were placed in three phenotypic categories based on leaf morphology. A, D, and G, Normal, plants that fully resembled wild-type phenotype. B, E, and H, Mild, plants that resemble the parental phenotype. C, F, and I, Severe, plants that show severe alterations of leaf shape and plant architecture. A through C, Six-leaves-stage plants grown in greenhouse; D through F, 10th leaf of same age from wild-type, mild, and severe PetE::KNAT1 T1 plants, respectively; G through I, inflorescence from wild-type, mild, and severe PetE::KNAT1 T1 plants, respectively; wild-type (J) and severe transgenic (K) adult plants of same age. Transgenic lettuce always flowers several days earlier than wild-type plants. Wild-type (L) and severe transgenic (M) plants at flowering time. PetE::KNAT1 inflorescence stem displays loss of apical dominance with formation of several floral branches of different length with respect to the more regular architecture of the wild-type. Size bar in C for A through C = 1 cm. Size bar in F for D through F = 1.2 cm. Size bar in I for G through I = 0.7 cm. Size bar in K for J and K = 2.5 cm. Size bar in M for L and M = 1.5 cm.
After transition from vegetative to reproductive phase, alteration of plant architecture was observed in the severe transgenics. In fact, inflorescence stem presented loss of apical dominance with formation of several floral branches of different length (Fig. 1, compare L with M). PetE:KNAT1 severe transformants showed an earlier flowering response as compared with wild type, consisting of around 20 d anticipation of stem elongation in autumn and 15 d in spring time (Fig. 1, J and K). The floral organ shape was morphologically normal, but severe plants showed a lower number of flowers per inflorescence and reduction of floral organs size (Fig. 1, G–I).
KNAT1 Ectopic Expression in Transgenic Lettuce T2 Lines Strongly Affects the Leaf Vascular System
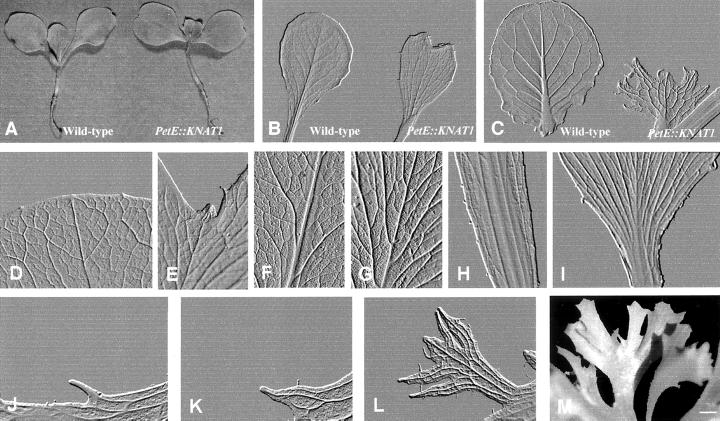
Seeds from 10 T1 self-pollinated plants showing the severe phenotypes were sown in greenhouse to produce homozygous lines. All the T2 plants presented the same dramatic phenotype observed in the parental individuals. The leaf venation pattern of the wild-type lettuce cv Luxor is characterized by a prominent midvein, several distinct size orders with smaller veins that diverge from the larger ones, and a closed vein reticulum in which the smallest veins form freely ending veinlets. Each discrete order of minor venation appears sequentially during leaf formation and expansion. The midvein provascular strand extends acropetally from the stem into the leaf primordium with the secondary veins extending from the midvein toward the margin as the leaf lamina is formed. The reticulum of tertiary and higher order veins is established during the intercalary expansion growth. PetE:KNAT1 plants showed alteration of venation pattern from the formation of the first leaf on (Fig. 2, A and B). In the early phase of seedling development, the first leaf showed a shortened midvein, which gave rise to a characteristic heart-shaped leaf phenotype (Fig. 2B). The difference in the venation pattern appeared increasingly pronounced during leaf growth and expansion and yet more dramatic when the following leaves were formed (Fig. 2C). PetE:KNAT1 adult leaves were small with an extremely short and thin midvein from which numerous secondary veins of similar size departed (Fig. 2I). The secondary veins elongated poorly before branching and connecting with other vascular strands. This gave rise to an extremely complex vascular network in which a distinct size order hierarchy was no longer distinguishable (Fig. 2G). The leaf tip is rounded and smooth in the wild type (Fig. 2D). On the contrary, in PetE:KNAT1 it appeared irregular (Fig. 2E) and almost hidden by overgrown lateral parts that originated by the faster development of the lateral vein network as compared with the midvein. The thick ultra-branched vein network extended toward the leaf margins where the vascularization was markedly enhanced with respect to control plants. As seen in Figure 2D, a regular network of tertiary thin and tiny veins represents the main vascular tissue at the margins of wild-type plants. On the contrary, PetE:KNAT1 leaf margins were irregular with thick broader vascular strands projected out of the leaf lamina and confluent into structures similar to neoformed leaflets (Fig. 2M). This leaf formation pattern appeared repeated, suggesting that an iterative differentiation program, driven by a de-regulated vascular elements formation, occurred. Figure 2, J through L, shows the typical pattern of leaf-like structures formation from the PetE:KNAT1 leaf margins: the initial event was triggered by the overgrowth of a single vein that protruded from the edge of the leaf (Fig. 2J), the tip of which presented meristematic features (Fig. 3, A and B). Additional vascular tissues subsequently contributed to form more complex structures with an ultra-branched vein network that gave rise to additional protruding elements showing the same iterative process of vascular differentiation.
Figure 2.
