Abstract
Molybdate, an oxidized form of molybdenum, facilitates molybdenum to be taken into cell, and thus to be included as a cofactor in the structure of enzymes necessary to ensure homeostasis. Although this compound provides the catalysis and electron transport of many biochemical reactions, it causes serious health problems in animals at high concentrations. For this reason, its recovery of water resources is one of the main subjects of scientific studies called bioremediaiton. One of the advantages of the remediation is that the biomass obtained from algae increases the amount of lipids, which are the raw material source for the biofuel production. For this purpose, the bioremediation abilities of Desmodesmus pannonicus and Scenedesmus aldavei algae were spectrophotometrically evaluated by using growth rate, chlorophyll-a, chlorophyll-b and total carotenoids for fourteen days. The bioremediation properties were also determined using Inductively coupled plasma - optical emission spectrometry (ICP-OES) analysis. D. pannonicus and S. aldavei algae have bioremediation capabilities up to 1 mg mL−1 Na2MoO4 concentration. The lipid content increased at all concentrations in S. aldavei and at 200 μg mL−1 in D. pannonicus. However, the Mo (VI) contents in dry mass changed depending on the increase of concentrations. Fourier Transform InfraRed Spectrometer analysis (FT-IR) was utilized to identify the alterations of specific functional groups such as carboxyl, amine, hydroxyl, and carbonyl in the samples. As a result, D. pannonicus and S. aldavei have great potential for Mo(VI) bioremediation. D. pannonicus and S. aldavei can tolerate Na2MoO4 up to 1 mg mL−1 concentrations and the lipid content used in biofuel production was increased during this process.
Keywords: Bioremediation, Chlorophyll-a content, Metal removal, Molybdate, Microalgae
Highlights
-
•
D. pannonicus and S. aldavei have a great potential for Mo(VI) bioremediation.
-
•
D. pannonicus and S. aldavei can be tolerant of NaMoO4 upto 1000 μg mL−1 concentrations.
-
•
The lipid content increase with Mo(VI) application.
-
•
Specific functional groups of the samples are changed with the application.
-
•
D. pannonicus and S. aldavei are very appropriate for the biotechnological usage.
1. Introduction
Molybdenum, being a nutrient included in the structure of enzymes necessary to perform many vital functions, exists in various oxidized forms, such as molybdate, depending on the pH [1]. Molybdate is taken into cells with two different carrier molecules and plays a vital role in ensuring homeostasis by binding to the molybdenum cofactor in algal and plant mechanisms [[2], [3], [4]]. In addition to being a part of the nitrogenase enzyme responsible for nitrogen fixation in Cyanobacteria, it also serves as an electron carrier in redox reactions in various bacterial metabolisms [5,6]. The metal has a value in various agricultural and industrial areas, such as use in ceramics, glass, metallurgy, food, pigments, fertilizers, catalysts, electronics, steel, and cosmetics [2,[7], [8], [9]]. Molybdenum can cause harmful health effects in animals because of its toxic effects on spermatogenesis and embryos. Chronic molybdenum poisoning in farm animals can lead to symptoms such as anemia, gastrointestinal disturbances, and bone disorders [10]. Molybdenum can lead to serious health problems above >10 ppm in the human body [11]. The contaminated metals should be remove from the drinking water resources.
In recent years, although electro-physicochemical methods have been researched to remove various heavy and toxic metals such as molybdate from water, these methods require a lot of cost and energy [12]. Also improved techniques produce waste products that are more harmful to the environment [13,14]. Phycoremediation is an enviro-friendly approach to enable a sustainable life instead of these methods. Algae, which constitute the primary material of phycoremediation, not only remove harmful compounds from the environment but also ensure the use of by-products in the industrial field and reduce the carbon footprint with their superior carbon dioxide capture performance [15,16]. By means of these creatures' superior adaptation properties, it is possible that the high-value biomass can be processed in biofuel production [17]. In addition, during heavy metal sorption from wastewater, algae remove not only the target metal but also N and P macronutrients being necessary for their primary metabolism due to including in the structure of compounds such as proteins, nucleic acids, and phospholipids [18].
Trenfield et al. [19] tested the toxicity of molybdenum in the microalgae Isochrysis galbana at three different temperatures: 24 °C, 28 °C, and 31 °C, but they found that Mo did not show toxicity to this species [20] found that the marine microalg Petalonia fascia had a high adsorption capacity (1376 ± 2 mg g−1) of the compound Mo(VI), at 20 °C and pH 1.0. They also found that hydroxyl and carboxylate functional groups were influential in the adsorption of molybdate anions. Tambat et al. [21] observed the molybdenum exposure on Chlorella sorokiniana TU5 and Picochlorum oklahomensis species for 14 days. The biomass of Chlorella sorokiniana TU5 and Picochlorum oklahomensis were 2.35 g L−1 and 115.30 mg L−1 and lipid yields were 579.3 mg L−1 and 65 mg L−1, respectively. They concluded that microalgal treatment can be used for molybdenum and other inorganic contaminants bioremediation at an industrial scale. Papapolymerou et al. [22] studied the effect of molybdenum metal on lipid distribution in the alga Microchloropsis gaditana. Tejada-Jimenez et al. (2024) investigated molybdenum metabolism in the alga Chlamydomonas reinhardtii. In the literature, the removal of the molybdate molecule from the environment has not been adequately explored and existent studies were not focused model organisms with high bioremediation properties in the research.
Desmodesmus pannonicus and Scenedesmus aldavei algae have been used in various biotechnological studies such as reducing carbon emissions, various bioremediation studies, and using the lipids they contain as biofuel [23,24,25]. However, the studies are so limited that the lipid content increase of these microalgae by some metals has not been surveyed. Also, the Mo uptake of these algae, which may be essential biosorbents for phycoremediation, and their effects on this metal have not been evaluated. This study hypothesizes that i) the algae Desmodesmus pannonicus and Scenedesmus aldavei, which contain important anionic compounds such as carboxyl, carbonyl, hydroxyl, and sulfhydryl in their cell walls, have a high ability to absorb the cationic Molybdenum metal ii) the amount of lipids in the cell rise according to Mo (VI) level.
