Abstract
Background
Microglial activation is an early phenomenon in Alzheimer’s disease (AD) that may occur prior to and independently of amyloid‐β (Aβ) aggregation. Compelling experimental evidence suggests that the apolipoprotein E ε4 (APOEε4) allele may be a culprit of early microglial activation in AD. However, it is unclear whether the APOEε4 genotype is associated with microglial reactivity in the living human brain. In individuals across the aging and AD spectrum, we tested the hypothesis that APOEε4 associates with microglial activation.
Method
We studied 118 individuals (79 cognitively unimpaired [CU], 23 with mild cognitive impairment [MCI], and 16 with AD dementia) from the Translational Biomarkers in Aging and Dementia (TRIAD) cohort. Individuals had available [18F]AZD4694 Aβ PET, [18F]MK6240 tau PET, [11C]PBR28 microglial activation PET, and magnetic resonance imaging (MRI), as well as APOE genotyping. To increase the reliability of our results, we only included high‐affinity binders for the [11C]PBR28 radiotracer. In a subgroup of 42 individuals with longitudinal clinical and MRI data, we further assessed longitudinal hippocampal atrophy and clinical deterioration.
Result
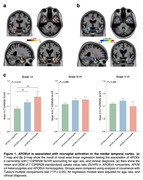
Voxel‐wise analysis revealed that APOEε4 carriership was associated with increased [11C]PBR28 uptake mainly in the medial temporal cortex (Figure 1A and B), and this effect of APOEε4 was independent of Aβ and tau accumulation. Region‐wise analyses demonstrated that APOEε4 carriers presented increased [11C]PBR28 SUVR relative to noncarriers only in Braak I‐II regions (Figure 1C), which further supports that APOEε4‐related microglial activation occurs specifically in medial temporal structures. Lastly, we found that [11C]PBR28 uptake in brain regions vulnerable to APOEε4 effects is associated with subsequent hippocampal atrophy and clinical decline over 2 years (Figure 2).
Conclusion
These results support a model in which APOEε4 plays a role in early AD progression by contributing to microglial activation in medial temporal regions. Our findings provide a rationale for the development of novel AD therapies targeting the interplay between ApoE and neuroinflammation.