Alteration of PetE::KNAT1 leaf shape and venation pattern at different developmental stages. A, One-leaf stage plants from lettuce wild-type and T2 PetE::KNAT1. Size bar in M = 0.3 cm. B through L, Embossed modified images of cleared leaves: first leaf from wild-type and T2 PetE::KNAT1 with the characteristic heart-shape due to a reduced elongation of the midvein; size bar in M = 1 mm (B); eighth leaf in expansion phase from wild-type and T2 PetE::KNAT1; size bar in M = 0.5 cm (C); D through I, detail of F and G leaf tip, H and I leaf base, and H and I midvein from which secondary veins depart from wild type (D, F, and H) and PetE::KNAT1 (E, G, and I). Size bar in M: for D and E = 90 μm, for F and G = 100 μm, and for H and I = 50 mm. J through M, Pattern of leaf-like structures formation from the PetE:KNAT1 leaf margins: sequential images (embossed modified images from cleared leaves) of outcoming structures that arise from PetE:KNAT1 leaf margin tissues. The first step involves the elongation of a single vein (J) that protrudes from the leaf edge in correspondence of groups of cells with meristematic features. The main vein subsequently branches (K) to form secondary strands that eventually protrude out from the main structure and give rise to leaf-like tissues in a reiterative process of morphogenesis (L). Size bar in M = 0.12 cm. M, Advanced leaf-like structures at the PetE::KNAT1 leaf margin; size bar = 0.2 cm.
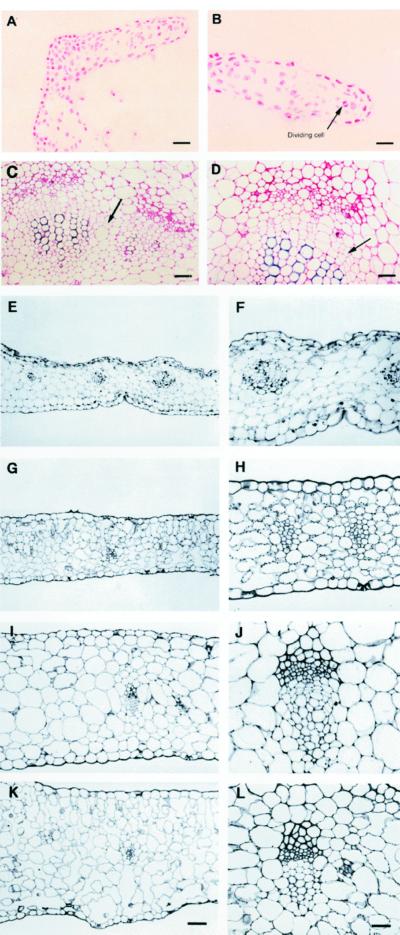
Figure 3.
Histological characterization of PetE::KNAT1 leaves. A, Transverse section (treated by the Feulgen reaction that stains only DNA) of PetE::KNAT1 leaf margin in correspondence to neoforming leaf-like structures. Size bar = 50 μm. B, Magnification of section in A. Cells present high nucleus/cytoplasm ratio and mitotic figures. Arrow indicates an example of cells in mitotic phase. Size bar = 40 μm. C, Transverse section of vascular bundles in inner sectors of a PetE::KNAT1 leaf. Arrow indicates interfascicular region between the two bundles. Size bar = 100 μm. D, Magnification of section in C. Arrow indicates elongated meristematic cells typical of cambium initiation tissues in the bundle between differentiated xylem and phloem elements. Size bar = 50 μm. E through L, Comparison of same leaf sectors from wild-type and PetE::KNAT1 plants of same age and same developmental stage. Light microscopy. E, Transverse section of PetE::KNAT1 leaf in close proximity to the margin. The mesophyll thickness is irregular, palisade cells are absent, and intercellular space is rare. F, Detail of a vascular bundle in transverse section of PetE::KNAT1 leaf in close proximity to the margin: irregular shape due to an anomalous and disorganized association of xylem and phloem elements. G, Transverse section of wild-type leaf in close proximity to the margin. Palisade cells are detectable and intercellular space is present. H, Detail of a vascular bundle in transverse section of wild-type leaf in close proximity to the margin. Regular association of xylem and phloem elements. I, Inner sector of PetE::KNAT1 leaf in close proximity to the midvein. The mesophyll is compact, palisade cells are absent, and intercellular space is rare. J, Detail of a vascular bundle in inner sectors of PetE::KNAT1 leaf in close proximity to the midvein: elongated cells typical of cambium initiation tissues are present inside the bundles between differentiated phloem and xylem elements regularly associated. K, Transverse section of inner sectors of wild-type leaf in close proximity to the midvein. Palisade tissue is fully developed and intercellular spaces are present. L, Detail of a vascular bundle in inner sectors of wild-type leaf in close proximity to the midvein. Phloem and xylem are regularly associated and cambial initial cells are never observed in wild-type leaves. Size bar in K for E through K = 60 μm. Size bar in L for F through L = 30 μm.
Cellular and Histological Alteration in PetE:KNAT1 Leaves
The mesophyll of PetE:KNAT1 leaves appeared rather homogeneous with tightly packed cells when compared with wild type, as palisade and lacunose tissues were not clearly distinguishable and intercellular air spaces were rare (Fig. 3, E and I). The shape of the vascular bundles proximal to the leaf margin of the transgenic plants was irregular due to an anomalous and disorganized association of xylem and phloem elements (Fig. 3F). In addition, a very high number of secretory cells were interspersed within the phloem (Fig. 3F). The cells in the digits of the leaf-like structures arising from the leaf margins exhibited lower chloroplast content and meristematic features such as high nucleus/cytoplasm ratio and the presence of mitotic figures (Fig. 3, A and B). The mesophyll thickness was very irregular with average values lower than controls in the leaf margins, whereas no significant difference was observed in more internal sectors (Table I). When leaf bundle distance was calculated, the number of bundles per surface, as well as the average surface of bundles, was generally higher in the leaf of PetE: KNAT1 lettuce than controls (Table I). Bundles in the inner part of the adult transgenic leaves, closer to the midvein, appeared to have a more regular structure when compared with the marginal ones (Fig. 3J). It is most interesting that dividing cells with features typical of the cambium initials were observed between differentiated phloem and xylem elements inside the bundles (Fig. 3, D and J) and across the interfascicular region (Fig. 3C). Comparable sectors of wild-type leaves of the same or different age never showed any cell similar to cambium initiation cells, as they are usually only associated with the secondary growth of stems.
Table I.