The scarcity of studies has led to the necessity of studying new algae species and the existing lipid amounts of these algae species in order to prevent molybdenum pollution. In addition, the low toxicities of molybdenum on algae display that the bioremediation method may be suitable for the recovery of this metal. This study aims to determine the effects of molybdenum exposure on growth parameters and lipid contents of Desmodesmus pannonicus and Scenedesmus aldavei algae, as well as to reveal the cellular changes caused by the exposure and to evaluate the molybdenum absorption abilities of living organisms.
2. Material and methods
2.1. Culture conditions
The microalgae strain Desmodesmus pannonicus SAU001 and Scenedesmus aldavei SAU002 was cultured in Sakarya University Science Faculty Algal Physiology Laboratory to evaluate the uptake capability of Molybdenum (MoVI). Both the microalgae strains were incubated under autotrophic conditions in BG11 medium (pH:7.5; [26]) under the conditions of available radiation in 5000 lux 12:12 h light/dark cycle at 27 °C until reaching 500 mL culture. Cultures were reinoculate at 0.9–1 McFarland turbidity and Na2MoO4 (0, 200, 400, 600, 800 and 1000 μg mL−1) were added to the culture medium as final concentration. The application concentrations were selected over the environmental concentrations according to Darham et al. [27] to evaluate bioremediaiton capacity.
2.2. Cell Growth and Pigment Analysis
The optical density (OD750), chlorophyll-a, chlorophyll-b, and total carotenoid levels were daily (every 24 h) measured with the help of multiplate reader spectrophotometer to determine growth rate until 14th day [26]. This time was reflected to the all growth stages in culture media, and at the final day, these microalgae showed the most growth in an exponential phase. Chlorophyll-a, chlorophyll-b and total carotenoid content were calculated according to Riitche, (2008). Cellular growth was measured to determine the correlation of Mo(VI) removal with microalgae cell biomass every 24 h during the entire growth phase. All experiments included in Cell Growth and Pigment Analysis were performed with four replicates, and data were expressed as mean ± SE. EC50 values were calculated on the fifth day by Origin 8.5 program.
2.3. Analytical analysis
Analytical analyses were performed according to the modified method from Tambat et al. [21]. Adsorption of Molybdenum metal compounds and other metal ions were directly related to microalgae biomass. By reaching the logarithmic growth phase, maximum microbial biomass was obtained on the 14th day. At the end of the 14th day, 30 mL of the broth cultures were collected and centrifuged for 10 min at 5000 rpm using a centrifuge. The supernatant and microalgae biomass pellets were carefully separated in pre-measured tubes. After drying, biomass in pellets were processed with 5 mL aqua regia. Both supernatants and obtained from pellets were filtered using filter paper with a pore size of 0.22 μm. Sample analyses were done using an inductively coupled plasma optical emission spectrometer (ICP-OES, Thermo Scientific) and before analysis, the sensitivity and stability of the machine were optimized up to analytical range 8 ppm; the samples obtained from pellets were diluted up to 25-fold from the initial concentration for the analysis of metal ion concentration and the supernatants were measured directly. All experiments in analytical analysis were performed with six replicates and data were expressed as mean ± SE.
2.4. FTIR (Fourier Transform InfraRed Spectrometer analysis) spectroscopy
FTIR spectroscopic analyses were conducted according to the modified method from Tambat et al. [21] with Shimadzu IRAffinity-1S FT-IR to identify specific functional groups in the samples, such as carboxyl, amine, hydroxyl, and carbonyl.The pellets were prepared with a small amount of Mo-adsorbed cultures of Desmodesmus pannonicus SAU001 and Scenedesmus aldavei SAU002. At the end of the 14th day, 30 mL of the broth cultures were collected and centrifuged for 10 min at 5000 rpm using a centrifuge. The dried biomass was carefully crushed by mortars and pestles to mix with KBr at a ratio of 1:20. The analyses were carried out in the frequency range of 400–4000 cm−1 for transmission spectra.
2.5. Lipid estimation
Lipid estimation analyses were performed from the dried biomass obtained from the algal cultures at the 14th day, according to Tambat et al. [21]. At the final day, these microalgae showed the most growth in an exponential phase and the assessment is most appropriate to lipid estimation due to being the end of logistic growth. After measuring the algal biomass, the samples were extracted in 3.9 ml of a 1:2 v/v ratio of chloroform and methanol at 60 °C for 1 h. After incubation, 1.3 mL of chloroform and 2.0 mL of ddH2O (>18.3 MΩ) were mixed with samples to separate phase effectively and centrifuged at 4500 rpm and lower phases were collected in a pre-weighed glass tube and dried overnight in the hot air oven at 60 °C. The lipid contents were calculated according to Sim et al. [28]. All experiments included in lipid estimation were performed with six replicate and data were expressed as mean ± SE.
2.6. Statistical analysis
The experimental design was conducted to decrease mistakes and probability in assays with replicates given each procedure. The significances of a difference between mean values were determined by one-way ANOVA using the Tukey postdoc test in the SPSS 20.0 statistical program for Windows based a 95 % confidence level. Also, the Na+ effect on lipid content and Molybdenum bioremediation were analysed by Spearman and Pearson correlation. In the graphical presentations, the standart errors were given, and the alterations were marked with asterisks.
3. Results and discussion
3.1. Effects of molybdenum toxicity on growth parameters
The OD750 absorbance, chlorophyll-a, chlorophyll-b and total carotenoid pigments have been measured to determine the growth curves of Desmodesmus pannonicus and Scenedesmus aldavei algae under Na2MoO4 toxicity for 14 days, and the alterations being differed from the control group were given in Fig. 1, Fig. 2, Fig. 3, Fig. 4.
Fig. 1.
The (a) OD750 absorbance, (b) total caretoniod contents of Desmodesmus pannonicus algae under Na2MoO4 toxicity for 14 days.
Fig. 2.
The (a)chlorophyll-a and (b) chlorophyll-b pigments of Desmodesmus pannonicus algae under Na2MoO4 toxicity for 14 days.
Fig. 3.
The (a) OD750 absorbance, (b) total caretoniod contents of Scenedesmus aldavei algae under Na2MoO4 toxicity for 14 days.
Fig. 4.
The (a)chlorophyll-a and (b) chlorophyll-b pigments of Scenedesmus aldavei algae under Na2MoO4 toxicity for 14 days.
OD750 absorbance values displayed that Desmodesmus pannonicus alga decreased in all concentrations between the fourth day and the twelfth day, but the values increased significantly compared to the control at the 200, 400, 600, and 800 μg mL−1 concentrations after the 12th day. Na2MoO4 restricted the growth of Desmodesmus pannonicus at 1000 μg mL−1 concentration on 13th and 14th days (Fig. 1a). EC50 value is 438.99 ± 52.68 μg mL−1 for Desmodesmus pannonicus and 348.69 ± 71.78 μg mL−1 for Scenedesmus aldavei. Trenfield et al. [19] reported that EC50 value of Molybdenum was 6000 μg mL−1 on Isochrysis galbana. Mandal et al. [29] reported that Mo supplementation showed a positive effect on biomass yield of Chlorella sp. NC-MKM. The biomass yield of Chlorella sp. NC-MKM rise of 65 % is recorded at 0.09-μM Mo and 37 % 0.06 μM Mo supplemented condition compared to the control. These studies support the result in which the biomass increase can be achived with Mo addition and molybdenum was a low effect on the microalgae.
While Chlorophyll-a showed a significant change in all concentrations, the amount of Chlorophyll-b increased at the concentration of 200 μg mL−1 (Fig. 2a and b). The total carotenoids decreased at all concentrations (Fig. 1b). In the Na2MoO4 application on Scenedesmus aldavei, the growth decreased in all concentrations (Fig. 3a). The Chlorophyll-a content increased at 200 and 400 μg mL−1 concentrations on the 14th day but decreased at other concentrations (Fig. 4a). Chlorophyll-b values generally decreased with application (Fig. 4b). While the total carotenoid content increased at a concentration of 200 μg mL−1 between the 3rd and 9th days, a significant decrease was observed with the application on the other days (Fig. 4b). Papapolymerou et al. [22] found that molybdenum metal had a toxic effect on the growth of the alga Microchloropsis gaditana by affecting chlorophyll-a and other pigments. This may be due to the effect of the metal on the enzymes in chlorophyll and pigment synthesis or because this metal directly disrupts the structure of the pigments. The decrease in these vital pigments directly affects carbohydrate synthesis and inhibits growth.
There is an inverse relationship between pigments, especially caretonid and chlorophyll pigments, due to their ability to transform into each other. In the life process, the nutrient uptake depletion and other physiological conditions caused the decrease of chlorophyll content but carotenoid production may continue. For this reason, instantaneous measurements may not detect chlorophyll pigment in culture. In Fig. 4, therefore, starting from the ninth day, carotenoids increased instead of chlorophyll pigments decreased [30,31].
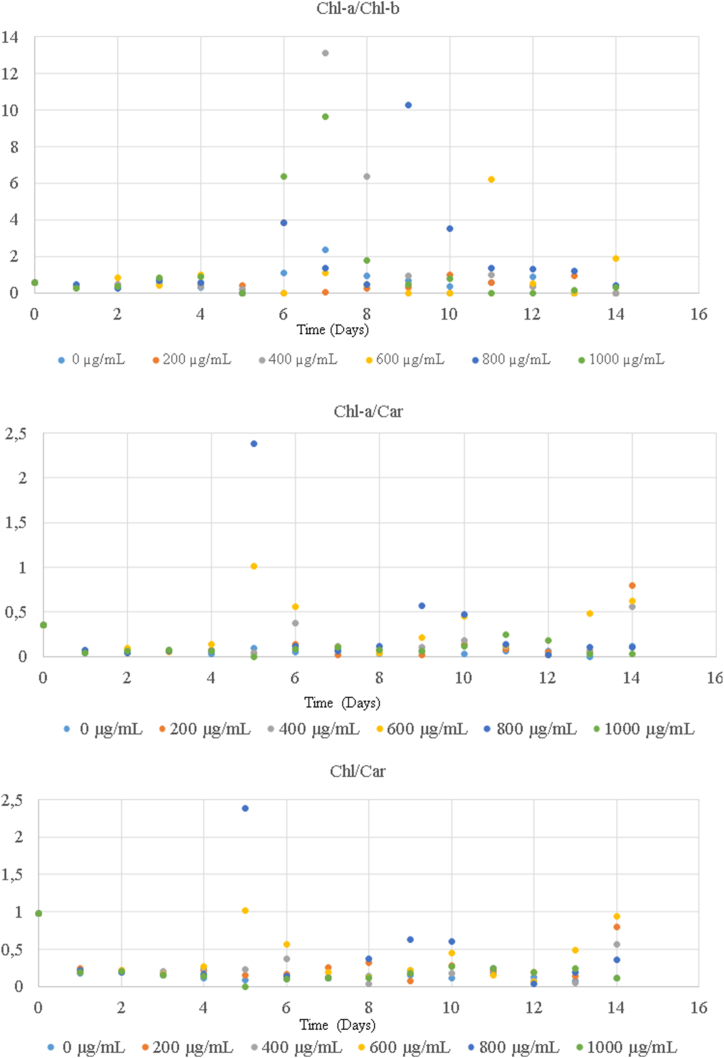
The ratio of carotenoid to chlorophyll molecules is noteworthy as molecular probes in evaluating of plant growth and performance. Carotenoides use light energy at different wavelengths from the chlorophyll pigment spectrum and transfer it to this pigment. It also plays a role in extinguishing the chlorophyll pigment before ROS formation. For this reason, the amount and activity of the pigment are highly affected by the environmental conditions [32]. Additionally, the excess of chl-a/chl-b, which is one of the indicators of stress against developmental stages, fertilizers, chemicals, moisture, and other environments, is positively correlated with the ratio of PSII molecule to the light-harvesting chlorophyll-protein complex [33]. In this study, the chl-a/chl-b ratio algae increased in Scenedesmus aldavei with the application. Due to a linear relationship between the formation of light-harvesting complex II (LHCII) aggregates and non-photochemical quenching, the chl:car ratio can be used to assess increase in LHCII (34,[24], [34], [35], [36], [37]). The values of some datasets published online are 2.99 in the LOPEX dataset and 3.45 in the HAWAII dataset [38]. These indicators imply that the dataset used in this study includes measurements under high-stress environments (Fig. 5, Fig. 6).