Morphometric features of PetE::KNAT1 and control lettuce leaves
| Leaf Portion | Lamina Thicknessa | Average Bundle Distance | No. Bundle | Bundle Average Surface | No. Stomata |
|---|---|---|---|---|---|
| μm | mm−1 | μm | mm−1 | ||
| PetE::KNAT1b | 150 ± 3c | 186 ± 11 | 6.1 ± 0.5 | 630 ± 67 | 0 |
| Controlb | 201 ± 9 | 246 ± 18 | 4.8 ± 0.2 | 457 ± 34 | 3.3 ± 0.4 |
| PetE::KNAT1d | 481 ± 20 | 431 ± 23 | 2.3 ± 0.1 | 3210 ± 360 | 0.9 ± 0.1 |
| Controld | 530 ± 23 | 429 ± 18 | 2.3 ± 0.1 | 2496 ± 390 | 1.9 ± 0.1 |
Measured at level of interbundles portion.
Leaf's margin sectors.
se.
Leaf's inner sectors close to the midvein.
Stomata were not found in the marginal sectors of PetE:KNAT1 leaves, and a very low stomata number was counted in their inner sectors (Table I).
Levels of KNAT1 Transcript in PetE:KNAT1 Lettuce
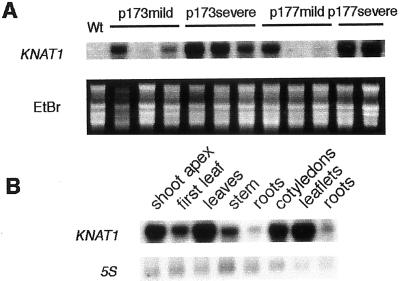
To determine whether RNA levels correlated with phenotypic severity, RNA from leaves was isolated from individual T1 173 and 177 plants and northern analysis was performed by using the KNAT1 cDNA as hybridization probe. All the severe phenotypes strictly correlated to high levels of KNAT1 expression (Fig. 4A), whereas the abundance of KNAT1 transcript was generally lower in the mild phenotypes, though some rare exceptions were observed (two examples of lack of correlation are shown in Fig. 4A).
Figure 4.
RNA gel-blot analysis of PetE::KNAT1 transgenic lines. A, Total RNA was isolated from rosette leaves of wild-type cv Luxor lettuce or T1 PetE::KNAT1 transformants with mild or severe leaf phenotype from two independent lines (p173 and p177). Ten micrograms of RNA was loaded per lane and probed with KNAT1 cDNA. Ethidium bromide staining is shown as control for loading. B, Total RNA from different tissues of PetE::KNAT1 homozygous 12-leaves-stage plants (first six lanes) or 4-leaves-stage plants (last three lanes). Ten micrograms of RNA was loaded per lane and probed with KNAT1 cDNA. 5S RNA was used as control for loading.
The Pea Plastocyanin Promoter Drives KNAT1 Expression Mostly in the Green Tissues of Transgenic Lettuce
Northern analysis was performed on different organs of PetE:KNAT1 seedlings and adult plants to verify the tissue specificity of the pea plastocyanin promoter (Fig. 4B). Although the strongest expression of the transgene was observed in all the green tissues at different developmental stages, a very weak expression was also detected in roots. To further inquire about tissue specificity conferred by the PetE plastocyanin promoter, transgenic lettuce plants harboring the PetE:β-glucuronidase (GUS) construct were produced and histochemical GUS localization was carried out. A strong activity was observed in the green tissues in accordance with the abundant products signaled in PetE:KNAT1 plants (data not shown). Moreover, GUS activity was detected in the tissue of transition between hypocotyl and root, whereas no staining was revealed in the remaining root. This fact may account for the weak signal observed in the northern analysis performed on PetE:KNAT1 plants.
PetE:KNAT1 Leaves Show Altered in Vitro Morphogenesis Response to Hormones and Delay in Senescence
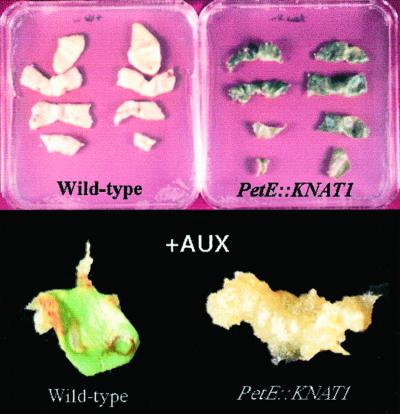
Explants from wild-type and PetE:KNAT1 leaves were cultivated at different concentration of auxin (naphthalene acetic acid [NAA]) and cytokinin (6-benzyne amino purine) to investigate whether the morphogenic response to hormones was altered in the transgenics. In the presence of increasing concentrations of cytokinin, no morphogenic response was observed in wild-type and transgenic plants (data not shown). On the contrary, remarkable differences appeared in the presence of NAA or in hormone-free medium (Fig. 5). After 9 d culture in NAA (1 mg L−1), leaf explants from PetE:KNAT1 plants with severe phenotype produced proliferating callus, whereas wild-type explants produced mainly differentiated roots. After several days, roots started to form from PetE:KNAT1 callus, probably due to the poor expression of the transgene in non-green tissues.
Figure 5.
Altered response to hormones of PetE::KNAT1 leaf explants. Leaf explants from wild-type lettuce and PetE::KNAT1 T2 homozygous plants were cultivated in vitro for 30 d in the absence of hormones (top) or in the presence of 1 mg L−1 NAA for 10 d. After 30 d without hormones, PetE::KNAT1 explants are still bright green, whereas wild-type leaves underwent to complete necrosis. In the presence of auxin, PetE::KNAT1 explants completely transform into callus, whereas wild-type leaves give rise exclusively to roots after 10 d.
In the absence of any hormone, PetE:KNAT1 leaf explants displayed a delay in chlorophyll degradation and maintained a bright green color, whereas wild-type leaf explants underwent complete necrosis in the same culture conditions (Fig. 5).