Fig. 5.
The pigment ratios (a) chla/chlb ratio of Desmodesmus pannonicus (b) chla/car ratio of Desmodesmus pannonicus (c) chl/car ratio of Desmodesmus pannonicus.
Fig. 6.
The pigment ratios (a) chla/chlb of Scenedesmus aldavei (b) chla/car of Scenedesmus aldavei (c)chl/car of Scenedesmus aldavei.
3.2. Molybdenum bioremediation and FTIR analysis
The data of the analyses performed on the 14th day in both supernatants and samples obtained from biomass to investigate the ability of Desmodesmus pannonicus and Scenedesmus aldavei algae to biosorb Mo (VI) were given in Fig. 7, Fig. 8, respectively. While Desmodesmus pannonicus removed the Mo(VI) 60.49 % at 800 μg mL−1 and Scenedesmus aldavei has a great potential at all concentrations between 98 and 99 % and Na+cations might affect the molybdenum bioremediation (Supp Material). Furthermore, the increase of OH− due to Molybdate can be a problem, but it may be buffered in the culture resolution.
Fig. 7.
The bioremediation of Mo(VI) metals in (a) sample of Desmodesmus pannonicus dried biomass (b) sample from Desmodesmus pannonicus supernatant.
Fig. 8.
The bioremediation of Mo(VI) metals in (a) sample of Scenedesmus aldavei dried biomass (b)sample from Scenedesmus aldavei supernatant under Na2MoO4 toxicity.
Kafshgari et al. [39] investigated that Molybdenum (VI) biosorption process in marine algae Cystoseria indica. They observed that the maximum Mo (VI) biosorption capacity was 18.32 mg g−1. When the initial concentration of Mo (VI) solution was increased from 30 to 95 mL min−1, the adsorption capacity changed from 18.32 (61 %) to 30.19 (38 %) mg g−1. Lou et al. [40] performed the Comparative semi-automated analysis (CAG gel) to describe the Mo(VI) adsorption equilibrium in Laminaria japonica and the maximum adsorption capacity for Mo(VI) was calculated as 65.90 mg g−1. Johansson et al. [41] found that algal-based biosorbents from Gracilaria and Oedogonium had adsorbtion capacities of Mo as 67.4–78.5 mg g−1, respectively. Tambat et al. [21] found that Chlorella sorokiniana TU5 and Picochlorum oklahomensis had bioremediation ability for molybdenum and the max removal efficiency of dry algae biomass was calculated as 57.8 % with 115.65 mg L−1 initial Mo(VI) concentration. These studies supports the our result that Scenedesmus aldavei and Desmodesmus pannonicus algae had a great potential removal of Mo(VI).
Molybdenum uptake should have been important for surviving. It is considered that this metal was important to adjust the osmotic regulation, cell membrane nature, and enzymatical progress.
Microorganisms such as bacteria, fungi, or algae can absorb heavy metals at very low concentrations due to the functional groups they contain, such as amides, amines, carbonyls, and carboxyls. These functional groups make them effective in biosorption applications. The biosorption process is influenced by factors such as the characteristic enzymatic profiles of microorganisms, steric and conformational factors, as well as the number and presence of reactive sites. Some microorganisms can convert heavy metals into less toxic compounds and utilize them for their growth [42].
FTIR analysis was used to identify specific functional groups in the samples, such as carboxyl, amine, hydroxyl, and carbonyl. When examining the spectra, the peak intensity between 2985 and 3690 cm −1 corresponds to the stretching vibrations of O-H and N-H bonds, respectively [43]. The 2810-2980 cm−1 peaks represent -CH3 asymmetric and -CH3 symmetric stretching vibrations [[44], [45]]. Bands in the 1715–1770 cm −1 range are related to C=O stretching vibrations, peaks between 1575 and 1710 cm−1 indicate the presence of the amide I protein band, and 1480- 1575 cm−1 bands indicate the presence of the amide II protein band, while peaks between 1265 and 1484 cm−1 belong to the amide III protein band [46]. The 1136–1191 cm −1 range confirms the presence of -COH groups of polysaccharides and P=O stretching vibrations of nucleic acids. The peaks at 951-1136 cm−1 again show the presence of C-O-C and C-OH groups of polysaccharides. The peak intensity between 400 and 950 cm−1 corresponds to the peaks of the C-H group outside the vibrational plane [[47], [48], [49]]. The fingerprint region of distinctive spectra of Scenedesmus aldavei and Desmodesmus pannonicus were presented in Fig. 9 and Table 1.
Fig. 9.
FT-IR spectra of a) Desmodesmus pannonicus control b) Scenedesmus aldavei control c) Desmodesmus pannonicus Na2MoO4 under teratment d) Scenedesmus aldavei under Na2MoO4 teratment.
Table 1.
Assignments of absorbance bands in FT-IR spectra.
| Wavelengths (cm−1) | Functional group | Desmodesmus pannonicus (control) | Scenedesmus aldavei (control) | Desmodesmus pannonicus (Na2MoO4 application) | Scenedesmus aldavei (Na2MoO4 application) |
|---|---|---|---|---|---|
| 2985–3690 | Water stretching (-OH); Protein stretching (-NH) | 3280 | 3280 | 3280 | 3280 |
| 2810–2980 | -CH3 asymmetric and -CH3 symmetric | 2922–2851 | 2922–2851 | 2922–2851 | 2922–2851 |
| 1715–1770 | Stretching (C=O) | 1743 | 1743 | 1743 | 1743 |
| 1575–1710 | Amid I protein band | 1638 | 1638 | 1638 | 1638 |
| 1480–1575 | Amid II protein band | 1536 | 1536 | 1536 | 1536 |
| 1265–1484 | Amid III protein band | 1363 | 1391 | 1378 | 1394 |
| 1136–1191 | Polysaccharides carbohydrate (-COH); Nucleic acid with stretching of phosphodiester (P=O). | 1144 | 1150 | 1147 | 1150 |
| 951–1136 | Polysaccharides carbohydrates (C-O-C and C-OH) | 1026 | 1026 | 1026 | 1023 |
| 400–950 | Outside the vibrational plane (CH) | 826–560 | 826–572 | 826–560 | 826–572 |
3.3. Lipid estimation
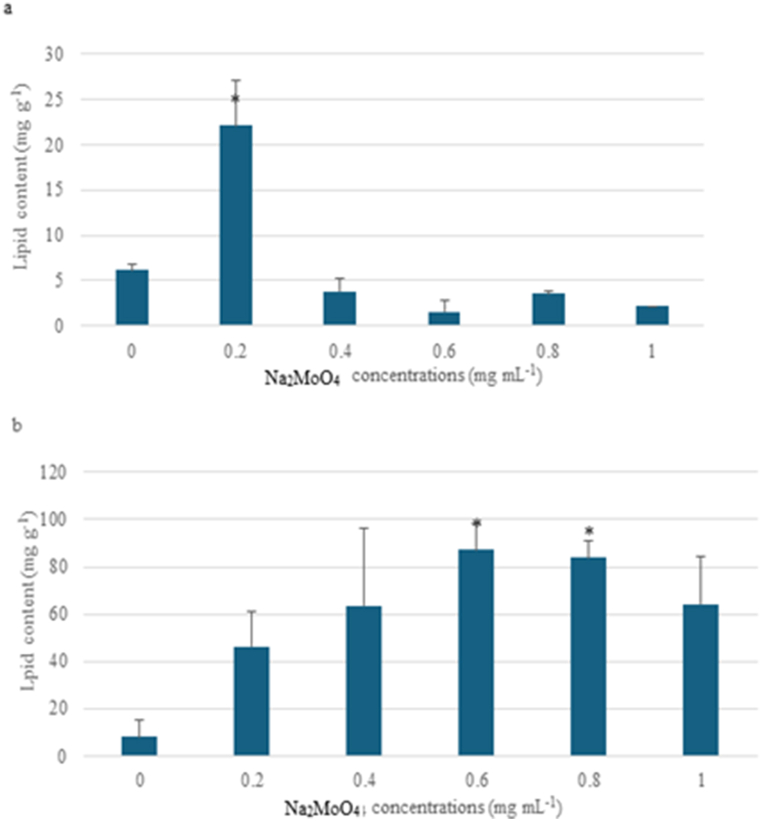
Algae have great potential for biofuel use due to their high lipid content. When the control groups were compared, it was observed that the lipid content of Scenedesmus aldavei (8.13 mg g−1) algae was more than Desmodesmus pannonicus (6.14 mg g−1). While the highest lipid content (22.07 mg g−1) was at 200 μg mL−1 concentration for Desmodesmus pannonicus, the highest lipid content (87.15 mg g−1) was at 600 μg mL−1 concentration for Scenedesmus aldavei (Fig. 10). Na+ cations effected the Molibdenum bioremediation at 0.01 confidence level (Sup Material)
Fig. 10.
Lipid content of a) Desmodesmus pannonicus b) Scenedesmus aldavei under Na2 MoO4 toxicity.
Rathore et al. [50] observed that molybdenum deficiency caused a 1.4-fold increase in total lipid of Anabaena doliolum. Molybdenum deficiency caused an increase in the cell content of digalactosyl diacylglycerol and phosphatidylglycerol, and a decrease in the lipids of monogalactosyl diacylglycerol and sulfocinovosyl diacylglycerol. They have reported that the unsaturated C18 fatty acids concentration was lower in Mo-deficient cells. Mandal et al. [29] found that Chlorella sp. NC-MKM algae had an increase in lipid yield (70.3 % increase) and neutral lipid amount (24.32 % increase) with Mo application compared to the control. Tambat et al. (2021) studied that molybdenum effected the lipid yield of Chlorella sorokiniana TU5 and Picochlorum oklahomensis as 232.5 (23.25 %) and 246.5 (24.65 %) mg g−1, respectively. However, they reported that high molybdenum concentrations had no effect on the accumulation of lipid content in microalgae cells due to the stress conditions experienced by molybdenum. Papapolymerou et al. [22] found that Molybdenum metal caused an increase in lipid contents on the alga Microchloropsis gaditana at 19.9 mg g−1 concentration compared to the control. These studies support the results that molybdenum caused an alteration in the lipid amount of Scenedesmus aldavei and Desmodesmus pannonicus algae. The molybdenum metal should have intrupted the lipid synthesis process and enzymes.
The study methodology provides sufficient data to understand the bioremediation capabilities of algae. While the growth parameters are fully elucidated to determine the effects of the material on algae, FT-IR analysis reveals the current changes caused by molybdenum metal in algal biomass. ICP-OES is sufficient to understand the presence of metal in existing biomass. The study methodology adapted and developed for algae can be used in further studies.