The Cytokinins Content Is Increased in the Leaves of PetE:KNAT1 Lettuce Plants
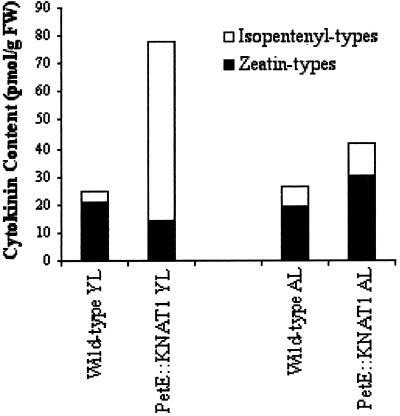
The content of isopentenyladenine (IP), isopentenyleadenosine (IPA), zeatine (Z), zeatine riboside (ZR), dihydrozeatin (DHZ), and dehydrozeatinriboside (DHZR) was measured (Dewitte et al., 1999) in the first leaf and in fully expanded adult leaves derived from wild-type and PetE:KNAT1 plants. The content of total Z types (Table II) in non-transformed lettuce plants was slightly higher in first leaves than in the adult samples, whereas the total content of isopentenyl types in first leaves was lower than that of adult leaves. However, the overall cytokinin content did not significantly differ between young first and adult leaves (24.9 and 26.7 pmol g−1 fresh weight, respectively). On the contrary, PetE:KNAT1 lettuce plants displayed a 2.5-fold higher accumulation of the total cytokinins in the young leaves with respect to adult samples. It is interesting that the total content of cytokinins was 3.13- and 1.58-fold higher than the wild type in PetE:KNAT1 young and adult leaves, respectively (Fig. 6). Isopentenyl-type cytokinins in PetE:KNAT1 young leaves were 16-fold higher than controls (Fig. 6), whereas Zs were slightly lower than those of the wild type. Therefore, the higher content of total cytokinins observed in PetE:KNAT1 young leaves with respect to wild type was due to an accumulation of isopentenyl-type cytokinins. In a converse manner, Z-type and isopentenyl-type cytokinins equally contributed to the 1.58-fold higher content of total cytokinins in PetE:KNAT1 adult leaves with respect to non-transformed lettuce.
Table II.
Cytokinin contenta in control (Luxor) and PetE::KNAT1 plants
| Leaf Stageb | Genotype | Zeatin Typesc | Isopentenyl Typesd |
|---|---|---|---|
| First | Luxor | 21.1 ± 0.2e | 3.8 ± 0.3 |
| Adult | Luxor | 19.3 ± 0.5 | 7.1 ± 0.1 |
| First | PetE::KNAT1 Luxor | 14.6 ± 0.5 | 63.4 ± 1.1 |
| Adult | PetE::KNAT1 Luxor | 30.6 ± 1.8 | 11.3 ± 0.1 |
Cytokinin content in picomoles per gram fresh wt of tissue.
First, First young leaves; Adult, fully expanded leaves. Ten first leaves from 10 plants from control or transgenics and randomly selected fragments from 10 fully expanded leaves from three different PetE::KNAT1 or wild-type plants were pooled.
Zeatin types include Z, ZR, DHZ, and DHZR.
Isopentenyl types include IP and IPA.
se (±) was calculated on four replicates.
Figure 6.
Analysis of cytokinin content in PetE::KNAT1young and adult leaves. Z-type (Z, ZR, DHZ, and DHZR) and isopentenyl-type (IP and IPA) cytokinin content was measured in leaves from adult and young leaves of wild-type and PetE::KNAT1 plants (graphic version of the data presented in Table II).
In summary, KNAT1 overexpression under the control of PetE promoter resulted in an accumulation of Z-type and isopentenyl-type cytokinin in adult leaves and in a strong accumulation of IP and IPA in young leaves.
Isopentenyl-Type Cytokinins Accumulate in Vascular Tissues of PetE:KNAT1 Plants in Proximity of the Neoforming Leaf-Like Structures
Immunocytolocalization of the cytokinins IP and Z with affinity-purified antisera (Dewitte et al., 1999) was carried out on aldehyde-fixed tissues of young leaves in expansion phase and adult leaves from PetE:KNAT1 and wild-type lettuce plants.
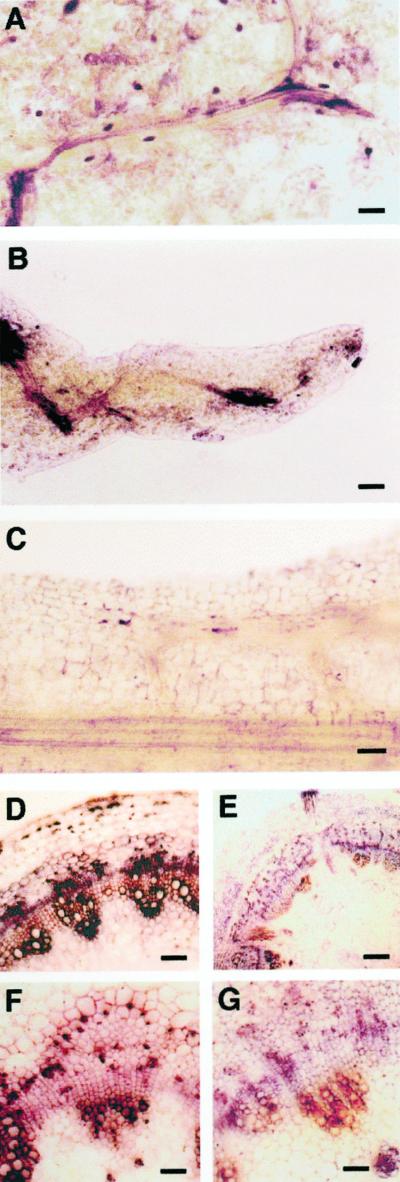
As for PetE:KNAT1 expanding leaves, strong signals corresponding to the IP-type cytokinins were observed in the vascular system, whereas IPs were barely detectable in the wild-type leaf vascular tissues (Fig. 7), which was in agreement with the analytical IP measurement (Table II). IP-type cytokinins accumulated in and/or were transported through the veins to the leaf margins where de novo leaf-like structures formed and developed, with a dense signal also observed in parenchyma cells located at the leaf margin. Figure 7 shows that IP-stained parenchyma cells are often found associated with neoforming vascular tissues in close vicinity to vascular elements at high IP level content. A significant accumulation of IP-type cytokinin, mainly located in the bundles, was also observed in the vases when transverse sections of the stems were analyzed (Fig. 7, D and F). In fully expanded leaves, IP-type signal in the inner tissues of the leaves did not significantly differ between transformed and control plants, although IP accumulation remained high in the vases of developing digits in PetE:KNAT1 leaves with respect to the margins of controls (data not shown). Wild-type and PetE::KNAT1 leaves showed no detectable IP in the epidermis layer, with the exception of stomata cells that accumulated high IP levels (data not shown). Z-type immunolocalization in transgenics and wild type resulted in comparably high saturating signals that impaired detection of quantitative differences (data not shown).