Additionally, Song et al. [51] reported that phytohormones and cold devices were effective in microalgal lipid production and the removal of HMs, and thus the effects of various heavy metals are reduced in microalgae. Also Song et al. [52] found chemicals such as glycine betaine increased both biomass content and lipid by balancing the antioxidant content. Considering the result of these studies, future studies need to be planned considering both phytohormones and different environmental conditions. Future studies need to be planned considering both phytohormones and different environmental conditions.
4. Conclusion
The growth curves such as OD750 absorbance, total carotenoid, chlorophyll-a, and chlorophyll-b pigments of Desmodesmus pannonicus and Scenedesmus aldavei algae under Na2MoO4 toxicity differed from the control group for 14 days. Desmodesmus pannonicus and Scenedesmus aldavei algae have bioremediation capability up to 1 mg mL−1 Na2MoO4 concentration and the absorption of Mo(VI) ion content increased with the applications. Lipid contents were increased with the application in Scenedesmus aldavei at all concentrations and Desmodesmus pannonicus at 200 μg mL−1 concentrations. These concentration range presents these microorganisms have more bioremediation potential than literature. FTIR analysis showed that the specific functional groups such as carboxyl, amine, hydroxyl, and carbonyl in the samples were changed with the applications. As a result, high concentrations of molybdate affected both the growth rates and micro and macronutrient uptake capacities of the two algae.
CRediT authorship contribution statement
Celal Caner: Writing – original draft, Investigation. Dilara Erdaği: Investigation, Funding acquisition. Büşranur Şeker: Investigation. Hüseyin Altundağ: Writing – original draft. Gizem Çeti̇n: Investigation. Hatice Tunca: Writing – review & editing, Writing – original draft, Supervision, Resources, Investigation, Formal analysis, Data curation, Conceptualization.
Financial interests
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.
Ethical approval
The study does not contain animal or human experiments.
Data availability statements
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Funding
This study was supported by Sakarya University Research Projects under Grant no. TUBİTAK 1919B012307149.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Nie Z., Li J., Liu H., Liu S., Wang D., Zhao P., Liu H. Adsorption kinetic characteristics of molybdenum in yellow-brown soil in response to pH and phosphate. Open Chem. 2020;18(1):663–668. [Google Scholar]
- 2.Rana M., Bhantana P., Sun X.C., Imran M., Shaaban M., Moussa M.…Hu C.X. Molybdenum as an essential element for crops: an overview. Int. J. Sci. Res. Growth. 2020;24(18535) [Google Scholar]
- 3.Roychoudhury A., Chakraborty S. Plant Nutrition and Food Security in the Era of Climate Change. Academic Press; 2022. Cobalt and molybdenum: deficiency, toxicity, and nutritional role in plant growth and development; pp. 255–270. [Google Scholar]
- 4.Tejada-Jimenez M., Leon-Miranda E., Llamas A. Chlamydomonas reinhardtii—a Reference microorganism for eukaryotic molybdenum metabolism. Microorganisms. 2023;11(7):1671. doi: 10.3390/microorganisms11071671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Darnajoux R., Bradley R., Bellenger J.P. In vivo temperature dependency of molybdenum and vanadium nitrogenase activity in the heterocystous cyanobacteria Anabaena variabilis. Environ. Sci. Technol. 2022;56(4):2760–2769. doi: 10.1021/acs.est.1c05279. [DOI] [PubMed] [Google Scholar]
- 6.Pau R.N., Klipp W., Leimkühler S. Transition Metals in Microbial Metabolism. CRC Press; 2022. Molybdenum transport, processing and gene regulation; pp. 217–234. [Google Scholar]
- 7.Gao S., Cui X., Kang S., Ding Y. Sustainable applications for utilizing molybdenum tailings in concrete. J. Clean. Prod. 2020;266 [Google Scholar]
- 8.Liu X.R., Sheng X.X., Yuan X.Y., Liu J.K., Sun X.W., Yang X.H. Research on correlation between corrosion resistance and photocatalytic activity of molybdenum zinc oxide modified by carbon quantum dots pigments. Dyes Pigments. 2020;175 [Google Scholar]
- 9.Shaaban K.S., Alotaibi B.M., Alrowaili Z.A., Al-Buriahi M.S., Ashour A., Yousef S. Thermal and mechanical studies of cerium molybdenum borosilicate glasses and glass–ceramics. Silicon. 2023;15(12):5233–5243. [Google Scholar]
- 10.Ermakov V., Safonov V., Dogadkin D. Characteristic features of molybdenum, copper, tungsten and rhenium accumulation in the environment. Innovat. Infrastruct. Solut. 2021;6:1–7. [Google Scholar]
- 11.Wrzecińska M., Kowalczyk A., Cwynar P., Czerniawska-Piątkowska E. Disorders of the reproductive health of cattle as a response to exposure to toxic metals. Biology. 2021;10(9):882. doi: 10.3390/biology10090882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yadav M., Singh G., Jadeja R.N. Pollutants and Water Management: Resources, Strategies and Scarcity. 2021. Physical and chemical methods for heavy metal removal; pp. 377–397. [Google Scholar]