Figure 7.
Immunocytolocalization of the cytokinins IP in PetE::KNAT1 leaves and stem. Affinity-purified rabbit antisera were used for immunolocalization of IP in aldehyde-fixed tissues of expanding leaves from PetE:KNAT1 and wild-type lettuce plants. Samples were mounted in a phosphate-buffered saline (PBS)/glycerine mixture (1:1, v/v) and were immediately observed under a photomicroscope (Leica). A, PetE:KNAT1 leaf vascular strands close to the leaf margin. IP strongly accumulate at the vein branching point, in parenchymal cells associated with vascular strands, and in the numerous secretory cells that are present in transgenic leaves (dark elliptical dots in the figure). Size bar = 100 μm. B, PetE:KNAT1 outgrowing structure that forms from the leaf margin. IP accumulates in the vascular bundles at the base of the neoforming structures. Size bar = 100 μm. C, Longitudinal section of a wild-type leaf margin. IP is barely detectable in every tissue. Size bar = 60 μm. D, Transverse section of PetE:KNAT1 stem proximal to the shoot apex. IP is strongly accumulated in vascular bundles. Size bar = 50 μm. E, Transverse section of wild-type stem proximal to the shoot apex. IP is barely detectable in vascular tissues. Size bar = 200 μm. F, Transverse section of PetE:KNAT1 stem proximal to the transition zone between shoot and root. IP is still high in vascular bundles elements. Size bar = 25 μm. G, Transverse section of wild-type stem proximal to the transition zone between shoot and root. IP is still barely detectable in vascular tissues. Size bar = 25 μm.
DISCUSSION
Overexpression of KNAT1in Arabidopsis induces lobes that initiate in the position of leaf serrations together with the occasional formation of ectopic meristems in the sinus regions close to veins (Chuck et al., 1996). It has been hypothesized that these lobes are extensions of serrations rather than abnormal outgrowths and that they may result from a reduced growth rate of the sinus relative to the serration. Our data of KNAT1 overexpression in lettuce strongly support a different view of the mechanism by which KNAT1 acts to alter leaf shape and structure. In PetE:KNAT1 transgenics, the very first event after leaf initiation was a strong reduction of midvein elongation with the secondary and tertiary veins branching precociously and lacking a recognizable vein size order. At the leaf margins, the tips of the veins of PetE::KNAT1 transgenics formed leaf-like primordia, as also observed in the Arabidopsis 35S::KNAT1 transformants. However, instead of arresting their growth, they further developed into leaf-like structures during and after the leaf expansion phase in a reiterative process of leaf morphogenesis. In lettuce, this seems to be a stronger effect of KNAT1 overexpression in comparison with Arabidopsis and might be due to the very strong expression of KNAT1 conferred by the PetE promoter. A similar promoter-dependent effect has been observed in rice when effective overexpression of knox genes was only achieved with an actin promoter (Sentoku et al., 2000).
The absence or reduction of the palisade parenchyma layer is a common feature observed in Arabidopsis and lettuce transgenics. This, together with the induction of meristem-like structures and the maintenance/appearance of cambium initiation cells in vascular strands of adult leaves, confirms a role of KNAT1as an antagonist to cell differentiation. This hypothesis is further reinforced by the reduction in number of stomata and their complete disappearance in the vicinity of the PetE:KNAT1 leaf margins. Stomatal formation requires a series of tightly regulated cell divisions and multiple differentiation steps.
Several traits in PetE:KNAT1 plants, like early flowering response and loss of apical dominance with formation of several floral branches of different length, resemble those induced by an overproduction of cytokinins (Hewelt et al., 1994; Faiss et al., 1997; Roeckel et al., 1997; McKenzie et al., 1998; Rupp et al., 1999). PetE:KNAT1 leaf explants formed callus instead of roots in the presence of auxin alone and displayed a strong delay in senescence in the absence of any hormone in the culture medium. Moreover, PetE:KNAT1 transgenics showed a significantly higher overall content of Z-type and IP-type cytokinins than did the wild type. In adult leaves, cytokinins were almost double those measured in the wild type, although the Z still represented the main class of cytokinins, as observed in the wild type. It is most interesting that the first leaves of PetE:KNAT1 transgenics exhibited a strong increase in IP-type cytokinins and the Z/IP ratio was completely inverted with respect to wild type. Cytokinin synthesis and metabolic pathways are largely unknown, and it has only recently been suggested that meristematic tissues might be the primary sites of cytokinin synthesis. That rises the question whether the phenotypic alterations observed in PetE::KNAT1 are the effect or the cause of cytokinin accumulation.
The accumulation of IP-type cytokinins in the first leaf of the transgenics, where secondary meristems are not yet detectable but the venation pattern is already altered, strongly supports the view that KNAT1 could act through the modification of the specific cytokinin content. In the transgenics, IP accumulates in those tissues where major phenotypic alterations are observed (vascular bundles and the procambium extending toward the base of the neoforming meristematic digits). On the contrary, green tissues that highly express KNAT1 under the control of PetE promoter are mildly altered. This suggests that KNAT1 induces the observed phenotypes through cytokinins (directly or indirectly) that are transported to the sites of action. Auxin and cytokinins are known to interact synergistically and antagonistically to control many aspects of plant growth and differentiation (Coenen and Lomax, 1997). Several mutants and transgenically modified plants that over- or under-produce auxin and cytokinin show vascular differentiation abnormalities, leading to the hypothesis that both these hormones can influence the degree and type of vascular differentiation (for review, see Klee and Lanahan, 1995).
PetE:KNAT1 leaf veins elongate poorly before branching to give rise to new vascular strands leading to an ultra-branched altered vein network. This behavior is reminiscent of the phenomenon of loss of apical dominance in the stem and it could be due to the same kind of antagonistic interaction between auxin fluxes and cytokinin acting in the vascular elements of the stem and the leaf vein network. Cambium initiation cells, usually located in stem bundles undergoing secondary growth, have been found in association with differentiated vascular strands in the inner sectors of PetE::KNAT1 leaves. This is consistent with the indeterminate growth of PetE::KNAT1 leaf veins. Vascular cambium seems to originate from meristematic cells that differ from those of the primary meristem, as they are strongly committed to the initiation of vascular tissues (Steeves and Sussex, 1989). The presence of secondary meristems in PetE::KNAT1 leaves suggests that a specific cytokinin content and distribution can have a key role in the determination of whether vascular strands undergo limited or indefinite growth.
IPA is hypothesized to be the precursor of all forms of cytokinins, and the reactions of hydroxylation and reduction to form active Zs are thought to be very rapid (Letham, 1994). In this respect, the accumulation of IP-type cytokinins (more than 3-fold higher than Z) in young leaves of PetE::KNAT1 might suggest an involvement (direct or indirect) of KNAT1 in regulating specific steps of cytokinin metabolism such as the inhibition of the enzymatic conversion of IP into Z. On the other hand, PetE:KNAT1 adult leaves present a Z/IP ratio comparable with the wild type and the overall higher content of cytokinin suggests an increase of cytokinin synthesis or accumulation. This finding could reflect a difference in cytokinin metabolism in young versus fully expanded leaves and the accumulation of IP might be due to a limited availability of the enzymes that convert IP to Z in young tissues compared with adult ones. Moreover, KNAT1 could modify cytokinin metabolism through its interaction with other members of the KN1-like family, which are differentially expressed during leaf development. An indirect effect of KNAT1 on cytokinin metabolism and/or a need for other tissue-specific protein components could account for the lack of a root growth inhibition phenotype observed in Arabidopsis 35S::KNAT1 plants (Lincoln et al., 1994; Chuck et al., 1996).
Z has been shown to accumulate in old leaves of tobacco SAG::Kn1 in which senescence processes are delayed. This suggested a specific role of Z-type cytokinins in this process and is confirmed by our data concerning the delay of senescence displayed by explants from PetE:KNAT1 adult leaves that contain increased levels of IP- and Z-type cytokinins. The precise role of distinct cytokinin types in cell differentiation is still unknown. However, the results of this work suggest that a varying ratio between Z- and IP-type cytokinins may affect diverse processes during leaf development and growth. Since the endogenous KNAT1 down-regulation in the shoot apical meristem of Arabidopsis is strongly associated with leaf primordia initiation, an attractive hypothesis is that a tightly controlled IP/Z cytokinin ratio in local areas of shoot apical meristem may be necessary for the formation of leaf primordia. In this respect, kn-1 like genes could trigger the morphogenic response through regulating the local concentrations of IP versus Z.
The use of inducible systems and the isolation of the immediate target genes of knox transcription factors are required to further substantiate a direct role for KNAT1 in regulating cytokinins levels. We anticipate that these transgenic lettuce lines will be useful in further studies to uncover novel steps of cytokinins metabolism and the roles of cytokinins in cell differentiation.
MATERIALS AND METHODS
Plant Growth Conditions and Transformation
Lettuce (Lactuca sativa cv Luxor) seeds were cultivated in a growth chamber with 16-h light and 8-h dark periods at 23°C. Seven-day-old cotyledon explants were transformed by cocultivation with an LBA4404 Agrobacterium tumefaciens strain harboring the binary vector pVDH282 (a pBIN19 derivative), which contained the Arabidopsis KNAT1 cDNA under the control of the pea plastocyanin promoter PetE (Pwee and Gray, 1993; Helliwell et al., 1997). The transformation procedure was as described by Curtis et al. (1994). Transgenic plants were selected on 50 mg L−1 kanamycin-selective medium. Two PetE:KNAT1 plants displaying leaf shape alteration and single-copy gene insertion were selected (p173 and p177), 70 seeds from each primary transformant were germinated in soil without any selection, and they were analyzed from the germination onward. T2 seeds from T1 homozygous plants with a dramatic phenotype were germinated in greenhouse and were used for the further characterization of PetE:KNAT1 phenotype.
In Vitro Morphogenesis and Senescence Assays
Leaf explants of wild type and PetE:KNAT1 from 1-month-old in vitro-grown plants were plated on different concentrations of cytokinin and auxin alone (1 mg L−1 NAA) or in combination. For senescence assays, leaf explants from wild type and transgenics were plated in the absence of hormones for 30 d.
RNA Gel-Blot Analysis
Total RNA (10 μg) was electrophoresed on glyoxal and agarose (1% [w/v]) gel and alkaline downward-transferred onto a nylon membrane (Hybond N+, Amersham, Buckinghamshire, UK) as described in Frugis et al. (1999b). Filters were incubated in 0.5 m sodium phosphate, pH 7.2, 5% (w/v) SDS, and 10 mm EDTA overnight at 65°C with an α-32P dCTP-labeled full-length KNAT1 cDNA probe. Blots were washed in 0.1× hybridization solution for 5 min at room temperature and then for an additional 5 min at 55°C and were then exposed to film (BIOMAX, Eastman-Kodak, Rochester, NY).