- 13.Yang L., Hu W., Chang Z., Liu T., Fang D., Shao P.…Luo X. Electrochemical recovery and high value-added reutilization of heavy metal ions from wastewater: recent advances and future trends. Environ. Int. 2021;152 doi: 10.1016/j.envint.2021.106512. [DOI] [PubMed] [Google Scholar]
- 14.Kumar R., Liu C., Ha G.S., Kim K.H., Chakrabortty S., Tripathy S.K.…Jeon B.H. A novel membrane-integrated sustainable technology for downstream recovery of molybdenum from industrial wastewater. Resour. Conserv. Recycl. 2023;196 [Google Scholar]
- 15.Ankit, Bauddh K., Korstad J. Phycoremediation: use of algae to sequester heavy metals. Hydrobiol. (Sofia) 2022;1(3):288–303. [Google Scholar]
- 16.Koul B., Sharma K., Shah M.P. Phycoremediation: a sustainable alternative in wastewater treatment (WWT) regime. Environ. Technol. Innovat. 2022;25 [Google Scholar]
- 17.Asiandu A.P., Wahyudi A. Phycoremediation: heavy metals green-removal by microalgae and its application in biofuel production. J. Environ. Treat. Techniq. 2021;9(3):647–656. [Google Scholar]
- 18.de Siqueira Castro J., Calijuri M.L., Mattiello E.M., Ribeiro V.J., Assemany P.P. Algal biomass from wastewater: soil phosphorus bioavailability and plants productivity. Sci. Total Environ. 2020;711 doi: 10.1016/j.scitotenv.2019.135088. [DOI] [PubMed] [Google Scholar]
- 19.Trenfield M.A., van Dam J.W., Harford A.J., Parry D., Streten C., Gibb K., van Dam R.A. Aluminium, gallium, and molybdenum toxicity to the tropical marine microalga Isochrysis galbana. Environ. Toxicol. Chem. 2015;34(8):1833–1840. doi: 10.1002/etc.2996. [DOI] [PubMed] [Google Scholar]
- 20.Carnevale B., Blanes P., Sala L., Bellu S. Removal of molybdate anions from contaminated waters by brown algae biomass in batch and continuous processes. J. Chem. Technol. Biotechnol. 2016;92(6):1298–1305. doi: 10.1002/jctb.5124. [DOI] [Google Scholar]
- 21.Tambat V.S., Tseng Y.S., Kumar P., Chen C.W., Singhania R.R., Chang J.S.…Patel A.K. Effective and sustainable bioremediation of molybdenum pollutants from wastewaters by potential microalgae. Environ. Technol. Innovat. 2023;30 [Google Scholar]
- 22.Papapolymerou G., Karapanagiotidis I.Τ., Katsoulas N., Metsoviti M.N., Gkalogianni E.Z. Biomass productivity of Microchloropsis gaditana cultivated in a variety of modes and effect of cobalt and molybdenum on its lipid distribution. Green Energy Sustain. 2023;3(2) [Google Scholar]
- 23.Allard B., Templier J. Comparison of neutral lipid profile of various trilaminar outer cell wall (TLS)-containing microalgae with emphasis on algaenan occurrence. Phytochemistry. 2000;54(4):369–380. doi: 10.1016/s0031-9422(00)00135-7. [DOI] [PubMed] [Google Scholar]
- 24.Kumar R., Goswami G., Debnath D., Sinha A., Das D. Screening and evaluation of novel microalga Desmodesmus pannonicus CT01 for CO2 sequestration potential and aqua feed application. Biomass Convers. Bio. 2024;14(5):6347–6358. [Google Scholar]
- 25.Balouch H., Zayadan B.K., Sadvakasova A.K., Kossalbayev B.D., Bolatkhan K., Gencer D.…Allakhverdiev S.I. Prospecting the biofuel potential of new microalgae isolates. Int. J. Hydrogen Energy. 2023;48(50):19060–19073. [Google Scholar]
- 26.Rippka R., Deruelles J., Waterbury J.B., Herdman M., Stanier R.Y. Generic assignments, strain histories and properties of pure cultures of cyanobacteria. Microbiology. 1979;111:1–61. [Google Scholar]
- 27.Darham S., Syed-Muhaimin S.N., Subramaniam K., Zulkharnain A., Shaharuddin N.A., Khalil K.A., Ahmad S.A. Optimisation of various physicochemical variables affecting molybdenum bioremediation using antarctic bacterium, arthrobacter sp. Strain AQ5-05. Water. 2021;13(17):2367. [Google Scholar]
- 28.Sim S.J., Joun J.M., Hong M.E., Patel A.K. Split mixotrophy: a novel mixotrophic cultivation strategy to improve mixotrophic effects in microalgae cultivation. Bioresour. Technol. 2019;291 doi: 10.1016/j.biortech.2019.121820. [DOI] [PubMed] [Google Scholar]
- 29.Mandal M.K., Saikia P., Chanu N.K., Chaurasia N. Modulation of lipid content and lipid profile by supplementation of iron, zinc, and molybdenum in indigenous microalgae. Environ. Sci. Pollut. Control Ser. 2019;26:20815–20828. doi: 10.1007/s11356-019-05065-6. [DOI] [PubMed] [Google Scholar]
- 30.Varaprasad D., Raga Sudha N., Nazaneen Parveen S., Chandrasekhar T. Effect of various solvents on chlorophyll and carotenoid extraction in green algae: Chlamydomonas reinhardtii and Chlorella vulgaris. Annal Plant Soil Res. 2019;21(4):341–345. [Google Scholar]
- 31.Rinawati M., Sari L.A., Pursetyo K.T. Chlorophyll and carotenoids analysis spectrophotometer using method on microalgae. IOP Conf. Ser. Earth Environ. Sci. 2020;441(1) [Google Scholar]
- 32.Srivastava R. Physicochemical, antioxidant properties of carotenoids and its optoelectronic and interaction studies with chlorophyll pigments. Sci. Rep. 2021;11(1) doi: 10.1038/s41598-021-97747-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Terashima I., Hikosaka K. Comparative ecophysiology of leaf and canopy photosynthesis. Plant Cell Environ. 1995;18:1111–1128. 1995. [Google Scholar]
- 34.Embry J.L., Nothnagel E.A. Leaf senescence of postproduction poinsettias in low-light stress. J. Am. Soc. Hortic. Sci. 1994;119:1006–1013. [Google Scholar] [CrossRef] [Green Version] [Google Scholar]