Immunolocalization of Z and IP
Preparation and specificity testing of rabbit antiserum against Z and IP were performed as described by Dewitte et al. (1999). For pre-embedding immunolocalization, leaf tissues of PetE: KNAT1 and wild-type lettuce (n = 10 for each sample) were fixed in a 0.5% (v/v) gluteraldehyde and 3% (w/v) paraformaldehyde mixture in PBS (135 mm NaCl, 2.7 mm KCl, 1.5 mm KH2PO4, and 8 mm K2HPO4, pH 7.2). Thick sections (18–20 μm) were cut with a vibratome (VT1000E, Leica Microsystems, Wetzlar, Germany), collected in PBS on ice, and were pre-incubated (3 × 10 min) in blocking buffer (0.1% [v/v] fish gelatin, 0.5% [w/v] bovine serum albumin, 1% [v/v] normal goat serum, 20 mm Gly, and PBS) and then in a 0.07% (w/v) saponin/PBS solution for 20 min. Afterward, sections were incubated with primary antibody against Z and IP, in a dilution of 1:200 and 1:100, respectively, in blocking buffer at 4°C overnight, followed by 1 h at room temperature. After rinsing with PBS, alkaline phosphatase (AP)-conjugated sheep anti-rabbit immunoglobulins (1:100; Boehringer Mannheim, Basel) were used as secondary antibodies. Nitroblue tetrazolium and 5-bromo-4-chloro-3-indolylphosphate (Boehringer, Germany) were provided as substrates for the alkaline phosphatase (AP) and the reaction product was monitored after 5 min at room temperature. Samples were mounted in a PBS/glycerine mixture (1:1, v/v) and were immediately observed under a photomicroscope (Leica). Controls were the omission of the primary antibody and the replacement of primary antibody with blocked antibody prepared by incubation with Z and IP at several concentrations.
Light Microscopy
Leaves at different stages were fixed in formaldehyde-acetic acid (3.7% [w/v] formaldehyde, 50% [w/v] ethanol, and 5% [w/v] acetic acid in water), dehydrated in an ethanol series, and embedded. Sections were cut at 2 μm with a tungsten knife, mounted on slides, and stained with 0.1% (w/v) toluidine blue and periodic acid-Schiff's reagent.
Leaf Vascular Network Observations
For accurate description of leaf vascular alteration in PetE:KNAT1 transgenics, leaves at the same developmental stage from wild-type and PetE:KNAT1 plants were excised, cleared in 70% (w/v) ethanol, and photographed with a stereo microscope (Nikon-SMZ-U, Nikon, Tokyo) on a dark background. Slides were scanned with a SprintScan 35 Plus (Polaroid Corporation, Cambridge, MA) and the derived computer imported pictures were transformed in three-dimensional images with the emboss function of Adobe Photoshop 3.0 program (Adobe Systems, Mountain View, CA).
Extraction, Purification, and Analysis of Cytokinins
The extraction, purification, and measurement of cytokinins (IP, IPA, Z, ZR, DHZ, and DZHR) were carried out according to Dewitte et al. (1999). Ten first leaves (young leaves) derived from 10 distinct wild-type and PetE:KNAT1 plants were excised and frosted in liquid nitrogen. Randomly selected fragments of 10 fully expanded leaves (adult leaves) located in the rosettes of three individual transgenic and wild-type plants were pooled. One hundred milligrams of fresh weight was used to analyze the cytokinin content.
ACKNOWLEDGMENTS
We especially thank Luigi Santini for his precious support and technical assistance, Leen de Mos Seed Company for providing Luxor lettuce seeds, the Van Der Have Company for the PetE::KNAT1 construct, Dr. Mathias Zeidler for the helpful suggestions and comments on the manuscript, and Dr. Peter Hare for the English critical review of the discussion.
Footnotes
This work was supported by a grant from the European Community and by the Agriculture, Agro-Industry including Fisheries project “Lettuce for the Next Century” (contract no. 92–250). G.F. and D.G. were supported by postdoctoral fellowships from the Italian National Council of Research.
LITERATURE CITED
- Chan RL, Gago GM, Palena CM, Gonzalez DH. Homeoboxes in plant development. Biochim Biophys Acta. 1998;1442:1–19. doi: 10.1016/s0167-4781(98)00119-5. [DOI] [PubMed] [Google Scholar]
- Chuck G, Lincoln C, Hake S. KNAT1 induces lobed leaves with ectopic meristems when overexpressed in Arabidopsis. Plant Cell. 1996;8:1277–1289. doi: 10.1105/tpc.8.8.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coenen C, Lomax TL. Auxin-cytokinin interactions in higher plants: old problems and new tools. Trends Plant Sci. 1997;9:351–356. doi: 10.1016/S1360-1385(97)84623-7. [DOI] [PubMed] [Google Scholar]
- Curtis IS, Power JB, Blackhall NW, de Laat AMM, Davey MR. Genotype-independent transformation of lettuce using Agrobacterium tumefaciens. J Exp Bot. 1994;279:1441–1449. [Google Scholar]
- Dewitte W, Chiappetta A, Azmi A, Witters E, Strnad M, Rembur J, Noin M, Chriqui D, Van Onckelen H. Dynamics of cytokinins in apical shoot meristems of a day-neutral tobacco during floral transition and flower formation. Plant Physiol. 1999;119:111–122. doi: 10.1104/pp.119.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faiss M, Zalubilova J, Strnad M, Schmulling T. Conditional transgenic expression of the ipt gene indicates a function for cytokinins in paracrine signaling in whole tobacco plants. Plant J. 1997;12:401–415. doi: 10.1046/j.1365-313x.1997.12020401.x. [DOI] [PubMed] [Google Scholar]
- Frugis G, Giannino D, Mele G, Nicolodi C, Innocenti AM, Chiappetta A, Bitonti MB, Dewitte W, Van Onckelen H, Mariotti D. Are homeobox Knotted-like genes and cytokinins the leaf architects? Plant Physiol. 1999a;119:1–3. doi: 10.1104/pp.119.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frugis G, Mele G, Giannino D, Mariotti D. MsJ1, an alfalfa heat shock inducible DnaJ-like gene, is tissue specific and transcriptionally regulated during cell cycle. Plant Mol Biol. 1999b;40:397–408. doi: 10.1023/a:1006215231492. [DOI] [PubMed] [Google Scholar]
- Gehring WT. Homeoboxes in the study of development. Science. 1987;236:1245–1252. doi: 10.1126/science.2884726. [DOI] [PubMed] [Google Scholar]
- Hareven D, Gutfinger T, Parnis A, Eshed Y, Lifschitz E. The making of a compound leaf: genetic manipulation of leaf architecture in tomato. Cell. 1996;84:735–744. doi: 10.1016/s0092-8674(00)81051-x. [DOI] [PubMed] [Google Scholar]
- Helliwell CA, Webster CI, Gray JC. Light-regulated expression of the pea plastocyanin gene is mediated by elements within the transcribed region of the gene. Plant J. 1997;12:499–506. doi: 10.1046/j.1365-313x.1997.00499.x. [DOI] [PubMed] [Google Scholar]
- Hewelt A, Prinsen E, Schell J, Van Onckelen H, Schmulling T. Promoter tagging with a promoterless ipt gene leads to cytokinin-induced phenotypic variability in transgenic tobacco plants: implications of gene dosage effects. Plant J. 1994;6:879–891. doi: 10.1046/j.1365-313x.1994.6060879.x. [DOI] [PubMed] [Google Scholar]
- Hewelt A, Prinsen E, Thomas M, Van Onckelen H, Meins F., Jr Ectopic expression of maize knotted1 results in the cytokinin-autotrophic growth of cultured tobacco tissues. Planta. 2000;210:884–889. doi: 10.1007/s004250050693. [DOI] [PubMed] [Google Scholar]
- Kessel M, Gruss P. Murine developmental control genes. Science. 1990;249:374–379. doi: 10.1126/science.1974085. [DOI] [PubMed] [Google Scholar]
- Klee HJ, Lanahan MB. Transgenic plants in hormone biology. In: Davies PJ, editor. Plant Hormones: Physiology, Biochemistry and Molecular Biology. Ed 2. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1995. pp. 340–353. [Google Scholar]
- Letham DS. Cytokinins as phytohormones: sites of biosynthesis, translocation, and function of translocated cytokinin. In: Mok DWS, Mok MC, editors. Cytokinins, Chemistry, Activity and Function. Boca Raton, FL: CRC Press; 1994. pp. 57–80. [Google Scholar]
- Lincoln C, Long J, Yamaguchi J, Serikawa K, Hake S. A knotted1-like homeobox gene in Arabidopsis is expressed in the vegetative meristem and dramatically alters leaf morphology when overexpressed in transgenic plants. Plant Cell. 1994;6:1859–1876. doi: 10.1105/tpc.6.12.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie MJ, Mett V, Stewart Reynolds PH, Jameson PE. Controlled cytokinin production in transgenic tobacco using a copper-inducible promoter. Plant Physiol. 1998;116:969–977. doi: 10.1104/pp.116.3.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ori N, Juarez MT, Jackson D, Yamaguchin J, Banowetz GM, Hake S. Leaf senescence is delayed in tobacco plants expressing the maize homeobox gene knotted1 under the control of a senescence-activated promoter. Plant Cell. 1999;11:1073–1080. [PMC free article] [PubMed] [Google Scholar]
- Pwee KH, Gray JC. The pea plastocyanin promoter directs all-specific but not full light-regulated expression in transgenic tobacco plants. Plant J. 1993;3:437–449. doi: 10.1046/j.1365-313x.1993.t01-26-00999.x. [DOI] [PubMed] [Google Scholar]
- Reiser L, Sanchez-Baracaldo P, Hake S. Knots in the family tree: evolutionary relationships and functions of knox homeobox genes. Plant Mol Biol. 2000;42:151–166. [PubMed] [Google Scholar]
- Roeckel P, Oancia T, Drevet J. Effects of seed-specific expression of a cytokinin biosynthetic gene on canola and tobacco phenotypes. Transgenic Res. 1997;6:133–141. doi: 10.1023/a:1018425720949. [DOI] [PubMed] [Google Scholar]
- Rupp HM, Frank M, Werner T, Strnad M, Schmulling T. Increased steady-state mRNA levels of the STM and KNAT1 homeobox genes in cytokinin overproducing Arabidopsis thaliana indicate a role for cytokinins in the shoot apical meristem. Plant J. 1999;18:557–563. doi: 10.1046/j.1365-313x.1999.00472.x. [DOI] [PubMed] [Google Scholar]
- Sentoku N, Sato Y, Matsuoka M. Overexpression of rice OSH genes induces ectopic shoots on leaf sheaths of transgenic rice plants. Dev Biol. 2000;220:358–364. doi: 10.1006/dbio.2000.9624. [DOI] [PubMed] [Google Scholar]
- Sinha NR, Williams RE, Hake S. Overexpression of the maize homeobox gene KNOTTED-1 causes a switch from determinate to indeterminate cell fates. Genes Dev. 1993;7:787–795. doi: 10.1101/gad.7.5.787. [DOI] [PubMed] [Google Scholar]
- Smith LG, Greene B, Veit B, Hake S. A dominant mutation in the maize homeobox gene, Knotted-1, causes its ectopic expression in leaf cells with altered fates. Development. 1992;116:21–30. doi: 10.1242/dev.116.1.21. [DOI] [PubMed] [Google Scholar]
- Steeves TA, Sussex IM. Pattern in Plant Development. Ed 2. Cambridge, UK: Cambridge University Press; 1989. [Google Scholar]
- Tamaoki M, Kusaba S, Kano-Murakami Y, Matsuoka M. Ectopic expression of a tobacco homeobox gene, NTH15, dramatically alters leaf morphology and hormone levels in transgenic tobacco. Plant Cell Physiol. 1997;38:917–927. doi: 10.1093/oxfordjournals.pcp.a029252. [DOI] [PubMed] [Google Scholar]
- Vollbrecht E, Veit B, Sinha N, Hake S. The developmental gene Knotted-1 is a member of a maize homeobox gene family. Nature. 1991;350:241–243. doi: 10.1038/350241a0. [DOI] [PubMed] [Google Scholar]