- 35.Melanıe A.T., Joost W., Dam A.J.H., Davıd Parry. Aluminium, gallium, and molybdenum toxicity to the tropical marine microalga Isochrysis galbana. Environ. Toxicol. Chem. 2015;34:1833–1840. doi: 10.1002/etc.2996. [DOI] [PubMed] [Google Scholar]
- 36.Ritchie R.J. Universal chlorophyll equations for estimating chlorophylls a, b, c, and d and total chlorophylls in natural assemblages of photosynthetic organisms using acetone, methanol, or ethanol solvents. Photosynthetica. 2008;46:115–126. [Google Scholar]
- 37.Tang Y.L., Wen X.G., Lu Q.T., Yang Z.P., Cheng Z.K., Lu C.M. Heat stress induces an aggregation of the light-harvesting complex of photosystem II in spinach plants. Plant Physiol. 2007;143:629–638. doi: 10.1104/pp.106.090712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sonobe R., Yamashita H., Mihara H., Morita A., Ikka T. Estimation of leaf chlorophyll a, b and carotenoid contents and their ratios using hyperspectral reflectance. Rem. Sens. 2020;12(19):3265. [Google Scholar]
- 39.Kafshgari F., Keshtkar A.R., Mousavian M.A. Study of Mo (VI) removal from aqueous solution: application of different mathematical models to continuous biosorption data. Iran. J. Environ. Health Sci. Eng. 2013;10:1–11. doi: 10.1186/1735-2746-10-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lou Z., Wang J., Jin X., Wan L., Wang Y., Chen H.…Xiong Y. Brown algae based new sorption material for fractional recovery of molybdenum and rhenium from wastewater. Chem. Eng. J. 2015;273:231–239. [Google Scholar]
- 41.Johansson C.L., Paul N.A., de Nys R., Roberts D.A. Simultaneous biosorption of selenium, arsenic and molybdenum with modified algal-based biochars. J. Environ. Manag. 2016;165:117–123. doi: 10.1016/j.jenvman.2015.09.021. [DOI] [PubMed] [Google Scholar]
- 42.Kapahi M., Sachdeva S. Bioremediation options for heavy metal pollution. J Health Pollut. 2019;9(24) doi: 10.5696/2156-9614-9.24.191203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Waqar R., Rahman S., Iqbal J., Kaleem M., Minhas L.A., Ullah N., Kausar F., Chalgham W., Al-Misned F.A., El-Serehy H.A., et al. Biosorption potential of Desmodesmus sp. for the sequestration of cadmium and lead from contaminated water. Sustainability. 2023;15 doi: 10.3390/su151511634. [DOI] [Google Scholar]
- 44.Moraes Leonardo C., Figueiredo Rute C., Ribeiro-Andrade Rodrigo, Pontes-Silva Augusto V., Arantes Mônica L., Giani Alessandra, Figueredo Cleber C. High diversity of microalgae as a tool for the synthesis of different silver nanoparticles: a species-specific green synthesis. Coll. Inter. Sci. Commun. 2021;42 doi: 10.1016/j.colcom.2021.100420. [DOI] [Google Scholar]
- 45.Moraes L.C., Figueiredo R.C., Ribeiro-Andrade R., Pontes-Silva A.V., Arantes M.L., Giani A., Figueredo C.C. High diversity of microalgae as a tool for the synthesis of different silver nanoparticles: a species-specific green synthesis. Coll. Inter. Sci. Commun. 2021;42 [Google Scholar]
- 46.Jing W., Li G., Guangzhu S., Xing Y., Ming L. The role of adsorption in microalgae biological desalination: salt removal from brackish water using Scenedesmus obliquus. Desalination. 2020;493 doi: 10.1016/j.desal.2020.114616. [DOI] [Google Scholar]
- 47.Ferraro G., Toranzo R.M., Bagnato C., Jousse M.G., Areco M.M., Bohé A.…Curutchet G. Native Desmodesmus sp. and Chlorella sp. isolated from the Reconquista River display a different binding preference for Cu (II) and Zn (II) J. Environ. Manag. 2021;293 doi: 10.1016/j.jenvman.2021.112835. [DOI] [PubMed] [Google Scholar]
- 48.Gislayne S., Santos J., Glauber C., Aluísio A.C., Glauco V.P.B., Ramón R.P.G., Ulisses N.M., Wolia C.G. Biotechnological investigation of Pediastrum boryanum and Desmodesmus subspicatus microalgae species for a potential application in bioenergy. Algal Res. 2023;75(2023) [Google Scholar]
- 49.Singh R.P., Yadav P., Kumar A., Hashem A., Avila-Quezada G.D., Abd_Allah E.F., Gupta R.K. Salinity-induced physiochemical alterations to enhance lipid content in oleaginous microalgae Scenedesmus sp. BHU1 via two-stage cultivation for biodiesel feedstock. Microorganisms. 2023;11:2064. doi: 10.3390/microorganisms11082064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rathore D.S., Kumar A., Kumar H.D. Lipid content and fatty acid composition in N 2-fixing cyanobacterium Anabaena doliolum as affected by molybdenum deficiency. World J. Microbiol. Biotechnol. 1993;9:508–510. doi: 10.1007/BF00386284. [DOI] [PubMed] [Google Scholar]
- 51.Song X., Kong F., Liu B.F., Song Q., Ren N.Q., Ren H.Y. Lipidomics analysis of microalgal lipid production and heavy metal adsorption under glycine betaine-mediated alleviation of low-temperature stress. J. Hazard Mater. 2024 doi: 10.1016/j.jhazmat.2024.135831. [DOI] [PubMed] [Google Scholar]
- 52.Song X., Kong F., Liu B.F., Song Q., Ren N.Q., Ren H.Y. Antioxidants alleviated low-temperature stress in microalgae by modulating reactive oxygen species to improve lipid production and antioxidant defense. Bioresour. Technol. 2024 doi: 10.1016/j.biortech.2024.131451. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.